Abstract
Diabetes is a major predictor of in-stent restenosis, which is associated with fibroproliferative remodeling of the vascular wall due to increased transforming growth factor-β (TGF-β) action. It is well established that thrombospondin1 (TSP1) is a major regulator of TGF-β activation in renal and cardiac complications of diabetes. However, the role of the TSP1-TGF-β pathway in macrovascular diabetic complications, including restenosis, has not been addressed. In mesangial cells, high glucose concentrations depress protein kinase G (PKG) activity, but not PKG-I protein, thereby downregulating transcriptional repression of TSP1. Previously, we showed that high glucose downregulates PKG-I protein expression by vascular smooth muscle cells (VSMCs) through altered NADPH oxidase signaling. In the present study, we investigated whether high glucose regulation of PKG protein and activity in VSMCs similarly regulates TSP1 expression and downstream TGF-β activity. These studies showed that high glucose stimulates both TSP1 expression and TGF-β bioactivity in primary murine aortic smooth muscle cells (VSMCs). TSP1 is responsible for the increased TGF-β bioactivity under high glucose conditions, because treatment with anti-TSP1 antibody, small interfering RNA-TSP1, or an inhibitory peptide blocked glucose-mediated increases in TGF-β activity and extracellular matrix protein (fibronectin) expression. Overexpression of constitutively active PKG, but not the PKG-I protein, inhibited glucose-induced TSP1 expression and TGF-β bioactivity, suggesting that PKG protein expression is insufficient to regulate TSP1 expression. Together, these data establish that glucose-mediated downregulation of PKG levels stimulates TSP1 expression and enhances TGF-β activity and matrix protein expression, which can contribute to vascular remodeling in diabetes.
Keywords: transforming growth factor-β, fibronectin
diabetes is associated with an increased incidence of macrovascular disease including in-stent restenosis (ISR) (7). ISR is an inflammatory and fibroproliferative response of vascular cells to the stenting procedure and also to the stent materials. One of the pathological characteristics of ISR involves vascular smooth muscle cell (VSMC) proliferation and accumulation of extracellular matrix proteins, resulting in vascular reocclusion. Hyperglycemia is known to induce transforming growth factor-β (TGF-β) and reactive oxygen species, which are key factors in neointimal remodeling in ISR (24, 41).
TGF-β is a pluripotent growth factor that drives fibroproliferative responses critical to restenosis (8, 27) and to many diabetic complications, including diabetic nephropathy, cardiomyopathy, and macrovascular disease (2, 38, 50). TGF-β is secreted by most cells in an inactive form, termed latent TGF-β, which must be converted to its active form to exhibit biological activity. We showed previously that the matricellular protein thrombospondin1 (TSP1) is an activator of latent TGF-β (6). Activation by TSP1 occurs through direct binding between TSP1 and the latent TGF-β complex. One of the sites critical for maintenance of latency is the conserved LSKL sequence in the latency-associated peptide (LAP) NH2-terminal domain of latent TGF-β. TSP1 binds to the LSKL sequence in the LAP domain to activate latent TGF-β (51). A peptide of this sequence, LSKL, has been used as a competitive antagonist of TSP1-mediated TGF-β activation in vitro and in several in vivo disease models, including diabetes and hypertension (2, 35).
TSP1 expression is upregulated in both Type 1 and Type 2 diabetic patients (40, 43, 44). Increased TSP1 protein levels and mRNA levels were shown in large vessels from diabetic Zucker rats, suggesting that TSP1 plays an important role in the development of diabetic vascular complications (40). Glucose stimulation of TSP1 expression is also observed in cultured vascular cells (smooth muscle cells, fibroblasts, and endothelial cells) (3, 32, 40). Moreover, TSP1 is highly upregulated following stenting and evidence suggests a role for TSP1 in restenosis (9, 18, 28).
TSP1 expression can be differentially regulated by glucose in a cell type-specific manner (3). We showed previously that high glucose stimulation of TSP1 levels in mesangial cells occurs through glucose-mediated downregulation of cGMP-dependent protein kinase (PKG) activity. PKG protein levels in mesangial cells were not affected by high glucose treatment, but PKG activity was decreased by glucose: the decreased PKG activity is the result of diminished nitric oxide-dependent cGMP generation and decreased activation of PKG through cGMP binding to the regulatory domain of PKG (45, 48). PKG represses the expression of upstream stimulatory factor 2 (USF2), a glucose-responsive transcription factor that binds to the TSP1 gene promoter. Under high glucose conditions, the downregulation of PKG activity relieves the repression of glucose-dependent USF2 expression and TSP1 transcription, leading to increased TGF-β activation in mesangial cells (46). In VSMCs, Raman et al. (32) showed that TSP1 expression is upregulated by high glucose treatment through the hexosamine pathway. We previously demonstrated that high glucose treatment of VSMCs reduced PKG-I levels (both mRNA and protein) through PKC-dependent activation of NADPH oxidase-derived superoxide production (25). This suggested that PKG might also be involved in regulation of TSP1 expression by VSMCs.
In the present studies, we determined whether glucose-mediated alteration of PKG activity is involved in regulation of the TSP1-TGF-β axis in VSMCs to better understand how a high glucose environment might trigger TGF-β action critical for fibroproliferative responses important for diabetic macrovascular remodeling. Data from these studies show that glucose stimulates TGF-β bioactivity in VSMC occurs through TSP1-dependent activation of latent TGF-β in a PKG-dependent manner. However, in contrast to glucose-treated mesangial cells, high glucose treatment regulates PKG at the mRNA and protein level in VSMCs, rather than at the level of nitric oxide-cGMP activation of PKG. These studies establish a molecular link between glucose, altered NADPH oxidase levels, and the VSMCs synthetic phenotype key to restenosis in diabetes through control of PKG and its downstream effects on the TSP1-TGF-β axis.
MATERIALS AND METHODS
Materials.
