Abstract
Mustn1 (Mustang, musculoskeletal temporally activated novel gene) was originally identified in fracture callus tissue, but its greatest expression is detected in skeletal muscle. Thus, we conducted experiments to investigate the expression and function of Mustn1 during myogenesis. Temporally, quantitative real-time PCR analysis of muscle samples from embryonic day 17 to 12 mo of age reveals that Mustn1 mRNA expression is greatest at 3 mo of age and beyond, consistent with the expression pattern of Myod. In situ hybridization shows abundant Mustn1 expression in somites and developing skeletal muscles, while in adult muscle, Mustn1 is localized to some peripherally located nuclei. Using RNA interference (RNAi), we investigated the function of Mustn1 in C2C12 myoblasts. Though silencing Mustn1 mRNA had no effect on myoblast proliferation, it did significantly impair myoblast differentiation, preventing myofusion. Specifically, when placed in low-serum medium for up to 6 days, Mustn1-silenced myoblasts elongated poorly and were mononucleated. In contrast, control RNAi-treated and parental myoblasts presented as large, multinucleated myotubes. Further supporting the morphological observations, immunocytochemistry of Mustn1-silenced cells demonstrated significant reductions in myogenin (Myog) and myosin heavy chain (Myhc) expression at 4 and 6 days of differentiation as compared with control and parental cells. The decreases in Myog and Myhc protein expression in Mustn1-silenced cells were associated with robust (∼3-fold or greater) decreases in the expression of Myod and desmin (Des), as well as the myofusion markers calpain 1 (Capn1), caveolin 3 (Cav3), and cadherin 15 (M-cadherin; Cadh15). Overall, we demonstrate that Mustn1 is an essential regulator of myogenic differentiation and myofusion, and our findings implicate Myod and Myog as its downstream targets.
Keywords: RNA interference
our laboratory has previously reported on the discovery and expression patterns of Mustn1 (Mustang, musculoskeletal temporally activated novel gene), encoding for a small 82 amino acid nuclear protein (16). Specifically, Mustn1 was identified through our transcriptional profiling experiments on bone regeneration (7) and its expression was subsequently shown to be temporally regulated during this process, with the highest levels observed at 5 days postfracture in osteoprogenitor cells of the periosteum, as well as osteoblasts and chondrocytes of the healing callus (16). In normal adult tissues, Mustn1 expression was predominantly detected in skeletal muscle and tendon, suggesting that it represents a musculoskeletal-specific gene. Characterization of the Mustn1 promoter uncovered four AP-1 domains, one of which is responsible for substantial transcriptional activation (15). Further characterization revealed that in both proliferating and differentiating C2C12 cells, the key AP-1 site required only c-Fos, Fra-2, or JunD for transcriptional activation, supporting the idea that these immediate early gene products are important for promoting Mustn1 expression. Lastly, we recently reported on Mustn1's functional contribution to chondrogenic proliferation and differentiation (5).
Given that Mustn1 expression is observed within progenitor cell populations during bone regeneration as well as in adult skeletal muscle and that its promoter contains functionally significant AP-1-binding motifs, we were interested in investigating the expression pattern and functional significance of Mustn1 during adult myogenesis, a process that requires muscle progenitor cell (myoblast) proliferation, differentiation, and fusion.
In vitro, removal of proliferative stimuli will cause myoblasts to abort their proliferative cycle and initiate the myogenic differentiation cascade. The mononucleated myoblasts will take on an elongated, spindle-shaped appearance, migrate, and align to one another. The subsequent process of myofusion involves dissociation of the cell membrane at the focal contact points and longitudinal fusion of the cytoplasm, resulting in the formation of multinucleated, fiberlike myotubes (2). These cellular processes recapitulate those occurring during skeletal myogenesis in vivo and are ultimately controlled by molecular events involving a group of transcription factors, the myogenic regulatory factors (MRFs) (19, 21). While the MRFs Myod and Myf5 are characterized as determination factors, defining satellite cells (the primary muscle stem cell population) to the myogenic lineage, the MRFs Myog and Mrf4 are characterized as late myogenic differentiation factors because their genomic inactivation results in a severe loss of differentiated skeletal muscle while having no effect on myoblast number (8, 18). Though the expression of Myog and Mrf4 specifies myoblast differentiation, completion of the differentiation process is indicated by expression of contractile proteins such as myosin heavy chain (Myhc) (19). The myofusion events are required for the generation of multinucleated myofibers, and their subsequent hypertrophy is characterized by the expression of genes such as calpain 1 (Capn1), caveolin 3 (Cav3), and cadherin 15 (Cadh15) (4, 17, 20).
Armed with this information, we investigated the developmental expression of Mustn1 in skeletal muscle and defined its functional contribution to myoblast proliferation, differentiation, and myofusion by testing the hypothesis that Mustn1 is expressed during skeletal muscle development and that its downregulation would affect myogenic differentiation. Results from these experiments demonstrate that Mustn1 is expressed during embryogenesis in the somites and developing muscles, but its expression peaks during the third month of life and beyond, consistent with the expression pattern of Myod. Silencing of endogenous Mustn1 using RNA interference (RNAi) had no effect on myoblast proliferation; however, profound effects on myoblast differentiation were observed, with myoblasts elongating poorly and failing to fuse, leading to robust downregulation of differentiation and fusion markers as well as a complete absence of multinucleated myotubes. Taken together, these findings indicate that Mustn1 is a novel protein critical for normal myogenic progenitor cell (myoblast) function because its repression leads to a complete loss of myofusion, ultimately resulting in an absence of multinucleated myotubes.
