Abstract
Extracellular matrix (ECM) remodeling occurs during normal homeostasis and also plays an important role during development, tissue repair, and in various disease processes. ECM remodeling involves changes in the synthesis, deposition, and degradation of ECM molecules. ECM molecules can be degraded extracellularly, as well as intracellularly following endocytosis. Our data show that the ECM protein fibronectin is an important regulator of ECM remodeling. We previously showed that agents that inhibit the polymerization of fibronectin into ECM fibrils promote the loss of preexisting fibronectin matrix and accelerate fibronectin endocytosis and degradation. In this paper we show that inhibition of fibronectin polymerization leads to the loss of collagen I matrix fibrils and a corresponding increase in the levels of endocytosed collagen I. In contrast, manipulations that stabilize fibronectin matrix fibrils, such as caveolin-1 depletion, stabilize collagen I matrix fibrils and cause a decrease in ECM collagen I endocytosis. Our data also show that endocytosis of ECM collagen I is regulated by both β1 integrins and Endo180/urokinase plasminogen activator associated protein (uPARAP). Unexpectedly, Endo180/uPARAP was also shown to promote the endocytosis of fibronectin from the ECM. These data demonstrate that fibronectin polymerization regulates the remodeling of ECM collagen I, in part, by regulating collagen I endocytosis. Furthermore, these data show that processes that regulate ECM deposition coordinately regulate the removal of proteins from the ECM. These data highlight the complexity of ECM remodeling. This multifaceted regulatory process may be important to ensure tight regulation of ECM fibronectin and collagen I levels.
Keywords: extracellular matrix, integrin, endocytosis, Endo180, urokinase plasminogen activator-associated protein
extracellular matrix (ECM) remodeling is a critical process that occurs during development and tissue repair. ECM remodeling also occurs in a variety of pathological conditions, such as hypertension, restenosis following angioplasty, heart failure, fibrosis, and cancer (1, 4, 39, 68). ECM synthesis, deposition, and degradation are all components of ECM remodeling. The balance between these processes determines whether net accumulation or loss of ECM occurs. Different ECM proteins, and combinations of proteins, can have distinct effects on the phenotype of cells, affecting such important processes as cell survival, growth, differentiation, and migration (2, 42, 55, 59, 64). Additionally, ECM fragments produced by proteolysis can accumulate in vivo and contribute to changes in cell behavior (3, 24, 44, 63). ECM fragments can have properties distinct from the intact parental proteins (8, 14, 41). Hence, mechanisms that limit the accumulation of ECM fragments are likely to be important for regulating a variety of cell processes.
A variety of extracellular proteases regulate ECM degradation. Prominent among these proteases are matrix metalloproteinases (MMPs). MMPs are important regulators of cell migration and growth (1, 16, 25). MMPs are also known to release bioactive matrix fragments following cleavage of certain ECM proteins (14, 17, 20). Degradation of ECM proteins can also occur intracellularly in the lysosomes following endocytosis. A number of ECM molecules are known to be endocytosed and degraded in the lysosomes, including collagen I, fibronectin, vitronectin, thrombospondin, and proteoglycans (11, 12, 18, 34, 43, 52). Endocytosis may be an important mechanism that limits the accumulation of extracellular ECM fragments. Several receptors have been shown to regulate endocytosis of ECM molecules, including integrins, proteoglycans, and Endo180/urokinase plasminogen activator associated protein (uPARAP) (11, 12, 31, 38, 43).
Collagen I is the major ECM protein in the body. Dynamic regulation of collagen I production and degradation is a key event in tissue repair. Degradation of collagen I by MT1-MMP has been shown to be critical for cell migration in three-dimensional matrices (25). Interestingly, newly synthesized collagen has also been shown to be necessary for smooth muscle cell (SMC) and epithelial cell migration (47, 56). In addition, fibronectin-directed collagen I deposition has also been shown to be important for myofibroblast migration (55).
We and others have previously shown that the deposition (polymerization) of fibronectin into the ECM regulates the deposition of type I collagen (53, 55, 61). In the absence of fibronectin and fibronectin polymerization, collagen I fibrils are not deposited into the ECM, despite the presence of soluble collagen I (55, 61). Our previously published data also show that agents that inhibit the deposition of fibronectin into the ECM result in turnover of fibronectin and collagen I matrix fibrils (53). Fibronectin that is lost from the ECM is endocytosed in a β1 integrin and caveolin-1-dependent process and degraded in the lysosomes (51, 52). Recent studies have shown that the mannose receptor family member Endo180/uPARAP can bind to soluble collagen I and mediate its endocytosis (12, 62). Certain β1 integrins can also bind to collagen and have been implicated in phagocytosis of collagen-coated beads (32, 50). Most studies that have examined the endocytosis of collagen I have assayed the uptake of either soluble collagen or collagen-coated beads. The ability of integrins and Endo180/uPARAP to endocytose cell-derived polymerized collagen matrices has not been explored. In addition, the role of fibronectin polymerization in regulating the endocytosis of matrix collagen I is unknown. In this paper, we examined the role of fibronectin polymerization and the involvement of β1 integrins and Endo180/uPARAP in regulating the endocytosis of matrix collagen I. These studies add important new information concerning the role of fibronectin polymerization in regulating ECM remodeling and the multiple receptor systems that are involved in regulating collagen I uptake.
MATERIALS AND METHODS
Immunological Reagents and Chemicals
Antibodies to β1 (Ha2/5), β3 (2C9.G2), and αv integrins (H9.2B8) and ERK1/2 were from BD Biosciences (San Diego, CA). Antibodies to Endo180 were a generous gift from Dr. Niels Behrendt (The Finsen Laboratory, Copenhagen, Denmark). Texas red (TR) and Alexa Fluor 488 (AF488) conjugation kits and CellTracker green CMFDA were from Invitrogen (Carlsbad, CA). GM6001 and the control compound were from Calbiochem (EMD Chemicals, Gibbstown, NJ). Collagenase II was from Worthington (Lakewood, NJ).
Proteins
Human fibronectin was purified from Cohn%s fractions 1 and 2 (a generous gift from Dr. Ken Ingham, American Red Cross, Bethesda, MD) as previously described (37). Rat fibronectin was purified from rat serum on columns of gelatin-Sepharose (Pharmacia, Piscataway, NJ). pUR4 (also known as FUD, functional upstream domain) and the control III-11C peptides were purified from bacterial lysates on nickel-agarose columns as previously described (58). Recombinant vitronectin was produced in bacteria as previously described (67). Rat collagen type I (collagen I) was purchased from UBI (Lake Placid, NY) or BD Biosystems. AF488 and TR-conjugated fibronectin were made according to the manufacture%s protocol (Molecular Probes/Invitrogen). For labeling of collagen I with TR, collagen was first diluted to 1 mg/ml in 0.01 N acetic acid, dialyzed into 0.1 M phosphate buffer (pH 7.6), and then dialyzed into 0.05 M carbonate buffer (pH 9) containing 0.4 M NaCl. TR was added to the collagen in carbonate buffer and incubated at room temperature for 30 min. Unreacted dye was removed by exhaustive dialysis. Labeled collagens were spun at 100,000 g at 4°C for 1 h to remove insoluble aggregates. The supernatant was stored in 0.01 N acetic acid at 4°C.