Anti-α-smooth muscle actin antibody was purchased from Biocarta (San Diego, CA). Antifibronectin antibody was purchased from BD Transduction Laboratories (San Diego, CA). Monoclonal antibody 133 raised against human platelet TSP1 stripped of TGF-β activity was described previously (42). Anti-TSP antibody (clone A4.1) was purchased from Lab Vision (Fremont, CA). Rabbit polyclonal antiserum against rat fibronectin was purchased from Life Technologies (Gaithersburg, MD). Anti-PKG I antibody was purchased from StressGen Biotechnology (Victoria, BC, Canada). Secondary antibody was purchased from Jackson ImmunoResearch (West Grove, PA). Anti-vasodilator-stimulated phosphoprotein (VASP) and anti-VASP-phosphospecific (Ser239) antibodies were purchased from Calbiochem (La Jolla, CA). LSKL and SLLK peptides were synthesized and purified by AnaSpec (San Jose, CA). Small interfering RNA (siRNA) for TSP1 and control siRNA were purchased from Ambion (Austin, TX). Cell culture media were purchased from Invitrogen (Carlsbad, CA). Elastase was purchased from Elastin Products (Owensville, MO). Collagenase type IV was purchased from Worthington Biochemical (Lakewood, NJ). Luciferase assay reagent and passive lysis buffer were purchased from Promega (Madison, WI). Human recombinant TGF-β and monoclonal anti-TGF-β1,2,3 antibody were purchased from R&D Systems (Minneapolis, MN). DetaNONOate was purchased from Alexis Biochemicals (San Diego, CA). The cGMP analog 8-(p-chlorophenylthio)-cGMP (8-pCPT-cGMP) was purchased from Sigma. The expression vector for PKG catalytic domain was described previously (16).
Cell culture.
Mouse aortic smooth muscle cells (VSMCs) were isolated from the thoracic and abdominal aortas of mice (C57BL/6J) as described previously (34). The mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). All protocols were approved by the Institutional Animal Care and Use Committee of both University of Kentucky and University of Alabama at Birmingham. Briefly, aortas from mice were digested in medium containing 0.1 mg/ml elastase and 180 U/ml collagenase type IV for 2 to 3 h until a single cell suspension was obtained. Cells were then plated in culture flasks with DMEM media containing 10% FBS, 5 mM glucose, 50 μg/ml streptomycin, and 50 U/ml penicillin. VSMCs prepared by this method were not contaminated with fibroblasts or endothelial cells as evidenced by a greater than 90% positive immunostaining for smooth muscle α-actin with fluorescein isothiocyanate-conjugated anti-α-actin antibody (Sigma). Cells were used at passages 1–3 in these studies.
Immunoblotting.
Mouse VSMCs (p2) were cultured and made quiescent in serum and insulin-free DMEM media containing 5 mmol/l glucose for 48 h. Cells were treated with serum-free DMEM media containing 5 mM or 30 mM glucose for different time periods. Mannitol (30 mmol/l) was used as osmolarity control. After treatment, conditioned media were collected. The protein concentrations in the conditioned media were measured by the Bio-Rad protein assay. Protein concentrations of the media or the cell lysates did not differ between the various treatment groups, and β-actin levels in immunoblots of cell lysates did not show differences in cellular protein over the assay time (data not shown). Equal amounts of protein in the conditioned media were loaded to 8% SDS-PAGE gel and transferred to nitrocellulose membranes to detect TSP1 and fibronectin protein levels using anti-TSP1 or antifibronectin antibodies as described previously (47). Equal loading and transfer of protein samples were also assayed by staining the blots with Ponceau S. In addition, cells were harvested after treatments. Cell lysates were prepared and protein concentrations were measured. Equal amounts of protein in cell extract were subjected to 10% SDS-PAGE and transferred to nitrocellulose membranes to detect PKG-I levels with a polyclonal anti-PKG antibody (47). The enhanced chemiluminescence detection system (Pierce) was used for visualization of immunoreactive bands. β-Actin was used as a loading control. Immunoblots were analyzed by scanning densitometry and quantified by Quantity One gel analysis (Bio-Rad).
For transfection studies, VSMCs were cultured and transiently transfected with 0.8–1 μg of expression vector for the catalytic domain of PKG (PKG-CD, pcDNA1-CD) or empty vector (16) using Lipofectamine 2000 transfection reagent (Invitrogen). In another set of experiments, cells were plated in six-well plates and infected with adenoviral vector for bovine PKG-I (Ad.PKG-I) or control adenoviral vector (adenoviral green fluorescent protein) [Human Adenoviral-type 5 (DE1/E3), Vector Biolabs, Philadelphia, PA] at dosage of 20 multiplicity of infection of virus of virus/well. After overnight infection, cells were treated with 5 mM or 30 mM glucose in the absence or presence of nitric oxide donor (5 μM DetaNONOate) or cGMP analog (1 μM 8-pCPT-cGMP) for 24 h. TSP1 protein levels in the conditioned media were determined as described above by immunoblotting. The infection efficiency was >90%.
TGF-β bioassay.
Total and active TGF-β levels in the conditioned media were assayed using the plasminogen activator inhibitor-1 (PAI-1)/luciferase bioassay as described previously (1). Mink lung epithelial cells stably expressing the firefly luciferase reporter gene under the control of the TGF-β-response element of PAI-1 promoter were plated in 24-well plates at a density of 2.5 × 105 cells/well with DMEM containing 10% FBS, 2 mM l-glutamine, 1% penicillin-streptomycin, and 200 μg/ml G418. Cells were allowed to attach for 4 h and washed with serum-free media. Conditioned media and TGF-β standards were added to the cells and incubated overnight. To measure total TGF-β, conditioned media samples were heat activated for 3 min at 100°C. After incubation, cell lysates were prepared and luciferase activity was measured using a luminometer. The mean values of triplicate samples were converted into concentrations of TGF-β (pM) using a standard curve obtained with human recombinant TGF-β1 (Quantikine, R&D Systems). Specificity of the reporter activity was determined by neutralizing TGF-β activity in conditioned media with an anti-TGF-β antibody.
RNA isolation, Northern blot assay, and real-time PCR assay.
Quiescent cells were treated for 24 h with serum-free media containing either 5 or 30 mM glucose. After treatment, total RNA was extracted and the Northern blot assay for PKG-I mRNA abundance was performed as described previously (25). In addition, real-time PCR was performed to determine the changes in PKG-I mRNA levels in VSMCs after 24 h of 5 or 30 mM glucose treatment. Primers were synthesized by Integrated DNA Technologies, with sequences as follows: forward 5′-AAACTCCACAAATGCCAGTCGGTG-3′ and reverse 5′-TTTAGTGAACTTCCGGAACGCCTG-3′. Real-time PCR analyses were performed using SYBR Green PCR Master Mix kit with a MyiQ real-time PCR thermal cycler (Bio-Rad). Total RNA was converted to cDNA for PCR reaction. The conditions for PCR were 94°C for 10 min, followed by 35 cycles of 94°C for 30 s, and 55°C for 30 s. All reactions were performed in triplicate in a final volume of 25 μl. A standard curve was generated using 18S RNA primers. The quantities of PKG-I and internal control 18S RNA were determined from the standard curve using the MyiQ software, and PKG-I mRNA levels were normalized to 18S levels. All experiments were repeated three times.
