Abstract
CFTR has been recognized to function as both an anion channel and a key regulator of Slc26 anion transporters in heterologous expression systems. Whether this regulatory relationship between CFTR and Slc26 transporters is seen in native intestine, and whether this effect is coupled to CFTR transport function or other features of this protein, has not been studied. The duodena of anesthetized CFTR-, NHE3-, Slc26a6-, and Scl26a3-deficient mice and wild-type (WT) littermates were perfused, and duodenal bicarbonate (HCO3−) secretion (DBS) and fluid absorptive or secretory rates were measured. The selective NHE3 inhibitor S1611 or genetic ablation of NHE3 significantly reduced fluid absorptive rates and increased DBS. Slc26a6 (PAT1) or Slc26a3 (DRA) ablation reduced the S1611-induced DBS increase and reduced fluid absorptive rates, suggesting that the effect of S1611 or NHE3 ablation on HCO3− secretion may be an unmasking of Slc26a6- and Slc26a3-mediated Cl−/HCO3− exchange activity. In the absence of CFTR expression or after application of the CFTR(inh)-172, fluid absorptive rates were similar to those of WT, but S1611 induced virtually no increase in DBS, demonstrating that CFTR transport activity, and not just its presence, is required for Slc26-mediated duodenal HCO3− secretion. A functionally active CFTR is an absolute requirement for Slc26-mediated duodenal HCO3− secretion, but not for Slc26-mediated fluid absorption, in which these transporters operate in conjunction with the Na+/H+ exchanger NHE3. This suggests that Slc26a6 and Slc26a3 need proton recycling via NHE3 to operate in the Cl− absorptive mode and Cl− exit via CFTR to operate in the HCO3− secretory mode.
Keywords: cystic fibrosis transmembrane conductance regulator, intestine, bicarbonate secretion, sodium absorption, anion exchange
cystic fibrosis (CF) patients suffer from a variety of gastrointestinal and pancreobiliary problems (13, 36, 37). The affected epithelia (pancreas, biliary tract, intestine) all have a severe HCO3− secretory defect (see Ref. 51 for review). Because HCO3− secretion is essential for normal intestinal mucus secretion (17), the HCO3− secretory defect in CF is thought to be of major importance for the overall CF pathology (41, 42, 51). The identified apical transport systems involved in intestinal HCO3− secretion are the CFTR channel and members of the Slc26 anion transport family (8, 27, 49, 56, 59, 61). These same transporters are also expressed in the pancreobiliary system (see Refs. 11, 24 for review). In a heterologous expression system, transport activity of Slc26 transporters is strongly increased by an interaction of their STAS domain with the R domain of CFTR in a PKA-regulated fashion (29). Because of the crypt-predominant expression of CFTR (3, 12, 55) and the villus-predominant expression of Slc26a6 (PAT1) (53) and Slc26a3 (DRA) (61), it is unclear whether this type of regulation is the predominant one in the intestine. In addition to the molecular interaction between Slc26 and CFTR, CFTR transport activity may be necessary as a shunt pathway for Cl− exit during Slc26-mediated HCO3− secretion (21, 38).
Na+ and fluid absorption, on the other hand, is upregulated in nasal, airway, and colonic CF epithelia and may contribute to the obstruction of ductal systems and the intestine (6, 22, 23, 28, 30). Murine small intestinal Na+ absorptive rate, which is largely mediated via Na+/H+ exchanger isoform NHE3 (18), was normal in CFTR-deficient murine intestine (7). NHE3-mediated intestinal salt absorption requires the coordinated action of Scl26a6 and Slc26a3 to achieve net NaCl absorption (50, 62). The same Slc26 transporters are involved in intestinal HCO3− secretion (56, 59, 61). The current models that postulate CFTR-dependent Cl−/HCO3− exchange activity of Slc26a3 and Slc26a6 (11, 29) cannot explain why intestinal salt and fluid absorption in CFTR-deficient intestine is not different from that in wild type (WT) (Ref. 7 and the current study).
To understand the importance of CFTR for Slc26-dependent HCO3− secretion and salt and fluid absorption in the native intestine, we studied duodenal HCO3− secretion and fluid absorption in anesthetized mice during pharmacological inhibition and/or genetic ablation of each of the involved transport proteins named above.
MATERIALS AND METHODS
Chemicals and solutions.
All the reagents were purchased from Sigma-Aldrich (Deisenhofen, Germany) unless mentioned otherwise. Forskolin was purchased from Alexis Biochemicals (Lörrach, Germany). Carbachol was purchased from Calbiochem (Darmstadt, Germany). S1611 was kindly provided by Sanofi-Aventis (Frankfurt, Germany). Stock solutions of all the reagents were prepared and stored at −20°C. Forskolin was dissolved in 100% DMSO (10−2 M) and used at a final concentration of 100 μM. Heat-stable Escherichia coli enterotoxin STa was dissolved in saline (10−5 M) and used at a final concentration of 0.1 μM. Carbachol was dissolved in water (100 mM) and used at a final concentration of 1 mM. 16,16-Dimethyl PGE2 (PGE2) was dissolved in absolute ethanol (10−2 M) and used at a final concentration of 10 μM. S1611 was dissolved in 100% DMSO (10−2 M) and used at a final concentration of 20 μM. CFTR(inh)-172 was dissolved in 100% DMSO (10−2 M) and used at a final concentration of 20 μM.
Animals.
All studies were approved by the Hannover Medical School Committee on investigations involving animals and the “Regierungspräsidium.” Experiments were performed with WT and gene-deficient knockout (KO) mouse models of CFTR, NHE3, Slc26a3, and Slc26a6 (44, 47, 48, 63). Mice were bred at the animal care facility at Hannover Medical School under standard temperature and light conditions and were allowed free access to food and water. Care was taken to match the mice not only as sex-matched littermates but also in terms of an equal number of male and female pairs of WT and KO mice in each group of experiments. For experiments that only used WT mice (with pharmacological inhibitors or for expression studies), WT mice from the different backgrounds were used in equal numbers. We had tried to breed all strains on the same background, but this failed because in our hands CFTR-deficient mice showed an unacceptably high mortality rate on the C57BL/6N background. On the other hand, the NMRI background is not optimal for ion transport studies in the intestine and was reserved for CFTR-deficient mice.
Surgical procedure: in vivo duodenal or jejunal loop experiment.