MATERIALS AND METHODS
Cell culture.
C2C12 cells and human embryonic kidney (HEK)-293 cells were obtained from American Type Culture Collection and maintained in proliferation medium consisting of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. All cells were incubated at 37°C and 5% CO2. To induce myogenic C2C12 differentiation, proliferation medium was replaced with differentiation medium (DMEM supplemented with 2% horse serum) on confluent myoblasts.
In situ hybridization.
All methods and animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Stony Brook University and met or exceeded all federal guidelines for the humane use of animals in research. In situ hybridization was conducted as previously reported by our laboratory (6, 16). Briefly, riboprobes were created by using T7 and Sp6 primers to amplify sense and antisense RNA probe sequences. These products were then purified and labeled using the DIG RNA labeling kit (Roche). The original PCR product was degraded using RNase free DNase 1 (Qiagen). This reaction was stopped using 0.2 M EDTA (pH 8.0). The final riboprobes were then purified using sephadex G-50 (Qiagen) quick spin columns. Embryos were dissected out on 10.5 and 11.5 days postcoitum (dpc) and fixed with 4% paraformaldehyde for 1 h at room temperature, permeabilized with proteinase K, bleached with H2O2, and stored at −20°C in 100% methanol. Embryos were then rehydrated through a methanol gradient and hybridized overnight at 65°C in a probe concentration of 250 ng/ml, followed by a wash with MABT, blocked in MABT + 20% goat serum + 2% Boehringer blocking reagent (BM 1096 176) and incubated overnight in 1:2,000 dilution anti-DIG 2° antibody. Finally, the embryos were again washed with MABT and expression was visualized with a solution of NTMT (40 mM Tris, 100 mM NaCl, 40 mM MgCl, 0.2% Tween 20)-BCIP (5-bromo-4-chloro-3-indolyl phosphate). Photomicrographs were taken under bright field using a Zeiss Discovery.V8 Stereo microscope with an AxioCam MRc digital camera.
Rat embryo sections were purchased (ready for use) from Novagen while adult mouse skeletal muscles were carefully dissected, flash frozen, and sectioned (8 μm). Before hybridization, all tissue sections were fixed in 4% paraformaldehyde. Protein digestion was then accomplished by incubation in 1 N HCl, followed by incubation with varying concentrations of proteinase K (1 to 100 μg/ml, Roche). The sections were then acetylated with 0.5% acetic anhydride in PBS (pH 8.0) for 10 min with continuous stirring. Before hybridization, riboprobes in hybridization buffer were heated at 80°C for 3 min, followed by quick cooling in ice water. The hybridization mixture contained a riboprobe (1.0 ng/μl), 50% deionized formamide, 10% dextran sulfate, 2× SSC, 0.02% SDS, and 0.01% salmon sperm DNA. The slides were incubated for 16 h at 60°C in a humid atmosphere. Following hybridization, the sections were washed and the same anti-DIG detection assay was used. Finally, the sections were rinsed with tap water, mounted, and viewed with a Zeiss microscope (Axiovert 200) and photographed using an AxioCam MRc digital camera.
RNA interference.
RNAi was performed to silence Mustn1 in C2C12 myoblasts using the retroviral delivery of short hairpin RNA (shRNA) into the host cells. Four Mustn1 shRNA constructs (19 or 20 bp in length and specific to the mouse Mustn1 coding region) were evaluated relative to a control RNAi (green fluorescent protein, GFP), and the one showing the most Mustn1 suppression was selected for further analysis. All experimental procedures were carried out following instructions from the Retroviral GeneSuppressor System provided by IMGENEX. Briefly, to prepare the RNAi constructs, four different oligonucleotide sequences as well as a control (GFP, 5′-CATACGGAAAACTTACCC-3′) were selected according to the guidelines stated in the kit's manual and cloned into the pSuppressorRetro retroviral vector. To produce the viruses, the plasmids were used individually with the pCL-Eco plasmid (kit provided) to cotransfect HEK-293 cells using the FuGENE 6 Transfection Kit (Roche) following the manufacturer's instruction. Viruses were collected from the culture media as instructed and purified using 0.45-μm filters.
The viruses carrying the Mustn1 shRNA were then used individually to infect proliferating C2C12 cells (∼30% confluent). Similarly, viruses carrying the control GFP-specific shRNA were also used to infect C2C12 cells (control cell line). All cells were cultured in the presence of 400 μg/ml G418 (Invitrogen), and single colonies were isolated from the infected cell lines and maintained separately to identify which one showed 1) the most dramatic Mustn1 suppression and 2) no interferon response, relative to the control cell line. In addition to the parental-uninfected and GFP control-infected cells, the Mustn1 RNAi (5′-TATTCAGCCGCAACCGCAC-3′) cell line showing the greatest degree of knockdown was used for all subsequent analyses.
Quantitative real-time polymerase chain reaction.
Total RNA samples were prepared with RNeasy Mini Kit (Qiagen) and treated with DNase I (Qiagen) to remove residual genomic DNA. RNA quality was determined by gel electrophoresis, and concentration was measured with a NanoDrop ND-1000 (NanoDrop). Quantitative real-time polymerase chain reaction (Q-PCR) was carried out with QuantiTect SYBR Green RT-PCR Kit (Qiagen) and the LightCycler system (Roche) following a standard protocol used previously in our laboratory (5, 6, 10, 11, 15, 28). Genes of interest and their primers are listed in Table 1. All data were normalized to a housekeeping gene (i.e., 18S rRNA or β-actin). Each experiment was performed in triplicate to determine standard deviation. Statistical significance was determined by one-way ANOVA with Tukey's post hoc test.