PKG enzyme activity assay.
After treatment, cells were harvested and homogenized. Supernatants were assayed for PKG activity by measuring the incorporation of P32 from [γ-P32]ATP into a specific PKG substrate, the BPDEtide: RKISASEFDRPL (AnaSpec) as described previously (4). Briefly, 10 μl of extract was assayed for PKG activity in 100 μl assay buffer with 0.2 mM [γ-P32]ATP, 100 μM of a BPDEtide, and 1 μM PKI (PKA inhibitor). Assays were conducted at 30°C for 5 min. The reaction was terminated by aliquoting samples to P81 phosphocellulose paper and washing in 75 mM phosphoric acid. Then the paper was dried and counted in 10 ml scintillation fluid. PKG activity was expressed as nanomoles of peptide phosphorylated per minute per milligram of cell extract protein.
Statistical analysis.
Data are expressed as means ± SD. Statistical evaluation of the data was performed using ANOVA or Student's t-test as appropriate, considering the P value of <0.05 as significant.
RESULTS
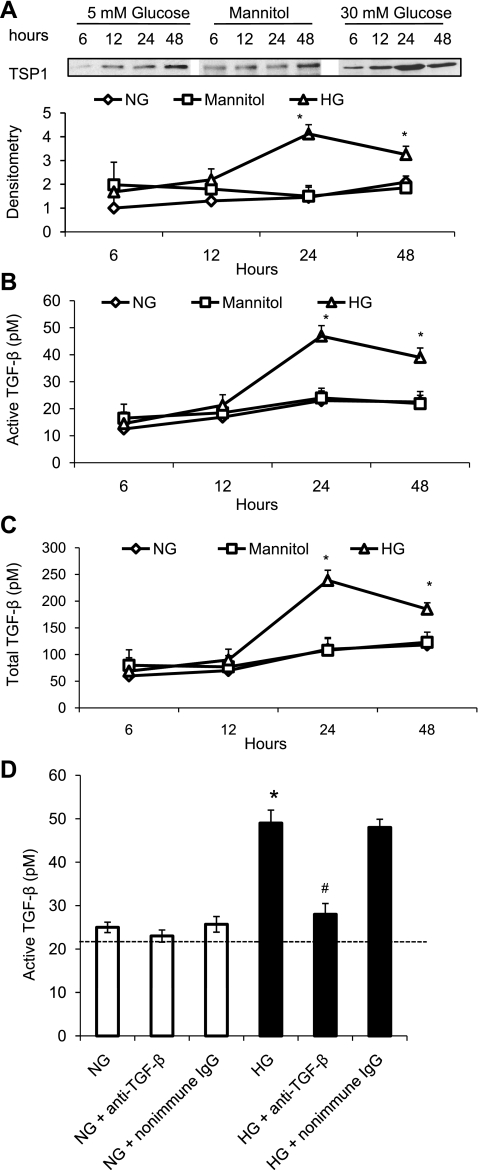
High glucose concentrations upregulate TSP1 protein levels, TGF-β bioactivity, and TGF-β protein production in a time-dependent manner in VSMCs.
We previously demonstrated that high glucose concentrations stimulate TSP1 expression and TGF-β bioactivity in mesangial cells (31). In current studies, we evaluated whether high glucose concentrations upregulate TSP1 expression and TGF-β bioactivity in mouse VSMCs. The results showed that high glucose (30 mM) treatment upregulated TSP1 expression in a time-dependent manner (Fig. 1A). The maximum response occurred at 24 h of 30 mM glucose treatment. Mannitol (30 mM) had no effect on TSP1 expression, suggesting that glucose stimulation of TSP1 expression is independent of osmotic changes. Similarly, TGF-β bioactivity and TGF-β protein production were also upregulated by high glucose treatment in a time-dependent manner (Fig. 1, B and C). This assay utilizes the TGF-β response element of the PAI-1 promoter to measure TGF-β activity (1). To distinguish possible glucose-dependent from TGF-β-dependent effects on this PAI-1 promoter reporter construct, PAI-1 reporter luciferase activity stimulated by conditioned media of cells treated with either normal or high glucose for 24 h was assessed in the presence of anti-TGF-β neutralizing antibody. We found that ∼92% of high glucose-induced PAI-1 promoter activity was diminished by anti-TGF-β treatment (Fig. 1D). However, under normal glucose conditions, only ∼5% of the PAI-1 promoter activity was inhibited by anti-TGF-β antibody treatment, suggesting that basal levels of PAI-1 promoter activity are TGF-β independent, whereas nearly all of the reporter activity under high glucose conditions is due to TGF-β stimulation of this site of the PAI-1 promoter rather than due to stimulation by glucose itself. In addition, cell number was determined after 24 h of glucose treatment, and under these conditions, there was no statistically significant difference in cell number between VSMCs grown in either normal or high glucose concentrations. Since the maximum effect was achieved at 24 h of high glucose treatment, the following studies were performed after 24 h of glucose treatment.
Fig. 1.
High glucose (HG) stimulates thrombospondin1 (TSP1) and transforming growth factor-β (TGF-β) expression in vascular smooth muscle cells (VSMCs). Mouse VSMCs (p2) were cultured and made quiescent in serum and insulin-free DMEM media containing 5 mmol/l glucose for 48 h. Cells were treated with serum-free DMEM media containing 5 mM or 30 mM glucose for indicated time points. After treatment, conditioned media were collected and equal amounts of protein in the conditioned media were used to analyze TSP1 protein levels by immunoblotting (A). Equal loading and transfer of protein samples were assayed by staining the blots with Ponceau S. The data shown are representative of three separate experiments. Relative TSP1 levels were determined by scanning densitometry of immunoblots. Results are means ± SD. TGF-β levels in the conditioned media were measured by plasminogen activator inhibitor-1 (PAI-1)/luciferase bioassay (B and C). To distinguish possible glucose-dependent from TGF-β-dependent effects on this PAI-1 promoter reporter construct, PAI-1 reporter luciferase activity stimulated by conditioned media of cells treated with either 5 mM or 30 mM glucose for 24 h was assessed in the presence of anti-TGF-β neutralizing antibody (10 μg/ml) (D). Results are expressed as means ± SD of triplicate determinations of three separate experiments. NG, normal glucose. *P < 0.05 vs. NG; #P < 0.05 vs. HG.