The mice were anesthetized by spontaneous inhalation of isoflurane (Forene; Abbott Germany, Wiesbaden, Germany) via a mask, and the experiments were otherwise performed as previously described (54, 57). In brief, a catheter was placed in the left carotid artery for continuous infusion of (in mM) 200 Na+, 100 CO3−2, 5 × 10−3 K+, and 5 × 10−3 Cl− at a rate of 0.30 ml/h in WT, CFTR KO, NHE3 KO, and Slc26a6 KO mice. This treatment was shown previously to correct the systemic acid-base balance in isoflurane-anesthetized mice (57). Slc26a3 KO mice were infused with an isotonic mixture of (in mM) 147 Na+, 147 Cl−, 5.4 × 10−3 K+, 0.9 × 10−3 Ca2+, and 1 × 10−3 Mg2+ due to their tendency to have a more alkaline blood pH. Table 1 shows the blood gas analysis for the Slc26a3 WT and KO mice after completion of the experiment. After the pancreatic and biliary ducts were ligated, ∼1 cm of the proximal duodenum, the complete duodenum, or ∼5 cm of the proximal jejunum was perfused for the experiments. A small polyethylene tube (PE-100; inner diameter 1 mm) with a distal flange was advanced to the duodenal bulb or the proximal jejunum and secured by a ligature; another flanged polyethylene tube (PE-200; inner diameter 2 mm) was secured in the distal end of the perfused segment by ligature to allow for drainage. The isolated segment with an intact blood supply was gently flushed and then perfused (Perfusor compact; Braun) at a rate of 15 ml/h with 154 mM NaCl. Effluents from the isolated segment were visually free of bile and blood throughout all experiments. Animals were maintained at 37°C using a heating pad controlled by a rectal thermistor probe.
Table 1.
Blood gas analysis of Slc26a3 WT and KO mice taken at the end of the experiment
| pH | Pco2 | Po2 | So2, % | HCO3−, mM | ABE, mM | SBE, mM | SBC, mm | |
|---|---|---|---|---|---|---|---|---|
| Slc26a3 (+/+) | 7.44 ± 0.02 | 34.46 ± 1.76 | 124.50 ± 5.15 | 98.50 ± 0.16 | 22.85 ± 0.53 | −0.27 ± 0.56 | −0.73 ± 0.55 | 24.23 ± 0.48 |
| Slc26a3 (−/−) | 7.46 ± 0.03 | 35.46 ± 3.28 | 124.77 ± 5.15 | 98.54 ± 0.18 | 25.77 ± 2.36 | 1.85 ± 1.62 | 1.54 ± 1.93 | 26.85 ± 1.76 |
Both wild-type (WT) and knockout (KO) groups of mice were infused with a different mixture of salt solution for the correction of systemic acidosis caused by anesthesia (see materials and methods). There was no significant difference between the 2 groups during the experiment, although the Slc26a3 KO mice were slightly alkalotic preoperatively. The other mouse strains do not have differences in their preoperative blood gas values and were perfused with the same solutions as the WT mice. So2, O2 saturation; ABE, actual base excess; SBE, standard base excess; SBC, standard bicarbonate.
Measurement of luminal alkalinization.
The rate of luminal alkalinization was determined by back titration of the perfusate to pH 5.00 with 2 mM HCl by using pH-stat equipment (PHM82 Standard pH meter; Radiometer, Copenhagen, Denmark) as described previously (55, 56). For Cl−-free experiments, sodium gluconate was used as a NaCl replacement, and pH 6.30 was used for end-point titration. To correct for the intrinsic buffer capacity of perfusate solutions, equal amounts of the isotonic saline or sodium gluconate solution (without having been perfused through the intestine) were subjected to the same end-point titration as the solutions that had been perfused through the intestine, and the respective value of the nonperfused solution was subtracted from the value of the respective solution after it had been perfused through the intestine. After each experiment, the length of the duodenal or jejunal test segment was measured after being excised from the gut. HCO3− outputs were determined every 10 min, and the rates of luminal alkalinization are expressed as micromoles of base secreted per centimeter of intestine per hour (μmol·cm−1·h−1).
Measurement of fluid absorption.
For duodenal or jejunal fluid absorption, ∼5 cm of intestine were perfused at a rate of 3 ml/h with 154 mM NaCl. After the surgery, mice were left for stabilization for 30 min for washout of intestinal contents and fluid resuscitation after surgical trauma. After the recovery period, the basal fluid absorption was measured for another 30 min exactly as previously described for murine jejunum (54). Pharmacological agents were added to the perfusate at the indicated concentrations.
Short-circuit current measurements in isolated intestinal segments.
Isolated duodenal and jejunal mucosae were placed in Ussing chambers, and short-circuit current (Isc) measurements were performed according to identical protocols described previously (4). The aim of the Ussing chamber experiments was to test whether inhibition of NHE3 with the specific NHE3 inhibitor S1611 would produce an electroneutral increase in HCO3− secretion (as would be expected if the sole action of the substance was to inhibit NHE3) or whether the stimulation of an electrogenic process was involved.
Quantitative PCR protocol.
Mucosa from duodenum of WT mice were scraped, and RNA was isolated with the NucleoSpin RNAII total RNA isolation kit (Macherey & Nagel). Reverse transcription was performed with SuperScript III RNaseH reverse transcriptase (Invitrogen). PCR primers were designed using the computer program Primer Express (Applied Biosystems) and are shown in Table 2. Real-time PCR reactions were carried out using SyberGreen PCR master mix (Applied Biosystems) in the Applied Biosystems 7300 Real-Time PCR System as described elsewhere (4). PCR extension was performed at 60°C with 40 repeats. Data were analyzed using Sequence Detection software version 1.2.3 (Applied Biosystems) and exported to Microsoft Excel. Relative quantification was carried out using β-actin, villin, and cytokeratin 18 as reference genes.
Table 2.
Primer pairs used for quantitative PCR and length of amplified gene products
| Gene | Forward Primer | Reverse Primer | Length, bp | Accession No. |
|---|---|---|---|---|
| Cytokeratin 18 | GGAATATGAAGCCCTCTTGAAC | TCTGCCATCCACGATCTTAC | 169 | NM_010664 |
| β-Actin | AGAGGGAAATCGTGCGTGAC | CAATAGTGATGACCTGGCCGT | 138 | NM_007393 Guilietti et al. |
| Villin | TCATACTCAAGACTCCGTCC | TACCACTTGTTTCTCCGTCC | 119 | NM_009509 Broere et al. |
| NHE3 | AGGCCACCAACTATGAAGAG | AGGGGAGAACACGGGATTATC | 110 | NM_00108160 Broere et al. |
| CFTR | TTCTTCACGCCCCTATGTCGA | GCTCCAATCACAATGAACACCA | 145 | NM_021050 |
| Slc26a3 | TTCCCCTCAACATCACCATCC | GTAAAATCGTTCTGAGGCCCC | 110 | NM_021353 |
| Slc26a6 | GGCTCCTGGGTGATCTGTTA | CCAAACATAGGAGGCAATCC | 100 | NM_134420 Alvarez et al. |
Statistical analysis.