Table 1.
Primers and conditions used for quantitative real-time PCR
| Target Gene | Accession No. | Primer Sequence | Amplicon Size, bp | Tm, °C |
|---|---|---|---|---|
| 18S | AY248756 | Forward: CGCGGTTCTATTTTGTTGGT | 219 | 60 |
| Reverse: AGTCGGCATCGTTTATGGTC | ||||
| β-Actin | NM_007393 | Forward: AGACCTTCAACACCCCAG | 166 | 60 |
| Reverse: AGGTCCAGACGCAGGATG | ||||
| Capn1 | NM_007600 | Forward: GGTGAAGTGGAGTGGAAAGG | 226 | 60 |
| Reverse: TGCCCTCGTAAAATGTGGTA | ||||
| Cav3 | NM_007617 | Forward: ACGGTGTATGGAAGGTGAGC | 203 | 60 |
| Reverse: TGAGTAGATGTGGCTGATGC | ||||
| Des | NM_010043 | Forward: GCGGCTAAGAACATCTCTGA | 116 | 60 |
| Reverse: TCCATCATCTCCTGCTTGG | ||||
| Cadh15 | NM_007662 | Forward: CCCAACTAAGGGGCTCTCTC | 150 | 60 |
| Reverse: ATTCTCCCACCACTCCTGACT | ||||
| Mustn1 | NM_181390 | Forward: AAGAAGAAGCGGCCCCCT | 190 | 60 |
| Reverse: CTTTGGGCTTCTCAAAGAC | ||||
| Myh4 | NM_010855 | Forward: CAAGTCATCGGTGTTTGTGG | 175 | 60 |
| Reverse: GGCCATGTCCTCAATCTTGT | ||||
| Myod | NM_010866 | Forward: GCCTGAGCAAAGTGAATGAG | 184 | 60 |
| Reverse: GGTCCAGGTGCGTAGAAGG | ||||
| Myog | NM_031189 | Forward: GGAAGTCTGTGTCGGTGGAC | 150 | 60 |
| Reverse: CGCTGCGCAGGATCTCCAC |
Tm, temperature.
Cell proliferation.
Proliferation of the parental, GFP control, and Mustn1 RNAi cell lines was measured using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS) kit (Promega). Briefly, the cells were seeded in triplicate in 24-well plates at a density of 8,000 cells/well. At the specified times, cells were treated with MTS reagent for 30 min before dilution of the respective conditioned media 1:2 in double-distilled H2O and measurement of the absorption at 490 nm on SmartSpec 3000 (Bio-Rad). Measurements were taken every 24 h for 5 days starting at 2 h after plating. Statistical significance was determined by one-way ANOVA with Tukey's post hoc test.
Myotube quantification.
Photos of parental, GFP control, and Mustn1 RNAi cell lines were taken at 4 and 6 days following induction of myogenic differentiation through exposure to low-serum medium. Five individual phase-contrast views, one from the center and four corners (located at approximately the middle of the radius) of a 10-cm culture dish, were selected and photographed for each cell line. All elongated cells with a myotube-like morphology in each view were manually counted and averaged with the corresponding standard deviations calculated. Statistical significance was determined by one-way ANOVA with Tukey's post hoc test.
Immunocytochemistry.
The parental, GFP control, and Mustn1 RNAi cell lines were stained with antibodies against Myog and Myhc [MF20, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City (1)]. Initially, all cell lines were seeded on BD BioCoat Collagen I Coated Coverslips (BD Biosciences) and at 48 and 96 h after the beginning of myogenic differentiation were fixed with 4% paraformaldehyde for 15 min, washed with 1× PBS, then permeabilized with 0.1% Triton X-100 and washed again with 1× PBS. After blocking with 4% horse serum for 1 h, primary antibodies (1:1,000) were applied and incubated overnight at 4°C, then washed in 1× PBS and incubated with the corresponding Cy2-conjugated secondary antibody (1:200, Chemicon) for 1 h. Finally, all coverslips were rinsed with 1× PBS, mounted with permanent mounting media (VectaMount, Vector Laboratories), and observed and photographed using fluorescent microscopy with a Zeiss Axiovert 200 and an AxioCam MRc digital camera.
RESULTS
Mustn1 expression during skeletal muscle development.