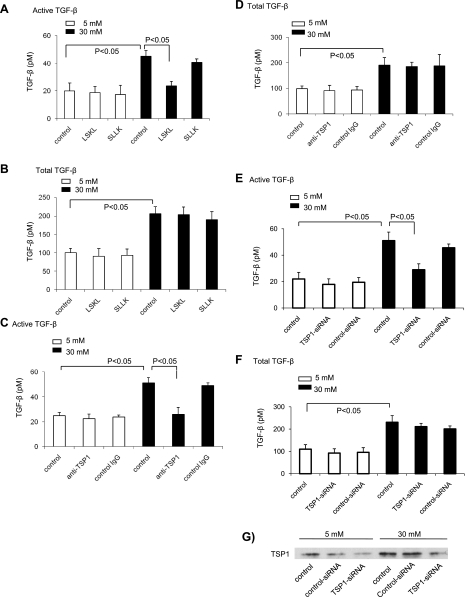
Glucose stimulation of TGF-β bioactivity in VSMCs is TSP1 dependent.
We demonstrated that TSP1 activates latent TGF-β, both in vitro and in vivo (10, 36, 37). We tested whether glucose stimulation of TGF-β bioactivity in VSMCs also occurs through a TSP1-dependent mechanism. Peptide antagonists of TSP1-mediated TGF-β activation, a neutralizing anti-TSP1 antibody, and siRNA-mediated TSP1 knockdown were used to test this hypothesis. Incubation of the inhibitory peptide (LSKL) (Fig. 2A) or the neutralizing anti-TSP1 antibody (Fig. 2C) with VSMCs cultured in 30 mM glucose media reduced the stimulatory effect of high glucose on TGF-β bioactivity. TGF-β activity was reduced to levels observed in VSMCs cultured with 5 mM glucose media (basal levels). In another set of experiments, siRNA for TSP1 and control siRNA were delivered to VSMCs by nucleofection (Amaxa) according to the manufacturer's instructions. siRNA-TSP1 significantly knocked down TSP1 levels in VSMCs (∼70–80% reduction, Fig. 2G). siRNA-mediated TSP1 knockdown reduced glucose-induced active TGF-β levels (Fig. 2E). Levels of total TGF-β were not affected by the inhibitory peptide, siRNA-TSP1, or the anti-TSP1 antibody (Fig. 2, B, D, and F). Furthermore, TGF-β activity in cultures grown under basal glucose conditions was not reduced by the antagonist peptide, the neutralizing antibody, or by siRNA-TSP1. Control peptide (SLLK), nonimmune IgG, or control siRNA did not affect glucose-stimulated levels of TGF-β activity or total TGF-β levels. Together, these data indicate that glucose simulation of TGF-β bioactivity in VSMCs occurs through a TSP1-dependent mechanism.
Fig. 2.
LSKL peptide, anti-TSP1 antibody, and small interfering RNA (siRNA)-mediated TSP1 knockdown block glucose-induced TGF-β activity. Quiescent VSMCs (p2) were treated with TSP1 antagonist peptide (10, 15, 36) (LSKL at 5 μmol/l) or control peptide (SLLK at 5 μmol/l) (A and B), monoclonal anti-TSP1 antibody (20 μg/ml) or nonimmune IgG (20 μg/ml) (C and D) for 24 h. In addition, another set of cells was transfected with siRNA-TSP1 or control siRNA and then treated with 5 mM or 30 mM glucose for 24 h (E and F). After treatment, conditioned media were collected to measure TGF-β levels by using PAI-1/luciferase bioassay. For total TGF-β, the samples were heat activated for 3 min at 100°C. Results are expressed as means ± SD of triplicate determinations of three separate experiments. The knockdown of TSP1 protein was confirmed by immunoblotting (G). A representative blot is shown.
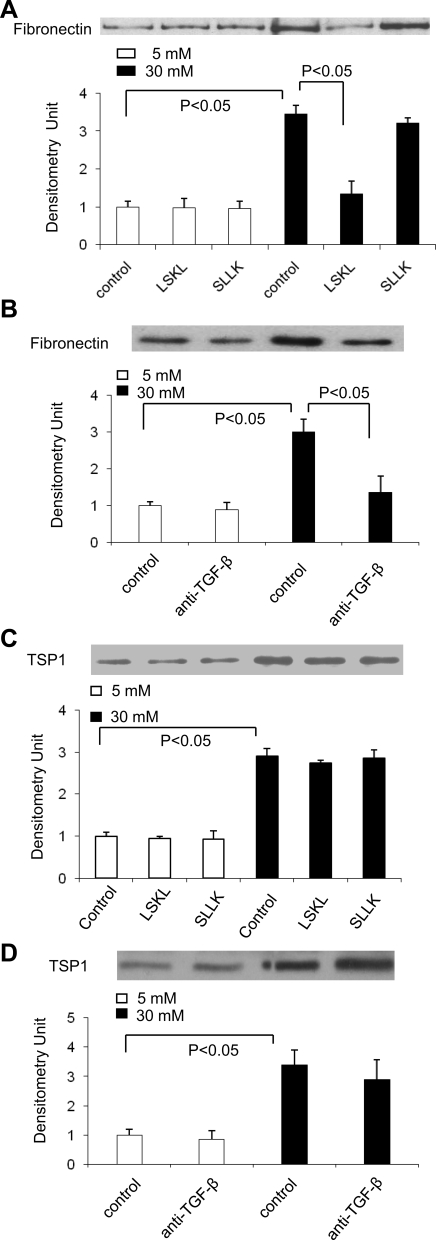
Glucose-induced extracellular matrix protein expression is dependent on TSP1-mediated activation of TGF-β in VSMCs.
Fibronectin expression is increased in aortic smooth muscle cells from diabetic rats and also in the tunica media of aortas from both type 1 and type 2 diabetic patients (23, 33). Therefore, we examined whether high glucose treatment stimulates fibronectin expression in VSMCs and whether blocking TSP1-dependent TGF-β activity inhibits glucose-dependent stimulation of fibronectin synthesis. The results showed that glucose stimulated fibronectin expression in VSMCs, and this increased expression was inhibited by either LSKL peptide (Fig. 3A) or a pan-specific anti-TGF-β1,2,3 neutralizing antibody (Fig. 3B). Control peptide, SLLK, or nonimmune IgG did not affect fibronectin levels secreted by VSMCs cultured in serum-free media containing either 5 or 30 mM glucose. These data show that glucose-stimulated fibronectin expression is dependent on TSP1 stimulation of latent TGF-β activation.
Fig. 3.
