Abstract
Cholinergic bronchoconstriction is mediated by M2 and M3 muscarinic receptors (MR). In heart and urinary bladder, MR are linked to caveolin-1 or -3, the structural proteins of caveolae. Caveolae are cholesterol-rich, omega-shaped invaginations of the plasma membrane. They provide a scaffold for multiple G protein receptors and membrane-bound enzymes, thereby orchestrating signaling into the cell interior. Hence, we hypothesized that airway MR signaling pathways are coupled to caveolae as well. To address this issue, we determined the distribution of caveolin isoforms and MR subtype M2R in murine and human airways and investigated protein-protein associations by fluorescence resonance energy transfer (FRET)-confocal laser scanning microscopy (CLSM) analysis in immunolabeled murine tissue sections. Bronchoconstrictor responses of murine bronchi were recorded in lung-slice preparations before and after caveolae disruption by methyl-β-cyclodextrin, with efficiency of this treatment being validated by electron microscopy. KCl-induced bronchoconstriction was unaffected after treatment, demonstrating functional integrity of the smooth muscle. Caveolae disruption decreased muscarine-induced bronchoconstriction in wild-type and abolished it in M2R−/− and M3R−/− mice. Thus M2R and M3R signaling pathways require intact caveolae. Furthermore, we identified a presumed skeletal and cardiac myocyte-specific caveolin isoform, caveolin-3, in human and murine bronchial smooth muscle and found it to be associated with M2R in situ. In contrast, M2R was not associated with caveolin-1, despite an in situ association of caveolin-1 and caveolin-3 that was detected. Here, we demonstrated that M2R- and M3R-mediated bronchoconstriction is caveolae-dependent. Since caveolin-3 is directly associated with M2R, we suggest caveolin-3 as novel regulator of M2R-mediated signaling.
Keywords: bronchus, caveolin, fluorescence resonance energy transfer, smooth muscle, muscarinic
regulation of airway diameter is under prominent control of ACh released from parasympathetic nerve fibers acting on G protein-coupled muscarinic receptors (MR) situated on airway smooth muscle cells (ASMC; Ref. 10). Five molecularly distinct MR subtypes (M1R–M5R) are known. We (38) previously identified M2R and M3R as being essential for ACh-induced constriction of murine bronchial SMC. Their stimulation leads to activation of different signaling pathways in bronchial SMC, all converging onto bronchoconstriction (3, 28). Under physiological conditions, M2R binding sites outnumber M3R binding sites to a species-dependent extent, but functionally there is always a dominance of M3R in bronchoconstriction (14). However, there is evidence that the functional relevance of M2R increases under pathological conditions. Airway diseases such as chronic obstructive pulmonary disease (COPD) and asthma are associated with bronchial hyperreagibility and prejunctional M2R dysfunction (4). Whether postjunctional M2R are dysfunctional as well is not known. Hence, it is of relevance to identify key regulatory molecules of the airway MR signaling pathways.
Several G protein-coupled receptors are not randomly distributed along the cell surface but aggregate at specialized membrane compartments termed “caveolae.” These are 50- to 100-nm flask-shaped invaginations of the plasma membrane, containing caveolins (Cav) as their major structural proteins (33). Three Cav isoforms have been identified, Cav-1, Cav-2, and Cav-3, among which the presence of either Cav-1 or Cav-3 is essential for the formation of caveolae (33). Cav-1 is widely expressed, including ASMC (13, 19, 31), whereas Cav-3 is found primarily in striated (skeletal and cardiac) muscle and certain SMC (35). In the heart and urinary bladder, MR subtypes are functionally coupled to caveolins. In rat cardiac ventricular myocytes, Feron et al. (8) showed an agonist-dependent translocation of the M2R into caveolae where a muscarinic radioligand was coimmunoprecipitated together with Cav-3. In urinary bladder SMC, the contractile response to carbachol, a MR agonist, is decreased by 67% in Cav-1 gene-deficient mice (21).
Hence, we hypothesized that the airway MR signaling pathways are coupled to caveolae as well. To address this issue, we 1) determined the distribution of Cav-1, Cav-3, and M2R in ASMC by immunohistochemistry (IHC), Western blotting, and laser-assisted microdissection with subsequent RT-PCR; 2) assessed protein-protein associations and the molecular composition of caveolae in situ by confocal laser scanning microscopy (CLSM) and fluorescence resonance energy transfer (FRET) analysis in tissue sections subjected to double-labeling indirect immunofluorescence (17); and 3) addressed the functional role of caveolae/caveolins in bronchoconstriction by videomicroscopy of precision-cut lung slices (PCLS; Ref. 23) from wild-type, M2R- and M3R-deficient mice before and after caveolae disruption by methyl-β-cyclodextrin (MCD), a cholesterol-depleting agent (34). The efficiency of MCD treatment was validated by electron microscopy.
MATERIALS AND METHODS
Murine and human tissue.
1) Videomorphometry, electron microscopy, laser-assisted microdissection, and RT-PCR analysis were performed on M2R- and/or M3R-deficient (M2R−/−, M3R−/−) male mice (10–20 wk old) and their corresponding wild-type mice (M2R+/+, M3R+/+). Each line was kept under specified pathogen-free conditions. The generation of M2R−/− and M3R−/− mice has been described previously (11, 44). 2) IHC, Western blotting, and FRET-CLSM analysis were performed on male and female FVB mice (10–25 wk old, kept under normal conditions). 3) For validation of M2R antibody specificity, a 31-wk-old female M2/3R double-knockout (M2/3R−/−) mouse and a 12-wk-old male wild-type mouse with the same genetic background [129/J1 (25%) × 129SvEv (50%) × CF1 (25%)] were used. The generation of this mutant mouse strain has been described previously (38). 4) Validation of Cav-1 and Cav-3 antibody specificity was performed on tissue from two 17- to 18-wk-old male Cav-1-deficient mice (Cav-1−/−) and two corresponding 20-wk-old wild-type mice. The generation of Cav-1−/− mice with a genetic background of C57BL/6 × SV129 has been described previously (33). For videomorphometric analysis, mice were killed by cervical dislocation. Apart from that, all animals were killed by isoflurane (Abbott, Wiesbaden, Germany) inhalation.
The human tissue (n = 5) for PCR analysis was obtained from organ donors (n = 5) whose lung had finally not been used for transplantation because of other reasons. Samples were shock-frozen and stored at −80°C until use. For IHC, 10% formalin-fixed human lung samples (n = 5) were postfixed with Zamboni (1.85% formaldehyde and 15% saturated picric acid solution in 0.1 M phosphate buffer) overnight at 4°C, cryoprotected (18% saccharose containing 0.1 M phosphate buffer), and shock-frozen in liquid N2. The protocols were reviewed and approved by the local animal welfare authority, Regierungspräsidium Giessen, Germany.
Antibodies and peptides.
