Abstract
The fetal lung vasculature forms in tandem with developing airways. Whereas saccular airway morphogenesis is arrested in bronchopulmonary dysplasia (BPD), the potential vascular phenotype in BPD at this stage of development is less well-understood. As inflammation increases the risk of BPD and induces arrest of saccular airway morphogenesis, we tested the effects of Escherichia coli LPS on fetal mouse lung vascular development. Injecting LPS into the amniotic fluid of Tie2-lacZ endothelial reporter mice at embryonic day 15 stimulated angiogenesis in the saccular stage fetal lung mesenchyme. LPS also increased the number of endothelial cells in saccular stage fetal mouse lung explants. Inflammation appeared to directly promote vascular development, as LPS stimulated pulmonary microvascular endothelial cell angiogenesis, cell migration, and proliferation in vitro. Whereas LPS did not increase expression of VEGF, angiopoietin-1 (Ang-1), Tie2, fetal liver kinase-1 (Flk-1), fms-like tyrosine kinase-1 (Flt-1), PDGFA, PDGFB, heparin-binding EGF-like growth factor (HB-EGF), or connective tissue growth factor (CTGF), LPS did stimulate the production of the angiogenic CC chemokines macrophage inflammatory protein-1α (MIP-1α) and monocyte chemoattractant protein-1 (MCP-1). Both MIP-1α and MCP-1 increased angiogenesis in fetal mouse lung explants. In addition, inhibitory antibodies against MIP-1α and MCP-1 blocked the effects of LPS on fetal lung vascular development, suggesting these chemokines are downstream mediators of LPS-induced angiogenesis. We speculate that an inflammation-mediated surge in angiogenesis could lead to formation of aberrant alveolar capillaries in the lungs of patients developing BPD.
Keywords: bronchopulmonary dysplasia, innate immunity, branching morphogenesis, inflammation
lung morphogenesis requires precise apposition of airways with blood vessels (50). During the earliest stages of embryonal lung development, the primitive airways arise from the ventral endoderm (10). Newly formed airways develop into a mature three-dimensional structure via multiple iterations of branching morphogenesis. Lung vascular development parallels airway branching, with peribronchial arteries growing and dividing alongside airways (21, 40). After branching completes, the alveolar capillary bed forms within the fetal lung mesenchyme through a combination of angiogenesis and vasculogenesis (3, 41). Alveolar capillary bed formation occurs during the canalicular [16–25 wk in humans, embryonic day 16 (E16) in mice] and saccular (25–36 wk in humans, E17 to postnatal day 5 in mice) stages of lung development (14). During capillary formation, epithelial-expressed growth factors including VEGF attract developing capillaries to the epithelial basement membrane (1, 46). In the later alveolar stage of development (after 36 wk in humans, postnatal day 5 in mice), epithelial-endothelial interactions promote alveolar septa formation (23, 38). Failure to normally complete these steps in lung morphogenesis can lead to defective gas exchange and respiratory disease.
Disruption of normal lung development in preterm infants causes bronchopulmonary dysplasia (BPD; Ref. 27). In extremely preterm infants born during the canalicular stage, lung morphogenesis must continue while exposed to the extrauterine environment (11). In up to 50% of infants born before 27 wk gestation, lung development fails to proceed normally, leading to BPD (5, 31). Infants with BPD have reduced lung volumes and defective gas exchange (26) and can develop pulmonary hypertension (33). Previous studies measuring vascular growth factors in the lungs of patients developing BPD have reported conflicting results (2, 12, 20, 29, 30). Some insights into lung vascular pathology in BPD have come from biopsy and autopsy specimens (13). Histological examination of lungs of BPD patients revealed dilated capillaries located in the lung interstitium and not intimately approximated to the alveolar epithelia. The mechanisms leading to these abnormally shaped and positioned vessels are not known. Lung vascular development has been most extensively studied in alveolar stage animal models, where both hypoxia and hyperoxia cause emphysema with reduced alveolar number and alveolar capillary density (28, 32). Whether these models emulate BPD pathogenesis is not completely clear, as infants born during the late saccular and alveolar stages of development (after 32 wk gestation) rarely develop BPD (5). The critical mechanisms leading to BPD, including potential derangement of the developing lung vasculature, likely originate in the earlier canalicular and saccular stages of lung development.
Clinical observations clearly show that inflammation is a major risk factor for BPD (9, 42, 48). Women with chorioamnionitis (inflammation of placenta, uterus, and amniotic membranes) are more likely to deliver prematurely, and infants exposed to inflammation either before birth or following delivery more commonly develop BPD. Whereas inflammation arrests airway morphogenesis in animal models, we know less about how inflammation affects the developing lung vasculature. We first examined the lung vasculature in infants that died with BPD. Using a mouse model of chorioamnionitis and in vitro models of saccular lung development, this study then tested the effects of inflammation on lung vascular development during the canalicular and saccular stages of lung development. We next asked whether the effects of inflammation on vascular development were caused by changes in vascular growth factors or by angiogenic inflammatory mediators. Our data show that inflammation stimulated angiogenesis in the fetal lung mesenchyme and that this surge in vascular development was mediated by angiogenic inflammatory chemokines. Our findings offer a new perspective on the vascular hypothesis of BPD and identify potential novel targets in BPD pathogenesis.
MATERIALS AND METHODS
Mice, cell culture, and reagents.