Fibronectin expression is upregulated by TSP1-dependent TGF-β under high glucose conditions. VSMCs (p2) were treated with 5 mM or 30 mM glucose in the presence of peptides [LSKL (5 μmol/l) and SLLK (5 μmol/l)] or monoclonal anti-TGF-β1,2,3 antibody (10 μg/ml) and control IgG for 24 h. Then conditioned media were collected and equal amounts of protein in the conditioned media were used to analyze fibronectin (A and B) and TSP1 (C and D) protein levels by immunoblotting. Equal loading and transfer of protein samples were assayed by staining the blots with Ponceau S. The data shown are representative of three separate experiments. Relative levels of TSP1 or fibronectin were determined by scanning densitometry of immunoblots. Results are means ± SD of three experiments.
In contrast to fibronectin, the expression of TSP1 by VSMCs cultured in either 5 mM or 30 mM of glucose was not affected by either the antagonist peptide (Fig. 3C) or anti-TGF-β1,2,3 antibody treatment (Fig. 3D), suggesting that glucose regulation of TSP1 expression by VSMCs is primarily TGF-β independent.
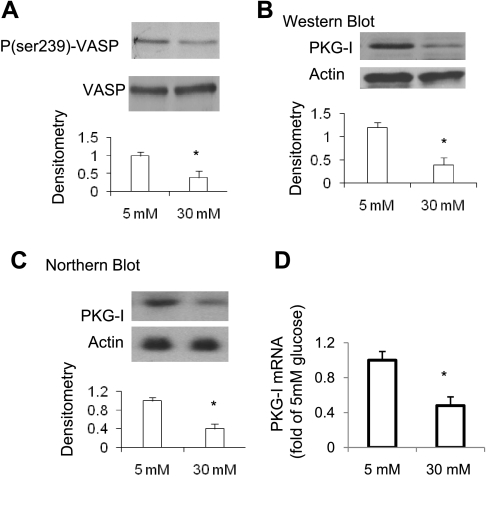
High glucose concentrations downregulate PKG activity and PKG levels (mRNA and protein) in VSMCs.
We showed that high glucose concentrations downregulate nitric oxide bioavailability in mesangial cells, leading to decreased cGMP levels and PKG activity without changes in PKG protein levels (45). In this study, we examined whether the PKG signaling pathway is similarly downregulated in VSMCs by high glucose treatment. Results showed that levels of phosphorylated-Ser239- VASP [reflecting PKG activity (39)] were markedly lower after 24 h of high glucose treatment (Fig. 4A). This was confirmed by measuring PKG enzyme activity (Table 1). Furthermore, 24 h of high glucose treatment reduced PKG mRNA levels as measured by either Northern blotting or real-time PCR analysis to ∼40% of basal levels (Fig. 4, C and D). PKG protein levels were reduced to a similar extent by high glucose treatment (Fig. 4B). These data are consistent with our previous findings that high glucose concentrations downregulate PKG mRNA/protein levels, as well as PKG activity, in VSMCs through an NADPH oxidase-dependent mechanism (25).
Fig. 4.
High glucose treatment reduces PKG levels (protein and mRNA) as well as PKG activity. Quiescent VSMCs (p2) were treated with 5 mM or 30 mM glucose for 24 h. Cells were harvested. A: phospho (p)-Ser239-vasodilator-stimulated phosphoprotein (VASP) levels in cell lysates were analyzed by immunoblotting and normalized to total VASP levels. B: PKG-I protein levels in cell lysates were analyzed by immunoblotting. A representative immunoblot is shown. Relative levels of PKG, p-VASP, or VASP were determined by scanning densitometry of immunoblots. Phospho-Ser239-VASP levels were determined following normalization to levels of total VASP and indicate PKG activity. C: PKG-I mRNA levels were analyzed by Northern blotting and normalized to β-actin levels (n = 3). D: PKG-I mRNA levels were analyzed by quantitative real-time PCR and normalized to 18S RNA. Results are means ± SD (n = 3). *P < 0.05 vs. 5 mM glucose.
Table 1.
PKG enzyme activity in VSMCs (fold change of NG + vector group)
| Treatment | Without NO or cGMP | + NO | + cGMP |
|---|---|---|---|
| NG + vector | 1 | 2.01 ± 0.07 | 2.32 ± 0.18 |
| HG + vector | 0.65 ± 0.13* | 1.19 ± 0.09* | 1.41 ± 0.15* |
| NG + PKG-CD | 2.11 ± 0.17† | 4.02 ± 0.14† | 4.07 ± 0.20† |
| HG + PKG-CD | 1.98 ± 0.08‡§ | 3.49 ± 0.20‡§ | 3.38 ± 0.24‡§ |
Values are means ± SD of three separate experiments. Vascular smooth muscle cells (VSMCs) were transiently transfected with pCDNA1-catalytic domain (CD) and control vector. After 24 h of normal (NG) or high glucose (HG) treatment, cells were harvested and PKG activity in the cell lysates was analyzed in the presence or absence of nitric oxide (NO; 5 μM DetaNONOate) or cGMP [1 μM 8-(p-chlorophenylthio)-cGMP] using the method as described in materials and methods.
P < 0.05 vs. NG + vector;
P < 0.05 vs. NG + vector;
P < 0.05 vs. HG + vector;
not significant, HG + PKG-CD vs. NG + PKG-CD.
Overexpression of constitutively active PKG-I (the catalytic domain of PKG-I) reduces glucose stimulation of TSP1 expression and TSP1-dependent TGF-β activation in VSMCs.