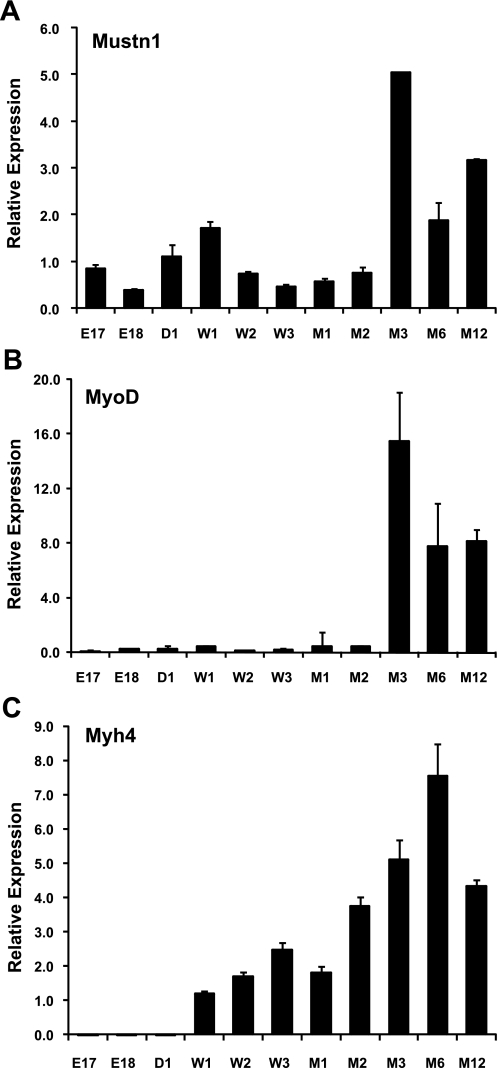
To investigate the temporal expression of Mustn1 during skeletal muscle development, we employed Q-PCR with RNA isolated from skeletal muscles of embryonic [E17 and E18 days dpc], newborn [day 1 (D1)], postnatal [weeks 1–3 (W1–3), months 1–2 (M1–2)], and adult [months 3–12 (M3–12)] mice. Results from these experiments show that Mustn1 expression is at relatively low levels during embryogenesis up to 2 mo of age but robustly increases at 3 mo of age (∼5-fold) and beyond (Fig. 1A). To relate the changes in Mustn1 expression with that of other myogenic related gene markers, we analyzed the temporal expression of Myod and myosin, heavy polypeptide 4 (Myh4; or MyHC-IIb). Myod displayed an almost identical profile as Mustn1, except with overall higher levels at M3 (∼15-fold) and beyond (Fig. 1B). In contrast, Myh4 displayed no detectable expression in the embryonic stages and at birth but then showed a steady increase beginning at W1 (∼1-fold) until M12 (∼4-fold) with a peak at M6 (∼7-fold) (Fig. 1C).
Fig. 1.
Temporal expression of Mustn1 during skeletal muscle development. Quantitative real-time PCR (Q-PCR) analysis of Mustn1 (A), Myod (B), and myosin, heavy polypeptide 4 (Myh4; C) was carried out using RNA isolated from quadriceps skeletal muscle from embryonic days 17 and 18 (E17 and E18), newborn day 1 (D1), postnatal weeks 1–3 (W1–W3), postnatal months 1 and 2 (M1–2), and adult months 3, 6, and 12 (M3, M6, and M12) mice. Error bars indicate SD derived from three independent PCR runs.
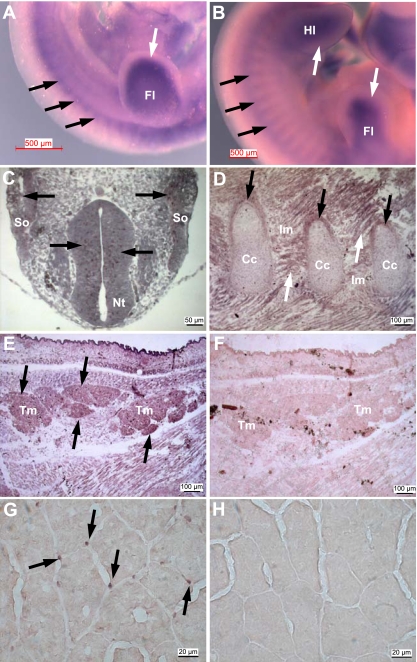
To localize the expression pattern of Mustn1 in developing and adult skeletal muscle, we undertook in situ hybridization analyses. Whole mount mouse embryos at both E10.5 and E11.5 dpc show robust Mustn1 expression in somites, as well as the fore- and hindlimb buds (Fig. 2, A and B, black and white arrows, respectively). Mustn1 expression was also detected as early as E10 dpc, specifically in neuroepithelium cells of the neural tube and somites (Fig. 2C) and later stages at E18 dpc in the perichondrium of developing ribs and intercostal muscles (Fig. 2D) in addition to the various dorsal skeletal muscles of the back including cross sections of the neck trapezius muscles (Fig. 2E). An adjacent control section hybridized with the sense probe shows no staining (Fig. 2F). Lastly, analyses of sections from healthy adult skeletal muscle (tibialis anterior) reveal Mustn1 expression localized to some peripherally located nuclei of skeletal muscle fibers (Fig. 2G) with no signal detected in the control sense hybridized section (Fig. 2H).
Fig. 2.
Mustn1 expression in developing and adult skeletal muscle. A and B: whole mount in situ hybridization of E10.5 and E11.5 mouse embryos, respectively, with the Mustn1 antisense probe. Black and white arrows indicate Mustn1 expression in somites and limb bud, respectively. Fl, forelimb; Hl, hindlimb. C–F: sections of an E10 (C) and E18 (D and E) day rat embryo hybridized to a Mustn1 antisense riboprobe. F: adjacent section to E but hybridized with the control sense riboprobe. Black arrows indicate Mustn1 expression in neuroepithelium and somites (So; C), perichondrium of developing ribs (D), and trapezius muscle (Tm; E). White arrows indicate Mustn1 expression in intercostal muscle (D). Nt, neural tube; Im, intercostal muscle; Cc, costal cartilage. G: adult skeletal muscle showing Mustn1 mRNA expression in nuclei at the periphery of adult myofibers, an area synonymous with the muscle satellite cell population (black arrows). H: the adjacent section to G was used as control and hybridized to the sense Mustn1 probe. Scale bar, 500 μm (A and B), 50 μm (C), 100 μm (D–F), and 20 μm (G and H).
