Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and typically fatal lung disease for which no effective therapy has been identified. The disease is characterized by excessive collagen deposition, possibly in response to dysregulated wound healing. Mediators normally involved in would healing induce proliferation of fibroblasts and their differentiation to myofibroblasts that actively secrete collagen. Curcumin, a polyphenolic compound from turmeric, has been shown to exert a variety of biological effects. Effects on IPF and associated cell types remain unclear, however. We accordingly tested the ability of curcumin to inhibit proliferation and differentiation to myofibroblasts by human lung fibroblasts, including those from IPF patients. To further examine the potential usefulness of curcumin in IPF, we examined its ability to reduce fibrosis in bleomycin-treated mice. We show that curcumin effectively reduces profibrotic effects in both normal and IPF fibroblasts in vitro and that this reduction is accompanied by inhibition of key steps in the transforming growth factor-β (TGF-β) signaling pathway. In vivo, oral curcumin treatment showed no effect on important measures of bleomycin-induced injury in mice, whereas intraperitoneal curcumin administration effectively inhibited inflammation and collagen deposition along with a trend toward improved survival. Intraperitoneal curcumin reduced fibrotic progression even when administered after the acute bleomycin-induced inflammation had subsided. These results encourage further research on alternative formulations and routes of administration for this potentially attractive IPF therapy.
Keywords: transforming growth factor-β, platelet-derived growth factor, myofibroblast, Smad, extracellular signal-regulated kinase
idiopathic pulmonary fibrosis (IPF) is a progressive disease characterized by inappropriate accumulation of fibroblasts and collagen-producing myofibroblasts in lung tissue. The resulting fibrotic changes in lung architecture lead to decreased gas exchange and pulmonary compliance (6). Median survival for IPF patients is <5 yr, and there are currently no Food and Drug Administration (FDA)-approved treatments for this condition (6). Standard treatment regimens include anti-inflammatory agents such as prednisone and various immunomodulatory agents. These regimens offer only modest effectiveness, and many have significant adverse effects (29, 32, 43). Lung transplantation provides the only possibility for IPF patients' long-term survival (6).
Current research suggests that IPF may begin with damage to lung alveoli and failure of alveolar reepithelialization after injury. Failure of reepithelialization allows lung fibroblasts to proliferate in the interstitium and then differentiate into myofibroblasts in response to normal wound healing signals such as transforming growth factor-β (TGF-β). The fibrotic changes seen in IPF largely reflect inappropriate deposition of collagen and extracellular matrix by these myofibroblasts (9, 19, 39, 40). With the failure of anti-inflammatory treatments and increased understanding of the underlying mechanisms of IPF, recent investigation of potential therapeutic strategies has focused on proliferation and differentiation mechanisms in lung fibroblasts.
Fibroblast to myofibroblast differentiation is characterized by de novo expression of α-smooth muscle actin (α-SMA) in response to TGF-β (10, 11, 41). Thus blockade of TGF-β activity and the signaling pathways through which it acts might significantly inhibit development of pulmonary fibrosis. Curcumin, the yellow pigment from the rhizomes of the Curcuma longa plant commonly known as turmeric, has long been used as a medicinal agent in many Asian countries (24, 35). This compound has been shown to exhibit a variety of potentially beneficial effects, including antioxidant and anti-inflammatory activities and promotion of wound healing (24). In particular, curcumin has been shown to inhibit processes essential to development of liver and pancreatic fibrosis. These processes include proliferation and differentiation of hepatic and pancreatic stellate cells as well as profibrotic expression of extracellular matrix genes and collagen deposition (17, 25, 48). These data suggest that curcumin may be an effective treatment for pulmonary fibrosis.
To better define the potential use of curcumin as a therapeutic option for pulmonary fibrosis, we investigated the effects of curcumin in IPF fibroblasts, including effects on TGF-β signaling pathways. Furthermore, we examined the antifibrotic effects of curcumin administration in a bleomycin-induced murine model of pulmonary fibrosis. Both oral and intraperitoneal routes of administration were employed, since oral bioavailability of curcumin is known to be limited in humans. We also examined the effects of delaying curcumin administration in the bleomycin model until after the acute inflammation had subsided.
MATERIALS AND METHODS
Reagents.
Curcumin (Sigma-Aldrich, St. Louis, MO) for in vitro studies was prepared at a concentration of 100 mM in DMSO (Sigma-Aldrich) and stored in aliquots at −30°C. Cell culture reagents were purchased from Invitrogen (Carlsbad, CA). HBSS, RPMI 1640, and DMEM were each purchased from Gibco (Grand Island, NY). Human TGF-β and PDGF were purchased from R&D Systems (Minneapolis, MN).
Human lung fibroblast cell culture.
All experiments were performed on normal human fetal lung fibroblasts (IMR-90; Institute for Medical Research, Camden, NJ) or fibroblasts grown from mechanically dissociated surgical lung biopsy of histologically normal or usual interstitial pneumonia (UIP) lung. All subjects provided written informed consent in accordance with the University of Michigan Institutional Review Board. Cells were used at passages 7–10. The fibroblasts were maintained in medium consisting of DMEM and supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% Fungizone (each from Invitrogen).
Human lung fibroblast cell counts.
At indicated times, media was removed, and cultures were washed and incubated in 500 μl of trypsin-EDTA. A 100-μl aliquot of the resulting suspension was diluted, and cells were counted using a Beckman Z2 Coulter Particle Count and Size Analyzer (Beckman Coulter).
Flow cytometric analysis.
Cells were trypsinized, washed, and fixed with 70% ethanol for 30 min at room temperature. After incubation, cells were washed with PBS and resuspended in staining buffer [propidium iodide (50 μg/ml) and RNase (0.01%)] in PBS. The cells were analyzed by FACSVantage flow cytometer using a CellQuest acquisition and analysis program (Becton Dickinson, San Jose, CA). Gating was set to exclude cell debris, cell doublets, and cell clumps.