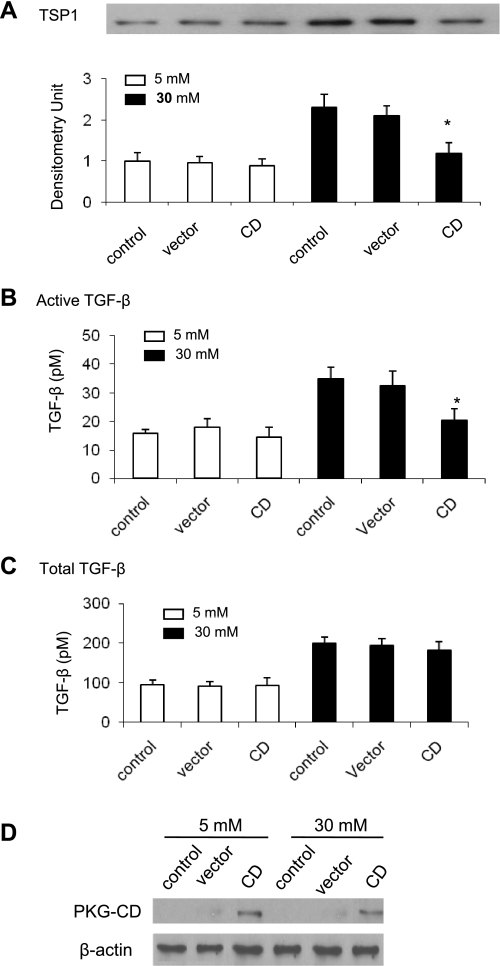
Mouse VSMCs were transiently transfected with the PKG catalytic domain or a empty vector control for 24 h (16). After transfection, conditioned media were collected to analyze TSP1 protein levels and to measure TGF-β activity. Cells were harvested for analysis of PKG activity. As shown in Table 1, overexpression of the PKG catalytic domain (PKG-CD) increased PKG activity in VSMCs under both normal and high glucose conditions to similar levels and, importantly, overcame high glucose-mediated deceases in PKG activity. Overexpression of PKG-CD significantly reduced glucose stimulation of TSP1 protein levels but had no effect on basal TSP1 protein levels (5 mM glucose treatment) (Fig. 5A). Similarly, glucose stimulation of TGF-β bioactivity was also blocked by overexpression of the catalytic domain of PKG, but total TGF-β production was not altered (Fig. 5, B and C). Transfection efficiency was confirmed by immunoblotting of PKG-CD in cell lysates (Fig. 5D). These data indicate that there is a correlation between reduced PKG activity and glucose induction of TSP1 expression and TSP1-dependent TGF-β activation by VSMCs.
Fig. 5.
Overexpression of the catalytic domain of PKG (PKG-CD) inhibits glucose stimulation of TSP1 expression and TSP1-dependent TGF-β activation in mouse VSMCs. Quiescent VSMCs (p2) were transiently transfected with pcDNA1-CD. After 24 h of glucose treatment, conditioned media were collected. A: TSP1 protein levels were analyzed by immunoblotting. Equal loading and transfer of protein samples were assayed by staining the blots with Ponceau S. The data shown are representative of three separate experiments. Relative TSP1 levels were determined by scanning densitometry of immunoblots. B and C: active TGF-β (B) or total TGF-β levels (C) in conditioned media were measured by using PAI-1/luciferase bioassay. For total TGF-β, the samples were heat activated for 3 min at 100°C. Results are means ± SD of triplicate determinations of three separate experiments. *P < 0.05 vs. 30 mM + vector. D: after treatment, cells were harvested. Expression of PKG-CD in cell lysates was analyzed by immunoblotting. A representative blot is shown. β-Actin was used as internal control.
Effect of nitric oxide donor and cGMP analog treatment on glucose stimulation of TSP1 protein levels in VSMCs.
Previously, we showed that high glucose treatment downregulated nitric oxide and cGMP levels, resulting in decreased PKG activity in mesangial cells. PKG-I protein levels were not altered by high glucose treatment. Addition of either a nitric oxide donor or a cGMP analog to high glucose-treated cultures to overcome glucose-mediated decreased PKG activity restored TSP1 levels to normal (45). Glucose-mediated downregulation of nitric oxide and cGMP levels also occurred in VSMCs (26, 29). In contrast to mesangial cells, PKG protein levels were also reduced by glucose treatment in VSMCs (Fig. 4 and Ref. 25). To determine whether nitric oxide or cGMP stimulation is in itself sufficient to modify PKG-mediated regulation of TSP1 expression in VSMCs under high glucose conditions, VSMCs were treated with either a nitric oxide donor (DetaNONOate) or a cGMP analog (8-pCPT-cGMP) for 24 h. After treatment, conditioned media were collected and TSP1 protein levels were analyzed by immunoblotting. Neither the nitric oxide donor nor the cGMP analog significantly altered TSP1 protein levels under either normal or high glucose conditions (Fig. 6A). This result is consistent with our findings that levels of PKG activity in nitric oxide or cGMP stimulated VSMCs under high glucose conditions were about 60% of nitric oxide or cGMP stimulated VSMCs under normal glucose conditions (Table 1). The fold PKG activation by the nitric oxide donor or the cGMP analog was similar under both normal and high glucose conditions, suggesting that the PKG enzyme sensitivity to nitric oxide/cGMP activation is not altered by high glucose. Rather the reduced PKG activity under high glucose conditions appears to be due to the nearly 60% reduction in PKG protein.
Fig. 6.
Effect of nitric oxide (NO) donor or cGMP analog on TSP1 protein levels under either normal or high glucose conditions. A: quiescent VSMCs were treated with nitric oxide donor (5 μM DetaNONOate) or cGMP analog [1 μM 8-(p-chlorophenylthio)-cGMP] for 24 h. B: VSMCs were cultured in 6-well plates, infected with adenoviral vector PKG-I (Ad.PKG-I) or control adenoviral vector-green fluorescent protein (Ad.control; 20 multiplicity of infection of virus/well) overnight, and then treated with 5 mM or 30 mM glucose in the presence or absence of nitric oxide donor or cGMP analog for 24 h. After treatment, conditioned media were collected for analysis of TSP1 protein levels by immunoblotting. Equal loading and transfer of protein samples were assayed by staining the blots with Ponceau S. The data shown are representative of three separate experiments. Relative TSP1 levels were determined by scanning densitometry of immunoblots. Results are means ± SD. *P < 0.05 vs. 5 mM glucose; #P < 0.05 vs. 30 mM glucose.
In contrast, when VSMCs cultured under high glucose conditions are engineered to express PKG-1 protein by infection with an adenovirus construct of the full-length PKG-1 protein containing both the regulatory and the catalytic domains, treatment with the nitric oxide donor or the cGMP analog is now able to reduce the elevated TSP1 protein levels observed under high glucose conditions (Fig. 6B). Expression of PKG-I protein without activation did not block 30 mM glucose stimulation of TSP1 expression, suggesting that PKG-I protein expression in itself is not sufficient to regulate TSP1 expression.
DISCUSSION
These studies show that glucose stimulates increased TGF-β activity and extracellular matrix production by VSMCs through increased TSP1 expression and consequent latent TGF-β activation. Glucose downregulates PKG mRNA and protein expression in VSMCs, which thus attenuates PKG-mediated repression of TSP1 expression. These findings suggest that glucose-mediated downregulation of PKG protein expression has important consequences for upregulation of the fibrotic TSP1-TGF-β pathway, and this is potentially important in diabetic macrovascular fibroproliferative remodeling.
