Abstract
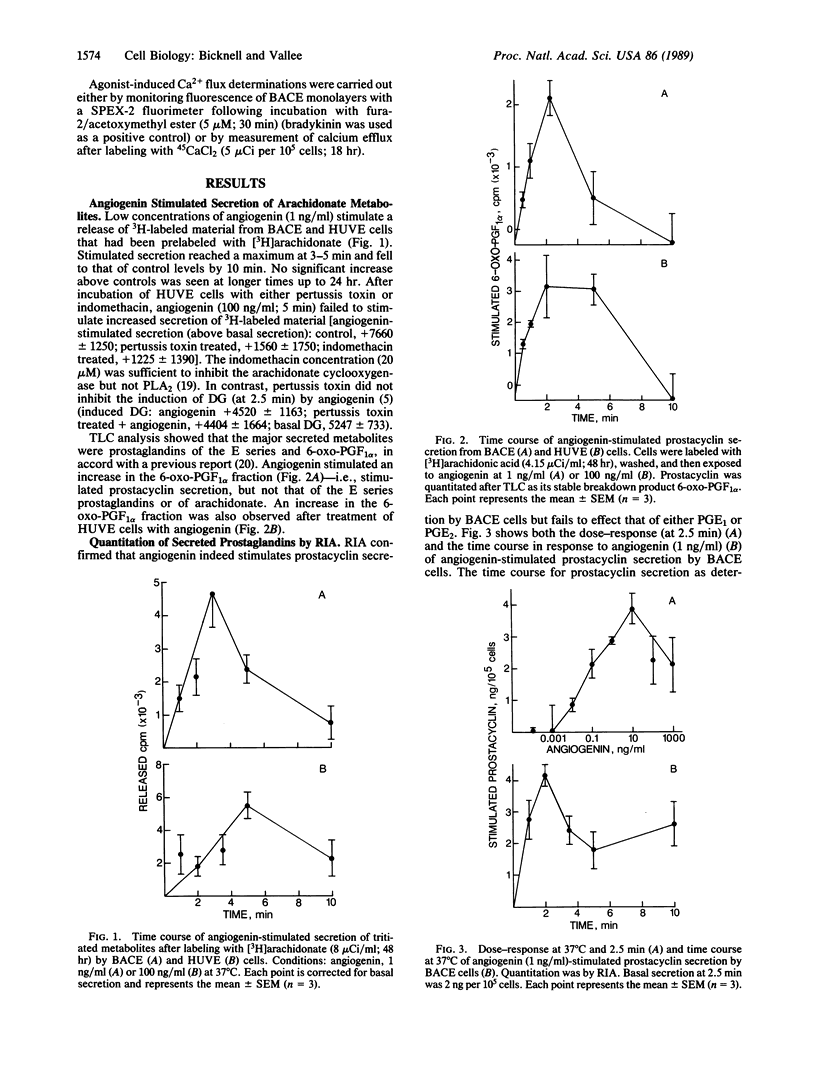
Angiogenin stimulates capillary and umbilical vein endothelial cell prostacyclin secretion but not that of prostaglandins of the E series. The response was quantitated by radioimmunoassay and by [3H]arachidonate labeling followed by analysis of the secreted prostaglandins. The stimulated secretion lasts for several minutes and is optimal at 2-4 min. The dose-response (peak at 1-10 ng/ml) is similar to that previously observed for activation of endothelial cell phospholipase C. Stimulated secretion was blocked by pretreatment with the inhibitors of prostacyclin synthesis, indomethacin and tranylcypromine, and also the specific inhibitor of phospholipase A2, quinacrine, as well as pertussis toxin and the diglyceryl and monoglyceryl lipase inhibitor RHC 80267. Stimulated secretion was also abolished in cells that were either pretreated for 48 hr with phorbol ester to down-regulate protein kinase C or incubated with the protein kinase inhibitor H7. Hydrolysis of phosphatidylinositol by phospholipase A2 appears to be the source of angiogenin-mobilized arachidonate; angiogenin-induced hydrolysis of phosphatidylcholine was not detected. Activation of phospholipase A2 occurs in the absence of an angiogenin-induced calcium flux. The results are discussed in terms of mechanisms of agonist-induced intracellular arachidonate mobilization and relevance to angiogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Force L. E., Becherer P. R. Histamine stimulate prostacyclin synthesis in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1435–1440. doi: 10.1016/0006-291x(80)90447-7. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin activates endothelial cell phospholipase C. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Ma A. L., Axelrod J. Phorbol esters and diacylglycerols amplify bradykinin-stimulated prostaglandin synthesis in Swiss 3T3 fibroblasts. Possible independence from protein kinase C. J Biol Chem. 1988 Apr 5;263(10):4764–4767. [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Zetter B. R., Antoniades H. N., Levine L. Platelet-dependent stimulation of prostacyclin synthesis by platelet-derived growth factor. Nature. 1980 Dec 11;288(5791):600–602. doi: 10.1038/288600a0. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Hoak J. C., Fry G. L., Haycraft D. L. Use of a radioimmunoassay to study thrombin-induced release of PGI2 from cultured endothelium. Thromb Res. 1979;14(4-5):781–786. doi: 10.1016/0049-3848(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Fleisher L. N., Tall A. R., Witte L. D., Miller R. W., Cannon P. J. Stimulation of arterial endothelial cell prostacyclin synthesis by high density lipoproteins. J Biol Chem. 1982 Jun 25;257(12):6653–6655. [PubMed] [Google Scholar]