Tie2-lacZ, Tie2-green fluorescent protein (GFP), BALB/cJ, and ROSA26-enhanced yellow fluorescent protein (EYFP) mice were obtained from The Jackson Laboratory. SCL-Cre-ER(T) mice (18) were obtained from Joachim Gothert. Breeding colonies were established for timed matings, with the day of vaginal plug discovery defined as E0. SCL-Cre-ER(T):ROSA26-EYFP mice (referred to subsequently as SCL-YFP) were injected with tamoxifen (1 mg ip) on E12, E13, and E14. All animal procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committees at Vanderbilt University and the University of Alabama at Birmingham.
Primary cultures of fetal lung mesenchymal cells were isolated from E16 BALB/cJ mouse lungs and cultured in DMEM with 10% fetal bovine serum as previously described (8, 15). Mesenchymal cells were used before reaching third passage. Subculturing primary mesenchymal cells >10 passages resulted in a more homogeneous population of α-smooth muscle actin-expressing myofibroblasts. These cells were also maintained in DMEM with 10% fetal bovine serum and studied between passages 10 and 15. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2. Pulmonary microvascular endothelial cells from conditionally immortalized simian virus 40 (SV40) mice (Immortomouse; Refs. 24, 35) were generously supplied by Mark de Caestecker. Cells were maintained in EGM-2 MV media (Lonza) with IFN-γ at 33°C. To ensure degradation of the SV40 T antigen, cells were shifted to 37°C in the absence of IFN-γ for 72 h and passaged once before study.
Ion exchange-purified Escherichia coli LPS (strain O55:B5) was purchased from Sigma. Rat anti-platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31) was purchased from Chemicon. Mouse monoclonal anti-CD34 was from Dako. Antibodies against total and phosphorylated Akt were from Cell Signaling Technology. Polyclonal antibodies against the mesenchymal markers NG2 and PDGF receptor-β (PDGFRβ) were generously supplied by William Stallcup. Purified VEGF, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and inhibitory antibodies against MCP-1 and MIP-1α were purchased from R&D Systems.
Human lung samples.
Formalin-fixed paraffin-embedded lung tissue sections were prepared from autopsy specimens of six patients with BPD and six age-matched controls. Ages at time of death were between 3 and 18 mo. Clinical diagnosis of BPD was made first by the attending physician (oxygen requirement for 28 days and at 36 wk corrected gestation) and confirmed by the pathologist performing the autopsy. Age-matched controls did not have clinical or pathological evidence of lung disease.
In vivo chorioamnionitis.
The mouse chorioamnionitis and saccular stage explant models have been described previously (8, 15, 36, 37). For chorioamnionitis studies, at least seven litters of mice were studied in each group.
Microscopy and image analysis.
Standard procedures were used for tissue processing, immunostaining, and wide-field and confocal imaging. For quantifying endothelial cell number, Tie2-lacZ whole lungs were fixed in 4% formaldehyde, 0.05% Tween 20, 0.1 M PIPES, pH 6.5–6.8 and then incubated at room temperature overnight in 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) staining solution [5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 1 mg/ml X-gal in PBS + 2 mM MgCl2]. Whole lungs were placed in 30% sucrose solution at 4°C overnight, frozen in optimum cutting temperature compound (OCT) using liquid nitrogen, sectioned, and affixed to glass slides. Sections were then postfixed in 0.4% formaldehyde and counterstained before imaging.
Photographs were taken of individual airways in randomly chosen coronal or axial sections from Tie2-lacZ whole lungs using a Nikon TE2000-U inverted microscope and a Nikon QICAM digital camera. MetaMorph version 6.2r4 software (Universal Imaging) was used to identify and count individual lacZ-positive endothelial cells. Endothelial cells surrounding the airway epithelia were counted to calculate the number of endothelial cells per unit of area for each individual airway, which was measured by tracing each airway. Bronchial airways (lined with columnar epithelia) were excluded from analysis. Between 3 and 7 airway were analyzed in each image, and ∼60 images were obtained from each litter of fetal mice. At least 7 litters of fetal mice were analyzed at each time point and condition. Concealing the identity of each slide ensured that the investigator was blinded to the identity of each frozen section throughout the process of slide selection, imaging, and image analysis. Similar analysis was performed on Tie2-GFP explants, with visible airways along the explant margin photographed for analysis. For each time point and condition, 50–80 airways from a total of at least 5 separate explants were imaged and quantified. Statistical analysis of different treatment conditions was performed using ANOVA or unpaired t-tests where appropriate.
Endothelial cell proliferation, migration, and angiogenesis.
Cell proliferation was measured by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Roche). Cell migration through collagen-coated modified Boyden chambers was performed as previously described (7). The number of cells migrating through the filter with LPS in the media was normalized to control for each experiment. To measure artificial wound healing, pulmonary microvascular endothelial cells were grown to confluency and “wounded” using a sterile 200-μl pipette tip. Images were obtained at 6 distinct locations along the wound immediately after wounding and then 24 h later. The remaining area of the wound not covered with cells after 24 h was measured and subtracted from the initial wounded area. For each experiment, the fraction of wound area that was healed was normalized to control. Data were compared by ANOVA. To measure angiogenesis, pulmonary microvascular endothelial cells (150,000 cells per well) were cultured on a basement membrane matrix (Growth Factor Reduced Matrigel; BD Biosciences). Images of each well were obtained and analyzed using Histometrix (Andor Bioimaging) to determine total endothelial tube length. For each experiment, the total endothelial tube length was normalized to control. Data were compared by unpaired t-test.