Silencing of Mustn1 in skeletal muscle myoblasts prevents differentiation.
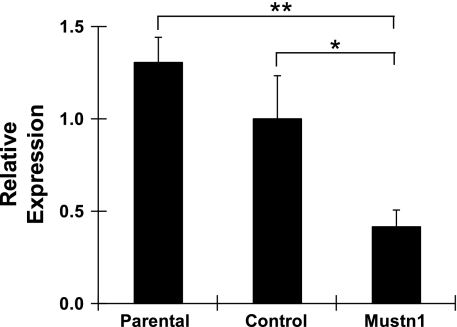
To functionally perturb Mustn1, we employed retroviral delivery of shRNA. Specifically, we used four shRNAs targeting selected regions of Mustn1 mRNA, as well as a control retrovirus (targeting GFP). On the basis of preliminary experiments with C2C12 cells, we selected one shRNA that showed maximal reduction of Mustn1 and no induction of interferon response gene expression (2′,5′-oligoadenylate synthetase 1 or OAS1, data not shown) for further experiments. Figure 3 shows that this shRNA was able to significantly reduce Mustn1 mRNA levels by ∼60% in comparison to those of control (P < 0.05) or parental cells (P < 0.01) at day 4 of differentiation. The control shRNA targeting GFP had no effect on Mustn1 expression, similar to that observed in parental cells (Fig. 3).
Fig. 3.
Validation of Mustn1 silencing. The expression level of Mustn1 mRNA from day 4 differentiated C2C12 cells was detected by Q-PCR. All values were normalized to that of the parental cell line. Mustn1 is expressed at normal level in the parental and control [green fluorescent protein (GFP)-RNA interference (RNAi) treated] cell lines, but in lower amounts in the Mustn1 (Mustn1-RNAi treated) cells. Error bars indicate SD (n = 3). *P < 0.05, **P < 0.01, determined by one-way ANOVA with Tukey's post hoc test.
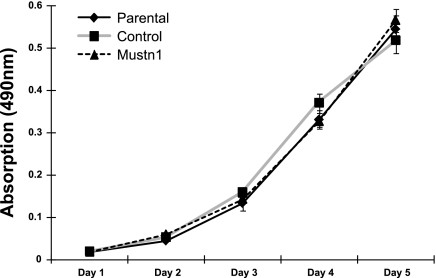
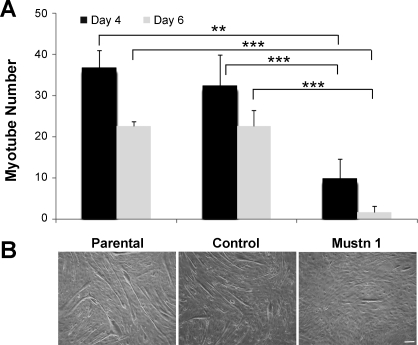
To investigate the effects of Mustn1 silencing on skeletal muscle myoblasts, we examined their capacity for proliferation and differentiation on the C2C12 myoblast cell line. Silencing of Mustn1 had no effect on the proliferative capacity of myoblasts compared with control-infected and parental cells up to 5 days in growth medium (Fig. 4). We did, however, observe robust inhibition of myogenic differentiation. Specifically, as a measure of differentiation, we examined the elongation of myoblasts to form myotube-like structures at 4 and 6 days following incubation in differentiation media. Results from these experiments reveal that the number of myotube-like structures in the Mustn1-silenced cells was significantly decreased by ∼73% and 93% in comparison to the parental cells and by ∼70% and 93% in comparison to the control-infected cells on day 4 and 6, respectively (Fig. 5A). There was no significant difference between control-infected and parental cells at either time point (Fig. 5A). Figure 5B depicts representative photos of the three cell lines at day 6 illustrating the presence of numerous, large myotube-like structures in both parental and control cells. In contrast, differentiation in Mustn1-silenced cells resulted in a very small number of elongated myotube-like structures which were much smaller in size than their control and parental counterparts (Fig. 5B).
Fig. 4.
Mustn1 silencing has no effect on C2C12 proliferation. Proliferation rate comparisons of the C2C12 parental, control, and Mustn1 RNAi cells using the MTS assay over a 5-day course. Error bars indicate SD derived from three independent measurements per time point. There was no statistical significance between any cell line at any time point as determined by one-way ANOVA with Tukey's post hoc test.
Fig. 5.
Mustn1 silencing suppresses myogenic differentiation and myotube formation. A: average number of myotubes between the parental, control, and Mustn1 RNAi cultures at 4 and 6 days following stimulation of differentiation. Error bars indicate SD (n = 5). **P < 0.01, ***P < 0.001, determined by one-way ANOVA with Tukey's post hoc test. B: photomicrographs showing the presence of myotubes in the three cell lines evaluated at day 6. Scale bar, 100 μm.