- Form D. M., Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983 Feb;172(2):214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse I., Tai H. H. Stimulations of arachidonate release and inositol-1,4,5-triphosphate formation are mediated by distinct G-proteins in human platelets. Biochem Biophys Res Commun. 1987 Jul 31;146(2):659–665. doi: 10.1016/0006-291x(87)90579-1. [DOI] [PubMed] [Google Scholar]
- GREEN K., SAMUELSSON B. PROSTAGLANDINS AND RELATED FACTORS: XIX. THIN-LAYER CHROMATOGRAPHY OF PROSTAGLANDINS. J Lipid Res. 1964 Jan;5:117–120. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Gryglewski R. J., Bunting S., Moncada S., Flower R. J., Vane J. R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins. 1976 Nov;12(5):685–713. doi: 10.1016/0090-6980(76)90047-2. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
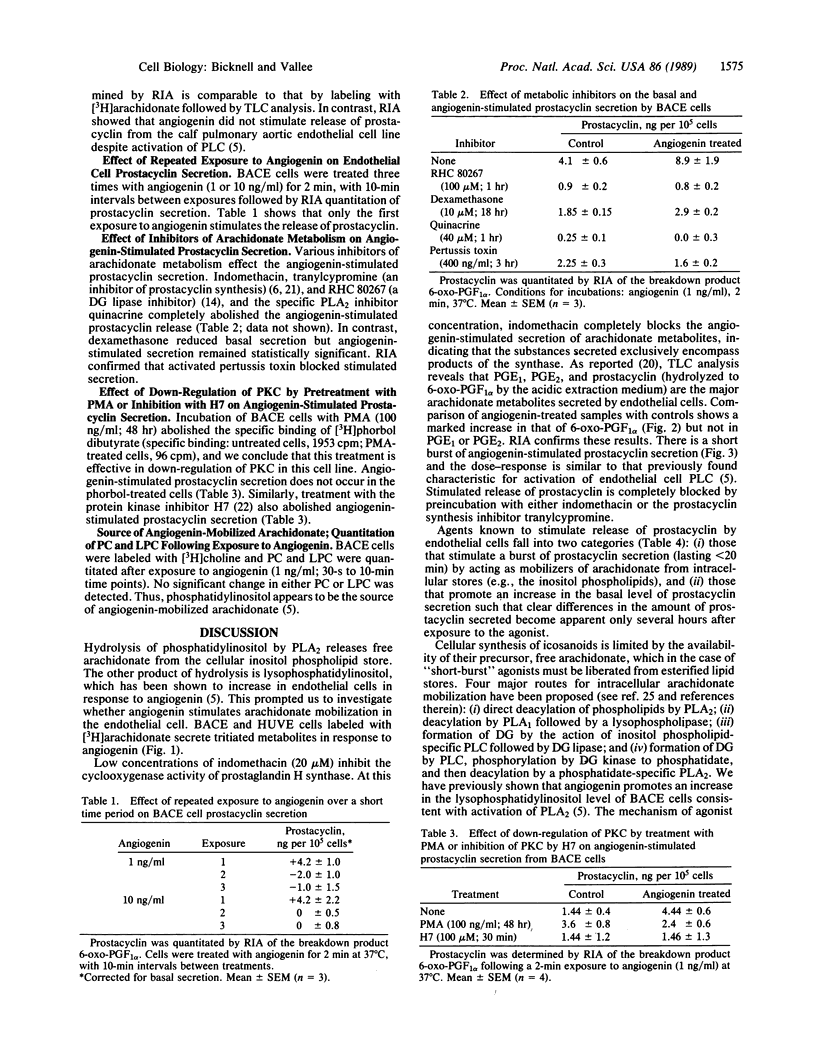
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hirata F., Matsuda K., Notsu Y., Hattori T., del Carmine R. Phosphorylation at a tyrosine residue of lipomodulin in mitogen-stimulated murine thymocytes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4717–4721. doi: 10.1073/pnas.81.15.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hong S. L. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb Res. 1980 Jun 15;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Kawakami M., Ishibashi S., Ogawa H., Murase T., Takaku F., Shibata S. Cachectin/TNF as well as interleukin-1 induces prostacyclin synthesis in cultured vascular endothelial cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):482–487. doi: 10.1016/s0006-291x(86)80198-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Checani G. C., Deykin D. Stimulation of Ca2+-activated human platelet phospholipase A2 by diacylglycerol. Biochem J. 1987 Dec 15;248(3):779–783. doi: 10.1042/bj2480779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The initial action of thrombin on platelets. Conversion of phosphatidylinositol to phosphatidic acid preceding the production of arachidonic acid. J Biol Chem. 1981 May 25;256(10):5037–5040. [PubMed] [Google Scholar]