Cell Culture
We previously described the isolation of fibronectin-null (FN) cells from fibronectin-null embryos (54). These cells were adapted to grow in defined media to establish a model system in which all cell- and serum-derived fibronectin was eliminated (54). We characterized these cells as myofibroblasts (FN-null MF) based on their expression of some SMC marker proteins (SM calponin and SM α-actin) but not others (SM22 and desmin) and on their ability to contract collagen gels (22, 55). Stable FN-null MF cell lines expressing caveolin-1 small interfering RNA (siRNA) (shcav) and control cells expressing siRNA to luciferase (shluc) were previously described (52). Rat aortic SMCs were obtained from Cell Applications (San Diego, CA) and maintained in serum-containing media (Cell Applications). Endo180 null and littermate control cells were generous gifts from Dr. Bugge (NIH, Bethesda, MD) (12). Endo180 null and control cells were spontaneously immortalized by using procedures similar to those used to produce 3T3 cells (57). For some experiments, Endo180 null and control cells were used before immortalization. GD25 β1 integrin null cells and GD25 cells that reexpress human β1 integrin were gifts from Dr. Reinhardt Fassler (Max-Planck-Institute for Biochemistry, Martinsried, Germany) (65), and Dr. Susan LaFlamme (Albany Medical College, Albany, NY) (46) respectively.
Pulse-Chase Assays
Long-term pulse-chase assays.
FN-null MFs were incubated (“pulsed”) overnight with 10 μg/ml fibronectin and 5–10 μg/ml labeled collagen. Cells were washed and then incubated (“chased”) with culture medium lacking labeled fibronectin or collagen at 37°C for various lengths of time.
For some experiments, cells were incubated with the fibronectin polymerization inhibitor pUR4 during the chase to promote ECM turnover.
Short-term endocytosis assays.
GD25 and GD25 β1 reexpressing cells were incubated with 5 μg/ml fluorescently labeled collagen I for 2 h at 37°C. Cells were washed, fixed, and then processed for immunofluorescence.
Preparation of Fibronectin and Collagen I Matrices
Preassembled fibronectin and collagen matrices were prepared using a modification of our previously described procedure (51). Briefly, FN-null MFs were incubated overnight with 10 μg/ml AF488-fibronectin and TR-collagen I to allow assembly of a robust fibronectin and collagen containing matrix. Cells were incubated with lysis buffer (20 mN Na2HPO4, pH 9.6, 1% Nonidet P-40) at room temperature for 10 min. Dishes were gently washed three times with phosphate-buffered saline (PBS). Fibronectin and collagen matrix were largely preserved after extraction (supplemental Fig. S.1). Cells were seeded onto preassembled matrix and incubated for 24–48 h at 37°C. In experiments with fibronectin-producing cells, cells were incubated with the fibronectin polymerization inhibitor pUR4 to promote ECM turnover. For integrin function blocking assays, cells were preincubated with integrin inhibitory antibodies at room temperature for 15–30 min before being seeded onto preassembled matrix.
For studies with in vitro polymerized type I collagen, type I collagen that was stored in 0.01 N acetic acid was neutralized with 0.1 N NaOH then mixed with 2× DMEM on ice. The final mixture contained 2 mg/ml collagen, 20 μg/ml TR-collagen, and 1× DMEM. The mixture was directly applied on top of cells and then incubated at 37°C for 1 h to permit polymerization of the collagen. Culture medium was then added to the wells, and the cells were cultured for 48 h. The collagen gel was gently removed from the cell layers, and the cells were then treated with 0.02% trypan blue for 5 min before being processed for imaging studies.
Imaging Assays
After fixation, cells were examined by using an Olympus microscope equipped with epifluorescence or with an Olympus scanning confocal microscope. Confocal images were used to assess protein colocalization. To quantitate the levels of endocytosed collagen in long-term pulse-chase assays, cells were treated with 0.02% trypan blue for 3 min at room temperature to quench extracellular fluorescence (19, 60) before fixation. To quantitate the levels of endocytosed collagen from preassembled matrix, extracellular fluorescently labeled collagen was removed by treating cells with 3 mg/ml collagenase II in digestion buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM CaCl2, and 1 μM ZnCl2) for 10 min at 37°C before fixation. Representative confocal images and corresponding differential interference contrast (DIC) images of cells were collected. A MATLab (Mathworks, Natick, MA)-based program was used to trace cell outlines and to quantitate fluorescence intensity. Background fluorescence was determined and subtracted from each image. The fluorescence intensity within individual cells was normalized by cell area. Sample sizes were ≥130 cells for each condition. For confluent cells, the fluorescence intensity was measured and reported per field of view; 4–10 fields of view were examined per condition. Each experiment was normalized separately. The mean value of two independent experiments (± range) is reported.
Reverse Transcription Polymerase Chain Reaction
mRNA was isolated from cells using TRIzol as per the manufacturer%s instructions (Invitrogen). cDNA was prepared using a Superscript First Strand Synthesis kit as per the manufacturer%s instructions (Invitrogen). Reverse transcription polymerase chain reaction (RT-PCR) primers for amplification of Endo180 were based on the mouse Endo180 sequence: CTGGTCACCATCACCAACAG (forward) and TGACTTCATCCCCACTGAGC (reverse).
Electron Microscopy
FN-null MF were cultured in the presence and absence of fibronectin and in the presence of the R1R2 peptide that inhibits collagen I matrix deposition, or the control peptide, III-11C (55). After 24 h, cells were processed for electron microscopy (EM) by the EM Research Core at the University of Rochester. Cells were fixed overnight at 4°C in a 0.1 M sodium cacodylate-buffered fixative containing 4.0% paraformaldehyde and 2.0% glutaraldehyde. Cells were postfixed in 1.0% osmium tetroxide, dehydrated through a graded series of ethanol to 100%, and infiltrated overnight in liquid Spurr epoxy resin. Size 3 BEEM capsules were filled with fresh resin, inverted, and placed over the cells and polymerized at 70°C overnight. Samples were thin sectioned at 70 nm and mounted onto uncoated 200-mesh copper grids then stained with aqueous uranyl acetate and lead citrate. An Hitachi 7650 transmission electron microscope with an attached Gatan 11 megapixel Erlangshen digital camera was used to digitally capture images. The extracellular fibrils that are detected by EM in cells incubated with the R1R2 peptide likely contain fibronectin and type III collagen, as the R1R2 peptide does not block fibronectin or type III collagen fibril formation (55). In addition, fibronectin fibril formation can occur in the absence of type I collagen fibril formation (55).