Real-time quantitative RT-PCR amplification.
Real-time quantitative PCR was performed using the TaqMan SYBR Green PCR master mix protocol. The following gene-specific primers were used: cyclin D1 (forward, 5′-ACCTGGATGCTGGAGGTCTG-3′; reverse, 5′-GAACTTCACATCTGTGGCACA-3′) and β-actin (forward, 5′-GTGGGGCGCCCCCAGGCACCA-3′; reverse, 5′-GCTCGGCCGTGGTGGTGAAGC-3′). Each set was designed using Primer Express software (Applied Biosystems). Each averaged experimental gene expression sample was compared with the averaged control sample, which was set to 1.
Western blot analysis.
Cells were lysed in ice-cold lysis buffer containing 0.5 mM sodium fluoride, 2 mM sodium orthovanadate, and a 1:100 dilution of protease inhibitor. Samples were then clarified by centrifugation at 13,000 g for 10 min, mixed with a commercial sample buffer (Invitrogen), and heated at 95°C for 5 min. Samples were then electrophoresed on 4–20% Novex Tris-Glycine SDS-polyacrylamide gels (Invitrogen), after which the gels were transferred to polyvinylidene difluoride (PVDF) membranes (0.45 μm). Blots were incubated overnight at 4°C with the primary antibody followed by appropriate secondary antibody. They were then washed and signal-detected using a SuperSignal chemiluminescent substrate Western blotting reagent (Pierce Biotechnology) with chemiluminescence-sensitive film. Densitometric analysis was performed using National Institutes of Health (NIH) ImageJ software. Primary antibodies employed in the various studies were: monoclonal mouse anti-α-SMA (Dako, Carpinteria, CA), monoclonal mouse anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), and polyclonal rabbit anti-phospho-Smad2 and anti-phospho-Smad3 (Cell Signaling Technology, Danvers, MA).
α-SMA immunocytochemistry.
Fibroblasts were cultured overnight on sterile glass coverslips. Cells were washed, fixed with chilled methanol (−20°C) for 15 min on ice, then washed again, air dried, and kept at −20°C for 1 h. After equilibration in a humidified chamber, the cells were blocked in 1% BSA in PBS for 30 min. The cells were then incubated overnight at 4°C with monoclonal anti-α-SMA antibody (1:100 dilution; Dako). Following incubation, the cells were washed three times with PBS and then incubated with FITC-conjugated sheep anti-rabbit IgG (1:200 dilution; Sigma-Aldrich) for 1 h at room temperature. The cells were washed as before, and the coverslips were mounted on glass slides and visualized under a fluorescent microscope.
Measurement of ERK.
At each time point, cells were collected, and protein lysates were prepared using the Bio-Plex cell lysis kit (Bio-Rad, Hercules, CA). Briefly, 50 μl of cell lysate (adjusted to a concentration of 0.9 mg protein/ml) was plated in a 96-well filter plate coated with beads coupled to anti-total extracellular signal-regulated kinase (ERK) or anti-phospho-ERK1/2 antibody. The plate was incubated overnight at room temperature on a platform shaker at 300 rpm. After a series of washes to remove unbound proteins, biotinylated detection antibodies, each specific for a different epitope, were added to the reaction. Streptavidin-phycoerythrin was then added to bind to the biotinylated detection antibodies on the bead surface. Data were acquired with the Bio-Plex 200 system and analyzed with the Bio-Plex Manager software from Bio-Rad.
Bleomycin administration and treatment protocol.
C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were housed in specific pathogen-free conditions. Mice were pretreated (3 days) with curcumin (300 mg/kg) in 0.5% carboxymethylcellulose (Sigma-Aldrich) via oral gavage, curcumin (300 mg/kg) in 1% DMSO via intraperitoneal injection, or appropriate vehicle alone. Mice were then given bleomycin sulfate (BLM; Sigma-Aldrich) in saline (50 μl) intratracheally. For delayed treatment experiments, mice were first given BLM intratracheally as described and then administered vehicle or curcumin by intraperitoneal injection starting on day 10. Mice were treated once daily until the end of the study. All experiments were approved by the University of Michigan Committee on Use and Care of Animals.
Lung inflammatory cell analysis.
Mice were euthanized by CO2 asphyxia, the lung vascular bed was perfused with PBS, and the lungs were excised, minced, and digested enzymatically with digestion solution (RPMI 1640, 1 mg/ml collagenase, and 30 U/ml DNase) at 37°C for 30 min. The suspension was dispersed by repeated aspiration through a 10-ml syringe fitted with an 18-gauge blunt-tip needle. The cells were then washed twice with HBSS, resuspended in RPMI 1640, and filtered through a 100-μm nylon mesh. Lung leukocytes were purified by spinning through a 20% discontinuous Percoll gradient. Cells were spun onto glass slides using a cytocentrifuge (Shandon, Pittsburgh, PA) and stained with Diff-Quik (Fisher Scientific, Pittsburgh, PA). Aliquots of cells from lung tissue digests were stained with trypan blue, and the cells were counted using a hemocytometer.
Collagen assays.
Hydroxyproline levels were determined as described by Huaux et al. (15). Briefly, levels were determined by homogenizing the lung in glacial acetic acid (0.5 M). Samples were vacuum dried for 24 h and hydrolyzed in 6 N HCl overnight at 110°C. Hydroxyproline concentration was determined by colorimetric analysis at 550 nm. Data were expressed as micrograms of hydroxyproline per lung as determined by standard curve. Soluble collagen levels were performed using a Sircol assay kit according to manufacturer's protocol (Accurate Chemical and Scientific, Westbury, NY). Briefly, fibroblasts were plated onto a six-well plate. Fibroblasts were treated, supernatants were collected, and collagen levels were quantified at an absorbance of 540 nm.
Measurement of lung morphometry.