Descriptive statistics are means ± SE, with the number of experiments given in parentheses. Statistical comparisons were made using a two-tailed unpaired Student's t-test for single-value comparisons. Data were considered significant when the P value was <0.05.
RESULTS
Stimulating duodenal Slc26-mediated bicarbonate secretion without activating CFTR conductance.
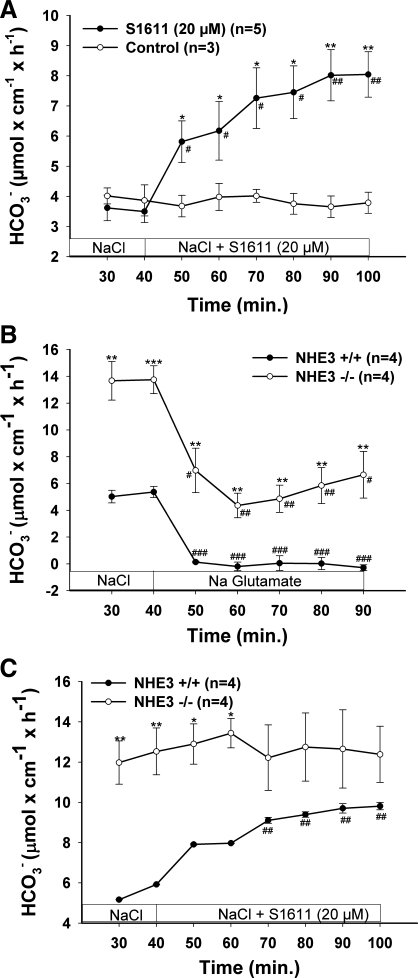
One difficulty in studying the regulation of Slc26 anion transport by CFTR in native intestine lies in the fact that basal HCO3− secretory rate is thought to be composed of an “electroneutral” (presumably mediated by Cl−/HCO3− exchange) and an “electrogenic” part (conductive HCO3− transport, probably CFTR itself) (58). Most stimuli of duodenal HCO3− secretion elicit an Isc response and therefore may involve direct activation of CFTR. For the purpose of our study, we needed a method to isolate Slc26-mediated HCO3− secretion without additional CFTR activation (above the CFTR activity already present in native intestine). One theoretical tool would be inhibition of the apical Na+/H+ exchanger isoform NHE3, thereby unmasking basal Cl−/HCO3− exchange activity. Indeed, the NHE3 inhibitor S1611 has been described to stimulate duodenal HCO3− secretion in the rat duodenum (16). To find out whether S1611 stimulates HCO3− secretion in murine duodenum in vivo in an NHE3-dependent fashion, we studied the effect of S1611 as well as NHE3 deletion on duodenal bicarbonate (HCO3−) secretion (DBS) in anesthetized mice. S1611 induced a significant increase in DBS (Fig. 1A). Likewise, in NHE3-deficient mice, basal DBS was significantly higher than in WT mice, and Cl− removal from the luminal perfusate caused a strong decrease in DBS in both NHE3-deficient and WT mice, demonstrating that a major part of DBS is mediated by anion exchangers in both NHE3 KO and WT mice (Fig. 1B). The application of S1611 stimulated DBS in WT but not in NHE3-deficient mice, indicating that its effect was NHE3 dependent (Fig. 1C).
Fig. 1.
Both pharmacological inhibition (A) and genetic deletion (B) of the Na+/H+ exchanger isoform NHE3 stimulated duodenal bicarbonate (HCO3−) secretion (DBS) in a Cl−-dependent fashion. A: pharmacological inhibition of NHE3 (via S1611) significantly stimulated DBS. B: NHE3-deficient duodenum displayed significantly higher DBS than wild-type (WT) duodenum, and S1611 caused no further stimulation in DBS (C). In both in NHE3-deficient and WT (and in S1611-pretreated, not shown) duodenum, the removal of luminal Cl− caused a strong decrease in DBS, indicating that the Slc26 Cl−/HCO3− exchangers are active in the presence and absence of NHE3. The residual HCO3− secretion after Cl− removal in NHE3-deficient duodenum reveals Cl−-independent HCO3− secretory pathways (at least in part direct CFTR-mediated HCO3− secretion, see Supplemental Material, Supplemental Fig. 3) are also present in murine duodenum. Results are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, significant difference between 2 groups. #P < 0.05; ##P < 0.01; ###P < 0.001, significant difference compared with basal response. The number of mice (n) euthanized for the experiments is given in parentheses.
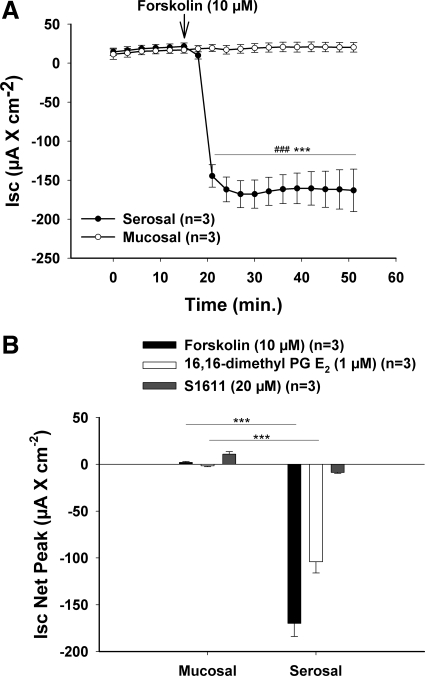
If S1611 stimulates CFTR activity, it is expected to elicit a short-circuit (Isc) response. We therefore studied different modes of stimulating DBS for the electrogenicity of the response in an Ussing chamber system. In equal concentrations, both 10 μM forskolin and 1 μM 16,16-dimethyl PGE2 (known CFTR activators) elicited an Isc response only when applied on the serosal side, whereas no response was seen when applied on the luminal side (Fig. 2). In contrast, 20 μM S1611 elicited a very small positive Isc response when added luminally (where it is supposed to act in inhibiting NHE3) and an equally small negative Isc response when added serosally. This behavior is inconsistent with CFTR activation. Further experiments were performed to characterize the effect of S1611 on duodenal NHE3 as well as Cl−/HCO3−exchange activity. These experiments, whose methodological details and results can be found in the Supplemental Material, were consistent with strong NHE3 inhibition but no intrinsic stimulation of Cl−/HCO3− activity by 20 μM S1611.
Fig. 2.