Western Blotting
Cells lysates were prepared as described (53). Equal amounts of proteins were analyzed under reducing conditions by SDS PAGE, transferred to PVDF membrane, and probed with antibodies that recognize Endo180. To ensure equal protein loading, the blots were also probed with antibodies that recognize Erk1/2. Blots were imaged and analyzed using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
RESULTS
Endocytosis of ECM Collagen I is Regulated by Fibronectin Polymerization
We previously showed that fibronectin and collagen I matrix fibrils are lost under conditions where fibronectin matrix polymerization is inhibited (53). We also showed that the major fate of ECM fibronectin that is lost from the matrix is endocytosis and lysosomal degradation (51, 52). In this study we used long-term pulse-chase studies and cell-derived matrix assays to study the endocytosis of ECM collagen. For pulse-chase studies, FN-null MF were incubated (pulsed) overnight with fluorescently labeled fibronectin and collagen I to allow them to assemble extensive ECM fibrils and then chased in the absence of exogenous fibronectin and collagen to trigger the turnover of ECM fibrils. Our published studies show that the great majority of the fibronectin that is present in the cell layers at the end of the pulse is ECM fibronectin (53). In the present study we also used assays in which cells were seeded onto preassembled cell-derived fibronectin and collagen-containing matrices or in which cells were incubated with in vitro polymerized collagen I fibrils. It should be emphasized that most other studies that have examined the ability of cultured cells to endocytose collagen I have examined the fate of soluble collagen I or collagen-coated beads. The assays that we have used in this study examine the fate of collagen I that has been polymerized into the ECM by cells or polymerized into collagen fibrils in vitro. Hence, these assays are ideal for studying the fate of ECM collagen I.
To determine whether endocytosed collagen I can be detected in FN-null MF, pulse-chase experiments were performed with TR-labeled collagen I and Alexa Fluor (AF)-488 labeled fibronectin. After the pulse, cells produce a robust fibronectin and collagen I matrix as shown by immunofluorescence microscopy (Fig. 1, A–C). When examined by transmission EM, extracellular fibrillar structures can be readily detected in cells cultured in the presence of fibronectin but not in cells cultured in the absence of fibronectin (Supplemental Fig. S.2). Furthermore, production of type I collagen and fibronectin fibrils required the presence of cells. Addition of fluorescently labeled fibronectin and collagen I to dishes in the absence of cells resulted in diffuse fluorescence signals but no detectable fibrils (Supplemental Fig. S.3).
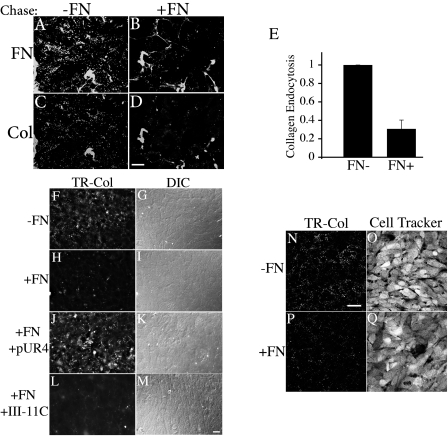
Fig. 1.
Endocytosis of collagen I and fibronectin (FN) from the extracellular matrix (ECM). A–F: FN-null myofibroblasts (MFs) were incubated with Texas red (TR)-collagen I and AF488-FN overnight. Cells were then either fixed immediately (A–C) or washed and then incubated for 24 h in media lacking fibronectin and collagen I (D–F). Collagen I (A) and fibronectin (B) fibrils elaborated by cells after the overnight pulse were visualized with an Olympus fluorescence microscope. The same fields of view are shown in A and B. The corresponding differential interference contrast (DIC) picture is shown in C. Endocytosed TR-collagen I (D) and AF488-FN (E) were visualized after 24 h of chase. F is an overlay of D and E. Bar, 20 μm. G–I and K–M: FN-null MFs (G–I) or SMC (K–M) were seeded onto preassembled matrix containing TR-collagen I and AF488-FN and cultured for 24 h at 37°C. Endocytosed TR-collagen I (G, K) and AF488-FN (H, L) are shown. I, M are overlay images; the yellow staining indicates colocalized collagen and fibronectin. Scale bar, G–I, 20 μm; K–M, 10 μm. For D–I and K–M, a large portion of the extracellular FN and collagen fibrils were lost during the chase. Confocal images were taken in a plane of focus that would best show the intracellular vesicles. J: in vitro polymerized collagen gels (containing TR-collagen) were formed on top of the cell layer as described in materials and methods. After 48 h, the collagen gel was gently removed. The cells were then treated with 0.02% trypan blue for 5 min before fixation to quench extracellular fluorescence. Endocytosed TR-collagen is shown. Scale bar, 10 μm. D–M are confocal images.
After the pulse, cells were incubated for 24 h in the absence of exogenously added soluble fibronectin and collagen to promote the turnover of ECM fibronectin and collagen. We found extensive intracellular collagen I (Fig. 1D) and fibronectin (Fig. 1E)-containing vesicles; many vesicles contained both collagen and fibronectin (Fig. 1F). To verify that the collagen I and fibronectin were endocytosed from the matrix, FN-null MF were seeded onto preassembled cell-derived matrices containing TR-collagen and AF488-FN and incubated for 24 h. Intracellular vesicles containing TR-collagen I (Fig. 1G) and AF488-FN (Fig. 1H) were readily detected. There was extensive colocalization of intracellular collagen I and fibronectin (Fig. 1I). To further demonstrate the ability of cells to endocytose type I collagen fibrils, we incubated FN-null MF in the presence of in vitro polymerized type I collagen. As shown in Fig. 1J, intracellular TR-collagen was readily detected in cells incubated with in vitro polymerized type I collagen. To establish that fibronectin-producing cells are also capable of endocytosing collagen I from preassembled matrices, SMCs were seeded onto fluorescently labeled collagen I- and fibronectin-containing matrices in the presence of the fibronectin polymerization inhibitor pUR4. We previously showed that fibronectin polymerization inhibitors can promote the turnover of ECM fibronectin and collagen I in cells that produce fibronectin (52, 53). As shown in Fig. 1, intracellular TR-collagen I (Fig. 1K) and AF-488 fibronectin (Fig. 1L) were readily detected in SMC cultured on preestablished matrices. These data show that cells can endocytose collagen from cell-polymerized ECM and from in vitro polymerized fibrils.