Quantitative lung morphometry was analyzed as previously described with modifications (38). Briefly, lungs were inflated by an intratracheal instillation of 10% formalin in PBS and then stored in 10% formalin in PBS for 24 h. Lung tissue was then removed and cut into 3-mm slices along the long axis. Two slices were randomly selected for morphometric analysis. The slices were embedded in paraffin, after which 6-μm histological sections were fixed to glass slides and stained with hematoxylin and eosin. Morphometry was performed using C.A.S.T. (Computer Assisted Stereology Toolbox) software (Olympus). Using a ×2 low power objective, the entire area of lung from each tissue section was outlined and included in the assessment area. The microscope objective was then changed to ×20, and a meander sampling protocol was initiated in which the software chose a random starting field from the selected lung area and subsequent fields were determined by a computer-controlled fixed step in the x- and y-position of the microscope stage. To assess the extent of open air spaces following bleomycin injury, a nine-point grid was imposed on each ×20 field. Individual points were then counted as residing in air space or over tissue depending on where they fell. If points fell within large airways, they were excluded from the analysis. An air space score was then calculated by dividing the number of points that resided in the air spaces of the lung by the sum of the air space and tissue points.
HPLC analysis of plasma.
Isolation of plasma and isocratic HPLC analysis was performed according to the method of Pak et al. (28) with minor modifications. Briefly, blood samples were collected from the right ventricle of euthanized mice at specific time points. Red blood cells were separated from plasma by centrifugation at 2,000 g for 10 min. Plasma was subsequently transferred to fresh 2-ml polypropylene tubes. Two hundred microliters of control plasma was spiked with various concentrations of curcumin (to create standard curve) or β-estradiol (internal standard) followed by two volumes of ethyl acetate. Samples were mixed for 5 min on a rocker and centrifuged at 5,000 rpm for 10 min at room temperature. The supernatant was transferred to a clean glass tube and dried under nitrogen at 40°C. Samples were reconstituted in 100 μl of mobile phase, and 50 μl was injected onto the HPLC system, a Waters 717, UV detector, and a Waters 515 pump (Milford, MA). A visible wavelength of 430 nm was used to detect curcumin and 280 nm to detect β-estradiol. Curcumin separation was accomplished by using the isocratic HPLC method. Samples were injected onto an Apollo reversed-phase C18 column, 150-mm × 3.9-mm × 5-μm particle size (Alltech Associates, Deerfield, IL). The column was operated at a flow rate of 1 ml/min at room temperature. The mobile phase consisted of 1% (wt/vol) citric acid solution, adjusted to pH 3.0 using a 45% potassium hydroxide solution, in HPLC grade water, which was mixed with tetrahydrofuran in a 50:50 (vol/vol) ratio. The solution was filtered through a 0.2-μm filter.
Statistical analysis.
Data are represented as means ± SE and were analyzed with the Prism 4.0 statistical program (GraphPad Software, San Diego, CA). Comparisons among experimental groups were performed with one-way ANOVA followed by Dunnett adjustment for multiple comparisons or one-sided Student's t-test as applicable. All data shown are averages from at least 3 independent experiments. Differences were considered significant if P was <0.05.
RESULTS
Curcumin inhibits proliferation of lung fibroblasts from normal and IPF patients.
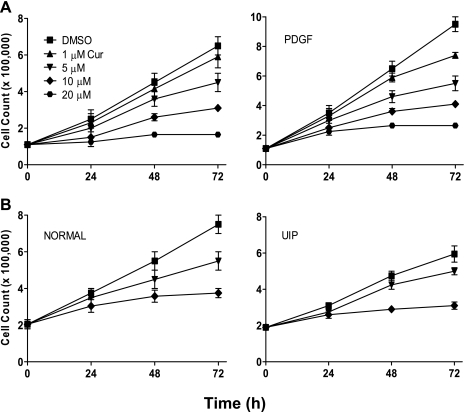
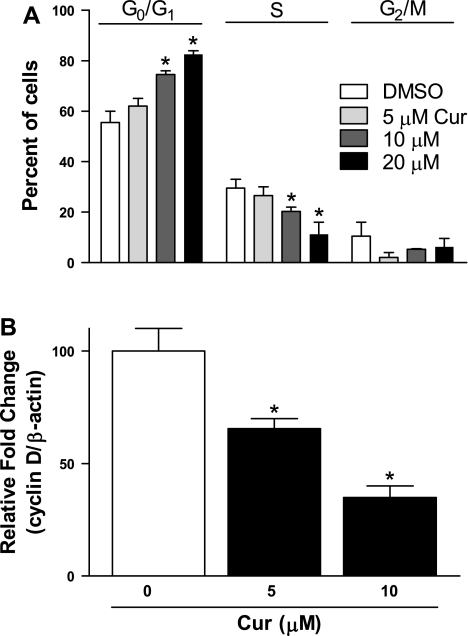
Fibroblast proliferation is thought to play a key role in the development of IPF; as such, blockade of this step would be important in inhibiting disease progression. We accordingly assessed potential antiproliferative effects of curcumin. Human lung fibroblasts were treated with curcumin at varying concentrations (0–20 μM) and for varying times (24, 48, and 72 h). Curcumin inhibited proliferation of all lung fibroblast types in a dose- and time-dependent manner. This inhibition was seen both in the absence (Fig. 1A, left) and the presence (Fig. 1A, right) of the fibroblast mitogen PDGF (5 ng/ml). It is significant that similar antiproliferative effects were seen in fibroblasts not only isolated from normal lungs (n = 4), but also from those of IPF patients (n = 4; Fig. 1B, left and right, respectively). To further examine these antiproliferative effects, we performed cell cycle analysis of human lung fibroblasts treated with curcumin. Cells initially synchronized by 48-h serum deprivation were treated with curcumin (0–20 μM) for an additional 48 h, after which the number of cells in each stage of the cell cycle was analyzed by flow cytometry. Results demonstrated a dose-dependent decrease in the number of cells in S phase with a proportional increase in G0/G1 phase cells (Fig. 2A). These results suggest blockage of cell cycle progression at G1 phase. We confirmed this effect of curcumin on the cell cycle by analyzing mRNA levels of cyclin D1, which increases during G1 to facilitate the onset of S phase (37). Addition of curcumin to cells caused a significant decrease in cyclin D1 mRNA levels, as would be expected when the cell cycle is blocked at G0/G1 (Fig. 2B).