S1611 does not stimulate electrogenic anion pathways. Isolated duodenal mucosa was placed in classic Ussing-chamber systems, forskolin (10 μM), 16,16-dimethyl PGE2 (1 μM), and S1611 (20 μM) were applied to the luminal or serosal bath, respectively, and the short-circuit current (Isc) response was measured. A: time course of the forskolin experiments. A strong Isc response was induced by forskolin when applied on the serosal side, whereas no response was observed when a similar concentration was applied on the mucosal side. B: net peak Isc response. S1611 elicited minimal Isc responses, and this minimal Isc response was positive when the substance was added to the luminal side (correct side for brush-border membrane NHE3 inhibition). This indicates that the S1611-stimulated HCO3− secretion does not occur via the activation of an anion conductance. Results are means ± SE. ***P < 0.001, significant difference between serosal and mucosal Isc response. ###P < 0.001, significant difference between basal and stimulated-state Isc response within the same group. The number of mice euthanized for the experiments is given in parentheses.
Together, these results suggest that the luminal addition of 20 μM of the NHE3 inhibitor S1611 most likely stimulates DBS by blocking NHE3-mediated proton secretion and unmasking Slc26-mediated Cl−/HCO3− exchange. This concentration was therefore used for further study.
Involvement of Slc26a6 and Slc26a3 in the S1611-mediated DBS response.
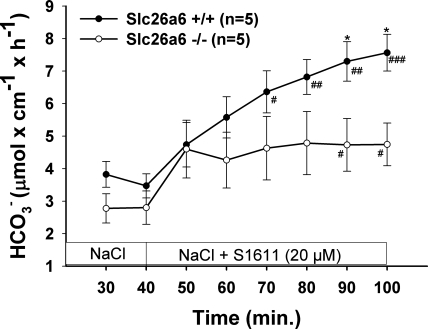
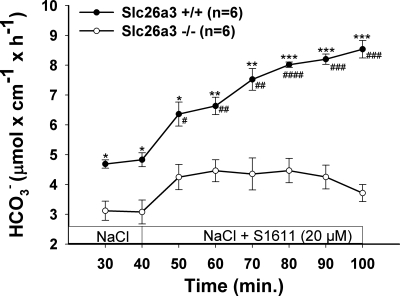
To verify the hypothesis that Slc26a6 and Slc26a3 mediate S1611-induced DBS, we studied the effect of 20 μM S1611 on DBS in Slc26a6- and Slc26a3-deficient mice. The S1611-induced increase in DBS was significantly reduced in Slc26a6-deficient mice (Fig. 3) and to a greater extent in Slc26a3-deficient mice (Fig. 4). Because the sum of the residual S1611-induced DBS in Slc26a6 and Slc26a3 duodenum was more than that in the WT, it is likely the two transporters can partly compensate for each other.
Fig. 3.
Slc26a6 is involved in S1611-stimulated DBS. S1611 (20 μM) caused a significant stimulation of DBS in WT duodenum, and this stimulatory effect was significantly lower in Slc26a6-deficient duodenum. Results are means ± SE. *P < 0.05, significant difference between 2 groups. #P < 0.05; ##P < 0.01; ###P < 0.001, significant difference compared with basal response. The number of mice euthanized for the experiments is given in parentheses.
Fig. 4.
Slc26a3 is involved in S1611-stimulated DBS. S1611 (20 μM) caused a significant stimulation of DBS in WT duodenum, and this stimulatory effect was significantly lower in Slc26a3-deficient duodenum. Results are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, significant difference between 2 groups. #P < 0.05; ##P < 0.01; ###P < 0.001, significant difference compared with basal response. The number of mice euthanized for the experiments is given in parentheses.
Involvement of CFTR in the S1611-mediated DBS response.
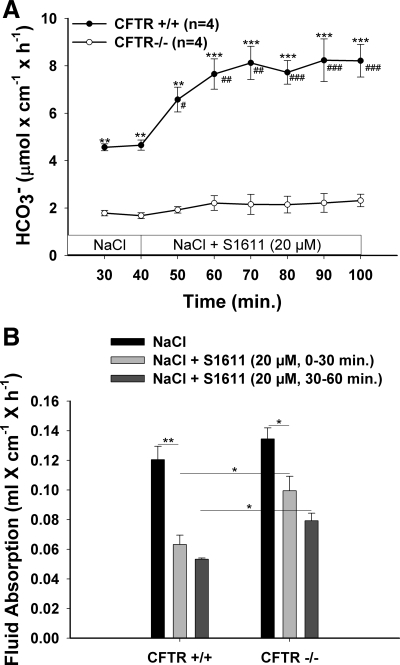
Interestingly, the S1611-induced DBS response was virtually absent in CFTR-deficient mice (Fig. 5A). This suggested that either CFTR expression is essential for Slc26-mediated DBS or NHE3 is not active at the slightly alkaline intracellular pH (pHi) of CFTR-deficient duodenocytes (Ref. 53 and Chen M, unpublished observations). We therefore tested the effect of 20 μM S1611 on duodenal fluid absorption in CFTR-deficient and WT mice. CFTR-deficient and WT mice had similar duodenal fluid absorptive rates, and S1611 inhibited fluid secretion in both the WT and CFTR-deficient duodenum (Fig. 5B). Interestingly, the inhibition of fluid absorption by S1611 required a longer time (or higher concentrations; data not shown) than in WT mice, possibly because of a slower permeation of the substance through the thicker and/or less permeable mucus gel barrier (Refs. 25, 35 and Seidler U, unpublished observations). Nevertheless, the data show that NHE3 is active in CFTR-deficient duodenum. The results further demonstrate that CFTR expression is absolutely required for the S1611-elicited, Slc26-dependent DBS response but not for (presumably) Slc26-mediated fluid absorption.
Fig. 5.
CFTR is involved in S1611-stimulated DBS but is not important for fluid absorption. A: in CFTR-deficient duodenum, basal DBS was significantly lower than in WT duodenum, and no significant S1611-stimulated DBS was observed. This indicates that CFTR expression is essential for Slc26-mediated HCO3− secretion. B: fluid absorption was not significantly different in CFTR-deficient duodenum, and S1611 inhibited fluid absorption in both WT and CFTR-deficient duodenum. The time course of inhibition of fluid absorption by S1611 was slower in CFTR-deficient than WT duodenum, yet a 60% inhibition was achieved in the second 30 min. Thus the reason for the lack of S1611-mediated stimulation of DBS was a necessity of CFTR for Slc26-mediated HCO3− secretion, not an inactivity of NHE3 in CFTR-deficient duodenum. Results are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, significant difference between 2 groups. #P < 0.05; ##P < 0.01; ###P < 0.001, significant difference compared with basal response. The number of mice euthanized for the experiments is given in parentheses.
Effect of the specific CFTR inhibitor CFTR(inh)-172 on the S1611-induced DBS response.