Our previous data show that the presence of soluble fibronectin and ongoing fibronectin matrix polymerization stabilize fibronectin and collagen I fibrils and decrease fibronectin endocytosis (51–53). In this study, we sought to determine whether the presence of soluble fibronectin similarly decreases collagen I endocytosis. To do this, cells with a preestablished fibronectin and collagen I matrix were incubated in the presence or absence of soluble fibronectin, and the effect on collagen I endocytosis was monitored by immunofluorescence microscopy. As shown in Fig. 2, there was a dramatic decrease in the amount of endocytosed fibronectin (Fig. 2B) and collagen I (Fig. 2D) in cells chased in the presence of soluble fibronectin. Quantitative analysis of immunofluorescence photographs show that there was a 70% decrease in collagen I uptake in cells incubated in the presence of unlabeled fibronectin compared with cells cultured in the absence of fibronectin (Fig. 2E). The ability of fibronectin to inhibit endocytosis of collagen I was due to its ability to become polymerized into the ECM, as soluble fibronectin failed to inhibit collagen I endocytosis when cells were coincubated with fibronectin and the fibronectin polymerization inhibitor pUR4 (Fig. 2J). The control peptide III-11C had no effect on collagen I endocytosis (Fig. 2L). Our previously published studies showed that fibronectin also failed to inhibit fibronectin endocytosis when cells were coincubated with fibronectin and pUR4 (51, 52). The presence of fibronectin and ongoing fibronectin polymerization during the chase period resulted in a corresponding increase in the stability of fibronectin and collagen I matrix fibrils (supplemental Fig. S.4). Fibronectin also inhibits the endocytosis of collagen I from in vitro polymerized collagen I fibrils (Fig. 2, N–Q). Taken together, these data show that fibronectin and fibronectin polymerization play an important role in regulating the endocytosis and turnover of ECM collagen I.
Fig. 2.
FN polymerization reduces collagen I endocytosis from matrix. A–M: FN-null MFs were incubated with TR-collagen I and AF488-FN overnight. Cells were washed and then incubated (chased) for 24 h in media lacking labeled fibronectin and collagen (A, C, F) or containing 10 μg/ml unlabeled FN (B, D, H, J, L). During the chase, cells were also incubated in the absence (A–I) or presence of 500 nM pUR4 (J, K) or III-11C (L, M) peptides. G, I, K, and M are DIC images corresponding to the left panel. For A–D, a large portion of the extracellular FN and collagen fibrils were lost during the chase. Confocal images were taken in a plane of focus that would best show the intracellular vesicles. For F, H, J, and L, cells were treated with 0.02% trypan blue for 5 min before fixation to quench extracellular fluorescence. Scale bar, A–D, 20 μm; F–M, 60 μm. E: quantitation of matrix collagen I endocytosis. Fluorescent images were quantitated as described in materials and methods. The fluorescence intensity is reported as intensity per field of view. The graph in E shows the fold change relative to the mean fluorescence intensity of endocytosed TR-collagen I in cells chased in the absence of FN, which was set equal to 1 in each individual experiment. N–Q: confluent cultures of FN-null MFs were incubated in the absence (N, O) or presence (P, Q) of 10 μg/ml fibronectin for 12 h. The cell culture media was removed, and 2 mg/ml collagen (containing 20 μg/ml TR-collagen) was added on top of the cells and allowed to polymerize as described in materials and methods. Cells were cultured in the absence (N, O) or presence (P, Q) of 10 μg/ml fibronectin for an additional 48 h. The collagen gel was gently removed. The cells were then treated with 0.02% trypan blue for 5 min to quench extracellular fluorescence. Scale bar, 10 μm. A–D and N–Q are confocal images.
Endocytosis of ECM Collagen I is Regulated by β1 Integrins
We previously showed that endocytosis of ECM fibronectin is regulated by β1 integrins (51). β1 Integrins are known to be important for the endocytosis of collagen-coated beads (32, 50). However, the role of β1 integrins in regulating endocytosis of ECM collagen I has not been previously reported. Our previously published data show that the absence of functional β1 integrins stabilizes fibronectin matrix fibrils and inhibits endocytosis of matrix fibronectin (51). In this study we sought to determine whether β1 integrins are also involved in regulating the stability of collagen I matrix fibrils and the endocytosis of matrix collagen I. To do this, we tested whether β1 integrin inhibitory antibodies could stabilize collagen I matrix fibrils and block the endocytosis of collagen I from preestablished matrix. As shown in Fig. 3, A–D, cells cultured in the presence of β1 integrin inhibitory antibodies showed reduced turnover of ECM collagen I fibrils compared with cells treated with the control antibody. In addition, the presence of β1 integrin inhibitory antibodies dramatically reduced the levels of endocytosed collagen I (Fig. 3G) compared with control antibody-treated cells (Fig. 3E). Quantitative analysis of immunofluorescence images show that there was a ∼60% reduction in collagen I endocytosis in cells incubated with β1 integrin inhibitory antibodies compared with control cells (Fig. 3I). We previously reported that β1 integrin inhibitory antibodies also blocked fibronectin endocytosis (51). Antibodies to αv (Fig. 3, J–M) or β3 integrins (F. Shi, unpublished data) had no effect on collagen I endocytosis. β1-Inhibitory antibodies similarly inhibited the ability of SMC to endocytose collagen I from the ECM (Fig. 3, N–Q). To confirm that β1 integrins play an important role in regulating endocytosis of matrix collagen I, we tested the ability of GD25 β1 integrin null fibroblasts to endocytose collagen I from preassembled ECM. GD25 cells show reduced loss of collagen I matrix fibrils and reduced levels of endocytosed collagen I (Fig. 3U) and fibronectin (Fig. 3V) from preassembled matrix compared with GD25 β1 integrin reexpressing cells (Fig. 3, R and S). Others have shown that β1 integrin inhibitory antibodies do not inhibit soluble collagen I uptake (12). Our data confirm this finding, as GD25 β1 null fibroblasts endocytose soluble collagen I similarly to GD25 β1 reexpressing cells (supplemental Fig. S.5). These data highlight important differences between the endocytosis of ECM collagen I and soluble collagen I.
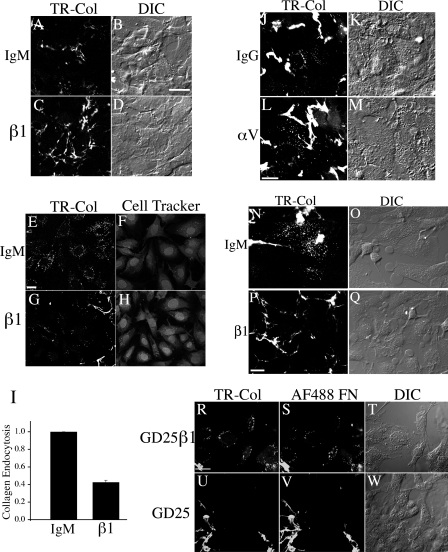
Fig. 3.