Fig. 1.
Curcumin (Cur) inhibits lung fibroblast proliferation. Human lung fibroblasts were treated with varying concentrations of curcumin. Cell numbers were then counted at 24, 48, and 72 h. A and B: IMR-90 fibroblasts, unstimulated (A, left) or stimulated with 5 ng/ml PDGF (A, right) and primary human lung fibroblasts (n = 4 each group) isolated from histologically normal lungs (B, left) and lungs of patients with usual interstitial pneumonia (UIP; B, right). Representative graphs from normal and idiopathic pulmonary fibrosis (IPF) fibroblasts are shown.
Fig. 2.
Curcumin inhibits lung fibroblast cell cycle progression at G0/G1. A: IMR-90 fibroblasts were synchronized by serum deprivation for 48 h and treated with indicated concentrations of curcumin or with vehicle (DMSO) for 48 h and then harvested and stained with propidium iodide. Stained cells were analyzed by FACS to assess cell cycle progression in each treatment group. *P < 0.05 vs. 0 μM. B: cyclin D1 mRNA levels in the presence or absence of curcumin were measured by real-time RT-PCR and expressed relative to mRNA levels of the housekeeping gene β-actin. *P < 0.05 vs. 0 μM.
Curcumin inhibits myofibroblast differentiation.
TGF-β-dependent differentiation of fibroblasts to myofibroblasts, which express α-SMA, is an essential step in development of fibrosis. To determine whether curcumin could inhibit this process, serum-starved fibroblasts from histologically normal patient lungs (n = 4) were treated with curcumin (0–20 μM) for 2 h and then incubated in the presence of TGF-β (2 ng/ml) for 24 h. Extent of differentiation was assessed by measurement of α-SMA using Western blotting and immunocytochemical analysis. TGF-β treatment resulted in enhanced expression of α-SMA that was inhibited by curcumin in a dose-dependent manner (Fig. 3, A–C).
Fig. 3.
Curcumin inhibits transforming growth factor-β (TGF-β)-induced myofibroblast differentiation. Human fibroblasts obtained from histologically normal lungs (n = 4) were pretreated with varying concentrations of curcumin for 2 h and incubated in the presence of TGF-β (2 ng/ml) for 24 h. A: levels of α-smooth muscle actin (α-SMA) were analyzed by Western blotting and normalized relative to the GAPDH loading control; representative blot is shown. B: quantitative values for each condition were obtained by densitometric analysis. *P < 0.05 vs. 0 μM. C: human fibroblasts (n = 3) were either untreated (Normal), treated with TGF-β (2 ng/ml), or treated with TGF-β plus curcumin (10 μM) as before. After 24 h, immunohistochemical analysis for α-SMA (FITC) was performed, and representative images are shown. Ctrl, control.
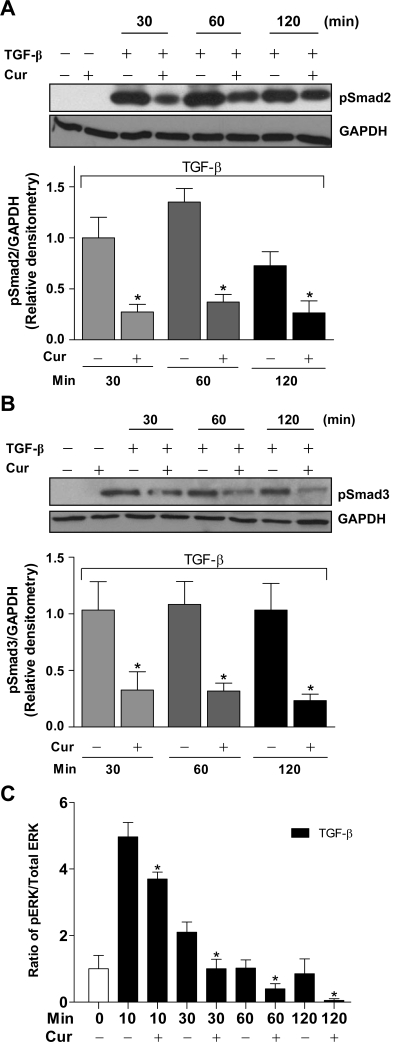
Curcumin inhibits TGF-β-induced phosphorylation of Smad2/3 and ERK1/2.
Activation of the TGF-β receptor results in phosphorylation of Smad2 and Smad3 as well as ERK1/2. To determine whether the ability of curcumin to inhibit TGF-β-induced myofibroblast differentiation reflected inhibition of these signaling pathways, we measured changes following different periods of TGF-β exposure in levels of phosphorylated Smad2, Smad3, and ERK1/2 in the presence of curcumin. Human fibroblasts obtained from histologically normal lungs were pretreated with curcumin at 20 μM for 2 h before TGF-β addition. At 30, 60, and 120 min following addition of TGF-β (2 ng/ml), cells were lysed, and amounts of phosphorylated signaling molecules were determined. Curcumin significantly reduced the amount of both phospho-Smad2 (Fig. 4A) and phospho-Smad3 (Fig. 4B) at all time points. Phosphorylation of ERK1/2 was maximal 10 min after TGF-β addition, declining to near control levels by 1 h (Fig. 4C). Curcumin again inhibited phosphorylation at all time points, with phosphorylation being essentially undetectable at 2 h.
Fig. 4.