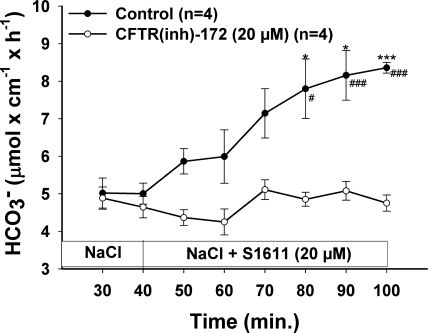
To find out whether the ion transport function of CFTR is necessary for the S1611-induced DBS, we applied S1611 in the presence of CFTR(inh)-172. CFTR(inh)-172 at 20 μM prevented the S1611-induced DBS response, indicating that CFTR-mediated transport is necessary for Slc26-mediated HCO3− secretion (Fig. 6).
Fig. 6.
CFTR protein function is important for the S1611-stimulated DBS. The graph clearly shows that not the absence of CFTR, as in case of CFTR knockout (KO) mice, but CFTR function is important for S1611-stimulated DBS, since CFTR(inh)-172 significantly blocked the S1611-stimulated DBS. Results are means ± SE. *P < 0.05; ***P < 0.001, significant difference between 2 groups. #P < 0.05; ###P < 0.001, significant difference compared with basal response. The number of mice euthanized for the experiments is given in parenthesis.
Involvement of Slc26a6 and Slc26a3 in small intestinal fluid absorption in vivo.
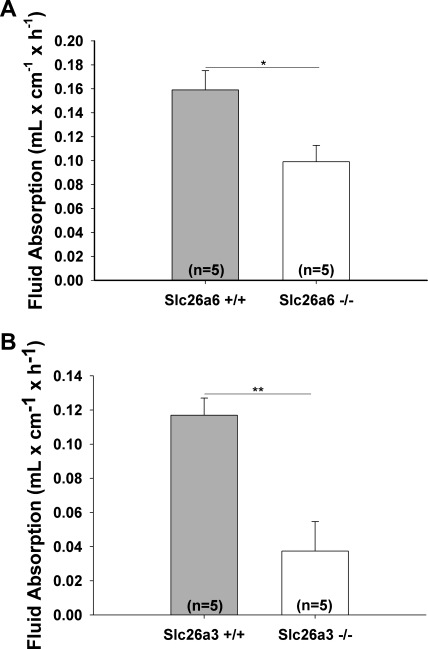
Both Slc26a6 (50) and Slc26a3 (62) have been shown to mediate intestinal Cl− absorption and are therefore believed to be essential for intestinal fluid absorption. We next verified the concept of the involvement of Slc26a6 and Slc26a3 in small intestinal fluid absorption. These experiments were performed in the proximal jejunum. We found a significant reduction of fluid absorption in Slc26a6-deficient small intestine (Fig. 7A) and a very strong inhibition in Slc26a3-deficient small intestine (Fig. 7B). This indicates that both transporters are involved in small intestinal salt and fluid absorption.
Fig. 7.
Both Slc26a6 and Slc26a3 are important in electroneutral salt absorption in the small intestine. Fluid absorption in the jejunum of Slc26a6 (A) and Slc26a3 (B) KO and WT mice were measured and was significantly reduced in both the KO mice. The reduction in the Slc26a3 KO mouse was much more pronounced, indicating this might be responsible for the major portion of salt being absorbed. Results are means ± SE. *P < 0.05; **P < 0.01, significant difference between 2 groups. The number of mice euthanized for the experiments is given in parentheses.
Effect of CFTR deletion or inhibition on small intestinal fluid absorption and secretion.
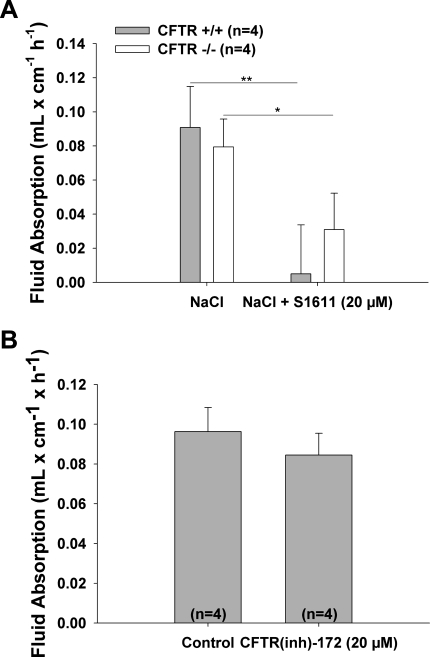
Amazingly, fluid absorptive rates were not different between CFTR-deficient and WT intestine in both the duodenum (Fig. 5B) and the jejunum (Fig. 8A). Since the experiments shown in Fig. 7 demonstrated that small intestinal fluid absorption is dependent on Slc26a6 and Slc26a3, the similar fluid absorptive rates in CFTR-deficient and WT mice suggest a normal operation of these transporters when coupled to NHE3 during fluid absorption, even in the absence of CFTR. This was verified using CFTR(inh)-172 (Fig. 8B).
Fig. 8.
Genetic deletion or pharmacological inhibition of CFTR did not alter the fluid absorptive capacity of small intestine. A: basal fluid absorption in the jejunum of CFTR KO mice was similar to hat in WT mice, indicating that the coupling of NHE3 with Slc26 members was not altered and electroneutral salt absorption was not compromised. Similar data were obtained when the experiment was performed in the duodenum (Fig. 5B). B: results of pharmacological inhibition of CFTR were similar to those for genetic deletion, indicating that both the inhibition of function of CFTR and total absence of CFTR did not alter the electroneutral salt absorption. Results are means ± SE. *P < 0.05; **P < 0.01, significant difference between 2 groups. The number of mice euthanized for the experiments is given in parentheses.
Effect of CFTR deletion on HCO3− secretion elicited by other secretagogues.
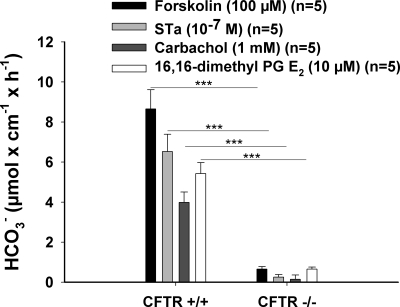
Other modes of activating duodenal HCO3− secretion also have been shown to involve Slc26 anion transporters, although they are not the exclusive transport systems (55, 59, 61). We therefore tested whether the principle of CFTR dependency of HCO3− secretion is also true for more physiological modes of HCO3− secretion. Figure 9 shows in vivo HCO3− secretory rates for cAMP-, cGMP-, and Ca2+-dependent secretagogues in CFTR-deficient murine intestine, as well as PGE2-mediated HCO3− secretion, which acts through several signaling pathways (60). None of the secretagogues elicited a HCO3− secretory response that was statistically different from zero in vivo. Thus the contribution of Slc26 transporters to the HCO3− secretory response requires CFTR expression for a diverse range of secretagogues.