Akt phosphorylation.
Pulmonary microvascular endothelial cells were treated with LPS for 10 min to 24 h and harvested in lysis buffer [50 mM Tris·HCl, pH 7, 2% SDS, 5% 2-mercaptoethanol, phosphatase inhibitor cocktail (containing sodium orthovanadate, sodium molybdate, sodium tartrate, and imidazole; Sigma), and protease inhibitor cocktail (Complete Mini; Roche)]. Cell lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Phosphorylated Akt was detected using an antibody specific for phosphorylated Ser473, peroxidase-conjugated secondary antibody, and chemiluminescence detection. The membranes were then stripped and reprobed with an antibody against total Akt. Cells treated for 30 min with insulin were used as a positive control.
Gene expression and chemokine measurement.
Gene expression measurement by real-time PCR was described previously (8). The 2−ΔΔCT method was used to compare gene expression levels between samples (4). Data were compared by ANOVA to test for significant differences. Tracheal aspirate samples were obtained from intubated preterm infants. All protocols were reviewed and approved by the Institutional Review Board at the University of Alabama at Birmingham. Eligible patients were delivered between 23 and 28 wk gestation and intubated on the first day of life. Tracheal aspirates were collected before 24 h of age. Endotracheal suctioning was performed using an enclosed inline suction catheter. One milliliter of sterile saline was instilled endotracheally, and fluid was suctioned into an enclosed, sterile specimen trap. The aspirated fluid was centrifuged at 2,000 g at 4°C for 5 min. The supernatant was then filtered through a 0.45-μm low protein binding syringe filter (Millipore), aliquoted, and stored at −80°C. Urea concentration in each aspirate was measured using the QuantiChrom Urea Assay Kit (Bioassay Systems). Samples were diluted to a final urea concentration of 0.1 mg/dl before use. Patient identifiers were not obtained, but presence or absence of maternal chorioamnionitis was recorded. All cases of chorioamnionitis were confirmed by placental pathology. MCP-1 and MIP-1α concentrations in each sample were measured by SearchLight Assay (Pierce Endogen). Each sample was measured in triplicate over multiple dilutions. For chemokine measurement in cell culture media, primary fetal mouse lung mesenchymal cells and myofibroblasts were cultured in serum-free DMEM in the absence or presence of LPS (250 ng/ml). Media samples were collected, and chemokine concentrations were analyzed by Luminex assay (Bio-Rad). Each sample was measured in triplicate.
RESULTS
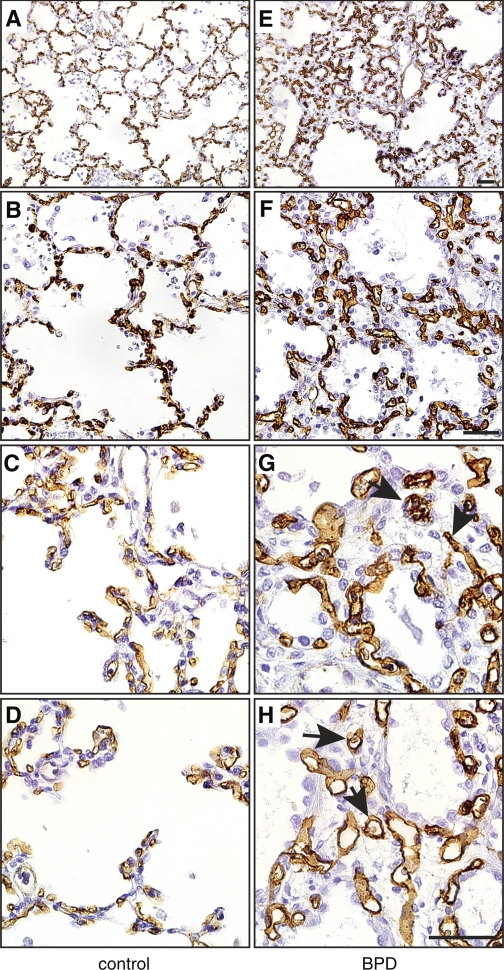
The lung vasculature develops abnormally in BPD. To better understand the vascular phenotype in BPD lungs, we first examined the vascular endothelia in lung sections from BPD and age-matched control autopsy samples. In control lungs, cells expressing the endothelial marker CD34 lined the alveolar septa (Fig. 1, A–D). In lungs from patients that died with BPD, abundant CD34 staining was seen throughout the lung interstitium (Fig. 1, E–H). Alveolar capillaries in BPD patients were often more dilated, with some vessels located deep within the interstitium and not adjacent to distal airways. These data suggest that patients with BPD have dysmorphic, malpositioned vessels and reduced numbers of alveolar septa.
Fig. 1.
Vascular endothelial cells in the lungs of patients with bronchopulmonary dysplasia (BPD). Autopsy samples from patients that died with BPD were immunostained with antibodies against CD34 to visualize vascular endothelial cells. Age-matched controls were obtained from patients that died without clinical or pathological evidence of lung disease. A and E: low magnification. B and F: medium magnification. C, D, G, and H: high magnification. Arrows in G and H indicate dilated capillaries in BPD samples in the lung interstitium. Images are representative of 6 control and 6 BPD samples tested. Scale bar, 50 μm.