Curcumin inhibits TGF-β-induced phosphorylation (p) of Smad2, Smad3, and extracellular signal-regulated kinase (ERK) 1/2 in lung fibroblasts. Human fibroblasts obtained from histologically normal lungs (n = 4) were treated with curcumin (20 μM) for 2 h followed by TGF-β (2 ng/ml) for varying periods. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies against pS465/467 Smad2 (A) and pS423/425 Smad3 (B). Blots were probed first with phosphospecific Smad antibodies, stripped, and reprobed with GAPDH. Mean densitometric data are also presented. *P < 0.05 vs. 0 μM at each respective time point. C: pERK and total ERK were measured by Bio-Plex, and their ratio was determined. *P < 0.05 vs. 0 μM at each respective time point.
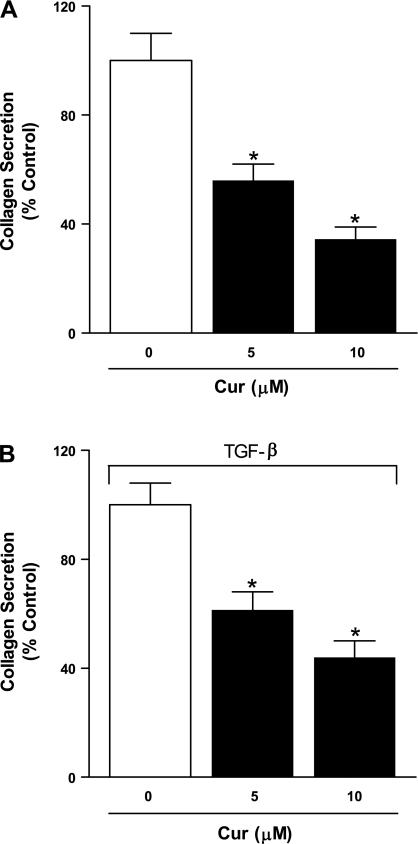
Curcumin inhibits collagen secretion by IPF fibroblasts.
The progressive lung damage observed in IPF is in large part due to the sustained and inappropriate deposition of collagen by myofibroblasts in the lung interstitium (33). To examine possible effects of curcumin on collagen production, fibroblasts isolated from IPF patients (n = 3) were treated with curcumin (5 or 10 μM) for 2 h and then incubated in the absence (Fig. 5A) and presence (Fig. 5B) of TGF-β (6 ng/ml) for 24 h in serum-free medium. TGF-β treatment led to increased collagen production (data not shown), whereas curcumin treatment significantly inhibited both baseline and TGF-β-induced collagen secretion in a dose-dependent manner.
Fig. 5.
Curcumin inhibits collagen secretion from IPF fibroblasts. Serum-starved fibroblasts from lungs of patients with IPF (n = 3) were pretreated with curcumin (5 and 10 μM) for 2 h and then incubated with fresh medium in the absence (A) or presence (B) of TGF-β (6 ng/ml) for 24 h. Soluble collagen levels were measured by Sircol assay. Values indicated were normalized to control for each experiment. *P < 0.05 vs. 0 μM.
Intraperitoneal, but not oral, administration of curcumin shows beneficial effects in bleomycin-induced lung injury.
Our results with curcumin treatment of lung fibroblasts from normal and IPF patients suggested that curcumin could be effective in vivo. We tested this hypothesis using a murine bleomycin-induced model of pulmonary fibrosis. Since oral bioavailability of curcumin may be limited, we conducted parallel experiments using either oral or intraperitoneal administration of curcumin.
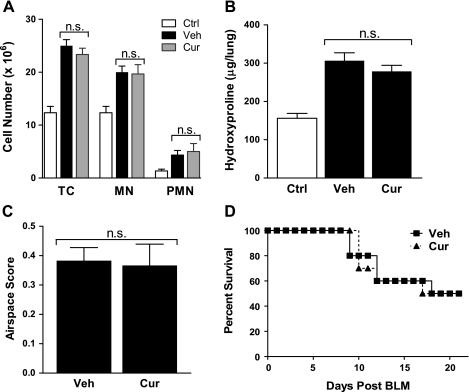
In one set of experiments, mice were pretreated with curcumin (300 mg/kg) or vehicle by oral gavage 72 h before a BLM (0.05 units) intratracheal injection. This was followed by daily administration of curcumin until the mice were killed. Inflammatory cells from digested lungs were quantified on day 4. There was no significant difference between the vehicle- and curcumin-treated mice in number of inflammatory cells following bleomycin injury (Fig. 6A). Collagen deposition in these animals was assessed by measuring concentrations of the collagen-specific amino acid hydroxyproline in lungs from mice on day 21. Bleomycin treatment increased lung hydroxyproline levels, but there was little or no difference in bleomycin effects between vehicle- and curcumin-treated animals (Fig. 6B). Morphometric analysis of the lung likewise demonstrated no significant difference between curcumin and vehicle treatment (Fig. 6C). Lastly, we did not observe a difference in mortality rate between vehicle- and curcumin-treated mice following a higher dose (0.075 units) of bleomycin (Fig. 6D).
Fig. 6.
Orally administered curcumin fails to inhibit bleomycin-induced lung injury or improve survival. C57BL/6 mice were pretreated with saline (Ctrl), carboxymethylcellulose (vehicle; Veh), or curcumin (300 mg/kg) by oral gavage (o.g.). After 72 h (day 0), mice were injected with bleomycin sulphate (BLM; 0.05 units), and daily o.g. treatment was continued. A: on day 4, mice (n = 5) were killed, and lung digests were analyzed for content of total cells (TC), mononuclear cells (MN), and polymorphonuclear neutrophils (PMN). B: on day 21, mice (n = 10) were killed, and whole lung was processed for hydroxyproline content and morphometric analysis (C; n = 5). D: mortality rate of curcumin-treated and control mice (n = 10) after bleomycin (0.075 units) instillation. n.s., Not significant.