Fig. 9.
CFTR is important for stimulated-state bicarbonate secretion by various secretagogues. DBS was measured before and after various secretagogues were applied luminally (concentrations given in parentheses), and the net peak response was compared between CFTR KO and WT mice. The DBS response to all secretagogues used was significantly decreased in the case of KO mice. Results are means ± SE. ***P < 0.001, significant difference between 2 groups. The number of mice euthanized for the experiments are given in parenthesis.
mRNA expression of CFTR, Slc26a6, Slc26a3, and NHE3 in murine duodenal mucosa.
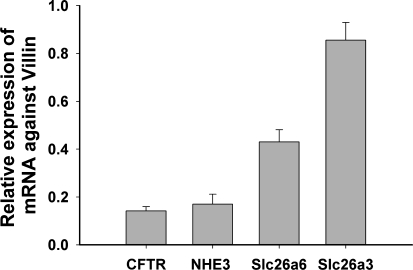
To estimate the relative expression of the four transporter genes of interest in the duodenal mucosa, we designed primers to amplify gene products of similar size for each transcript and performed quantitative PCR (Fig. 10). We used three different control genes, all of which have different aspects. Villin is a brush-border membrane protein, but due to the more elaborate microvillar system in the villi, it is expressed more strongly in the villus region and is therefore a perfect control gene for transporters like NHE3 or Slc26a6. Cytokeratin 18 is another epithelium-specific gene, but in the rodent small intestine it is expressed more strongly in the lower villus and crypt region (15). β-Actin is not epithelium specific, but we have always noticed that its crypt-villus expression pattern is similar to that of villin, suggesting a stronger expression in the villi. Figure 10 shows the expression of CFTR, NHE3 (Slc9a3), Slc26a3 (DRA), and Slc26a6 (PAT1) in relation to villin as control genes. CFTR and NHE3 were similarly expressed, and Slc26a3 and Slc26a6 were expressed more strongly. It is evident that the use of villin as a control gene slightly overestimates the transporters in the lower villus/crypt region (CFTR, Slc26a3), and the use of cytokeratin 18 slightly overestimates the villus-predominant genes like Slc26a6 (see Supplemental Fig. 4).
Fig. 10.
Relative mRNA expression of CFTR, NHE3, Slc26a6, and Slc26a3 in the murine duodenum. The quantitative PCR data shown were acquired in procedures performed in WT mice against villin. We also used β-actin and cytokeratin 18 as control genes (see Supplementary Material, Supplemental Fig. 4). CFTR and NHE3 showed similar expression levels with any of the control genes.
DISCUSSION
The severe pathology of secretory organs affected in CF have taught researchers and clinicians that the regulation of anion transport is an essential requirement for epithelial integrity (5, 10, 37, 39, 41, 42, 46). What has remained unclear is why a defect in an anion channel results in a vast variety of regulatory dysfunctions of other transport events (19, 34, 49, 52). Early physiological experiments suggested that the lack of a functional Cl− conductance altered membrane potential (9, 40, 45, 64) and Cl− recycling via the apical membrane with secondary consequences for other transport events (20, 21, 31). Soon it became clear that these alterations may fail to explain such dramatic events as a lack of agonist-induced HCO3− secretion or a lack of cAMP-mediated inhibition of both electroneutral and electrogenic pathways for salt and fluid absorption, processes that apparently do not involve CFTR-mediated transport at all (7, 21, 32, 34).
Experiments in heterologous expression systems demonstrated that CFTR interacts with other transporters and regulates their transport rates. In the case of Slc26 anion transporters, reciprocal activation of CFTR and Slc26 members is believed to occur via a direct molecular interaction of the two proteins (29). Data from heterologous expression system are conflicting, but some suggest the mere expression of WT CFTR, independent of its transport activity, may be enough to activate the expression and/or function of Slc26 transporters (26, 43). On the other hand, CFTR-deficient epithelia may have normal Slc26 expression levels (53, 59) and, as shown in this study, preserved activity in a subset of Slc26-mediated functions.
The focus of this study was on the importance of CFTR expression and/or function on Slc26-mediated anion absorption and secretion. The investigation of HCO3− secretion and fluid absorption was performed in the murine duodenum of mice deficient for each of the four major transporters that regulate small intestinal anion secretion and salt and fluid absorption, namely, CFTR (49, 55, 56), NHE3 (18), Slc26a3 (DRA) (61, 62), and Scl26a6 (PAT1) (50, 56, 59). The duodenum was chosen because in this tissue the rates of NHE3-mediated proton secretion and CFTR- and Slc26-mediated HCO3− secretion are fairly well balanced. In addition, all four transporters are expressed in balanced proportions, and the duodenum is the only murine intestinal segment with approximately equal expression of both Slc26a3 and Slc26a6.
To study the role of CFTR expression and/or transport activity on Slc26-mediated HCO3− secretion in the native intestine, we had to find a way to elicit a HCO3− secretion that was completely, or at least largely, mediated via the Slc26 anion exchangers. After testing a variety of stimulatory maneuvers, we selected the stimulation of HCO3− secretion by NHE3 blockade (16). The selective NHE3 inhibitor S1611 or genetic ablation of NHE3 resulted in a significantly higher basal DBS in WT mice, suggesting that NHE3 inhibition may “unmask” apical Cl−/HCO3− exchange activity. This assumption was strengthened by the finding that S1611 had no effect on HCO3− secretion in the NHE3-deficient intestine. To rule out a direct stimulation of CFTR, we studied its ability to elicit a negative short-circuit response in isolated duodenal mucosa, which was not found. We also studied the effect of S1611 on Cl−/HCO3− exchange activity (see Supplemental Material). However, S1611 elicits a transient pHi increase at neutral but not at acidic pHi (see Supplemental Material).
Slc26a6 (PAT1) ablation slightly attenuated, whereas Slc26a3 (DRA) ablation strongly inhibited, both basal and the S1611-induced DBS. These data confirm that both Slc26 isoforms are involved in duodenal Cl−/HCO3− exchange-mediated HCO3− secretion in vivo (27, 56, 58, 59, 61). The study of small intestinal fluid absorptive rates showed a reduction in upper jejunal fluid absorptive rates in Slc26a6 mice and a marked reduction in Slc26a3 rates, again confirming in vitro observations (50, 62). We did not succeed in generating Slc26a6/a3 double-deficient mice, but we nevertheless found evidence that the two Slc26 transporters may partially compensate for each other. For example, the fructose-induced increase in jejunal fluid absorption was significantly attenuated in Slc26a6-deficient jejunum (54) but was normal in Slc26a3-deficient jejunum (Singh AK, unpublished observations). Conversely, acid-induced duodenal HCO3− secretory rates were similar to those in WT littermates in Slc26a6-deficient duodenum (56) but were reduced in Slc26a3-deficient duodenum (Singh AK, unpublished observations). Thus different intracellular signaling pathways appear to differentially activate Slc26a6 and Slc26a3.