β1 Integrins regulate collagen I endocytosis from the ECM. A–M: FN-null MFs were seeded onto preassembled cell-derived matrices containing TR-collagen I in the presence of 25 μg/ml β1 integrin inhibitory (C, D, G, and H), 50 μg/ml αv inhibitory (L, M) or isotype control antibodies (A, B, E, F: IgM; J and K: IgG). Cells were cultured at 37°C for 22–36 h before fixation. A and C are confocal images taken in a plane of focus to best show extracellular collagen fibrils. E, G, J, and L are confocal images taken in a plane of focus to best visualize endocytosed collagen I; some extracellular fibrils can also be seen in these images. The cell outlines were visualized by loading cells with CellTracker green (F, H) or by DIC imaging (B, D, K, and M). Scale Bar, A–D, 20 μm; E–H, 20 μm; J–M, 20 μm. I: quantitation of ECM collagen I endocytosis from preassembled matrix. Fluorescent images were quantitated as described in materials and methods. The fluorescence intensity is reported as intensity per unit area. The graph in I shows the fold change relative to the mean fluorescence intensity of endocytosed TR-collagen I in cells treated with isotype control IgM, which was set equal to 1 for each individual experiment. N–Q: smooth muscle cells (SMC) were seeded onto preassembled cell-derived matrices containing TR-collagen I in the presence of 25 μg/ml β1 integrin inhibitory (P, Q) or isotype control antibodies (N, O). Cells were incubated for 39 h before fixation. For N, P confocal images were taken in a plane of focus to best show intracellular vesicular fluorescence. The presence of the β1 integrin inhibitory antibody causes a reduction in intracellular vesicles and a preservation of matrix fibrils, as shown in P. Corresponding differential interference contrast (DIC) images are shown in O and Q. Scale Bar, 20 μm. R–W: GD25 β1 integrin null cells (U–W) and GD25 β1 reexpressing cells (R–T) were seeded onto preassembled cell-derived matrices containing TR-collagen I and AF488-FN and cultured for 24 h at 37°C. Confocal images were taken in a plane of focus to best show intracellular vesicular fluorescence. The absence of β1 integrins results in reduced intracellular vesicles and increased stability of extracellular matrix fibrils, as shown in U and V. TR-collagen (R, U) and AF488-FN (S, V) are shown. T and W are corresponding DIC images. Scale bar, 25 μm.
Previous work has demonstrated that Endo180/uPARAP is an important regulator of soluble collagen I endocytosis (12, 62). To verify that the failure of GD25 cells to endocytose matrix collagen I was not due to the absence of Endo180/uPARAP, we used RT-PCR and Western blot analysis to determine whether GD25 cells express Endo180/uPARAP. As shown in Fig. 4, GD25 cells contain Endo180/uPARAP mRNA (Fig. 4A) and protein (Fig. 4B). GD25 β1 integrin reexpressing cells also express Endo180/uPARAP (Fig. 4). Taken together, these data show that β1 integrins play an important role in regulating matrix collagen I endocytosis.
Fig. 4.
Expression of Endo180 in various cell lines. A: RT-PCR was performed as described in materials and methods. Endo180 mRNA was detected in FN-null MFs (lane 1), GD25 (lane 2), and GD25-β1 reexpressing cells (lane 3). B: cell lysates were prepared from Endo180 null cells (null), wild-type control cells (WT), GD25 (β1-), and GD25-β1 reexpressing (β1+) cells and analyzed by Western blot analysis using an antibody to Endo180. ERK was used as loading control. Molecular mass standards in kDa are shown on the left.
Our published data show that fibronectin endocytosis is regulated by caveolin-1 (51, 52). We previously showed that knockdown of caveolin-1 preserved fibronectin matrix fibrils and reduced fibronectin endocytosis (51, 52). To determine whether caveolin-1 knockdown similarly affects collagen I endocytosis, we assessed the ability of FN-null MF expressing caveolin-1 siRNA (shcav) to endocytose collagen I from preestablished ECM. As shown in Fig. 5, there was a dramatic reduction in endocytosed fibronectin (Fig. 5D) and collagen I (Fig. 5E) and a corresponding preservation of ECM fibronectin and collagen I fibrils in shcav cells when compared with control siRNA cells (Fig. 5, A–C). There was also a dramatic reduction (64%) in collagen I endocytosis in shcav cells compared with control cells in long-term pulse-chase experiments (Fig. 5G).
Fig. 5.
Endocytosis of ECM collagen I is reduced in caveolin-1 knockdown cells. FN-null MFs expressing caveolin-1 small interfering RNA (siRNA) (shcav, D–F) or control cells (shluc, A–C) were seeded onto preassembled matrix containing TR-collagen I and AF488-FN. Cells were incubated for 40 h at 37°C before fixation. Cells expressing caveolin-1 siRNA show enhanced stability of extracellular matrix collagen (E) and FN fibrils (D). Few intracellular vesicles were detected in caveolin-1 siRNA expressing cells (D, E). There was a paucity of ECM fibrils and a corresponding increase in intracellular TR-collagen I (B) and AF488-FN (A) in cells expressing control siRNA. Arrows in B point to internalized TR-collagen I. C and F are corresponding DIC images. A, B, D, and E are confocal images. Bar, 20 μm. G: quantitation of ECM collagen I endocytosis in caveolin-1 knockdown cells. Long-term pulse-chase assay were performed in FN-null MFs expressing caveolin-1 siRNA (shcav) or control cells (shluc) as described in materials and methods. Cells were chased for 14–18 h in the absence of exogenously added fibronectin and collagen I. Fluorescent images were quantitated as described in materials and methods. The fluorescence intensity is reported as intensity per field of view. The graph in G shows the fold change relative to the mean fluorescence intensity of endocytosed TR-collagen I in cells expressing control siRNA (shluc), which was set equal to 1 in each individual experiment.
Endo180/uPARAP Regulates Collagen I Endocytosis From the ECM
Endo180/uPARAP has recently been shown to regulate the endocytosis of soluble collagens (11, 12, 62, 66). The role of Endo180/uPARAP in regulating the endocytosis of cell-derived ECM collagen I has not been previously studied. To determine whether Endo180 regulates the turnover of ECM collagen I, we seeded Endo180 null cells and cells derived from wild-type (WT) littermates on preassembled fibronectin and collagen I-containing matrices. As shown in Fig. 6, Endo180 null cells are defective in their ability to endocytose ECM collagen I (Fig. 6E). There was a corresponding preservation of ECM collagen I fibrils in Endo180 null cells. Surprisingly, Endo180 null cells were also impaired in their ability to endocytose ECM fibronectin. There was an increase in the stability of fibronectin matrix fibrils and a corresponding decrease in endocytosed fibronectin (Fig. 6D) in Endo180 null cells compared with WT cells (Fig. 6A). Endo180 cells are able to bind to fibronectin and assemble a robust fibronectin matrix (F. Shi, unpublished data). Endo180 null cells also express similar levels of α5- and β1 integrins as WT control cells [(12) and F. Shi, unpublished data]. Hence, the failure of Endo180/uPARAP to turnover and endocytose ECM fibronectin is not due to decreased β1 integrin levels or to the inability of Endo180/uPARAP null cells to interact with fibronectin.
Fig. 6.