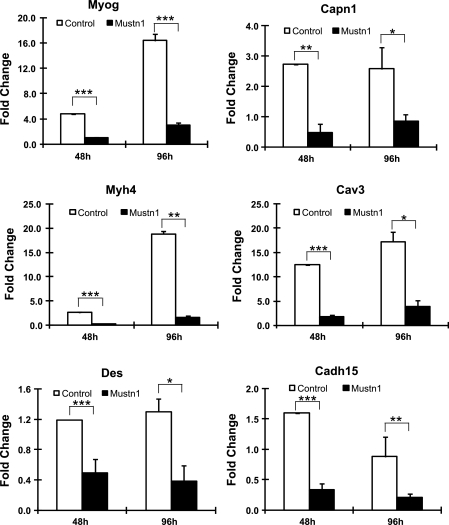
Since we observed such inhibitory effects on myogenic differentiation as a result of reduced Mustn1 levels, we decided to examine whether this reduction in myotube number and size is accompanied by changes in myogenic and myofusion marker gene expression. We initially employed Q-PCR to examine the temporal expression levels of a number of genes related to myogenic differentiation (Myog, Myh4, Des) and myofusion (Capn1, Cav3, Cadh15) (9). Figure 6 shows the measured expression levels of these genes at 48 and 96 h following addition of differentiation media between the control and Mustn1-silenced cells. All genes displayed significant decreases in mRNA expression levels in the Mustn1-silenced cells as compared with those in control, at both 48 and 96 h (Fig. 6). The most dramatic decrease in expression within the myogenic differentiation genes was observed for Myh4 with an ∼9 (P < 0.001) and 11.5-fold (P = 0.002) decrease in comparison to control at 48 and 96 h, respectively. Similarly, for the myofusion-related genes, the most dramatic decrease occurred with Cav3 with an ∼6.5 (P < 0.001) and 4.4-fold (P = 0.021) decrease seen at 48 and 96 h, respectively (Fig. 6).
Fig. 6.
Mustn1 silencing leads to downregulation of myogenic related gene expression. RNA from control (GFP) and Mustn1 RNAi-treated cells was isolated at 48 and 96 h after plating and subjected to Q-PCR. Genes tested represented myogenic differentiation markers [myogenin (Myog) desmin (Des), and Myh4] and myofusion-related genes [calpain 1 (Capn1), caveolin 3 (Cav3), and cadherin 14 (Cadh15); (9)]. Error bars indicate SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, determined by one-way ANOVA with Tukey's post hoc test.
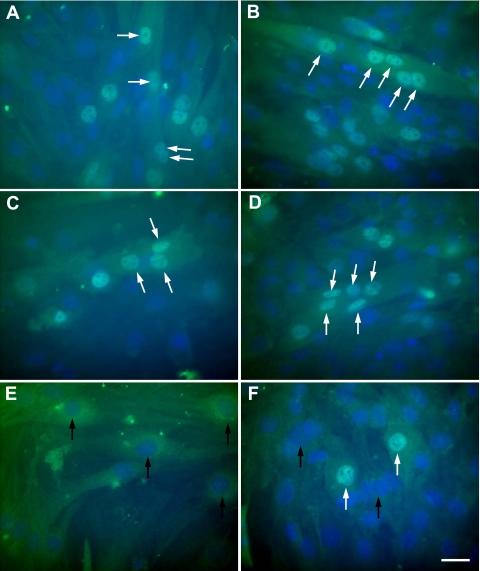
To further validate that the expression of myogenic differentiation markers was reduced in Mustn1-silenced cells, we undertook immunocytochemical analyses for Myog and Myhc. As seen in Fig. 7, silencing of Mustn1caused a considerable reduction in the protein expression of Myog and complete abolishment of myofusion. More specifically, the data show that both the parental and control-infected cells differentiated normally, with Myog expression observed in the nuclei beginning at 48 h (Fig. 7, A and C). Further differentiation (at 96 h) resulted in large, multinucleated myotubes with strong Myog expression (Fig. 7, B and D). In contrast, no Myog expression was detected in the Mustn1-silenced cells at 48 h and by 96 h some cells were Myog positive, but at much lower numbers (Fig. 7, E and F). Furthermore, the Myog-positive cells were not elongated and contained only a single nucleus (Fig. 7, E and F).
Fig. 7.
Mustn1 silencing decreases Myog expression during myogenic differentiation. The parental (A and B), control (C and D), and Mustn1 (E and F) RNAi-treated C2C12 cells were seeded on coverglasses and stained at day 2 (A, C, and E) and day 4 (B, D, and F) with an anti-myogenin antibody. White arrows indicate Myog-positive nuclei within individual myotubes. Black arrows denote the absence of Myog expression. Scale bar, 10 μm.
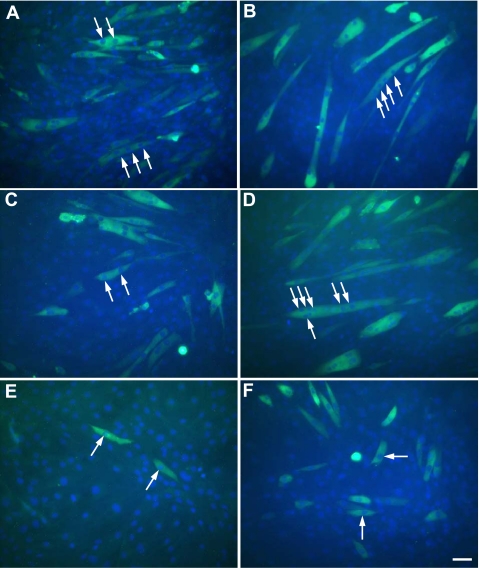
Myhc immunostaining further verified the Myog results and confirmed what we had observed with light microscopy, that the number and size of myotubes were indeed smaller in the Mustn1-silenced cells as compared with those in the parental and control-infected cells (Fig. 8). Both parental and control cells showed normal myogenic differentiation by expressing Myhc at 48 h (Fig. 8, A and C), and more elongated, multinucleated myotubes were observed at 96 h (Fig. 8, B and D). In contrast, the Mustn1-silenced cells displayed much lower Myhc expression and failed to initiate myofusion at both 48 and 96 h; failure to fuse was verified by the absence of multiple nuclei in these cells (Fig. 8, E and F).