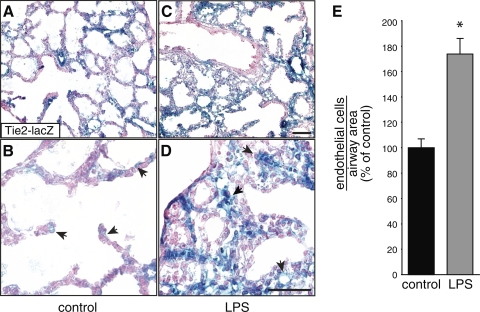
Because inflammation appears to play a role in early BPD pathogenesis, we tested the effects of chorioamnionitis on fetal lung vascular development. We (36, 37) have previously used a mouse chorioamnionitis model to show that inflammation can alter canalicular and saccular airway morphogenesis. To measure the effects of inflammation on fetal lung vascular development, we induced chorioamnionitis in Tie2-lacZ endothelial reporter mice and examined lacZ expression within the fetal lung mesenchyme 48 h later, as canalicular lungs develop into the saccular stage. LPS increased the number of lacZ-positive endothelial cells surrounding each distal airway when controlled for airway area (Fig. 2). Some endothelial cells localized to the lung interstitium, similar to the pattern seen in human BPD samples (Fig. 1). These in vivo data suggested that prenatal exposure to inflammation could stimulate vascular development in the canalicular and saccular stage mouse lung.
Fig. 2.
LPS increases Tie2-lacZ expression in vivo. Embryonic day 15 (E15) Tie2-lacZ mice were injected with sterile saline (A and B) or Escherichia coli LPS (C and D). After 48 h, the fetal lungs were isolated, fixed, and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal). Arrows indicate Tie2-lacZ-expressing endothelial cells. Higher magnification images are shown in B and D. Scale bar, 50 μm. E: the number of lacZ-positive endothelial cells surrounding each saccular airway was counted and normalized to airway area. (*P < 0.001; n = 154 control, n = 147 LPS; error bars ± standard error of the mean).
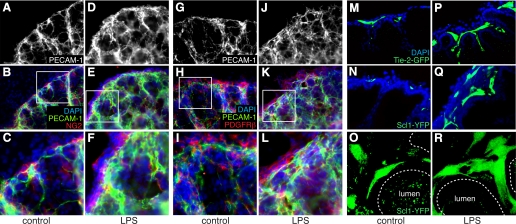
To study the effects of inflammation on vascular development independent of systemic and maternal immune systems, we used saccular stage E16 fetal mouse lung explants. In control explants, distinct PECAM-1-positive endothelial cells can be seen surrounding saccular airways with long, thin cellular processes (Fig. 3, A–C and G–I). Culturing explants in the presence of LPS increased PECAM-1 staining around developing airways, with large bands of endothelial cells throughout the mesenchyme (Fig. 3, D–F and J–L). This increase in endothelial cell number was likely due to angiogenesis, as we did not detect cells costaining with endothelial and mesenchymal markers. We further tested the effects of LPS using GFP and YFP reporter mice. In Tie2-GFP fetal lung explants, LPS increased the number of GFP-expressing endothelial cells surrounding saccular airways (Fig. 3, M and P). We obtained similar results using explants from SCL-YFP mice in which tamoxifen was used to label endothelial cells between E12 and E14 (Fig. 3, N and Q; Ref. 18). Confocal images from SCL-YFP explants show multiple endothelial cell projections in LPS-treated explants, consistent with increased angiogenesis (Fig. 3, O and R). Similar to our in vivo data, LPS increased lung vascular development in saccular stage lung explants.
Fig. 3.
LPS stimulates vascular formation in saccular stage fetal lung explants. A–L: saccular stage fetal mouse lung explants cultured under control conditions (A–C and G–I) or in the presence of LPS (D–F and J–L) for 72 h were immunostained with antibodies against platelet endothelial cell adhesion molecule-1 (PECAM-1) and the mesenchymal markers NG2 (B, C, E, and F) or PDGF receptor-β (PDGFRβ; H, I, K, and L). Higher magnification of areas indicated by boxes in B, E, H, and K are shown in C, F, I, and L. M–R: saccular stage explants from endothelial reporter Tie2-green fluorescent protein (GFP) (M and P) or SCL-Cre-ER(T):ROSA26-enhanced yellow fluorescent protein (EYFP) (SCL-YFP; N, O, Q, and R) were cultured in control media (M–O) or in the presence of LPS (P–R). Confocal images in M, N, P, and Q show increased GFP- and YFP-positive endothelial cells in LPS-treated explants. Maximal projection of z-series of images (O and R) show increased endothelial cell sprouts and cellular processes in LPS-treated SCL-YFP explants. DAPI, 4′,6′-diamidino-2-phenylindole.