It is known that PKG is a downstream signaling mediator of nitric oxide and cGMP. PKG is a serine/threonine kinase, consisting of a regulatory and a catalytic domain. Binding of cGMP by the regulatory domain leads to activation of the catalytic domain and increases PKG activity (48). We have demonstrated that PKG activity is downregulated by high glucose exposure. The mechanisms by which glucose downregulates PKG activity appear to be cell type specific. Our previous studies demonstrated that PKG protein levels in mesangial cells were not affected by high glucose exposure. Rather, the nitric oxide and cGMP level was decreased in mesangial cells under high glucose conditions, resulting in decreased PKG kinase activity (45, 48). In contrast, in VSMCs, glucose decreases steady-state mRNA levels as well as protein levels of PKG, suggesting that high glucose regulates PKG expression at the transcriptional or posttranscriptional levels. Moreover, we demonstrated that PKC-dependent activation of NAD(P)H oxidase-derived superoxide production is involved in glucose-mediated regulation of PKG protein in VSMCs (25). In VSMCs, we showed that high glucose treatment stimulated expression of p47phox, but not p22phox NADPH oxidase subunits, leading to upregulation of NADPH oxidase (25). In contrast, studies from Xia et al. (49) demonstrated that both p47phox and p22phox were increased by high glucose treatment in mesangial cells. Whether differential regulation of NADPH oxidase subunits by glucose in VSMCs and mesangial cells accounts for the difference in regulation of PKG message and protein between these two types of cells is unknown and needs to be investigated further.
As discussed above, high glucose treatment downregulates PKG protein and/or activity in a cell type-specific manner. This may lead to cell type-specific regulation of TSP1 expression by glucose. Previously, we showed that glucose stimulates TSP1 transcription through concomitant upregulation of the transcription factor USF2 and downregulation of PKG activity (46). Stenina and colleagues showed that glucose stimulation of TSP1 transcription by VSMCs is regulated by the hexosamine pathway downstream of glucose catabolism (32). In contrast to endothelium from the macrovasculature (11), glucose downregulates TSP1 expression by microvascular endothelial cells by posttranscriptional mechanisms (3). In our current studies, we now identify a role for PKG in downregulation of TSP1 expression by VSMCs and the attenuation of this pathway under high glucose conditions. It is not known whether PKG signaling and the hexosamine pathway are interrelated in their regulation of TSP1 expression under high glucose conditions. PKG has been shown to regulate glucose oxidation in skeletal muscle (52). It is possible that downregulation of PKG activity is necessary for stimulation of TSP1 expression by the hexosamine pathway. Interestingly, studies from Isenberg and colleagues (20, 21) demonstrated that TSP1 can inhibit nitric oxide-cGMP signaling in endothelial cells, vascular smooth muscle cells, and platelets. This could result in a feed-forward loop that could amplify the effects of high glucose on attenuation of PKG activity in VSMCs.
We previously showed that high glucose exposure reduced nitric oxide synthesis and cGMP generation in mesangial cells but without alteration of PKG protein levels. Therefore, addition of nitric oxide donor or cGMP analog to activate PKG inhibited high glucose-induced TSP1 expression and TGF-β activity in this cell type (45). However, in VSMCs, PKG protein levels were reduced under high glucose conditions. As expected, addition of a nitric oxide donor or a cGMP analog increased PKG activity to a lesser extent in high glucose-treated cells than in normal glucose-treated cells, resulting in reduced inhibition of high glucose-induced TSP1 expression by either nitric oxide or cGMP treatment. Consistent with the above result, high glucose-induced TSP1 expression was inhibited in VSMCs by overexpression of full-length PKG-I and activation by nitric oxide donor or cGMP analog. Overexpression of PKG-I in the absence of NO or cGMP treatment had no effect on glucose-induced TSP1 expression, implicating PKG enzymatic activity in glucose-induced TSP1 expression. Furthermore, transfection of VSMCs with the catalytic domain of PKG (constitutively active PKG) overcame the high glucose-mediated decrease in PKG activity, resulting in inhibition of glucose-induced TSP1 expression and TGF-β activity. In this study, TSP1 expression and TGF-β activity under normal glucose conditions are not regulated by PKG activity, suggesting that basal TSP1 expression is maintained through different mechanisms, such as the involvement of transcription factors YY-1 or ELL (22, 53). Taken together, our present studies identify a novel mechanism of high glucose-mediated downregulation of PKG activity and resultant upregulation of TSP1 expression in VSMCs.
TSP1 is a multifunctional protein that is produced by a variety of cells (5, 17, 19, 30). One of the major functions of TSP1 is its role as a regulator of latent TGF-β activation (6). TGF-β is a pluripotent growth factor which drives fibroproliferative responses critical to restenosis (8, 27) and to many diabetic complications, including diabetic nephropathy, cardiomyopathy, and macrovascular disease (2, 38, 50). TSP1-mediated activation of latent TGF-β has been shown to play an important role in a number of fibroproliferative diseases in multiple organ systems, including fibrotic diabetic complications in the myocardium and the kidney (2, 12–15). Recently, we demonstrated that stainless steel ions stimulate increased TSP1-dependent TGF-β activation in VSMCs, which plays a role in ISR (28). It is known that diabetes is a significant risk factor for ISR (7). Hyperglycemia has been shown to induce TGF-β and reactive oxygen species, which are key factors in neointimal remodeling in ISR (24, 41). Our current studies further demonstrated that high glucose treatment to mimic diabetic conditions stimulated TSP1-dependent TGF-β activation and accumulation of extracellular matrix proteins in VSMCs via decreased PKG activity, supporting the role of this pathway in the development of diabetic macrovascular fibroproliferative remodeling including ISR occurrence in diabetic patients.
In summary, we demonstrated that glucose stimulation of TGF-β bioactivity in vascular smooth muscle cells occurs through a TSP1-dependent mechanism following upregulation of TSP1 expression. Moreover, expression of PKG by VSMCs is suppressed by glucose exposure through NADPH oxidase-derived reactive oxygen species. These studies establish a molecular link between glucose, altered NADPH oxidase levels, and the VSMC synthetic phenotype through control of PKG and its downstream effects on the TSP1-TGF-β axis. These data suggest that this pathway plays an important role in the development of diabetic macrovascular fibroproliferative remodeling. Importantly, transfection of the catalytic domain of PKG to increase PKG activity may represent a therapeutic approach for attenuating the development of diabetic macrovascular complications.