Fig. 8.
Mustn1 silencing decreases myosin heavy chain (Myhc) expression during myogenic differentiation. The parental (A and B), control (C and D), and Mustn1 (E and F) RNAi-treated C2C12 cells were seeded on coverglasses and stained at day 2 (A, C, and E) and 4 (B, D, and F) with an anti-Myhc antibody. White arrows indicate multiple nuclei within individual myotubes. Scale bar, 50 μm.
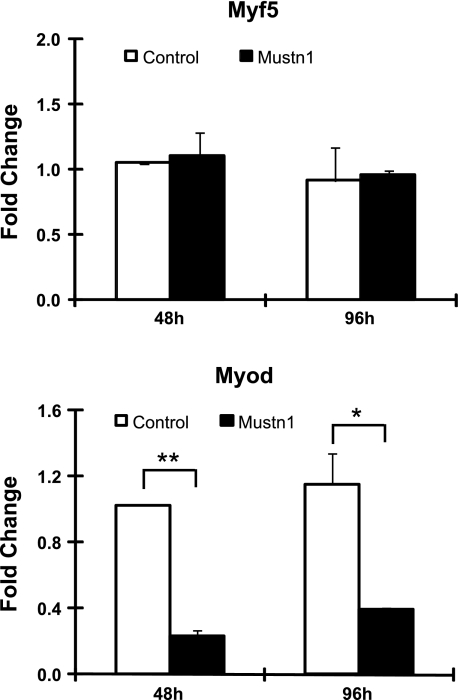
Given that Mustn1expression was reduced at both mRNA and protein levels, we investigated the mRNA expression pattern of the myogenic regulatory factors upstream of Myog, that is, Myf5, Pax3, and Myod. As can be seen in Fig. 9, there was a threefold reduction in Myod expression at both 48 and 96 h of differentiation in Mustn1-silenced cells compared with control and parental cells. This is in contrast to Myf5, whose expression profile was not different between the two cell lines at either time point (Fig. 9). Although attempts were made to investigate the expression of Pax3, we could not detect any endogenous Pax3 mRNA in samples used in the aforementioned analyses (data not shown). In addition, we also examined Pax3 expression in normal proliferating and differentiating C2C12 cells and again we did not detect endogenous mRNA expression (data not shown), consistent with previous reports (3, 13).
Fig. 9.
Mustn1 silencing leads to downregulation of Myod gene expression. RNA from control (GFP) and Mustn1 RNAi-treated cells was isolated at 48 and 96 h after plating and subjected to Q-PCR. Aside from Myod, Myf5 was also tested but did not reveal any differences in mRNA expression between the two different cell lines. Error bars indicate SD (n = 3). *P < 0.05, **P < 0.01, determined by one-way ANOVA with Tukey's post hoc test.
DISCUSSION
While our previous research had identified Mustn1 as a musculoskeletal-specific gene involved in bone development and regeneration (16) as well as chondrogenesis (5), the results described herein extend our knowledge of Mustn1 to now include its expression during skeletal muscle development and, more importantly, its critical role in the myogenic progenitor cell (myoblast) function. Given that skeletal muscle growth and hypertrophy require myoblast proliferation, differentiation, and fusion to existing fibers (26), our findings clearly support the importance of Mustn1 in myoblast differentiation, such that in its absence, myoblast differentiation is abrogated and myofusion fails to occur completely.
In the present study, we demonstrated that the absence of Mustn1 abrogated myogenic fusion and differentiation, thereby halting myogenesis in vitro. Mustn1 has also been identified by others as a contributor to muscle growth and development. Zheng et al. (27) in their work examining the transcriptional changes underlying divergent skeletal muscle growth rates of broiler and layer chickens identified Mustn1 as a gene important for skeletal muscle growth. A second study investigating the effects of an acute bout of resistance training on gene expression found that Mustn1 expression upregulated with eccentric contractions, but not concentric contractions, suggesting that Mustn1 may be involved in repair of eccentric-exercise-induced muscle damage (12). A third study that explored the callipyge mutation in sheep (results in postnatal skeletal muscle hypertrophy in the pelvic limbs and loins) identified Mustn1 as a differentially expressed gene displaying upregulated expression during hypertrophy in the sheep with the callipyge mutation (24). These findings are consistent with the present work demonstrating that Mustn1 plays a critical role in myoblast differentiation, an action that is essential for skeletal muscle growth, hypertrophy, and regeneration. In the present study, Mustn1 RNAi was expressed in a myoblast cell line, rather than primary myoblasts, though we hypothesize that these findings would be consistent with similar experiments conducted with primary myoblasts. Further studies are needed to confirm this hypothesis.