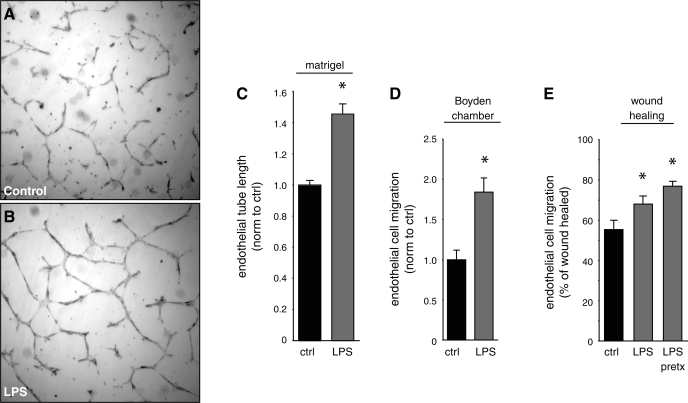
We next tested whether LPS could directly stimulate endothelial angiogenesis in cultured lung microvascular endothelial cells independent of other cell types. LPS increased the length of the endothelial tubes that formed after 20 h of culture in Matrigel (Fig. 4, A–C). Exposing lung microvascular endothelial cells to LPS also stimulated haptotactic cell migration and artificial wound healing (Fig. 4, D and E). In addition to the effects on tube formation and migration, LPS increased endothelial cell proliferation as measured by XTT assays by 15% (±2%; P < 0.001; n = 24). These data suggest direct addition of LPS to lung microvascular endothelial cells stimulates angiogenesis.
Fig. 4.
LPS stimulates microvascular endothelial cell angiogenesis and migration. A–C: mouse lung microvascular endothelial cells were cultured for 20 h in control Matrigel (A) or Matrigel containing E. coli LPS (250 ng/ml; B). C: the lengths of endothelial cell tubes were measured between branch points and normalized to control (*P < 0.001; n = 31). D: LPS increased migration of microvascular endothelial cells through Boyden chamber membranes. (*P < 0.001; n = 18). E: LPS increased wound healing in cultured microvascular endothelial cells. LPS was added either at the time of wounding (LPS) or 24 h before wounding (LPS pretx). Wound healing was measured 24 h after wounding (*P < 0.05; n = 41). Error bars ± standard error of the mean. Norm, normal; ctrl, control.
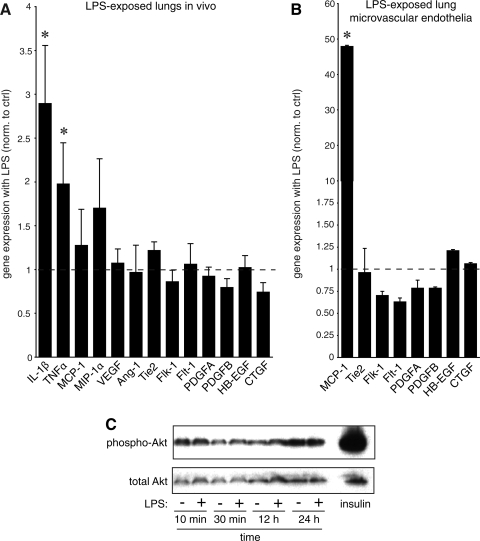
In stimulating vascular development, LPS could either increase expression of proangiogenic growth factors or induce inflammatory mediators with angiogenic properties. To first test the effects of LPS on expression of proangiogenic factors, we measured gene expression in fetal mouse lung tissue exposed to LPS in vivo (Fig. 5A). LPS increased expression of the inflammatory mediators IL-1β and TNFα but had no effect on the expression of VEGF, angiopoietin-1 (Ang-1), Tie2, fetal liver kinase-1 (Flk-1), fms-like tyrosine kinase-1 (Flt-1), PDGFA, PDGFB, heparin-binding EGF-like growth factor (HB-EGF), or connective tissue growth factor (CTGF). Similar results were obtained in LPS-treated lung microvascular endothelial cells (Fig. 5B). LPS increased MCP-1 expression but had no effect on expression of Tie2, Flk-1, Flt-1, PDGFA, PDGFB, HB-EGF, or CTGF (VEGF and Ang-1 were not detected in cultured cells). Treating microvascular endothelial cells with LPS also did not lead to increased phosphorylation of Akt (Fig. 5C), a downstream mediator of VEGF-stimulated angiogenesis (17). Whereas these data could not completely exclude increases in vascular growth factor expression, they suggested other mechanisms might be playing a role.
Fig. 5.
LPS stimulates inflammatory gene expression but not genes involved in growth factor-mediated vascular development. A: the effects of in vivo LPS exposure and chorioamnionitis on gene expression in fetal mouse lungs. RNA isolated from E18 mouse lungs were exposed in vivo to LPS for 72 h. Gene expression was measured by real-time PCR and compared with control RNA obtained from mice injected with saline. Only inflammatory genes were significantly increased (*P < 0.05; 6 controls and 6 LPS-exposed samples used). B: LPS increases monocyte chemoattractant protein-1 (MCP-1) expression in lung microvascular endothelial cells by real-time PCR (*P < 0.001; n = 3). No differences in endothelial markers or growth factors were detected. C: LPS did not stimulate Akt phosphorylation in lung microvascular endothelial cells. Blots shown are representative of 3 separate experiments. Insulin treatment was included as positive control. Error bars ± standard error of the mean. Ang-1, angiopoietin-1; Flk-1, fetal liver kinase-1; Flt-1, fms-like tyrosine kinase-1; HB-EGF, heparin-binding EGF-like growth factor; CTGF, connective tissue growth factor; MIP-1α, macrophage inflammatory protein-1α.