GRANTS
This work was supported by American Heart Association Grant 0435132N (to S. Wang), National Institutes of Health (NIH) Grant DK60658 (to J. E. Murphy-Ullrich), and NIH Grant HL-53426 (to T. M. Lincoln). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06 RR 15490 from the National Center for Research Resources, NIH.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol 171: 777–789, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Marinic TE, Krukovets I, Hoppe G, Stenina OI. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem 283: 5699–5707, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res 34: 245–259, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest 107: 929–934, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol 36: 1115–1125, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Carter AJ, Bailey L, Devries J, Hubbard B. The effects of uncontrolled hyperglycemia on thrombosis and formation of neointima after coronary stent placement in a novel diabetic porcine model of restenosis. Coron Artery Dis 11: 473–479, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain J. Transforming growth factor-beta: a promising target for anti-stenosis therapy. Cardiovasc Drug Rev 19: 329–344, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain J, Gunn J, Francis SE, Holt CM, Arnold ND, Cumberland DC, Ferguson MW, Crossman DC. TGFbeta is active, and correlates with activators of TGFbeta, following porcine coronary angioplasty. Cardiovasc Res 50: 125–136, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93: 1159–1170, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res 102: 1558–1565, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel C, Amann K, Hohenstein B, Bornstein P, Hugo C. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in experimental glomerulonephritis in mice. J Am Soc Nephrol 18: 788–798, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes 56: 2982–2989, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Daniel C, Takabatake Y, Mizui M, Isaka Y, Kawashi H, Rupprecht H, Imai E, Hugo C. Antisense oligonucleotides against thrombospondin-1 inhibit activation of TGF-beta in fibrotic renal disease in the rat in vivo. Am J Pathol 163: 1185–1192, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int 65: 459–468, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Dey NB, Boerth NJ, Murphy-Ullrich JE, Chang PL, Prince CW, Lincoln TM. Cyclic GMP-dependent protein kinase inhibits osteopontin and thrombospondin production in rat aortic smooth muscle cells. Circ Res 82: 139–146, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Donoviel DB, Amacher SL, Judge KW, Bornstein P. Thrombospondin gene expression is associated with mitogenesis in 3T3 cells: induction by basic fibroblast growth factor. J Cell Physiol 145: 16–23, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann J, Simon P, Zimmermann AK, Lemancyk M, Walter T, Beyer M, Hoffmeister HM, Ziemer G, Wendel HP. Thrombospondin 1 as possible key factor in the hemocompatibility of endocoronary prostheses. Biomaterials 26: 5240–5250, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hugo C, Pichler R, Meek R, Gordon K, Kyriakides T, Floege J, Bornstein P, Couser WG, Johnson RJ. Thrombospondin 1 is expressed by proliferating mesangial cells and is up-regulated by PDGF and bFGF in vivo. Kidney Int 48: 1846–1856, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 111: 613–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res 71: 785–793, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Chang SY, Yeom DH, Kim SA, Um SJ, Hong KJ. Weakening of the repressive YY-1 site on the thrombospondin-1 promoter via c-Jun/YY-1 interaction. Exp Mol Med 36: 300–310, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kanzaki T, Shiina R, Saito Y, Zardi L, Morisaki N. Transforming growth factor-beta receptor and fibronectin expressions in aortic smooth muscle cells in diabetic rats. Diabetologia 40: 383–391, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Khan R, Agrotis A, Bobik A. Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc Res 74: 223–234, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med 42: 852–863, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Muniyappa R, Srinivas PR, Ram JL, Walsh MF, Sowers JR. Calcium and protein kinase C mediate high-glucose-induced inhibition of inducible nitric oxide synthase in vascular smooth muscle cells. Hypertension 31: 289–295, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Nabel EG, Shum L, Pompili VJ, Yang ZY, San H, Shu HB, Liptay S, Gold L, Gordon D, Derynck R, Nabel GJ. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA 90: 10759–10763, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallero MA, Talbert Roden M, Chen YF, Anderson PG, Lemons J, Brott BC, Murphy-Ullrich JE. Stainless steel ions stimulate increased thrombospondin-1-dependent TGF-Beta activation by vascular smooth muscle cells: implications for in-stent restenosis. J Vasc Res 47: 309–322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandolfi A, Grilli A, Cilli C, Patruno A, Giaccari A, Di Silvestre S, De Lutiis MA, Pellegrini G, Capani F, Consoli A, Felaco M. Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: association with increased nitric oxide synthase expression and superoxide anion generation. J Cell Physiol 196: 378–385, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Phelan MW, Forman LW, Perrine SP, Faller DV. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med 132: 519–529, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol 157: 1353–1363, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem 282: 5704–5714, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen LM, Heickendorff L. Accumulation of fibronectin in aortas from diabetic patients. A quantitative immunohistochemical and biochemical study. Lab Invest 61: 440–446, 1989 [PubMed] [Google Scholar]
- 34.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23: 185–188, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 274: 13586–13593, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J Biol Chem 269: 26783–26788, 1994 [PubMed] [Google Scholar]
- 37.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol 122: 923–932, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44: 1139–1146, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem 273: 20029–20035, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 107: 3209–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Tardif JC, Gregoire J, L'Allier PL. Prevention of restenosis with antioxidants: mechanisms and implications. Am J Cardiovasc Drugs 2: 323–334, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol 122: 497–511, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ, 3rd, Lee MJ, Fried SK, McGehee RE, Jr, Peterson CA, Kern PA. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57: 432–439, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia 4: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem 277: 9880–9888, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem 279: 34311–34322, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of constitutively active cGMP-dependent protein kinase prevents glucose stimulation of thrombospondin 1 expression and TGF-beta activity. Diabetes 52: 2144–2150, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Wernet W, Flockerzi V, Hofmann F. The cDNA of the two isoforms of bovine cGMP-dependent protein kinase. FEBS Lett 251: 191–196, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Xia L, Wang H, Goldberg HJ, Munk S, Fantus IG, Whiteside CI. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Am J Physiol Renal Physiol 290: F345–F356, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama H, Deckert T. Central role of TGF-beta in the pathogenesis of diabetic nephropathy and macrovascular complications: a hypothesis. Diabet Med 13: 313–320, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem 279: 38032–38039, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Young ME, Leighton B. Fuel oxidation in skeletal muscle is increased by nitric oxide/cGMP–evidence for involvement of cGMP-dependent protein kinase. FEBS Lett 424: 79–83, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Feng X, Ban B, Liu J, Wang Z, Xiao W. Elongation factor ELL (Eleven-Nineteen Lysine-rich Leukemia) acts as a transcription factor for direct thrombospondin-1 regulation. J Biol Chem 284: 19142–19152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]