Endo180 regulates the endocytosis of collagen I from the ECM. Endo180 null (D–F) or WT control (A–C) cells were seeded onto preassembled matrix containing TR-collagen I and AF488-FN. Cells were cultured for 24 h before fixation. TR-collagen I (B, E) and AF488-FN (A, D) are shown. Confocal images were taken in a plane of focus that would best show intracellular vesicles. Arrows in B point to abundant internalized TR-collagen I in WT control cells. Abundant internalized fibronectin is also seen in WT cells (A). There were few intracellular vesicles and a corresponding increase in the stability of fibrillar fibronectin and collagen I in Endo180 null cells (D,E). C and F are corresponding DIC images. Bar, 20 μM.
Role of MMPs in Regulating Fibronectin and Collagen I Endocytosis From the ECM
Recent data indicate that MMPs cooperate with Endo180/uPARAP in regulating collagen I turnover (33, 62). In addition, cleaved or heat-denatured collagen I was shown to be a preferred ligand for endocytosis by Endo180/uPARAP (33). Therefore, we asked whether MMP inhibitors could block the turnover of collagen I and fibronectin matrix fibrils and the endocytosis of ECM collagen I. To determine the role of MMPs in regulating ECM fibril loss, a fibronectin pulse-chase experiment was performed in the presence and absence of the MMP inhibitor GM6001. As shown in Fig. 7, FN-null MF assembled a robust fibronectin (Fig. 7A) and collagen I (Fig. 7B)-containing matrix when pulsed overnight with fluorescently labeled ECM proteins. Cells were then incubated in the absence of added fibronectin and collagen and in the presence or absence of GM6001. GM6001 largely preserved fibronectin (Fig. 7G) and collagen I (Fig. 7H) matrix fibrils. In contrast, there was a dramatic loss of fibronectin (Fig. 7D) and collagen I (Fig. 7E) matrix fibrils in control treated cells. We next examined the effect of GM6001 on collagen I endocytosis. GM6001 caused a drastic reduction in endocytosed collagen I (Fig. 7, J–M). These data show that extracellular proteolysis of collagen I contributes to enhanced loss of matrix fibrils and subsequent endocytosis.
Fig. 7.
GM6001 inhibits collagen I matrix turnover and endocytosis. FN-null MFs were incubated with 10 μg/ml AF488-FN and 5 μg/ml (A–I) or 10 μg/ml (J–M) TR-collagen I overnight. A and B show extracellular matrix fibrils present in cells after the overnight incubation with fluorescently labeled fibronectin (A) or collagen (B) (“pulse”, A–C). Cells were washed and then incubated for 24 h in media lacking fibronectin and collagen I but containing 20 μM GM6001, a metalloproteinase inhibitor (G–I, L, M), or vehicle control (D–F, J, K). Images in D, E, G, and H were taken in a plane of focus to best show extracellular matrix fibrils. In J–M, cells were treated with 0.02% trypan blue to quench extracellular fluorescence prior to fixation as described in materials and methods. AF488-FN is shown in A, D, and G; TR-collagen I is shown in B, E, H, J, and L. Corresponding phase (C, F, I) or DIC (K, M) images are shown. A–I: bar, 80 μm. J–M: bar, 20 μm.
DISCUSSION
The ability of cells to produce, organize, and remodel their ECM is a key property that allows cells to respond to changes in their extracellular environment. Alterations in the composition and organization of the ECM can have a profound influence on cell migration, growth, and survival. In addition, fragments of ECM molecules generated during ECM remodeling can have effects on cell function that are distinct from those of the intact molecules (14, 41, 48, 69). Hence, understanding how cells remodel their ECM may provide insight into methods that could be used to maintain or improve tissue function in diseases associated with aberrant ECM remodeling. In this paper we show that the polymerization of fibronectin into the ECM regulates the turnover and endocytosis of ECM collagen I. This is the first demonstration that the deposition of one ECM molecule can affect matrix remodeling by regulating the endocytosis of other ECM molecules. In addition, we demonstrate that the endocytosis of ECM collagen I depends on two different receptors: β1 integrins and Endo180/uPARAP. Furthermore, we show that endocytosis of ECM collagen I is regulated by matrix metalloproteinases and caveolin-1. Our data highlight the complexity of collagen I matrix turnover. This complex multilayered regulation of collagen I turnover may ensure tight control over the accumulation of extracellular collagen I and collagen I fragments.
We previously showed that the polymerization of fibronectin into the ECM has important consequences for cells and can influence the stability of fibronectin, collagen I, collagen III, and thrombospondin 1 matrix fibrils (53, 55). We and others have shown that fibronectin polymerization can also promote cell migration and growth and regulate cell differentiation (7, 9, 21, 49, 55). Fibronectin polymerization also plays a key role in regulating vascular tone, angiogenesis, and vascular remodeling (9, 23, 70). Fibronectin may exert some of these effects by virtue of its ability to organize other molecules into fibrils in the ECM. For example, we and others have shown that the deposition of collagen I and III into ECM fibrils requires fibronectin polymerization (53, 55, 61). Furthermore, we previously showed that agents that disrupt fibronectin polymerization disrupt the deposition of other matrix molecules and can trigger the loss of ECM collagen I fibrils (53). In this paper we show that the loss of ECM collagen I fibrils that occurs when fibronectin polymerization is blocked is accompanied by an increase in collagen I endocytosis (Fig. 2). Interestingly, all agents tested that block the turnover of fibronectin matrix fibrils have a corresponding effect on the turnover and endocytosis of collagen I. Downregulation of caveolin-1, inhibition of β1 integrins, ongoing fibronectin matrix polymerization, and inhibition of MMPs all stabilize fibronectin and collagen I matrix fibrils and result in decreased endocytosis of collagen I (Figs. 2, 3, 5, and 7). Fibronectin is known to bind to the site in collagen I that is cleaved by collagenases to produce collagen one-quarter and three-quarter fragments (27, 30). Furthermore, fibronectin has been shown to protect collagen I from digestion by collagenase (36). Our data (Fig. 7) as well as published data show that MMP inhibitors can block the endocytosis of collagen I (33, 62). Hence, fibronectin may inhibit collagen I endocytosis by protecting it from cleavage by MMPs. Fibronectin may also affect collagen I endocytosis by mechanisms that are distinct from its effects on MMP cleavage. For example, our unpublished data show that fibronectin polymerization can decrease β1 integrin endocytosis. In this paper we show that β1 integrins positively regulate collagen I endocytosis (Fig. 3). Hence, the ability of fibronectin polymerization to inhibit β1 integrin endocytosis could contribute to its ability to decrease collagen I endocytosis.
Previous studies have shown that β1 integrins are involved in the endocytosis of collagen-coated beads (5, 32, 50). However, the ability of β1 integrins to regulate endocytosis of ECM collagen has not been previously demonstrated. Others have shown that β1 integrin inhibitory antibodies have no effect on the endocytosis of soluble collagen I (12). In support of this, our data show that GD25 β1 integrin null cells are not impaired in their ability to endocytose soluble collagen I (supplemental Fig. S.5). In contrast, there is a striking decrease in the endocytosis of collagen I from the ECM of cells that lack functional β1 integrins (Fig. 3). We previously showed that cells lacking β1 integrins can endocytose soluble fibronectin but are severely impaired in their ability to endocytose ECM fibronectin (51). Taken together, these data show that different receptor systems are involved in regulating ECM and soluble fibronectin and collagen 1 endocytosis. These data highlight the importance of distinguishing between endocytosis of soluble and polymerized forms of ECM proteins.