Surprisingly, S1611 induced virtually no increase in DBS in the absence of CFTR expression. The same was observed after luminal application of the CFTR(inh)-172. These observations demonstrate that CFTR transport activity, and not just its presence, is required for HCO3− export via the Slc26 anion exchangers. Since fluid absorptive rates were unaltered in CFTR-deficient, but decreased in Slc26a6-, Slc26a3-, and NHE3-deficient mice compared with the respective WT small intestine, the findings suggest a normal Slc26-mediated Cl−/HCO3− exchange activity as long as NHE3 is functioning as a proton recycling system, even in the absence of CFTR expression. However, when NHE3 is inhibited, CFTR function is required as a Cl− shunt pathway for the Slc26 exchangers to operate as HCO3− secretory transporters.
S1611 is a nonphysiological mode of stimulating duodenal HCO3− secretion. What about other modes of stimulation? As stated in RESULTS, S1611-mediated HCO3− secretion was the only stimulus that clearly induced an electroneutral HCO3− secretion, thus excluding the activation of anion conductance. We also tested a number of secretagogues that mimic the range of physiologically important signaling pathways for duodenal HCO3− secretion and involve Cl−-dependent and/or electroneutral transport pathways, which are modes of Slc26-mediated transport pathways for duodenal HCO3− secretion (52, 59, 61). All tested secretagogues elicited a robust HCO3− secretory response in the duodenum of anesthetized WT mice, and, somewhat to our surprise, none had significant stimulatory activity in the CFTR-deficient duodenum in vivo. Therefore, we are confident that a necessity for CFTR expression is present for all forms of Slc26-mediated duodenal HCO3− secretory activity.
Finally, the question remains under what physiological or pathophysiological conditions such a switch of the intestinal Slc26 exchangers from anion absorptive to HCO3− secretory mode occurs. NHE3 is inhibited by a large variety of hormones, enterotoxins, and neurotransmitters that operate via an intracellular elevation of cAMP, cGMP, or Ca2+ (14, 65). Physiological elevations of these second messengers, where tested, did not inhibit either Slc26a6 or Slc26a3 (1, 5a, 33). Isotope flux studies in muscle-stripped native small intestine of mouse (50) and rabbit (58) showed that the cAMP-mediated inhibition of mucosal to serosal Na+ flux was NHE3-dependent and was not accompanied by an equivalent inhibition of mucosal-to-serosal Cl− flux (50) but was accompanied by an increase in HCO3− secretion. The pathway for HCO3− secretion was not determined, but it is feasible that the HCO3− secretion was in part Slc26 mediated. This may indicate that during acute diarrheal disease, a part of the intestinal HCO3− loss is Slc26 mediated. Further studies are necessary to assess the regulation of the intestinal Slc26 members under pathophysiological conditions.
In summary, our data suggest that the Slc26 isoforms Scl26a3 (DRA) and Slc26a6 (PAT1) both participate in salt absorption in murine small intestine, with no apparent difference in absorptive rates in the presence as well as the absence of CFTR expression and or/function. However, a functionally active CFTR is an absolute requirement for Slc26-mediated intestinal HCO3− secretion. This suggests that Slc26a6 and Slc26a3 need proton recycling via Na+/H+ exchange to operate in the Cl− absorptive mode, as well as Cl− exit via CFTR to operate in the HCO3− secretory mode.
GRANTS
This work was funded by Deutsche Forschungsgemeinschaft Grant Se460/13-4 (to U. Seidler), a grant from the German Mucoviscidosis Foundation (to U. Seidler), a junior investigator (starter) grant from the Medical School Hanover “Hochschulinternen Leistungsförderung” program (to A. K. Singh), and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK 62809 (to M. Soleimani).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Alper SL, Stewart AK, Chernova MN, Zolotarev AS, Clark JS, Vandorpe DH. Anion exchangers in flux: functional differences between human and mouse SLC26A6 polypeptides. Novartis Found Symp 273: 107–119, 2006 [PubMed] [Google Scholar]
- 2.Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR. Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart. J Physiol 561: 721–734, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lunnemann M, Rottinghaus I, Krabbenhoft A, Engelhardt R, Rausch B, Weinman EJ, Donowitz M, Hubbard A, Kocher O, de Jonge HR, Hogema BM, Seidler U. Defective jejunal and colonic salt absorption and altered Na+/H+ exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflügers Arch 457: 1079–1091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan HC, Ruan YC, He Q, Chen MH, Chen H, Xu WM, Chen WY, Xie C, Zhang XH, Zhou Z. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J Physiol 587: 2187–2195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Chernova MN, Jiang LW, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants. Differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chinet TC, Fullton JM, Yankaskas JR, Boucher RC, Stutts MJ. Mechanism of sodium hyperabsorption in cultured cystic fibrosis nasal epithelium: a patch-clamp study. Am J Physiol Cell Physiol 266: C1061–C1068, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol Gastrointest Liver Physiol 270: G259–G267, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Cotton CU, Stutts MJ, Knowles MR, Gatzy JT, Boucher RC. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J Clin Invest 79: 80–85, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doring G, Gulbins E. Cystic fibrosis and innate immunity: how chloride channel mutations provoke lung disease. Cell Microbiol 11: 208–216, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 23: 104–114, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Doucet L, Mendes F, Montier T, Delepine P, Penque D, Ferec C, Amaral MD. Applicability of different antibodies for the immunohistochemical localization of CFTR in respiratory and intestinal tissues of human and murine origin. J Histochem Cytochem 51: 1191–1199, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Eggermont E. Gastrointestinal manifestations in cystic fibrosis. Eur J Gastroenterol Hepatol 8: 731–738, 1996 [PubMed] [Google Scholar]
- 14.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flint N, Pemberton PW, Lobley RW, Evans GS. Cytokeratin expression in epithelial cells isolated from the crypt and villus regions of the rodent small intestine. Epithelial Cell Biol 3: 16–23, 1994 [PubMed] [Google Scholar]
- 16.Furukawa O, Bi LC, Guth PH, Engel E, Hirokawa M, Kaunitz JD. NHE3 inhibition activates duodenal bicarbonate secretion in the rat. Am J Physiol Gastrointest Liver Physiol 286: G102–G109, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281: G1301–G1308, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Greger R. The membrane transporters regulating epithelial NaCl secretion. Pflügers Arch 432: 579–588, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Greger R, Mall M, Bleich M, Ecke D, Warth R, Riedemann N, Kunzelmann K. Regulation of epithelial ion channels by the cystic fibrosis transmembrane conductance regulator. J Mol Med 74: 527–534, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Grubb BR, Boucher RC. Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. Am J Physiol Gastrointest Liver Physiol 272: G393–G400, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. Am J Physiol Cell Physiol 266: C1478–C1483, 1994 [DOI] [PubMed] [Google Scholar]