Antibodies and their sources were as follows: FITC-conjugated anti-α-smooth muscle actin (anti-α-sma; IHC 1:500), monoclonal from mouse (clone 1A4; Sigma, Taufkirchen, Germany); anti-Cav-1α (IHC human 1:400, IHC mouse 1:800, Western blotting 1:1,000) and tetramethylrhodamine isothiocyanate (TRITC)-conjugated anti-Cav-1α (TRITC-anti-Cav-1α; IHC 1:100), polyclonal from rabbit (sc-894; TRITC; Santa Cruz Biotechnology, Heidelberg, Germany); anti-M2R (1:800 IHC), monoclonal from rat (MAB367; 1 mg/ml; Chemicon, Temecula, CA); anti-Cav-3 (IHC human 1:800, IHC mouse 1:500; Western blotting 1:4,000), polyclonal from rabbit (Affinity BioReagents); synthetic peptide corresponding to amino acid residues 1–18 of mouse Cav-3 sequence (IHC 100 μg/ml; Western blotting 8.3 μg/ml; Affinity BioReagents). Secondary antibodies used in this study for IHC were Cy3-conjugated donkey anti-rabbit-Ig (1:1,000; Chemicon), Cy5-conjugated donkey anti-rabbit-Ig, F(ab′)2 fragments (1:400; Dianova, Hamburg, Germany), and Cy3-conjugated donkey anti-rat-Ig (1:2,000; Dianova). Secondary antibody used in this study for Western blotting was horseradish peroxidase-conjugated goat anti-rabbit-IgG (1:10,000; Pierce, Rockford, IL).
Western blotting.
For protein detection of Cav-1 and Cav-3, lung homogenates of Cav-1+/+ and Cav-1−/− mice and FVB mice (n = 3 each) were used. Four-hundred-micrometer-thick vibratome-cut lung slices were homogenized in lysis buffer containing 10 mM Tris (pH 7.5), 50 mM NaCl, 1% Triton X-100, 60 mM octylglucoside (Sigma), and one Complete Mini Protease Inhibitor Cocktail tablet (Roche Diagnostics, Mannheim, Germany) per 10-ml buffer by a mixer mill (MM 300; Qiagen, Hilden, Germany). After the protein solutions were incubated at 4°C for 1 h, they were centrifuged for 5 min at 20,800 g. Equal amounts of proteins for each tissue were applied as judged by staining of gels with SimplyBlue SafeStain (Invitrogen, Carlsbad, CA). Ten-microliter appropriately diluted protein solution and 2 μl of 5× sample buffer (320 mM Tris·HCl, pH 6.8, 5% SDS, 50% glycerol, 0.25 mg/ml bromophenol blue, and 1% 2-mercaptoethanol) were boiled for 5 min at 95°C. The samples were subjected to 15% SDS-PAGE under reducing conditions and subsequently transferred by semidry blotting to a polyvinylidene fluoride (PVDF) membrane (Immobilon-P; Fisher Scientific) activated with methanol. The PVDF membranes were stained with Ponceau S to confirm the protein transfer from the gels to the membrane. After subsequent washing in 25 mM Tris-buffered saline with 0.05% Tween 20 (TTBS), unspecific binding sites were saturated by incubation with 10% nonfat dry milk (Sigma) in TTBS for 90 min at room temperature. The membrane was incubated overnight at room temperature with the primary antibody diluted in 5% nonfat dry milk in TTBS. The secondary antibody was diluted in 2.5% nonfat dry milk in TTBS and incubated for 1 h at room temperature. SuperSignal West Pico Chemiluminescent Substrate (Pierce) and an X-ray film (Amersham) were used for visualization. Controls for the specificity of antibodies used were done 1) for anti-Cav-3 antibody by incubation of this antibody with the corresponding peptide for 1 h at room temperature before use, and 2) for anti-Cav-1α by using lung slices from Cav-1-deficient mice. Controls for the specificity of the secondary reagents were performed by omitting the primary antibody.
IHC.
Unfixed tissue of FVB mice, Cav-1- and M2/3R-deficient mice, and their corresponding wild-type mice (n = 2 each) were used for IHC validation of antibody specificity. Lungs were inflated via the trachea with optimum cutting temperature (OCT) compound (Sakura, Zoeterwoude, The Netherlands) diluted with an equal amount of 0.1 M phosphate buffer (pH 7.4), orientated on a piece of filter paper, and shock-frozen in melting isopentane. Cryosections (10 μm) from murine and human lung samples were cut, fixed with acetone at −20°C, air-dried for 10 min, and incubated for 1 h in 5% normal goat serum containing 5% BSA in 0.005 M PBS. Primary antibodies were diluted in 0.005 M phosphate buffer containing 0.01% NaN3 and 4.48 g/l NaCl and applied overnight at room temperature. These antibodies were applied either singly or in combination for double-labeling immunofluorescence. Primary antibody combinations were as follows: rabbit anti-Cav-1α/rat anti-M2R; rabbit anti-Cav-3/rat anti-M2R; and rabbit (TRITC-) anti-Cav-1α/rabbit anti-Cav-3. After a washing step, Cy5-conjugated F(ab′)2 donkey anti-rabbit-Ig was applied overnight, and after a second washing step the slides were incubated with Cy3-conjugated donkey anti-rat-Ig for 1 h. In the case of TRITC-anti-Cav-1α as primary antibody, slices were incubated with TRITC-anti-Cav-1α overnight instead of Cy3-conjugated antibody. Sections were rinsed, postfixed for 10 min in 4% paraformaldehyde, rinsed again, and coverslipped with carbonate-buffered glycerol (pH 8.6) or Mowiol 4-88 (pH 8.6; Merck, Darmstadt, Germany) containing 0.1% DABCO (Sigma). In case of human tissue, slices from five donor lungs were incubated with mouse FITC-anti-α-sma overnight and postfixed before coverslipped. Slides were evaluated with an epifluorescence microscope (Zeiss, Jena, Germany) using appropriate filter sets and with a CLSM (Leica TCS SP2 AOBS; Leica, Bensheim, Germany) using an oil immersion objective lens with a magnification of ×63 and 1.4 numerical aperture.
FRET.
FRET is a nonradiative energy transfer between 2 fluorophores (a donor and an acceptor) that can be detected only if the 2 fluorophores are less than 10 nm apart. We used FRET combined with CLSM and double-labeling indirect immunofluorescence as a technique for measuring close spatial association of proteins in tissue sections (17). Sections of thoracic viscera from FVB mice were double-labeled for Cav-1/Cav-3, Cav-1/M2R, and Cav-3/M2R (n ≥ 4 each) as described above. For control of the species specificity of the secondary reagents, only the acceptor antibody (anti-Cav-1α or anti-Cav-3 antibody) and both secondary antibodies were applied. FRET was quantified by the acceptor photobleaching method using a CLSM (Leica TCS SP2 AOBS). In this method, FRET is detected by measuring the intensity of fluorescence of the donor before and after bleaching of the acceptor. The CLSM settings were as follows: detection of Cy3 and TRITC, 52% laser power at 543 nm, detection at 555–620 nm; and Cy5, 20% laser power at 633 nm, detection at 639–738 nm. A region of interest (ROI) was photobleached 5 times for Cav-1/Cav-3 and 10 times for Cav-1/M2R and Cav-3/M2R staining at 100% activity using the 633-nm laser and maximal zoom to destroy the acceptor fluorophore (Cy5). The difference to Cy3 or TRITC signal (ΔIF), respectively, was determined in the photobleached area where ΔIF = ΔDA − ΔDB, ΔDA is the fluorescence intensity of the donor after photobleaching of the acceptor, and ΔDB is the fluorescence intensity of the donor before photobleaching of the acceptor. The increase of fluorescence (ΔIF) for Cav-1/Cav-3, Cav-1/M2R, and Cav-3/M2R staining was measured in bronchi and heart atrium of 4–6 mice in at least 10 different ROI each. Heart atrium was applied as a positive control for the detection of association between the investigated proteins.
Laser-assisted microdissection and subsequent RT-PCR.