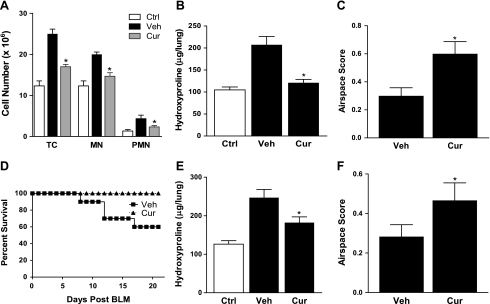
In the second set of experiments, curcumin or vehicle was administered intraperitoneally. The protocol was otherwise as described for oral administration. In contrast to oral administration, intraperitoneal administration resulted in marked differences between curcumin-treated mice and untreated controls. Total cells, mononuclear cells, and polymorphonuclear cells in lung tissue were reduced in curcumin-treated mice at day 4 (Fig. 7A), indicating reduced inflammation. In addition, lung hydroxyproline content at day 21 was significantly diminished, almost to baseline levels, in curcumin-treated mice (Fig. 7B). Morphometric analysis likewise demonstrated significant improvement in the extent of open air spaces in curcumin-treated mice (Fig. 7C). These observed improvements in inflammation and collagen deposition were also reflected by a trend in improved survival (Fig. 7D; P > 0.05).
Fig. 7.
Intraperitoneally administered curcumin suppresses bleomycin-induced lung injury and improves survival. C57BL/6 mice were intraperitoneally pretreated with saline (Ctrl), 1% DMSO (Veh), or curcumin (300 mg/kg). After 72 h (day 0), mice were injected with BLM (0.05 units), and daily intraperitoneal treatment was continued. A: on day 4, mice (n = 5) were killed and lung digests analyzed for TC, MN, and PMN. *P < 0.05 vs. Veh. B: on day 21, mice (n = 10) were killed, and whole lung was processed for hydroxyproline content and morphometric analysis (C; n = 5). *P < 0.05 vs. Veh. D: mortality rate of curcumin-treated and control mice (n = 10) after bleomycin (0.075 units) instillation. E: mice were not pretreated and began daily intraperitoneal treatments on day 10 after BLM (0.05 units) injury. Mice (n = 10) were killed on day 21, and whole lung was processed for hydroxyproline content and morphometric analysis (F; n = 5). *P < 0.05 vs. Veh.
To extend our findings further, we sought to determine whether curcumin treatment could be effective after bleomycin injury has occurred and acute inflammation has subsided. This mimics the minimal inflammation but active fibrosis seen in patients with IPF. We measured lung hydroxyproline as previously described for mice with BLM-induced lung injury but delayed intraperitoneal curcumin treatment until day 10. Figure 7, E and F, shows that even with delayed administration, curcumin significantly reduced lung hydroxyproline content and improved morphometric air space score in treated mice.
Plasma levels of curcumin are significantly greater via intraperitoneal compared with oral administration.
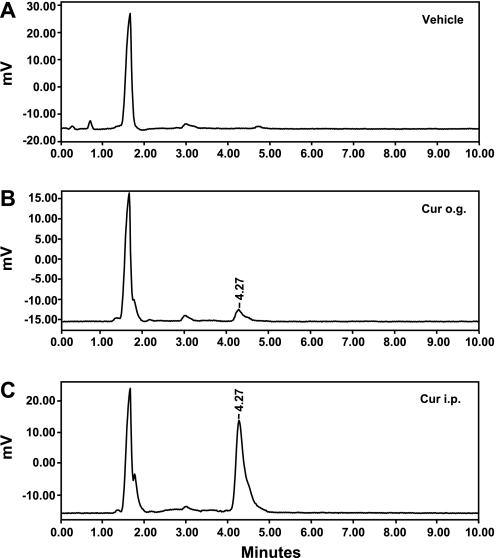
Because we found a great discrepancy between oral and intraperitoneal curcumin delivery in the effectiveness with which they reduced bleomycin-induced injury, we hypothesized that bioavailability of curcumin differed significantly according to the method of delivery. To test this hypothesis, we used HPLC to measure plasma curcumin levels in mice following oral or intraperitoneal administration of curcumin (each 300 mg/kg). No curcumin was found in control mice (Fig. 8A), whereas murine plasma obtained 2 h after an oral dose (Fig. 8B) had a concentration of 15.7 ± 3.8 ng/ml (∼43 nM). In contrast, intraperitoneal administration of curcumin (Fig. 8C) resulted in a much higher curcumin plasma level, 181 ± 23.1 ng/ml (∼506 nM). Thus intraperitoneal administration of curcumin provided increased plasma levels and a higher effective systemic dose than did oral administration. These results provide a rationale for the more profound biological effects we saw with intraperitoneal administration of curcumin following bleomycin lung injury.
Fig. 8.
Curcumin exhibits greater bioavailability after intraperitoneal administration than after oral administration. HPLC chromatograms were obtained from extraction of mouse plasma following no treatment (A; Vehicle), an o.g. dose of 300 mg/kg curcumin (Cur o.g.) 2 h previously (B), or injection of an intraperitoneal dose of 300 mg/kg curcumin (C; Cur i.p.) 2 h previously. Ethyl acetate was used to extract curcumin from murine plasma (200 μl). Each experiment was repeated 3 times, with a representative graph being presented.
DISCUSSION
In this study, we show that curcumin effectively inhibits fibroblast proliferation, differentiation to myofibroblasts, and secretion of collagen, important processes in the progression of pulmonary fibrosis. We also show that intraperitoneal but not oral curcumin inhibits fibrotic changes following bleomycin administration in mice and that this remains true even when curcumin administration is delayed until after acute inflammation has subsided. This delayed administration is potentially relevant to human disease, since patients do not typically seek treatment until some degree of fibrosis has already occurred. Furthermore, we explain the differing results seen with the two routes of curcumin administration by measuring the respective plasma levels. We find that oral bioavailability of curcumin is highly limited and that plasma levels are much lower following oral administration than following intraperitoneal administration of the same dose. This limited oral bioavailability of curcumin parallels previous findings in humans (8, 34, 35).