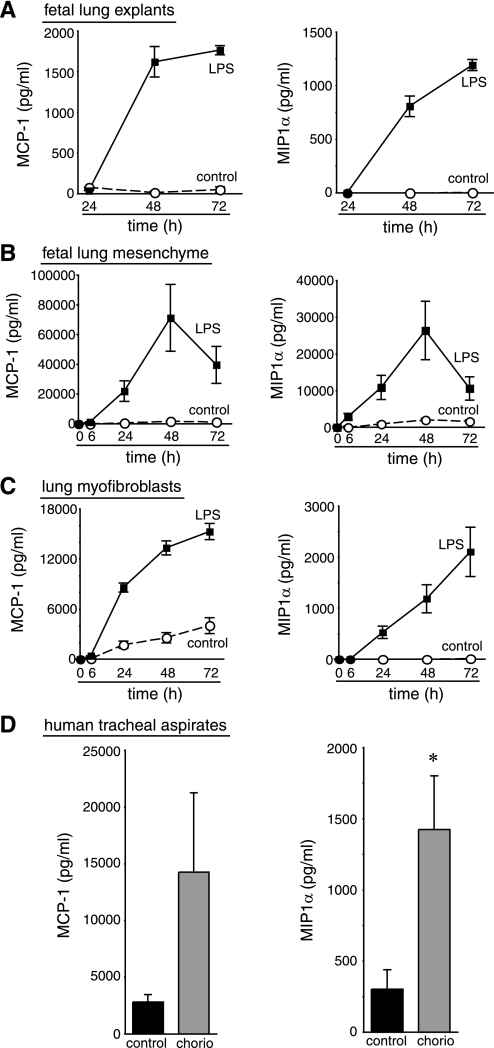
Because LPS did not induce angiogenic growth factor expression, we hypothesized that inflammatory mediators might stimulate lung vascular development. Among the many inflammatory mediators induced by LPS, the chemokines MCP-1 and MIP-1α have been reported to promote angiogenesis (43, 49). LPS increased expression of MCP-1 in cultured pulmonary microvascular endothelial cells (Fig. 5B). In addition, LPS stimulated the release of both MCP-1 and MIP-1α from fetal mouse lung explants, cultured fetal lung mesenchyme, and fetal-lung derived myofibroblasts (Fig. 6, A–C). We also measured increased MCP-1 and MIP-1α concentrations in the tracheal aspirate fluid of newborn extremely preterm infants exposed to chorioamnionitis compared with infants born preterm due to maternal indications (Fig. 6D). MCP-1 and MIP-1α therefore appear to be present when the fetal lung is exposed to inflammatory stimuli.
Fig. 6.
LPS stimulates release of MIP-1α and MCP-1. Fetal mouse lung explants (A), primary fetal mouse lung mesenchymal cells (B), and mouse lung myofibroblasts (C) were cultured in control media or in the presence of E. coli LPS (250 ng/ml). Media were collected at indicated time points, and chemokine concentrations were measured by Luminex assay. Each sample was measured in triplicate. D: MCP-1 and MIP-1α were detected in the tracheal aspirate fluid of newborn preterm infants exposed to maternal chorioamnionitis (chorio) by SearchLight assay (*P < 0.05; 7 control and 9 chorioamnionitis samples tested). Error bars ± standard error of the mean.
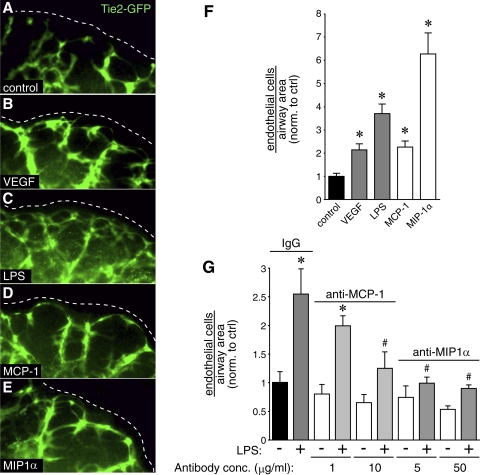
We used saccular stage Tie2-GFP explants to test whether MCP-1 and MIP-1α could stimulate vascular development in fetal mouse lung. Addition of either MCP-1 or MIP-1α increased Tie2-GFP expression surrounding developing airways (Fig. 7, D and E). Control images show increased GFP expression with LPS and VEGF treatment (Fig. 7, B and C). MCP-1 and MIP-1α also increased the number of endothelial cells surrounding each saccular airway (Fig. 7F). Neutralizing antibodies against MCP-1 and MIP-1α inhibited the effects of LPS on angiogenesis (Fig. 7G). Collectively, these data support the role of inflammatory chemokines in stimulating vascular development in fetal mouse lungs.
Fig. 7.
MCP-1 and MIP-1α stimulate vascular development in fetal mouse lung explants. A–E: saccular stage fetal lung explants from Tie2-GFP mice were cultured in the presence of VEGF, LPS, MCP-1, or MIP-1α. F: after obtaining confocal images of the Tie2-GFP explants, the number of endothelial cells surrounding each saccular airway was measured, corrected for airway area, and compared with control. (*P < 0.001; n = 12). G: antibodies against MCP-1 and MIP-1α inhibit LPS-mediated angiogenesis. Nonimmune rat IgG included as control. (*P < 0.05 compared with nonimmune IgG, #P < 0.05 compared with LPS with nonimmune IgG; n = 7). Error bars ± standard error of the mean. conc., Concentration.