Laser-assisted microdissection was used to isolate SMC from cryosections of tracheae and bronchioli of M3R+/+ mice (n = 4) using a MicroBeam System (P.A.L.M. Microlaser Technologies, Bernried, Germany). Tissues were prepared as described for IHC analysis. Serial cryosections (6 μm) were collected on membrane slides (P.A.L.M. Microlaser Technologies) previously radiated with UV light (254 nm) for 30 min. Within 1 h after preparing the sections, equal amount of tissue was collected into the lid of cups covered with mineral oil. RNA isolation and purification were performed using RNeasy Micro Kit (Qiagen) according to the manufacturer's protocol but omitting the DNA digestion step. Ten-microliter RNA were incubated at 70°C for 10 min. RT mix was added [2-μl 10× PCR buffer II, 4-μl MgCl2 (25 mM), 1-μl dNTPs (10 mM), 1-μl random hexamers (50 mM), 0.5-μl RNase inhibitor (20 U/μl), 1-μl murine leukemia virus RT (50 U/μl), 0.5-μl H2O; all reagents from Applied Biosystems, Darmstadt, Germany]. RNA was reverse-transcribed for 75 min at 43°C followed by inactivation of the RT by heating the RNA samples for 5 min at 99°C. For subsequent PCR, gene-specific intron spanning primer sets for Cav-1, Cav-3, and β-microglobulin (β-MG; Table 1) were used. Four-microliter cDNA, 2.5-μl 10× PCR buffer II, 2-μl MgCl2 (25 mM), 0.5-μl dNTPs (10 mM), 0.5 μl of each primer, 0.2-μl AmpliTaq Gold DNA Polymerase (5 U/μl; all reagents from Applied Biosystems), and 14.8-μl H2O were applied. Cycling conditions were 4 min at 95°C, 50 cycles with 20 s at 95°C, 20 s at 59°C, 20 s at 73°C, and a final extension at 72°C for 7 min. To control for smearing of RNA during the cutting procedure, areas of dried OCT compound similar in number and size to the samples of isolated SMC were applied. In addition, a cup containing mineral oil but no tissue was carried along. Control reactions for each primer pair included the absence of template. The PCR products were separated by electrophoresis on a 1.5% Tris-acetate-EDTA agarose gel.
Table 1.
Oligonucleotide primers used in RT-PCR analysis
| Gene | NCBI Acc. No. | Primer Sequence (5′ to 3′) | Product Length, bp |
|---|---|---|---|
| Mouse | |||
| Cav-1 | NM007616.3 | forward GCA CAC CAA GGA GAT TGA CC | 212 |
| reverse AGA TGA GTG CCA TTG GGA TG | |||
| Cav-3 | NM007617 | forward AGG ACA TTC ACT GCA AGG AG | 167 |
| reverse GTA CTT GGA GAC GGT GAA CG | |||
| M2R | AF264049 | forward TGT CTC CCA GTC TAG TGC AAG G | 369 |
| reverse CAT TCT GAC CTG ACG ATC CAA C | |||
| M3R | AF264050 | forward GTA CAA CCT CGC CTT TGT TTC C | 245 |
| reverse GAC AAG GAT GTT GCC GAT GAT G | |||
| β-MG | NM009735.3 | forward TGG TCT TTC TGG TGC TTG TC | 108 |
| reverse GTA TGT TCG GCT TCC CAT TC | |||
| Human | |||
| Cav-1 | BC082246 | forward ACG TAG ACT CGG AGG GAC AT | 137 |
| reverse CAG GTC GAT CTC CTT GGT GT | |||
| Cav-1 | BC082246 | forward GAG CTG AGC GAG AAG CAA GT | 178 |
| reverse CAG TGA AGG TGG TGA AGC TG | |||
| Cav-3 | NM001234.3 | forward GAT GGC AGA AGA GCA CAC AG | 227 |
| reverse ACA ACA GAC GGT AGC ACC AG | |||
| HPRT1 | NM000194.1 | forward TCCCAGCGTCGTGATTAGTG | 225 |
| reverse TCCAGCAGGTCAGCAAAGAA | |||
NCBI, National Center for Biotechnology Information; Cav, caveolin; M2R and M2R, muscarinic receptor subtypes; β-MG, β-microglobulin; HPRT1, hypoxanthine phosphoribosyl-transferase 1.
Real-time RT-PCR.
Total RNA from lung (n = 5), tracheal muscle (n = 4), and urinary bladder (n = 4) from M3R+/+ and M3R−/− mice and from human lung homogenates (n = 5) was isolated using the RNeasy method according to the manufacturer's protocol (Qiagen). Contaminating DNA was degraded using 1 unit of DNase I (Invitrogen, Karlsruhe, Germany) per microgram total RNA, and reverse transcription was done for 50 min at 42°C using 200 units of SuperScript II RT (Invitrogen, Karlsruhe, Germany) per microgram RNA. Real-time PCR was performed in an iCycler (Bio-Rad, Munich, Germany) using QuantiTect SYBR Green PCR Kit (Bio-Rad). Gene-specific intron spanning primer sets for M2R, M3R, and β-MG were used in mouse. Two independent primer pairs for Cav-1 and one for Cav-3 and hypoxanthine phosphoribosyltransferase (HPRT) were used in human (Table 1). The PCR conditions included initial denaturation in one cycle of 10 min at 95°C followed by 45 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C. All analyses were done in triplicate, and the mean cycle thresholds (CT) for M2R, M3R, and β-MG were calculated. The ΔCT of M2R (ΔCTM2R) and M3R (ΔCTM3R), respectively, compared with β-MG was calculated as follows:
Control reactions included the absence of DNA template and the absence of RT. The PCR products were separated by electrophoresis on a 2% Tris-acetate-EDTA agarose gel. Specificities of the amplified human Cav-3 and HPRT PCR products were verified by sequencing (MWG Biotech, Ebersberg, Germany).
Videomorphometry.
PCLS from M2R+/+, M2R−/−, M3R+/+, and M3R−/− mice (n ≥ 4 each) were obtained by using a slightly modified version of the protocol used by Martin et al. (23) that was described in detail earlier (28, 38). In summary, the lungs were perfused with HEPES-Ringer buffer containing heparin (1,000 IU) via the right ventricle. The airways were filled via the cannulated trachea with low melting point agarose (Sigma). Lungs were dissected and cooled immediately. Two-hundred-micrometer-thick PCLS were cut (vibratome VT1000 S; Leica) from the left lobe of the lung and incubated in MEM (GIBCO, Karlsruhe, Germany) at 37°C for 2–6 h to remove the agarose. Experiments were performed in a lung-slice perfusion chamber (Hugo Sachs Elektronik, March, Germany) mounted on an inverted microscope. Images of bronchi of 150–250 μm in diameter were recorded with a charge-coupled device (CCD) camera every 60 s and analyzed with Optimas 6.5 software (Stemmer Imaging, Puchheim, Germany). The area of the airway lumen after 10-min incubation in HEPES-Ringer buffer in the slide chamber was set as 100%. Bronchoconstriction and dilatation were expressed as a percentage decrease or increase of this area. After stimulation with muscarine, 5-HT (Sigma), and KCl, slices were washed and incubated with 10 mM MCD (Sigma) or vehicle (H2O) for 1 h at 37°C followed by restimulation with the same stimulants. Statistical analyses were performed throughout for the last minute of stimulation. Only those bronchi were included in the final data analysis, which responded to a stimulus of KCl (60 mM) with a reduction of luminal area of at least 20% in both recording series.