Our studies demonstrate that curcumin blocks proliferation not only in human lung fibroblast cell lines and primary fibroblast cultures from normal human lungs, but also in primary cultures from IPF patients. This is the first time that the antiproliferative effects of curcumin have been shown in fibroblasts from lungs of IPF patients or healthy adults. These antiproliferative effects appear to reflect blockade of cell cycle progression, as we show that curcumin causes accumulation of proliferating lung fibroblast cells in the G0/G1 phase of the cell cycle and causes a decrease in expression of cyclin D1, which is important for progression to G2. Previous reports have shown that curcumin can act at various points in the cell cycle through downregulation of cyclin D1, inhibition of DNA synthesis, activation of p53 and cyclin-dependent kinase (cdk) inhibitors, and inhibition of NF-κB (18, 20). A study in U-937 monocytes showed that the curcumin-induced reduction in cyclin D1 levels was due to inhibition of NF-κB translocation to the nucleus together with induction of B-cell translocation gene 2 (BTG2; Ref. 21). G1 cell cycle arrest has also been reported following curcumin treatment in PCC4 embryonal carcinoma cells (1).
Differentiation of human lung fibroblasts into potent collagen-expressing myofibroblasts is important in the generation of the fibrotic changes seen in IPF. We show that curcumin can block both TGF-β-induced differentiation of and collagen deposition by IPF fibroblasts. These results suggest that curcumin could effectively slow the continuing progression of IPF, an important step toward establishing the agent as a viable treatment option in this disease. Curcumin has similarly been shown to inhibit both proliferation (44) and TGF-β- or culture-induced differentiation of hepatic stellate cells (17, 48), pancreatic stellate cells (25), and fetal lung fibroblasts (13). Inhibition of collagen deposition following stellate cell differentiation has also been demonstrated (17, 25, 48).
Our investigation of the mechanism by which curcumin exerts these effects demonstrated that curcumin inhibited TGF-β-induced phosphorylation of Smad2, Smad3, and ERK1/2, all important steps in the signaling pathway of this profibrotic cytokine. Inhibition of induced ERK phosphorylation by curcumin was first demonstrated in Jurkat T cells (4) and has since been observed in a wide variety of cell types. In hepatic stellate cells, the major effector cells of liver fibrosis, curcumin inhibition of proliferation and activation is accompanied by a reduction in ERK phosphorylation but not in total ERK protein levels (5), whereas its ability to reduce connective tissue growth factor and collagen production is mimicked by inhibitors of ERK phosphorylation (3). Inhibitors of ERK phosphorylation also mimic the ability of curcumin to block TGF-β-induced differentiation of fetal lung fibroblasts into myofibroblasts (13). ERK is not unique in this respect, however, as these authors and others have obtained similar results with blockage of other MAPK signaling pathways. Curcumin inhibition of Smad2/3 phosphorylation has similarly been observed in connection with inhibition of TGF-β-induced collagen expression by renal fibroblasts (7). Curcumin has also been shown to counteract the ability of TGF-β to downregulate expression of the nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPARγ) in hepatic stellate cells (49). Antifibrotic effects of PPARγ activation have been demonstrated (2, 11, 26, 47), although this current study did not examine a possible role for PPARγ in the effects we observed.
Our in vivo studies in the bleomycin-induced murine model of pulmonary fibrosis show that intraperitoneal but not oral administration of curcumin significantly inhibits development of fibrotic changes, with improvements in inflammation, collagen deposition, and survival. Importantly, collagen deposition was inhibited even when administration was delayed until acute inflammation had subsided. We note that inflammation is typically minimal or absent at the time IPF patients present for treatment, raising the possibility that such delayed administration may be relevant to treatment of human disease. Not only is our study the first to demonstrate that curcumin is effective during what has been referred to as “the therapeutic period,” but also very few agents of any type have proven effective at this time (27).
HPLC measurement of curcumin levels in mouse plasma after oral and intraperitoneal administration provide an explanation for the lack of effect seen with oral curcumin, as we found a plasma concentration of curcumin >10 times as great following intraperitoneal injection as following oral dosing. This is in accord with results in humans, where use of oral curcumin treatment is hampered by poor bioavailability (8, 34, 35). A recent dose escalation study of oral curcumin toxicity and bioavailability in human volunteers reported very low concentrations (30–60 ng/ml) or unmeasurable curcumin in plasma even at the highest doses (22). In addition, two clinical studies detected only low nanomolar or no curcumin in plasma after consumption of, respectively, up to 2.2 g of Curcuma extract or 3.6 g of curcumin daily for as much as 4 mo (34, 36). Studies in humans have confirmed that the bulk of orally consumed curcumin is metabolized quickly in the intestine (16) or excreted in fecal matter (34, 36). There is currently no strong evidence that oral administration of curcumin can provide in plasma the levels that have been shown to be effective in cultured cells (35).
Interestingly, previous studies in rats have shown beneficial effects of early oral curcumin administration in bleomycin- or amiodarone-induced pulmonary fibrosis (30, 31, 42, 45). There is no obvious explanation for the difference from our negative results, even with early administration. Although intestinal metabolism of curcumin is lower in the rat than in the human (16), bioavailability remains very low even following high doses of 500 mg/kg (46). It is possible to suppose that the vehicle in which curcumin is administered affects oral bioavailability to some extent or that curcumin from different sources may not be fully equivalent. Neither possibility seems particularly likely, however, in view of the consistency in human data from several different groups.