DISCUSSION
Our results show that inflammation stimulates vascular development in the canalicular-to-saccular stage mouse lung. The bacterial endotoxin LPS increased the number of vascular endothelial cells surrounding developing saccular airways both in vivo and in vitro. This effect was likely attributable to increased proangiogenic inflammatory mediators and not to changes in vascular growth factors expression. Among the inflammatory mediators released in response to LPS, the chemokines MCP-1 and MIP-1α stimulated angiogenesis when added to cultured fetal lung explants. Antibodies against MCP-1 and MIP-1α inhibited the increase in vascular formation with LPS, supporting their role in inflammation-mediated angiogenesis. The effects of inflammation on vascular formation also appeared to be cell autonomous, as LPS stimulated proliferation, migration, and tube formation in lung microvascular endothelial cells in vitro. Collectively, our data support an inflammation-mediated surge in angiogenesis when the saccular stage mouse lung is exposed to LPS.
The increased vascular development on exposure to LPS in fetal mouse lung parallels the pathological effects of inflammation in other models. During cancer progression, both MCP-1 and MIP-1α stimulate angiogenesis and neovascularization, allowing tumor survival and growth (39, 49). Inflammation also promotes neovascularization and angiogenesis in autoimmune diseases, including psoriatic arthritis, ankylosing spondylitis, and rheumatoid synovitis (44). In atherosclerotic vascular disease, MCP-1 stimulates angiogenesis and collateral vessel formation in addition to recruiting inflammatory cells (34, 47). In mediating these effects, chemokines can either recruit VEGF-expressing macrophages or directly stimulate chemokine receptors on the endothelium (19, 22, 43). Our data suggest a direct effect in fetal mouse lung, as LPS stimulated angiogenesis in cultured lung explants devoid of circulation, did not increase VEGF expression, and did not stimulate Akt phosphorylation.
LPS binds Toll-like receptor 4 and activates the NF-κB pathway. Translocation of activated NF-κB subunits to the nucleus induces expression of inflammatory genes and promotes cell survival. Alternatively, LPS can promote apoptosis of some cell types by non-NF-κB mechanisms. When LPS stimulates their release, CC chemokines bind G protein-coupled receptors, leading to changes in intracellular Ca2+ concentrations and mitogen-activated protein kinase activity. These pathways regulate cell division, adhesion, and migration in multiple cell types (6, 45). Future studies will determine which pathways are required for LPS-mediated changes in fetal lung vascular development.
The physiological consequences of a surge in fetal lung angiogenesis are not clear. The initial effects may be beneficial, as patients exposed to chorioamnionitis often have less severe respiratory distress immediately following birth. Alternatively, increased angiogenesis could be detrimental, forming leaky blood vessels in the lung mesenchyme. As increased vascular permeability accompanies angiogenesis (16), new vessel formation could produce the interstitial pulmonary edema commonly described in preterm infants (25). Inflammation-mediated angiogenesis in the fetal lung could contribute to BPD pathogenesis by disrupting the normal relationships between developing airways and capillaries. If inflammatory mediators such as MIP-1α and MCP-1 stimulate angiogenesis independent of epithelial VEGF, lung capillaries may form without close approximation to airway epithelia. This could increase the distance between air spaces and capillaries, producing a potential barrier for gas exchange. Such dysmorphic capillary orientation is commonly observed in the lungs of patients with BPD (Ref. 13; Fig. 1). Inflammation during the perinatal period could also affect capillary remodeling later in lung development when the preterm lung is often exposed to hyperoxia, mechanical trauma, or repeated episodes of inflammation.
Lung injury in neonatal rodents reduces the number of alveolar septa and capillaries, supporting the role of abnormal vascular development in BPD pathogenesis (28, 32). This loss of alveolar septa and capillaries following injury is at least partially due to epithelial dysfunction and reduced VEGF production. Our data suggest that inflammation inhibits vascular development independent of the airway epithelia and VEGF expression. Therefore, our findings expand on this vascular hypothesis by demonstrating the effects of inflammation on earlier canalicular and saccular stages of lung vascular development, which correspond to the developmental stages of infants most at risk of BPD. The mechanism and developmental timing of lung injury may have distinctly different effects on vascular development. We propose that during the early stages of BPD pathogenesis, inflammation actually accelerates vascular development, possibly destabilizing developing capillaries. This change in vascular programming could lead to defects later in development as the alveolar capillary bed remodels and matures. The vascular abnormalities in BPD may not therefore be limited to fewer alveolar septa and capillaries, but may involve more complex cellular pathophysiology (11, 13). More detailed understanding of the basic mechanisms regulating lung capillary morphogenesis may shed additional light on the processes leading to altered vascular and alveolar development in BPD.
GRANTS
This work was supported by Fellowships from Ikaria (J. D. Miller and J. T. Benjamin) and Discovery Laboratories (J. D. Miller), American Lung Association Biomedical Research Grant RG-10721-N (L. S. Prince), March of Dimes Basil O'Connor Award FY07-555 (L. S. Prince), and National Heart, Lung, and Blood Institute Grants HL-086324 (L. S. Prince) and HL-097195 (L. S. Prince).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Brian Halloran, Teodora Nicola, William Andrews, and Robert Lott for their experimental assistance. We also are grateful to our colleagues Judy Aschner, Mark de Caestecker, Ambra Pozzi, Scott Baldwin, Timothy Blackwell, and Namasivayam Ambalavanan for their helpful insight and comments.