Electron microscopy.
For conventional electron microscopy, PCLS from four M3R+/+ mice were collected after videomorphometric experiments and treatment with MCD (n = 11) or vehicle (n = 9). Slices were fixed for 2–3 h in a fixative consisting of 1.5% glutardialdehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), washed in 0.1 M phosphate buffer and 3 × 5 min in 0.1 M Tris·HCl buffer, osmicated for 40 min in aqueous 1% OsO4, washed 3 × 5 min in 0.05 M maleate buffer (pH 5.2), stained en block for 1 h in 1% uranyl acetate in 0.05 M maleate buffer (pH 6.0), washed again 3 × 5 min in 0.05 M maleate buffer (pH 5.2), dehydrated in ascending concentrations of ethanol, and embedded in Epon. Sections of ∼80-nm thickness were cut on an ultramicrotome (Reichert Ultracut E; Leica), stained with alkaline lead citrate and examined in an EM 902 transmission electron microscope (Zeiss).
Statistical analysis.
Nonparametric statistical tests were used. Differences between experimental and control groups in the FRET experiments and differences among mRNA expression levels in RT-PCR were analyzed with the Kruskal-Wallis test and, if P ≤ 0.05, followed by Mann-Whitney U test using SPSS software version 11.5 (SPSS Software, Munich, Germany). Data of videomorphometric experiments are presented as means ± SE of 6–11 slices obtained from 4 animals each. Matched pairs were evaluated applying Wilcoxon rank sum test. Nonmatched pairs were compared by Mann-Whitney U test. Differences were considered as statistically significant when P ≤ 0.05, P ≤ 0.01, or P ≤ 0.001.
RESULTS
Cav-1 and Cav-3 are present in murine and human bronchial SMC and epithelial cells.
Cav-1 and Cav-3 immunoreactivities were located predominantly in the plasma membrane of murine bronchial SMC (Fig. 1, A and B). In addition, we found Cav-1 immunoreactivity in basal cells of mouse airways, as previously described (18), in vascular endothelial cells and in unidentified cells in the lamina propria, probably fibroblasts. Cav-3 immunoreactivity was detected additionally in the apical part of a subset of columnar airway epithelial cells. Furthermore, double immunostaining for M2R/Cav-1, M2R/Cav-3, and Cav-1/Cav-3 revealed colocalization of all these proteins in the plasma membrane of ASMC (Fig. 1, A″, B″, and C″).
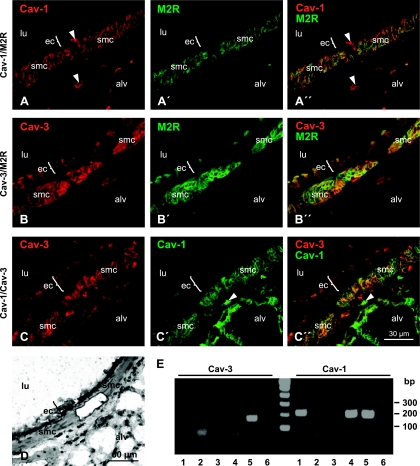
Fig. 1.
Protein and mRNA detection of caveolins Cav-1 and Cav-3 in murine bronchial smooth muscle cells (smc). A and C: double-labeling immunofluorescence of murine bronchi, confocal laser scanning microscopy (CLSM). A and C′: Cav-1 immunoreactivity is located in the plasma membrane of bronchial smc. B and C: bronchial epithelial cells (ec) and smc display labeling for Cav-3. Smooth muscle cells also exhibit plasma membrane staining for Cav-3. A′ and B′: muscarinic receptor subtype M2R immunoreactivity is found predominantly in the plasma membrane of bronchial smc. Additionally, an unspecific labeling was detected in the cilia of the ciliated cells. A″, B″, and C″: merged images. Colocalization of the labeling results in yellow color. Arrowheads, endothelium; alv, alveolar region; lu, airway lumen. D and E: caveolin mRNA detection in laser-assisted microdissected murine airway smc (D) with subsequent RT-PCR (E) in tracheal (1) and bronchial (4 and 5) smc but not in control samples including oil (2), bronchial luminal regions proximate to the dissected smc (3), and without template (6).
Furthermore, the presence of Cav-1 and Cav-3 mRNA in murine ASMC was shown by laser-assisted microdissection of bronchial and tracheal SMC with subsequent RT-PCR analysis. PCR products of expected size emerged from RNA extracts of microdissected ASMC but not from controls. Products with size under 100 bp in each lane correspond to primer dimers (Fig. 1, D and E).
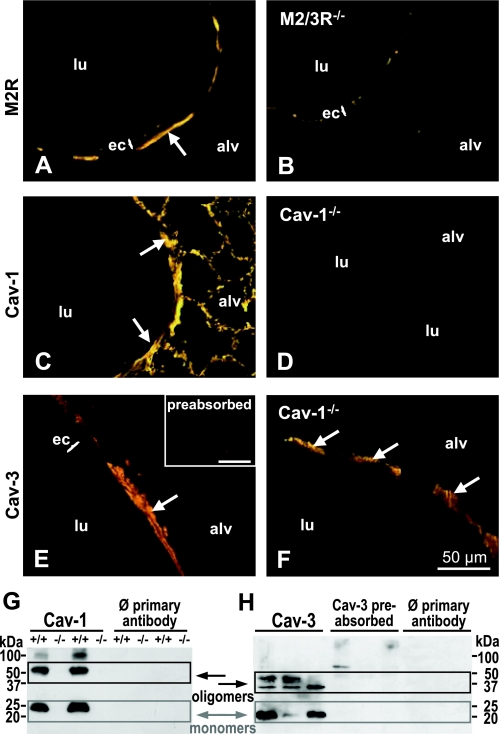
The specificity of anti-Cav-1α antibody was confirmed by the absence of Cav-1 immunolabeling in Cav-1-deficient mice (Fig. 2, C and D). In addition, specificity was validated in Western blot analysis. In lung homogenates of wild-type mice, Cav-1-immunoreactive bands with molecular mass of approximately 23, 50, and 90 kDa were detected. These products correspond to Cav-1 monomers and oligomers and were absent in lung homogenates from Cav-1-deficient mice (Fig. 2G). Cross-reactivity of the anti-M2R-antibody with proteins of the ciliated cells was detected as evidenced by immunofluorescence in the cilia of M2/3R-deficient mice after incubation with the anti-M2R-antibody (Fig. 2B). Still, the absence of M2R-immunolabeling in bronchial SMC of M2/3R-deficient mice confirmed the binding specificity of anti-M2R for bronchial SMC (Fig. 2, A and B). Cross-reactivity between the anti-Cav-3 antibody and Cav-1 was excluded by the presence of Cav-3 immunoreactivity in Cav-1−/− mice, and specificity of the antibody was validated by preabsorption with the corresponding peptide (Fig. 2, E and F). In Western blot analysis, the Cav-3 antibody detected three bands of approximately 21, 35, and 45 kDa in murine lung homogenates, corresponding to Cav-3 monomers and oligomers, respectively, that could be preabsorbed with the corresponding Cav-3 antigen (Fig. 2H).
Fig. 2.