Given the antifibrotic effects of curcumin demonstrated by this study, our results suggest that curcumin could potentially be an effective treatment for IPF. Attractiveness of this option is increased by the other beneficial therapeutic effects of curcumin such as antioxidant activity and promotion of wound healing. In addition, curcumin has demonstrated low toxicity (22, 34, 36), whereas the drugs currently being used for IPF often result in significant adverse effects. However, oral curcumin is not likely to be effective, and other therapeutic strategies should be considered. Delivery of nebulized curcumin directly to the lungs might be one such strategy, since use of nebulized pharmaceutical preparations is common and effective for treatment of other lung conditions such as asthma. A liposome-encapsulated curcumin formulation suitable for intravenous use has also been developed and shown to be effective in an animal model (23). We suggest that curcumin administered in either manner could potentially provide a safe and effective treatment for IPF.
GRANTS
This study was supported by NIH Grants HL-070068 and AI-079539, a University of Michigan Global REACH International Grant, and the Martin E. Galvin Fund and Quest for Breath Foundation (all to R. C. Reddy) and NIH Grant P50-HL-60289 (to T. J. Standiford).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Batth BK, Tripathi R, Srinivas UK. Curcumin-induced differentiation of mouse embryonal carcinoma PCC4 cells. Differentiation 68: 133–140, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chen A, Zheng S. Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-κB and ERK signalling. Br J Pharmacol 153: 557–567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YR, Tan TH. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17: 173–178, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Ping J, Liu C, Tan YZ, Chen GF. Study on effects of extracts from Salvia miltiorrhiza and Curcuma longa in inhibiting phosphorylated extracellular signal regulated kinase expression in rat's hepatic stellate cells. Chin J Integr Med 12: 207–211, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dempsey OJ, Kerr KM, Gomersall L, Remmen H, Currie GP. Idiopathic pulmonary fibrosis: an update. QJM 99: 643–654, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gaedeke J, Noble NA, Border WA. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int 66: 112–120, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 90: 1011–1015, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 345: 517–525, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factorβ and peroxisome proliferator-activated receptor γ. J Biol Chem 282: 22910–22920, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Peng J, Feng D, Chu L, Li X, Jin Z, Lin Z, Zeng Q. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-β1-induced α smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung 184: 33–42, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Huaux F, Gharaee-Kermani M, Liu T, Morel V, McGarry B, Ullenbruch M, Kunkel SL, Wang J, Xing Z, Phan SH. Role of eotaxin-1 (CCL11) and CC chemokine receptor 3 (CCR3) in bleomycin-induced lung injury and fibrosis. Am J Pathol 167: 1485–1496, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11: 105–111, 2002 [PubMed] [Google Scholar]
- 17.Kang HC, Nan JX, Park PH, Kim JY, Lee SH, Woo SW, Zhao YZ, Park EJ, Sohn DH. Curcumin inhibits collagen synthesis and hepatic stellate cell activation in-vivo and in-vitro. J Pharm Pharmacol 54: 119–126, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Karunagaran D, Joseph J, Kumar TR. Cell growth regulation. Adv Exp Med Biol 595: 245–268, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Keane MP, Strieter RM, Belperio JA. Mechanisms and mediators of pulmonary fibrosis. Crit Rev Immunol 25: 429–463, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv Exp Med Biol 595: 173–184, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kwon YK, Jun JM, Shin SW, Cho JW, Suh SI. Curcumin decreases cell proliferation rates through BTG2-mediated cyclin D1 down-regulation in U937 cells. Int J Oncol 26: 1597–1603, 2005 [PubMed] [Google Scholar]
- 22.Lao CD, Ruffin MT, 4th, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6: 10, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 104: 1322–1331, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci 78: 2081–2087, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Masamune A, Suzuki N, Kikuta K, Satoh M, Satoh K, Shimosegawa T. Curcumin blocks activation of pancreatic stellate cells. J Cell Biochem 97: 1080–1093, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L891–L901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40: 362–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pak Y, Patek R, Mayersohn M. Sensitive and rapid isocratic liquid chromatography method for the quantitation of curcumin in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 796: 339–346, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Pereira CA, Malheiros T, Coletta EM, Ferreira RG, Rubin AS, Otta JS, Rocha NS. Survival in idiopathic pulmonary fibrosis-cytotoxic agents compared to corticosteroids. Respir Med 100: 340–347, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Punithavathi D, Venkatesan N, Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol 131: 169–172, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punithavathi D, Venkatesan N, Babu M. Protective effects of curcumin against amiodarone-induced pulmonary fibrosis in rats. Br J Pharmacol 139: 1342–1350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richeldi L, Davies HR, Ferrara G, Franco F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 3: CD002880, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 64: 405–430, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10: 6847–6854, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 41: 1955–1968, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7: 1894–1900, 2001 [PubMed] [Google Scholar]
- 37.Sherr CJ. Cell cycle control and cancer. Harvey Lect 96: 73–92, 2000 [PubMed] [Google Scholar]
- 38.Sisson TH, Hansen JM, Shah M, Hanson KE, Du M, Ling T, Simon RH, Christensen PJ. Expression of the reverse tetracycline-transactivator gene causes emphysema-like changes in mice. Am J Respir Cell Mol Biol 34: 552–560, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thannickal VJ, Flaherty KR, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: emerging concepts on pharmacotherapy. Expert Opin Pharmacother 5: 1671–1686, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Venkatesan N, Punithavathi V, Chandrakasan G. Curcumin protects bleomycin-induced lung injury in rats. Life Sci 61: PL51–PL58, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Walter N, Collard HR, King TE., Jr Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc 3: 330–338, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-γ contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol 285: G20–G30, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Xu M, Deng B, Chow Yl, Zhao Zz, Hu B. Effects of curcumin in treatment of experimental pulmonary fibrosis: a comparison with hydrocortisone. J Ethnopharmacol 112: 292–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 853: 183–189, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun 350: 385–391, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng S, Chen A. Activation of PPARγ is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J 384: 149–157, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng S, Chen A. Disruption of transforming growth factor-β signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 292: G113–G123, 2007. [DOI] [PubMed] [Google Scholar]