Present address of J. T. Benjamin: Dept. of Pediatrics, Univ. of South Alabama, Mobile, AL 36604.
REFERENCES
- 1.Akeson AL, Greenberg JM, Cameron JE, Thompson FY, Brooks SK, Wiginton D, Whitsett JA. Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev Biol 264: 443–455, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res 53: 240–244, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Anderson-Berry A, O'Brien EA, Bleyl SB, Lawson A, Gundersen N, Ryssman D, Sweeley J, Dahl MJ, Drake CJ, Schoenwolf GC, Albertine KH. Vasculogenesis drives pulmonary vascular growth in the developing chick embryo. Dev Dyn 233: 145–153, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol 15: 56–61, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 8: 63–71, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Broxmeyer HE. CCR5 ligands modulate CXCL12-induced chemotaxis, adhesion, and Akt phosphorylation of human cord blood CD34+ cells. J Immunol 183: 7478–7488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin JT, Gaston DC, Halloran BA, Schnapp LM, Zent R, Prince LS. The role of integrin alpha8beta1 in fetal lung morphogenesis and injury. Dev Biol 335: 407–417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol 292: L550–L558, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bose CL, Laughon MM, Dammann CE. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed 93: F455–F461, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 133: 1611–1624, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol 30: 179–184, 2006 [DOI] [PubMed] [Google Scholar]
- 12.D'Angio CT, Maniscalco WM, Ryan RM, Avissar NE, Basavegowda K, Sinkin RA. Vascular endothelial growth factor in pulmonary lavage fluid from premature infants: effects of age and postnatal dexamethasone. Biol Neonate 76: 266–273, 1999 [DOI] [PubMed] [Google Scholar]
- 13.De Paepe ME, Mao Q, Powell J, Rubin SE, DeKoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med 173: 204–211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 16: 568–581, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res 59: 185–190, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039, 1995 [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gothert JR, Gustin SE, Hall MA, Green AR, Gottgens B, Izon DJ, Begley CG. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105: 2724–2732, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Green CE, Liu T, Montel V, Hsiao G, Lester RD, Subramaniam S, Gonias SL, Klemke RL. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One 4: e6713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan J, Beharry KD, Valencia AM, Strauss A, Modanlou HD. Soluble vascular endothelial growth factor receptor 1 in tracheal aspirate fluid of preterm neonates at birth may be predictive of bronchopulmonary dysplasia/chronic lung disease. Pediatrics 123: 1541–1547, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Hislop AA. Airway and blood vessel interaction during lung development. J Anat 201: 325–334, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 105: 1405–1407, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 53: 81–94, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Korhonen P, Laitinen J, Hyodynmaa E, Tammela O. Respiratory outcome in school-aged, very-low-birth-weight children in the surfactant era. Acta Paediatr 93: 316–321, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med 14: 2–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 289: L529–L535, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Lassus P, Ristimaki A, Ylikorkala O, Viinikka L, Andersson S. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med 159: 1429–1433, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981–1987, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network Pediatrics 107: E1, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 16: 557–567, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 154: 379–384, 384.e1–2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niiyama H, Kai H, Yamamoto T, Shimada T, Sasaki K, Murohara T, Egashira K, Imaizumi T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol 44: 661–666, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA 97: 2202–2207, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn 233: 553–561, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Prince LS, Okoh VO, Moninger TO, Matalon S. Lipopolysaccharide increases alveolar type II cell number in fetal mouse lungs through Toll-like receptor 4 and NF-κB. Am J Physiol Lung Cell Mol Physiol 287: L999–L1006, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Roth-Kleiner M, Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol 40: 113–134, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96: 34–40, 2000 [PubMed] [Google Scholar]
- 40.Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol 22: 157–165, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Schwarz MA, Caldwell L, Cafasso D, Zheng H. Emerging pulmonary vasculature lacks fate specification. Am J Physiol Lung Cell Mol Physiol 296: L71–L81, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol 26, Suppl 1: S57–S62; discussion S63–S64, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Stamatovic SM, Keep RF, Mostarica-Stojkovic M, Andjelkovic AV. CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. J Immunol 177: 2651–2661, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthriris Res Ther 10: 224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian Y, Lee MM, Yung LY, Allen RA, Slocombe PM, Twomey BM, Wong YH. Differential involvement of Galpha16 in CC chemokine-induced stimulation of phospholipase Cbeta, ERK, and chemotaxis. Cell Signal 20: 1179–1189, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 290: L209–L221, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, Schaper W, Piek JJ, Buschmann IR. Modulation of collateral artery growth in a porcine hindlimb ligation model using MCP-1. Am J Physiol Heart Circ Physiol 284: H1422–H1428, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97: 210–215, 1996 [PubMed] [Google Scholar]
- 49.Wu Y, Li YY, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol 181: 6384–6393, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto Y, Shiraishi I, Dai P, Hamaoka K, Takamatsu T. Regulation of embryonic lung vascular development by vascular endothelial growth factor receptors, Flk-1 and Flt-1. Anat Rec (Hoboken) 290: 958–973, 2007. [DOI] [PubMed] [Google Scholar]