The involvement of Mustn1 in hypertrophy is further supported by our previous in vitro results correlating Mustn1 expression with myogenic differentiation, myofusion, and myotube formation in C2C12 cells (15). Thus, to extend these diverse expression data and to be able to probe the role that Mustn1 plays in myogenesis, we sought to functionally perturb Mustn1 via silencing its expression in C2C12 myoblasts. The novel aspect to this work is the finding that a 60% decrease in Mustn1 mRNA expression led to a dramatic reduction in the number, elongation, and nucleation of myotubes. In fact, even in myotubes that were present, there were none that were elongated with multiple nuclei, indicating that the process of myofusion had been completely abolished. This observation was verified by examining the expression of genes related to the process of myofusion such as Capn1 (17), Cav3 (20), and Cadh15 (4), whose expression was severely reduced in the Mustn1-silenced cells. Given the dramatic decrease and delay in Des, Myod, and Myog expression, the robust alterations in genes associated with myofusion were not unexpected. Whether Mustn1 is affecting differentiation and myofusion independently, or the absence of myofusion is the result of significant reductions in the myogenic factors, has yet to be fully elucidated, though we would speculate it is the result of the latter given that Des, Myod, and Myog have all been demonstrated to be necessary for normal myogenic differentiation (14, 22) and a decrease in their expression would lead to impaired differentiation and subsequent myofusion.
It is worth noting that even though there were still low levels of both Myod and Myog mRNA present in the Mustn1-silenced cells at 48 and 96 h in differentiation medium, it was insufficient to rescue differentiation. The impairment in latter stages of differentiation was illustrated using both Q-PCR and immunocytochemistry, which revealed significant reductions in Myhc expression. Though some Mustn1-silenced cells expressed Myhc, no multinucleated myotubes were detected at either 2 days or 4 days of differentiation. Thus, these data suggest that Mustn1 affects the initial stages of myogenesis and it is reasonable to consider that myoblasts need to maintain Mustn1 above a critical level to allow the initiation of myogenic differentiation.
While the importance of Mustn1 in myogenic differentiation is clear from the present results, how Mustn1 is regulating this critical process has yet to be fully elucidated. Mustn1 is a nuclear protein and though its amino acid sequence does not indicate any known functional motifs (16), it is unclear if and how it may be directly affecting the expression of transcription factors like Myod and Myog, both of whose expression was severely reduced in the Mustn1-silenced cells. Clearly, studies aimed at elucidating the molecular mechanism(s) by which Mustn1 is regulating muscle differentiation are an important consideration and may lead to the identification of another critical regulatory protein essential for myogenic progenitor cell function. Furthermore, uncovering the mechanisms of action of Mustn1 may help to shed light on the complex pathways by which MRFs direct skeletal muscle growth and hypertrophy (21, 25).
GRANTS
This project was funded by National Institutes of Health Grant R21NS051222 (to M. Hadjiargyrou).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Frank Lombardo for help with in situ hybridization, Dr. David Komatsu for critically reading the manuscript, and Nubia Andrade for administrative help.
REFERENCES
- 1.Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol 95: 763–770, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science 230: 758–766, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, Morgan JE, Zammit PS. Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One 4: e4475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donalies M, Cramer M, Ringwald M, Starzinski-Powitz A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc Natl Acad Sci USA 88: 8024–8028, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gersch RP, Hadjiargyrou M. Mustn1 is expressed during chondrogenesis and is necessary for chondrocyte proliferation and differentiation in vitro. Bone 45: 330–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gersch RP, Lombardo F, McGovern SC, Hadjiargyrou M. Reactivation of Hox gene expression during bone regeneration. J Orthop Res 23: 882–890, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem 277: 30177–30182, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364: 501–506, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs 176: 67–78, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Komatsu DE, Bosch-Marce M, Semenza GL, Hadjiargyrou M. Enhanced bone regeneration associated with decreased apoptosis in mice with partial HIF-1alpha deficiency. J Bone Miner Res 22: 366–574, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 34: 680–688, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kostek MC, Chen YW, Cuthbertson DJ, Shi R, Fedele MJ, Esser KA, Rennie MJ. Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol Genomics 31: 42–52, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Shadrach JL, Wagers AJ, Lassar AB. Id3 is a direct transcriptional target of Pax7 in quiescent satellite cells. Mol Biol Cell 20: 3170–3177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators Myod and myogenin. J Cell Biol 124: 827–841, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Hadjiargyrou M. Identification and characterization of the Mustang promoter: regulation by AP-1 during myogenic differentiation. Bone 39: 815–824, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Lombardo F, Komatsu D, Hadjiargyrou M. Molecular cloning and characterization of Mustang, a novel nuclear protein expressed during skeletal development and regeneration. FASEB J 18: 52–61, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Moyen C, Goudenege S, Poussard S, Sassi AH, Brustis JJ, Cottin P. Involvement of micro-calpain (CAPN 1) in muscle cell differentiation. Int J Biochem Cell Biol 36: 728–743, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364: 532–535, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Parker MH, Seale P, Rudnicki MA. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat Rev Genet 4: 497–507, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 136: 137–154, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet 57: 16–25, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Singh J, Verma NK, Kansagra SM, Kate BN, Dey CS. Altered PPARgamma expression inhibits myogenic differentiation in C2C12 skeletal muscle cells. Mol Cell Biochem 294: 163–171, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Vuocolo T, Byrne K, White J, McWilliam S, Reverter A, Cockett NE, Tellam RL. Identification of a gene network contributing to hypertrophy in callipyge skeletal muscle. Physiol Genomics 28: 253–272, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Wei Q, Paterson BM. Regulation of Myod function in the dividing myoblast. FEBS Lett 490: 171–178, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54: 1177–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Zheng Q, Zhang Y, Chen Y, Yang N, Wang XJ, Zhu D. Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genomics 10: 87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone 39: 5–16, 2006 [DOI] [PubMed] [Google Scholar]