Validation of antibody specificity. A and B: the anti-M2R antibody labels airway smooth muscle cells (ASMC) in lung sections from wild-type (A) but not from M2R-deficient (B) mice. C: the anti-Cav-1 antibody labels ASMC and cells of the alveolar region in wild-type mice. D: this labeling is not present in Cav-1-deficient tissue. Smooth muscle cell staining with anti-Cav-3 antibody in a lung section from an FVB mouse (E) and a Cav-1-deficient mouse (F) is shown. Inset in E: preabsorption with the corresponding peptide abolished the anti-Cav-3 antibody labeling. Arrows: smooth muscle cells. Bar = 50 μm. G and H: Western blotting-based validation of Cav-1 and Cav-3 antibody specificities. Bands for Cav-1α detected in wild-type mice are not present in Cav-1-deficient mice (G). H: bands corresponding to the molecular mass of Cav-3 can be preabsorbed with the corresponding Cav-3 antigen. Controls for the specificity of the secondary reagents: Ø primary antibody.
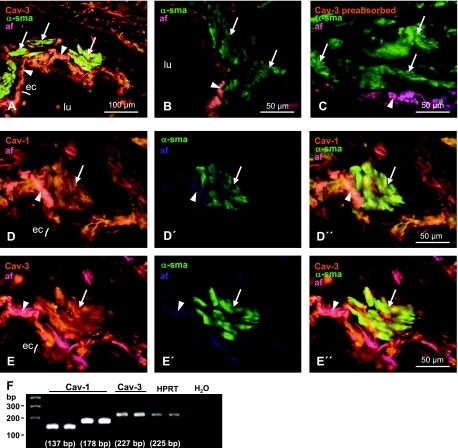
In human bronchi, Cav-1 and Cav-3 immunoreactivity were present in SMC and epithelium. (Fig. 3, D and E). Double immunostaining for α-sma, a SMC marker, with either Cav-1 or Cav-3 revealed colocalization of both Cav isoforms with α-sma in human bronchial SMC (Fig. 3, D″ and E″). Omitting the primary anti-Cav antibody and preabsorption of Cav-3 antibody with a corresponding peptide resulted in exclusive α-sma-labeling (Fig. 3, B and C). Autofluorescence was mainly observed in the basement membrane and was visualized by overlay in pink color (Fig. 3, A–E, D″, and E″) to distinguish from specific labeling for Cav-1 and Cav-3.
Fig. 3.
Detection of Cav-1 and Cav-3 in human bronchus. A–E: double-labeling immunohistochemistry, CLSM. A: cross-section of a human bronchus stained for Cav-3 (yellow) and α-smooth muscle actin (α-sma; green). Omitting the primary anti-Cav antibody (B) and preabsorption of Cav-3 antibody with a corresponding peptide (C) resulted in exclusive α-sma labeling in the smooth muscle. Cav-1 (D), Cav-3 (E), and α-sma immunoreactivities (D′ and E′) are present in human bronchial smc and epithelium, whereas α-sma immunoreactivity is restricted to smc (D′ and E′). To distinguish the specific labeling for Cav-1 (orange; D) and Cav-3 (orange; E) from autofluorescence (af) of elastic fibers, these fibers were visualized in blue using a short wavelength filter (D′ and E′). Images were merged, resulting in elastic fibers appearing in pink color (A–E, D″, and E″). Arrows, smooth muscle cells; arrowheads, basement membrane. F: expression of Cav-1 and Cav-3 mRNA detected in homogenates from human lungs (n = 5) by RT-PCR using different primer pairs (Table 1) performed in duplicates. Hypoxanthine phosphoribosyltransferase (HPRT) was used as housekeeping gene to control for the RT-PCR efficacy. H2O, without DNA template.
Furthermore, Cav-1 and Cav-3 mRNA were detected in homogenates of human lungs by RT-PCR analysis. PCR products of expected size emerged for Cav-1 and Cav-3 (Fig. 3F), and specificity of the amplified human Cav-3 PCR product was verified by sequencing. No bands were detected in control reactions when the RT was omitted or when no DNA template was present (Fig. 3F).
Cav-3 is associated with Cav-1 and M2R.
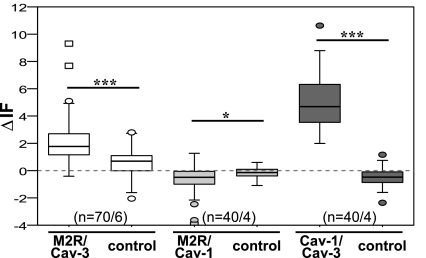
Conventional indirect double-labeling immunofluorescence with subsequent FRET-CLSM analysis, applying the acceptor bleaching method (17), was conducted to determine whether M2R, Cav-1, and Cav-3 are in close apposition in ASMC in situ. An increase of donor fluorescence (ΔIF) after acceptor bleaching indicates an association of both proteins. A false-positive FRET signal that can be caused by cross-reactivity of secondary antibodies was excluded by applying both secondary antibodies to sections incubated with only one primary antibody. Since M2R and the caveolins are membrane proteins, we measured ΔIF in cell surface regions.
Using FRET-CLSM analysis, we confirmed the association between M2R and Cav-3 in myocytes of the heart atrium as described for cardiac ventricular myocytes by Feron et al. (8) (Supplemental Fig. S1A, available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). Furthermore, in the same set of experiments, we detected an association between M2R/Cav-3 in bronchial SMC. For M2R/Cav-3, a significant increase in M2R fluorescence in the bleached area compared with control was observed (Fig. 4). In bronchial SMC and atrial cardiac myocytes, ΔIF for Cav-1/Cav-3 was also significantly higher compared with control, implying an association between Cav-1/Cav-3 as well (Fig. 4, Supplemental Fig. S1B). No increase in donor fluorescence and, therefore, no association was found for M2R/Cav-1 in bronchial SMC (Fig. 4) and atrial cardiac myocytes (ΔIF = −1.015 for M2R/Cav-1, ΔIF = −0.53 for controls; n = 50/5; P = 0.004).
Fig. 4.
Detection of close spatial association of M2R/Cav-3 and Cav-1/Cav-3 by double-labeling indirect immunofluorescence and CLSM-fluorescence resonance energy transfer (FRET) analysis in airway smooth muscle of murine bronchi in situ. Changes in donor fluorescence (ΔIF) in the membrane area of bronchial smc in experimental compared with control group are shown. For M2R/Cav-3 and Cav-1/Cav-3, ΔIF is higher in experimental groups than in controls. ***P ≤ 0.001, *P ≤ 0.01; n = number of regions of interest/animals. Box plots: percentiles 0, 25, median, 75, 100; extreme values (○), outlier (☐).
Bronchoconstriction requires intact caveolae.
Intrapulmonary bronchi were identified in 200-μm-thick PCLS. The resting diameter of bronchi included in this study was 216 ± 8 μm (mean ± SE) for M3R+/+ (n = 15), 205 ± 6 μm for M3R−/− (n = 16), 190 ± 9 μm for M2R+/+ (n = 22), and 178 ± 8 μm for M2R−/− mice (n = 14). Lung slices from all mouse strains reacted to stimulation with 1 μM muscarine, 1 μM 5-HT, and 60 mM KCl with immediate bronchoconstriction. All drugs were applied separately and induced a progressive reduction of the luminal area until they were washed out by perfusion with buffer after 15 min of incubation with muscarine and after 5-min incubation with 5-HT or KCl. In general, luminal area returned to initial values after 15 min of washing.