- Nakashima S., Hattori H., Shirato L., Takenaka A., Nozawa Y. Differential sensitivity of arachidonic acid release and 1,2-diacylglycerol formation to pertussis toxin, GDP beta S and NaF in saponin-permeabilized human platelets: possible evidence for distinct GTP-binding proteins involving phospholipase C and A2 activation. Biochem Biophys Res Commun. 1987 Nov 13;148(3):971–978. doi: 10.1016/s0006-291x(87)80227-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Parker J., Daniel L. W., Waite M. Evidence of protein kinase C involvement in phorbol diester-stimulated arachidonic acid release and prostaglandin synthesis. J Biol Chem. 1987 Apr 15;262(11):5385–5393. [PubMed] [Google Scholar]
- Pollock W. K., Irvine R. F., Rink T. J. Free Ca2+ requirements of agonist-induced thromboxane A2 synthesis in human platelets. Eur J Pharmacol. 1986 Dec 16;132(2-3):309–312. doi: 10.1016/0014-2999(86)90622-9. [DOI] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink T. J., Grigorian GYu, Moldabaeva A. K., Danilov S. M., Bühler F. R. Histamine-induced phosphoinositide metabolism in cultured human umbilical vein endothelial cells. Association with thromboxane and prostacyclin release. Biochem Biophys Res Commun. 1987 Apr 14;144(1):438–446. doi: 10.1016/s0006-291x(87)80529-6. [DOI] [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Rothhut B., Russo-Marie F., Wood J., DiRosa M., Flower R. J. Further characterization of the glucocorticoid-induced antiphospholipase protein "renocortin". Biochem Biophys Res Commun. 1983 Dec 28;117(3):878–884. doi: 10.1016/0006-291x(83)91678-9. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. In vitro protein kinase C phosphorylation sites of placental lipocortin. Biochemistry. 1988 Jun 14;27(12):4253–4258. doi: 10.1021/bi00412a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Silverman K. J., Lund D. P., Zetter B. R., Lainey L. L., Shahood J. A., Freiman D. G., Folkman J., Barger A. C. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun. 1988 May 31;153(1):347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Suffys P., Beyaert R., Van Roy F., Fiers W. Reduced tumour necrosis factor-induced cytotoxicity by inhibitors of the arachidonic acid metabolism. Biochem Biophys Res Commun. 1987 Dec 16;149(2):735–743. doi: 10.1016/0006-291x(87)90429-3. [DOI] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Blackshear P. J. Protein kinase C-mediated phosphorylation in intact cells. Methods Enzymol. 1987;141:412–424. doi: 10.1016/0076-6879(87)41087-2. [DOI] [PubMed] [Google Scholar]
- Ziche M., Jones J., Gullino P. M. Role of prostaglandin E1 and copper in angiogenesis. J Natl Cancer Inst. 1982 Aug;69(2):475–482. [PubMed] [Google Scholar]
- Zoeller R. A., Wightman P. D., Anderson M. S., Raetz C. R. Accumulation of lysophosphatidylinositol in RAW 264.7 macrophage tumor cells stimulated by lipid A precursors. J Biol Chem. 1987 Dec 15;262(35):17212–17220. [PubMed] [Google Scholar]