Recently, Endo180/uPARAP was shown to be an endocytic receptor for a variety of collagens, including collagen I, IV, and V (10–12, 33). Cells lacking Endo180 show a striking reduction in the endocytosis of soluble collagen I, IV, and V as well as denatured collagen (11, 12). The importance of Endo180/uPARAP in regulating ECM collagen endocytosis was not tested, although Endo180/uPARAP null cells seeded onto in vitro polymerized collagen showed reduced clearance of the underlying collagen (33). Our data show that Endo180/uPARAP is involved in regulating collagen I endocytosis from the ECM (Fig. 6). There was a striking reduction in matrix collagen I uptake in Endo180/uPARAP null cells, despite the presence of β1 integrins (Fig. 6E). Unexpectedly, Endo180/uPARAP null cells were also defective in endocytosing ECM fibronectin (Fig. 6D). In a previous report, Endo180/uPARAP null and WT cells were shown to endocytose equal amounts of soluble fibronectin (12). Our data show a striking defect in the ability of Endo180/uPARAP null cells to endocytose ECM fibronectin (Fig. 6), again highlighting mechanistic differences between receptor systems involved in regulating the endocytosis of soluble and polymerized ECM proteins. It is not currently known how Endo180/uPARAP regulates fibronectin endocytosis. We speculate that Endo180/uPARAP may interact with β1 integrins and modulate β1 integrin endocytosis.
Endo180/uPARAP is thought to be endocytosed by a clathrin-dependent mechanism. Electron microscopy studies have shown that Endo180/uPARAP can localize in clathrin-coated pits (28). In addition, the Endo180/uPARAP cytoplasmic tail contains a dihydrophobic motif that fits a consensus sequence for association with adaptor proteins that are part of the clathrin endocytic pathway (6). This dihydrophobic motif was shown to be important for Endo180/uPARAP endocytosis (26). Interestingly, our data show that caveolin-1 is involved in regulating ECM collagen uptake (Fig. 5). The exact role that caveolin-1 plays in regulating ECM collagen I uptake is not known. Our published data strongly suggest that β1 integrin-mediated fibronectin uptake occurs in caveolae (51). Hence, caveolae may similarly regulate β1 integrin-mediated collagen I endocytosis. Alternatively, the effect of caveolin-1 on collagen I uptake could be indirect. For example, caveolin-1 knockdown results in stabilization of ECM fibronectin fibrils (51). As discussed above, this could protect collagen I from MMP cleavage and hence reduce its endocytosis. β1 Integrins can also be endocytosed by a clathrin-dependent pathway (35, 40, 45), although this pathway does not appear to be involved in endocytosis of fibronectin (51).
Our EM studies show that extracellular fibrillar structures are present in cells cultured in the presence (Fig. S.2, B–D) but not in the absence of fibronectin (Fig. S.2A). Our published data show that cells do not organize collagen I matrix fibrils when fibronectin is absent, despite the presence of endogenous or exogenously supplied type I collagen (54, 55). However, our previous studies showed that fibronectin and type III collagen fibrils are able to form in cells in which collagen I fibril formation is inhibited (55). We previously characterized a peptide (R1R2) that inhibits type I collagen matrix deposition but does not block fibronectin or collagen III fibril formation (55). The presence of the R1R2 peptide resulted in sparser and thinner fibrils by EM (Fig. S.2D) than those formed by control cells (Fig. S.2, B and C). We did not find any evidence of banded collagen fibrils. This is consistent with published data showing that collagen deposited by cells in short-term cultures (such as we have used in our studies) does not form banded structures (15). However, the ECM deposited by cells under our culture conditions is organized into fibrillar structures as shown by our immunofluorescence (Figs. 1, 7, and S4) and EM images (Fig. S.2). Similar nonbanded collagen fibrils have also been identified by others (15).
Purified type I collagen can be organized into fibrils in the absence of cells. These in vitro polymerized collagen I fibrils have a banded appearance by EM (13, 29). Our data show that cells can endocytose collagen from in vitro polymerized type I collagen fibrils (Fig. 1J). Furthermore, endocytosis of in vitro polymerized collagen is also regulated by fibronectin (Fig. 2, N–Q) and fibronectin polymerization (F. Shi, unpublished data). These data suggest that similar methods may be used to endocytose banded collagen fibrils and the nonbanded collagen fibrils that are produced by the cells in our experiments.
The studies in this paper show that fibronectin polymerization stabilizes collagen I matrix fibrils and results in a corresponding decrease in collagen I endocytosis. These studies provide further evidence of the importance of fibronectin polymerization in regulating ECM remodeling and demonstrate that one of the mechanisms by which fibronectin regulates ECM remodeling is by regulating the endocytosis of ECM proteins. The studies in this paper also show that multiple receptor systems regulate collagen I endocytosis. How β1 integrins and Endo180/uPARAP cooperate to regulate ECM collagen I uptake is not known. There is no published data suggesting that integrins and Endo180/uPARAP associate with each other. It will be interesting to determine how these two receptor systems participate in regulating both fibronectin and collagen I endocytosis.
GRANTS
This research was supported by National Institutes of Health Grants HL-070261, GM-069729, and AG-033787.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Reinhardt Fassler, Dr. Susan LaFlamme, Dr. Bugge, and Dr. Behrendt for providing reagents used in this study, Dr. Peters (University of Wisconsin) for advice on labeling collagens, Karen Bentley (University of Rochester) for help with electron microscopy, Ken Foxx for developing the MATLab program for image analysis, and Andrew Serour for excellent technical assistance.