To clarify whether caveolae are involved in muscarine-induced bronchial constriction, PCLS that had already been analyzed in videomorphometric experiments were incubated with MCD, a caveolae disrupting agent, and bronchoconstrictor responses to the same stimuli were tested again. PCLS from M2R+/+ and M3R+/+ mice from these series (n = 11 each) were prepared for electron microscopic analysis to examine the effect of MCD treatment. To confirm that the vehicle itself affects neither ASMC contractility nor the number of caveolae, lung slices from the vehicle-treated control group were tested again videomorphometrically and collected for electron microscopy analysis as well (n = 9). We found a high number of caveolae in ASMC from vehicle-treated PCLS, mostly arranged in rows of several caveolae side by side (Fig. 5B). In PCLS treated with MCD, caveolae were completely absent from the majority of ASMC (Fig. 5C). In a few cells, their structure had only flattened, and only a very small number of structurally normal caveolae was observed.
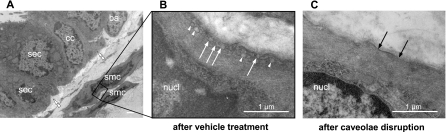
Fig. 5.
Transmission electron microscopy images of the airway wall of wild-type mice derived from precision-cut lung slices (PCLS) included in videomorphometric experiments. A: ciliated cells (cc), basal cells (ba), and secretory cells (sec) of lamina epithelialis mucosae, lamina propria with elastin (double arrowheads), and underlying tunica muscularis with bronchial smc. Bar = 1 μm. B: vehicle-treated murine ASMC containing areas with caveolae (arrows) in the plasma membrane and other subsurface vesicles (arrowheads). C: cell surface region of an equivalent bronchial smc after caveolae disruption by methyl-β-cyclodextrin (MCD). Black arrows point to plasma membrane without caveolae. nucl, Nucleus.
In line with our ultrastructural observations, the bronchoconstrictor responses of the PCLS were unchanged after vehicle treatment in all mouse strains except a minimal increase in response to KCl in M2R+/+ mice (Supplemental Fig. S2). A reduction of the initial luminal area was observed at the beginning of the second recording series when PCLS were treated with MCD (P < 0.001).
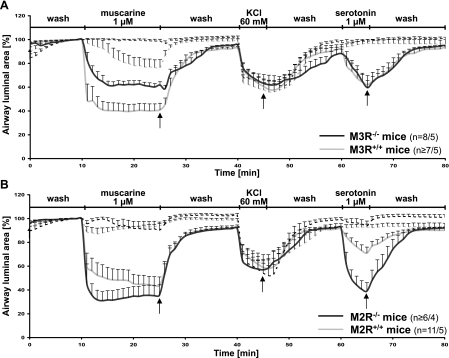
After vehicle treatment, the reduction of the bronchial luminal area due to stimulation with muscarine (1 μM) was significantly stronger in M3R+/+ than in M3R−/− mice (P = 0.009; Fig. 6A). Caveolae disruption by MCD strongly decreased the contractile response to muscarine in M3R+/+ mice (P = 0.004) and completely abolished it in M3R−/− mice (P < 0.001; Fig. 6A). Interestingly, the reduction of the response to muscarine in bronchi from M3R+/+ and M3R−/− mice accounted to the same extent. The differences in maximal luminal narrowing were 37 ± 4% in M3R+/+ and 36 ± 12% in M3R−/− mice (P = 0.093).
Fig. 6.
Bronchoconstrictor responses of M3R−/− and M3R+/+ mice (A) of M2R−/− and M2R+/+ mice (B) to stimulation with muscarine (1 μM), KCl (60 mM), and 5-HT (1 μM) after vehicle (—) or MCD treatment (- - -). Data represent luminal area with prestimulus value set as 100%. A: caveolae disruption reduces the response to muscarine in bronchi from M3R+/+ mice and fully abrogated in M3R−/− mice. No differences in response to KCl occur after MCD or vehicle treatment in either mouse strain. 5-HT-induced responses are fully abrogated by MCD in both strains. B: in M2R−/− and M2R+/+ mice, the muscarine-induced constriction is not significantly different from M2R+/+ mice and is reduced to less than 10% after MCD treatment. No differences in response to KCl occurred after MCD or vehicle treatment in either mouse strain. Responses to 5-HT were less distinct in M2R+/+ compared with M2R−/− mice but were reduced after caveolae disruption in both strains. Data are presented as means ± SE; n = number of bronchi/animals.
In M2R−/− mice, the muscarine-induced bronchoconstriction was not significantly different from M2R+/+ mice (P = 0.350; Fig. 6B). In both strains, it was strongly reduced to less than 10% after MCD compared with vehicle treatment (M2R−/−: P = 0.003; M2R+/+: P < 0.001; Fig. 6B). Again, the differences in maximal luminal narrowing were nearly identical: 50 ± 5% in M2R+/+ and 54 ± 8% in M2R−/− mice (P = 0.492).
No differences in response to KCl occurred after MCD or vehicle treatment in M3R+/+ and M3R−/− mice. Bronchoconstrictor responses to KCl in vehicle-treated PCLS from M3R+/+ and M3R−/− mice were comparable (P = 1.000) and were not affected by MCD treatment (M3R+/+: P = 0.867; M3R−/−: P = 0.505; Fig. 6A). This was also observed for M2R+/+ and M2R−/− mice (P = 0.884; M2R+/+: P = 0.748; M2R−/−: P = 0.755; Fig. 6B).
For 5-HT, bronchoconstrictor responses were comparable in M3R+/+ and in M3R−/− mice after vehicle treatment (P = 0.694) and were completely abolished after MCD treatment (M3R+/+: P = 0.006; M3R−/−: P = 0.007; Fig. 6A). In M2R−/− mice, responses to 5-HT were more pronounced compared with M2R+/+ mice (P = 0.007; Fig. 6B). Again, there were no statistical differences between the responses to 5-HT before and after vehicle treatment in each mouse strain (M2R−/−: P = 0.345; M2R+/+: P = 0.534; Supplemental Fig. S2, C and D). Responses to 5-HT were abolished in both mouse strains after MCD treatment (M2R+/+: P = 0.002; M2R−/−: P = 0.001; Fig. 6B).
M2R mRNA expression level is not altered in M3R−/− mice.
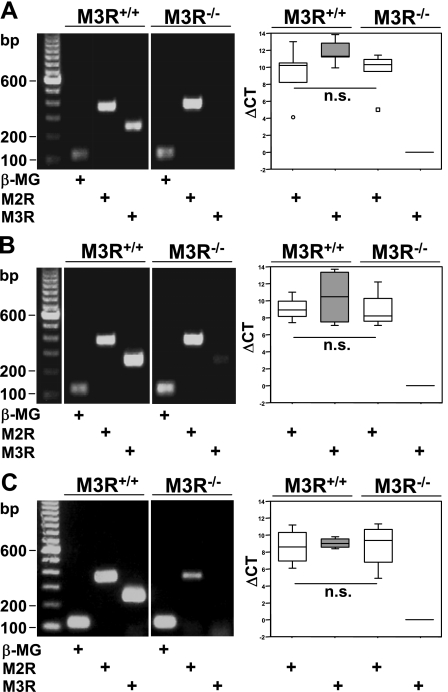
RT-PCR of total mRNA isolated from lung homogenates, tracheal muscle, and urinary bladder from M3R+/+ mice showed that the expression levels of M2R and M3R were comparably high in all these tissues (P = 0.222, P = 0.886, P = 0.886; Fig. 7). In M3R+/+ and M3R−/− mice, quantification of the relative expression of M2R mRNA showed comparably high expression levels (P = 0.841 in lung, P = 0.686 in tracheal muscle, P = 0.886 in urinary bladder; Fig. 7). PCR products were of the expected size. No bands were detected in control reactions when the RT was omitted or when no DNA template was present (data not shown).