- 23a.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25: 386–401, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hegyi P, Rakonczay Z, Jr, Farkas K, Venglovecz V, Ozsvari B, Seidler U, Gray MA, Argent BE. Controversies in the role of SLC26 anion exchangers in pancreatic ductal bicarbonate secretion. Pancreas 37: 232–234, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hinojosa-Kurtzberg AM, Johansson ME, Madsen CS, Hansson GC, Gendler SJ. Novel MUC1 splice variants contribute to mucin overexpression in CFTR-deficient mice. Am J Physiol Gastrointest Liver Physiol 284: G853–G862, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Ignath I, Hegyi P, Venglovecz V, Szekely CA, Carr G, Hasegawa M, Inoue M, Takacs T, Argent BE, Gray MA, Rakonczay Z., Jr CFTR expression but not Cl- transport is involved in the stimulatory effect of bile acids on apical Cl−/HCO3− exchange activity in human pancreatic duct cells. Pancreas 38: 921–929, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Finkbeiner WE, Widdicombe JH, McCray PB, Jr, Miller SS. Altered fluid transport across airway epithelium in cystic fibrosis. Science 262: 424–427, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Ko SBH, Zeng WZ, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunzelmann K. CFTR: interacting with everything? News Physiol Sci 16: 167–170, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kunzelmann K, Gerlach L, Frobe U, Greger R. Bicarbonate permeability of epithelial chloride channels. Pflügers Arch 417: 616–621, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Kunzelmann K, Mall M, Briel M, Hipper A, Nitschke R, Ricken S, Greger R. The cystic fibrosis transmembrane conductance regulator attenuates the endogenous Ca2+ activated Cl− conductance of Xenopus oocytes. Pflügers Arch 435: 178–181, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Lamprecht G, Gaco V, Turner JR, Natour D, Gregor M. Regulation of the intestinal anion exchanger DRA (downregulated in adenoma). Ann NY Acad Sci 1165: 261–266, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 277: G709–G716, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Malmberg EK, Noaksson KA, Phillipson M, Johansson ME, Hinojosa-Kurtzberg M, Holm L, Gendler SJ, Hansson GC. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol 291: G203–G210, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Modolell I, Guarner L, Malagelada JR. Digestive system involvement in cystic fibrosis. Pancreatology 2: 12–16, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Moyer K, Balistreri W. Hepatobiliary disease in patients with cystic fibrosis. Curr Opin Gastroenterol 25: 272–278, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflügers Arch 411: 58–68, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Planells-Cases R, Jentsch TJ. Chloride channelopathies. Biochim Biophys Acta 1792: 173–189, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Quinton PM. Cystic fibrosis: a disease in electrolyte transport. FASEB J 4: 2709–2717, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Quinton PM. The neglected ion: HCO3. Nat Med 7: 292–293, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Rakonczay Z, Jr, Hegyi P, Hasegawa M, Inoue M, You J, Iida A, Ignath I, Alton EW, Griesenbach U, Ovari G, Vag J, Da Paula AC, Crawford RM, Varga G, Amaral MD, Mehta A, Lonovics J, Argent BE, Gray MA. CFTR gene transfer to human cystic fibrosis pancreatic duct cells using a Sendai virus vector. J Cell Physiol 214: 442–455, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, Colledge WH. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet 4: 35–41, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Reddy MM, Quinton PM. Cl− permeability of sweat duct cell membranes: intracellular microelectrode analysis. Prog Clin Biol Res 254: 45–57, 1987 [PubMed] [Google Scholar]
- 46.Rottner M, Freyssinet JM, Martinez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res 10: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc Natl Acad Sci USA 90: 4166–4170, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 505: 411–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhoft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns MP, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflügers Arch 455: 757–766, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Seidler U, Singh A, Chen M, Cinar A, Bachmann O, Zheng W, Wang J, Yeruva S, Riederer B. Knockout mouse models for intestinal electrolyte transporters and regulatory PDZ adaptors: new insights into cystic fibrosis, secretory diarrhoea and fructose-induced hypertension. Exp Physiol 94: 175–179, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Sellers ZM, Childs D, Chow JYC, Smith AJ, Hogan DL, Isenberg JI, Dong H, Barrett KE, Pratha VS. Heat-stable enterotoxin of Escherichia coli stimulates a non-CFTR-mediated duodenal bicarbonate secretory pathway. Am J Physiol Gastrointest Liver Physiol 288: G654–G663, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, Barone SL, Engelhardt R, Zuo J, Seidler U, Soleimani M. Fructose-induced hypertension: essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int 74: 438–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119: 540–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh AK, Sjoblom M, Zheng W, Krabbenhoft A, Riederer B, Rausch B, Manns MP, Soleimani M, Seidler U. CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3− secretion. Acta Physiol (Oxf) 193: 357–365, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Sjöblom M, Nylander O. Isoflurane-induced acidosis depresses basal and PGE2-stimulated duodenal bicarbonate secretion in mice. Am J Physiol Gastrointest Liver Physiol 292: G899–G904, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Spiegel S, Phillipper M, Rossmann H, Riederer B, Gregor M, Seidler U. Independence of apical Cl−/HCO3− exchange and anion conductance in duodenal HCO3− secretion. Am J Physiol Gastrointest Liver Physiol 285: G887–G897, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger SLC26A6 in prostaglandin E2- but not forskolin-stimulated duodenal HCO3− secretion. Gastroenterology 130: 349–358, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Tuo BG, Wen GR, Seidler U. Phosphatidylinositol 3-kinase is involved in prostaglandin E2-mediated murine duodenal bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 293: G279–G287, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135: 1645.e3–1653.e3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang ZH, Wang T, Petrovic S, Tuo BG, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Yankaskas JR, Cotton CU, Knowles MR, Gatzy JT, Boucher RC. Culture of human nasal epithelial cells on collagen matrix supports. A comparison of bioelectric properties of normal and cystic fibrosis epithelia. Am Rev Respir Dis 132: 1281–1287, 1985 [DOI] [PubMed] [Google Scholar]
- 65.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.