REFERENCES
- 1.Ala-aho R, Kahari VM. Collagenases in cancer. Biochimie 87: 273–286, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells is dependent on the adhesive substrate. J Biol Chem 279: 35749–35759, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum 29: 252–265, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest 117: 568–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhide VM, Laschinger CA, Arora PD, Lee W, Hakkinen L, Larjava H, Sodek J, McCulloch CA. Collagen phagocytosis by fibroblasts is regulated by decorin. J Biol Chem 280: 23103–23113, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol 143: 267–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol 147: 619–630, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 29: 1074–1079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curino AC, Engelholm LH, Yamada SS, Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS, Bugge TH. Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol 169: 977–985, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.East L, McCarthy A, Wienke D, Sturge J, Ashworth A, Isacke CM. A targeted deletion in the endocytic receptor gene Endo180 results in a defect in collagen uptake. EMBO Rep 4: 710–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelholm LH, List K, Netzel-Arnett S, Cukierman E, Mitola DJ, Aaronson H, Kjoller L, Larsen JK, Yamada KM, Strickland DK, Holmbeck K, Dano K, Birkedal-Hansen H, Behrendt N, Bugge TH. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J Cell Biol 160: 1009–1015, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelman RA, Poppke DC, Piez KA. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J Biol Chem 254: 11741–11745, 1979 [PubMed] [Google Scholar]
- 14.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277: 225–227, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Goldberg B, Green H. An Analysis of collagen secretion by established mouse fibroblast lines. J Cell Biol 22: 227–258, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas TL. Endothelial cell regulation of matrix metalloproteinases. Can J Physiol Pharmacol 83: 1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 3: 589–601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausser H, Ober B, Quentin-Hoffmann E, Schmidt B, Kresse H. Endocytosis of different members of the small chondroitin/dermatan sulfate proteoglycan family. J Biol Chem 267: 11559–11564, 1992 [PubMed] [Google Scholar]
- 19.Hed J. The extinction of fluorescence by crystal violet and its use to differentiate between attached and ingested microorganisms in phagocytosis. FEMS Microbiol Lett 1: 357–361, 1977 [Google Scholar]
- 20.Heljasvaara R, Nyberg P, Luostarinen J, Parikka M, Heikkila P, Rehn M, Sorsa T, Salo T, Pihlajaniemi T. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res 307: 292–304, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hocking DC, Chang CH. Fibronectin matrix polymerization regulates small airway epithelial cell migration. Am J Physiol Lung Cell Mol Physiol 285: L169–L179, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Hocking DC, Sottile J, Langenbach KJ. Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem 275: 10673–10682, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hocking DC, Titus PA, Sumagin R, Sarelius IH. Extracellular matrix fibronectin mechanically couples skeletal muscle contraction with local vasodilation. Circ Res 102: 372–379, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Homandberg GA. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front Biosci 4: D713–D730, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114: 33–45, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Howard MJ, Isacke CM. The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J Biol Chem 277: 32320–32331, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Hynes RO. Fibronectins New York: Springer-Verlag, 1990 [Google Scholar]
- 28.Isacke CM, van der Geer P, Hunter T, Trowbridge IS. p180, a novel recycling transmembrane glycoprotein with restricted cell type expression. Mol Cell Biol 10: 2606–2618, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J 316: 1–11, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinman HK, McGoodwin EB, Martin GR, Klebe RJ, Fietzek PP, Woolley DE. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem 253: 5642–5646, 1978 [PubMed] [Google Scholar]
- 31.LaFlamme SE, Shi F, Sottile J. Integrin trafficking. In: Cell Junctions, edited by LaFlamme SE, Kowalczyk AP. Weinheim: Wiley-VCH, 2008, p. 89–107 [Google Scholar]
- 32.Lee W, Sodek J, McCulloch CA. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J Cell Physiol 168: 695–704, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem 282: 27037–27045, 2007 [DOI] [PubMed] [Google Scholar]
- 34.McKeown-Longo PJ, Hanning R, Mosher DF. Binding and degradation of platelet thrombospondin by cultured fibroblasts. J Cell Biol 98: 22, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Memmo LM, McKeown-Longo P. The alphavbeta5 integrin functions as an endocytic receptor for vitronectin. J Cell Sci 111: 425–433, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Menzel J, Borth W. Influence of plasma fibronectin on collagen cleavage by collagenase. Coll Relat Res 3: 217–230, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Miekka SI, Ingham KC, Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res 27: 1–14, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Murphy-Ullrich JE, Westrick LG, Esko JD, Mosher DF. Altered metabolism of thrombospondin by chinese hamster ovary cells defective in glycosaminoglycan synthesis. J Biol Chem 263: 6400, 1988 [PubMed] [Google Scholar]
- 39.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 190: 300–309, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell 13: 15–28, 2007 [DOI] [PubMed] [Google Scholar]
- 41.O%Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol 169: 191–202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panetti TS, McKeown-Longo PJ. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem 268: 11492–11495, 1993 [PubMed] [Google Scholar]
- 44.Poole AR, Nelson F, Dahlberg L, Tchetina E, Kobayashi M, Yasuda T, Laverty S, Squires G, Kojima T, Wu W, Billinghurst RC. Proteolysis of the collagen fibril in osteoarthritis. Biochem Soc Symp: 115–123, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Raub TJ, Kuentzel SL. Kinetic and morphological evidence for endocytosis of mammalian cell integrin receptors by using an anti-fibronectin receptor beta subunit monoclonal antibody. Exp Cell Res 184: 407–426, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Reverte CG, Benware A, Jones CW, LaFlamme SE. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J Cell Biol 174: 491–497, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocnik EF, Chan BM, Pickering JG. Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest 101: 1889–1898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol 301: 1179–1190, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Sechler JL, Schwarzbauer JE. Control of cell cycle progression by fibronectin matrix architecture. J Biol Chem 273: 25533–25536, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Segal G, Lee W, Arora PD, McKee M, Downey G, McCulloch CA. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J Cell Sci 114: 119–129, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Shi F, Sottile J. Caveolin-1 dependent integrin trafficking is a critical regulator of fibronectin turnover. J Cell Sci 121: 2360–2371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell 16: 757–768, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 13: 3546–3559, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sottile J, Hocking DC, Swiatek P. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J Cell Sci 111: 2933–2943, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Sottile J, Shi F, Rublyevska I, Chiang HY, Lust J, Chandler J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am J Physiol Cell Physiol 293: C1934–C1946, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Stenn KS, Madri JA, Roll FJ. Migrating epidermis produces AB2 collagen and requires continual collagen synthesis for movement. Nature 277: 229–232, 1979 [DOI] [PubMed] [Google Scholar]
- 57.Todaro G, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17: 299–313, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomasini-Johansson BR, Kaugman NR, Ensenberger MG, Ozeri V, Hanski E, Mosher DF. A 49-residue peptide from adhesin F1 of Streptococcus assembly. J Biol Chem 276: 23430–23439, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Tremble P, Chiquet-Ehrismann R, Werb Z. The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibronectin. Mol Biol Cell 5: 439–453, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Amersfoort ES, Van Strijp JA. Evaluation of a flow cytometric fluorescence quenching assay of phagocytosis of sensitized sheep erythrocytes by polymorphonuclear leukocytes. Cytometry 17: 294–301, 1994 [DOI] [PubMed] [Google Scholar]
- 61.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem 277: 37377–37381, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol 27: 6309–6322, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 12: 317–323, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Wenk MB, Midwood KS, Schwarzbauer JE. Tenascin-C suppresses Rho activation. J Cell Biol 150: 913–920, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. B1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol 132: 227–238, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wienke D, MacFadyen JR, Isacke CM. Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol Biol Cell 14: 3592–3604, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wojciechowski K, Chang CH, Hocking DC. Expression, production, and characterization of full-length vitronectin inEscherichia coli. Protein Expr Purif 36: 131–138, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Yuen SM, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol 154: 1069–1079, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev 22: 1231–1243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.