Fig. 7.
A–C: real-time RT-PCR. cDNA were derived from lung homogenate (A; n = 5), tracheal muscle (B; n = 4), and urinary bladder (C; n = 4) from M3R+/+ and M3R−/− mice each. Changes in cycle threshold (ΔCT) values were calculated for M2R and M3R using β-microglobulin (β-MG) as reference gene and PCR products of M2R, M3R, and β-MG separated on agarose gels. Images in A–C originate from the same gel, each, after excluding irrelevant samples. Box plots: percentiles 0, 25, median, 75, 100; extreme values (○), outlier (☐); n = number of animals; n.s., not significant.
DISCUSSION
We here identified Cav-3 as a novel caveolar protein in murine and human ASMC and demonstrate its association with M2R. Accordingly, the MR-dependent component of muscarinic bronchoconstriction requires the presence of caveolae.
The functional role of caveolae in MR-mediated bronchoconstriction was investigated by disruption of caveolae with MCD at a concentration comparable with that used in previously reported studies on mammalian arterial and airway SMC (5, 13, 29, 31). The efficiency of MCD treatment was confirmed by transmission electron microscopy. In line with observations on canine tracheal (13) and rat arteriolar SMC (29), caveolae were found only sporadically in MCD-treated mouse PCLS, and those persisting were mostly of flattened shape. These few remaining caveolae might be responsible for the minor residual effects of muscarine after MCD treatment. Other cellular structures remained unaffected, confirming the selectivity of MCD treatment in our experimental setup.
Test stimuli applied in videomorphometric experiments further validated efficacy and selectivity of MCD treatment. The bronchoconstrictor response to 5-HT is mediated by the receptor subtypes 5-HT2A and 5-HT1A (45). The 5-HT2A receptor coimmunoprecipitates with Cav-1 in a number of cell types (1), and knockdown of Cav-1 in C6 glioma cells nearly abolished the 5-HT-induced increase in intracellular Ca2+ ([Ca2+]i; Ref. 1), suggesting a coupling of the serotonergic pathway to Cav-1-containing caveolae. Accordingly, MCD treatment completely abolished the bronchoconstrictor response to 5-HT in our PCLS preparation, thereby providing evidence for an effective functional inactivation of caveolae-coupled signaling pathways under these conditions.
Nonselective damage of the plasma membrane by MCD-induced cholesterol depletion was excluded by the KCl experiments. KCl does not activate G protein-coupled receptor signaling pathways. Instead, it induces SMC contraction via membrane depolarization and activation of voltage-operated Ca2+ channels (VOCC) leading to increased cytosolic free Ca2+, activation of myosin light chain (MLC) kinase, phosphorylation of MLC, and contraction (32). There is considerable evidence that the function of VOCC is independent of caveolae (5, 22), and the presently observed resistance of the KCl-induced bronchoconstrictor response to MCD treatment further supports this notion. This intact membrane depolarization-induced contractile response excludes unspecific plasma membrane damage.
The functional role of the M3R in airway constriction is often highlighted, and bronchoconstrictor responses to methacholine and electrical stimulation of pulmonary vagal efferents are totally abolished in M3R−/− mice in whole animal experiments (9). The role of M2R becomes more evident in reductionist models. A contractile response to carbachol is still present in isolated tracheal segments and smooth muscle from M3R−/− mice, and the remaining constriction is conferred by M2R (36, 37). Accordingly, we presently observed a considerable muscarine-induced bronchoconstriction in PCLS from M3R−/− mice, although it was significantly reduced compared with the wild-type response. These results fully confirm earlier findings, and the remaining cholinergic bronchoconstriction is mediated by the M2R (38). Notably, in our experiments, levels of M2R mRNA were not elevated in whole lung preparations and tracheal muscle in M3R−/− mice. As several previous studies have demonstrated that the loss of one MR subtype does not affect the mRNA and protein expression level of the others (43), it is very unlikely that M2R takes over M3R function in its absence. In addition to its localization on smooth muscle, the M2R is also expressed by cholinergic neurons innervating the airway smooth muscle (10). Here, prejunctional M2R provide a negative feedback loop inhibiting ACh release (10). This inhibitory role of M2R does not become evident when PCLS are directly stimulated with muscarine, thereby bypassing neuronal ACh release. Accordingly, we observed no significant differences in the bronchoconstrictor response to muscarine (1 μM) in M2R−/− and corresponding wild-type mice. 5-HT, in contrast, causes release of ACh from cholinergic nerve terminals in the airways in addition to its direct stimulatory effect on muscular 5-HT receptors (24, 27, 42). In the mouse trachea, the indirect, ACh-mediated effect apparently dominates over direct serotonergic stimulation of ASMC (6, 25, 42). In mouse bronchi, however, serotonin exhibits full bronchoconstriction even in the absence of M3R (this study and Ref. 22), which argues for a predominant direct serotonergic effect on bronchial smooth muscle. Still, we observed an augmented serotonin-induced bronchoconstriction in M2R−/− mice, pointing toward an additional indirect, cholinergic effect with an M2R-mediated inhibitory feedback loop in this airway segment as well.
In the present study, we identified not only Cav-1, but also Cav-3 in mouse and human ASMC on mRNA and protein level. Up until now, the presence of Cav-3 in ASMC has only been described in tracheal muscle in rat (19). Whereas these findings suggest Cav-3 to be distributed throughout all mammalian species, other groups reported an absence of Cav-3 in isolated human ASMC (12, 13, 31). This discrepancy might be due to the different antibodies used for the detection and/or to alteration of Cav-3 expression by isolation of ASMC.
In our present experiments, caveolae disruption led to marked inhibition of the M2R- and the M3R-mediated bronchoconstriction to 1 μM muscarine in M3R−/− and M2R−/− mice, respectively, clearly demonstrating the dependence of both MR pathways on caveolae. Independently, further evidence for M2R coupling to caveolae through an association with Cav-3 is provided by double-labeling IHC combined with FRET-CLSM analysis. This is an accepted noninvasive method for investigation of protein-protein interactions in tissue sections in situ that was established and methodologically described recently (17). Nevertheless, since this method is based on indirect IHC, it does not allow to quantify extent of association and to compare this between different protein partners based on the extent of FRET effect measured. Here, we first assessed the capability of this technique to demonstrate association between Cav-3 and M2R in situ by analyzing cardiac myocytes. In rat cardiomyocytes, Cav-3 was coimmunoprecipitated with a muscarinic radioligand (8). Utilizing the FRET-CLSM approach, we demonstrated for the first time that M2R is directly associated with Cav-3 and not with Cav-1 in murine bronchial smooth muscle in situ. Collectively, the dependence of M2R-mediated bronchoconstriction on intact caveolae and the demonstrated association between Cav-3 and M2R strongly suggest that anchoring of M2R to caveolae via Cav-3 is important for initiation of M2R-mediated signaling. However, for the final proof of the functional relevance of this association, further experiments with Cav-3−/− mice are required. Unfortunately, this mouse strain was not provided to our group to perform these functional studies.
An involvement of caveolae in cholinergic airway constriction has also been proposed recently by Gosens and coworkers (13). In canine tracheal muscle strips, contraction force and [Ca2+]i increase were less sensitive to low ACh concentrations (<10−6 M) after caveolae disruption, and M3R was detected in caveolae-rich fractions from human and canine ASMC lysates (13). Their results indicate that caveolae and Cav-1 facilitate [Ca2+]i mobilization, leading to M3R-mediated ASMC contraction induced by submaximal concentrations of ACh (13). Whether an association comparable with M2R/Cav-3 also exists for M3R/Cav-1 and M3R/Cav-3 remains unclear because of the lack of specific antibodies for detection of M3R, as recently demonstrated by use of tissues from M3R−/− mice (15, 30).
A study from Novi et al. (26) demonstrates the need of homo- or heterodimerization of MR for activation of signaling pathways via β-arrestin recruitment with M2R/M3R forming the most efficient complex. Adapting this theory, it is tempting to speculate that the formation of MR homodimers instead of heterodimers in M2R−/− and M3R−/− mice might contribute to the difference we observed in these mouse strains compared with their corresponding wild-type strains.
Both Cav-1 and Cav-3 are known to form homo- and heterooligomers with Cav-2 (2). In our Western blot analysis of murine lung homogenates using specific antibodies against Cav-1 and Cav-3, we detected 3 bands each, also suggesting formation of homooligomers in bronchial SMC. These results correspond to a previous report on Cav-1 and Cav-3 forming ∼350-kDa homooligomers made of 14–16 monomers (39). Cav-1/Cav-3 heterooligomer formation was recently shown by coimmunoprecipitation in rat and mouse cardiac myocytes and in skeletal muscle from Cav-1-overexpressing mice (2, 41). In line with these results, we detected an in situ association between Cav-1 and Cav-3 in murine ASMC using the FRET-CLSM approach. Several proteins binding to Cav-3 in muscle cells are also able to interact with Cav-1 (7, 40), suggesting that Cav-3 is exchangeable with Cav-1. Nonetheless, an association between M2R and Cav-1 was neither detected in ASMC nor in cardiac atrial myocytes in our FRET-CLSM analysis, supporting the concept that functional differences exist between the caveolin isoforms (16).
In conclusion, the present study demonstrates the presence of Cav-3 in ASMC in situ. In this tissue, it interacts with the most abundant MR subtype, M2R. Taking into consideration that M2R- and M3R-mediated bronchoconstriction is caveolae-dependent and Cav-3 is directly associated with M2R, we suggest Cav-3 as novel regulator of MR-mediated signaling.
GRANTS
This study was supported by a postdoctoral stipend from the Excellence Cluster Cardio-Pulmonary System (ECCPS) and by a young scientist grant from the Medical Faculty of the Justus-Liebig-University Giessen to G. Krasteva.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Karola Michael for expert technical help with the figures.
REFERENCES
- 1.Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem 279: 34614–34623, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Capozza F, Cohen AW, Cheung MW, Sotgia F, Schubert W, Battista M, Lee H, Frank PG, Lisanti MP. Muscle-specific interaction of caveolin isoforms: differential complex formation between caveolins in fibroblastic vs. muscle cells. Am J Physiol Cell Physiol 288: C677–C691, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chilvers ER, Nahorski SR. Phosphoinositide metabolism in airway smooth muscle. Am Rev Respir Dis 141: S137–S140, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther 98: 59–69, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol 22: 1267–1272, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Eum SY, Norel X, Lefort J, Labat C, Vargaftig BB, Brink C. Anaphylactic bronchoconstriction in BP2 mice: interactions between serotonin and acetylcholine. Br J Pharmacol 126: 312–316, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem 271: 22810–22814, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Feron O, Smith TW, Michel T, Kelly RA. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J Biol Chem 272: 17744–17748, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J 18: 711–713, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med 158: S154–S160, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 96: 1692–1697, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gosens R, Stelmack GL, Dueck G, Mutawe MM, Hinton M, McNeill KD, Paulson A, Dakshinamurti S, Gerthoffer WT, Thliveris JA, Unruh H, Zaagsma J, Halayko AJ. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1406–L1418, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Gosens R, Zaagsma J, Meuers H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 379: 389–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kogo H, Ito SY, Moritoki Y, Kurahashi H, Fujimoto T. Differential expression of caveolin-3 in mouse smooth muscle cells in vivo. Cell Tissue Res 324: 291–300, 2006 [DOI] [PubMed] [Google Scholar]
- 17.König P, Krasteva G, Tag C, König IR, Arens C, Kummer W. FRET-CLSM and double-labeling indirect immunofluorescence to detect close association of proteins in tissue sections. Lab Invest 86: 853–864, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Krasteva G, Pfeil U, Drab M, Kummer W, Konig P. Caveolin-1 and -2 in airway epithelium: expression and in situ association as detected by FRET-CLSM. Respir Res 7: 108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasteva G, Pfeil U, Filip AM, Lips KS, Kummer W, Konig P. Caveolin-3 and eNOS colocalize and interact in ciliated airway epithelial cells in the rat. Int J Biochem Cell Biol 39: 615–625, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kummer W, Wiegand S, Akinci S, Wessler I, Schinkel AH, Wess J, Koepsell H, Haberberger RV, Lips KS. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res 7: 65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai HH, Boone TB, Yang G, Smith CP, Kiss S, Thompson TC, Somogyi GT. Loss of caveolin-1 expression is associated with disruption of muscarinic cholinergic activities in the urinary bladder. Neurochem Int 45: 1185–1193, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Löhn M, Furstenau M, Sagach V, Elger M, Schulze W, Luft FC, Haller H, Gollasch M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res 87: 1034–1039, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Martin C, Uhlig S, Ullrich V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur Respir J 9: 2479–2487, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Martin TR, Cohen ML, Drazen JM. Serotonin-induced pulmonary responses are mediated by the 5-HT2 receptor in the mouse. J Pharmacol Exp Ther 268: 104–109, 1994 [PubMed] [Google Scholar]
- 25.Moffatt JD, Cocks TM, Page CP. Role of the epithelium and acetylcholine in mediating the contraction to 5-hydroxytryptamine in the mouse isolated trachea. Br J Pharmacol 141: 1159–1166, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novi F, Stanasila L, Giorgi F, Corsini GU, Cotecchia S, Maggio R. Paired activation of two components within muscarinic M3 receptor dimers is required for recruitment of beta-arrestin-1 to the plasma membrane. J Biol Chem 280: 19768–19776, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Olszewski MA, Zhang XY, Robinson NE. Pre- and postjunctional effects of inflammatory mediators in horse airways. Am J Physiol Lung Cell Mol Physiol 277: L327–L333, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Pfaff M, Powaga N, Akinci S, Schutz W, Banno Y, Wiegand S, Kummer W, Wess J, Haberberger RV. Activation of the SPHK/S1P signalling pathway is coupled to muscarinic receptor-dependent regulation of peripheral airways. Respir Res 6: 48, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potocnik SJ, Jenkins N, Murphy TV, Hill MA. Membrane cholesterol depletion with beta-cyclodextrin impairs pressure-induced contraction and calcium signalling in isolated skeletal muscle arterioles. J Vasc Res 44: 292–302, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pradidarcheep W, Stallen J, Labruyère WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 379: 397–402, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 288: C769–C783, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 54: 431–467, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Smart EJ, Anderson RG. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol 353: 131–139, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271: 15160–15165, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Stengel PW, Gomeza J, Wess J, Cohen ML. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther 292: 877–885, 2000 [PubMed] [Google Scholar]
- 37.Stengel PW, Yamada M, Wess J, Cohen ML. M3-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol 282: R1443–R1449, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, Haberberger RV. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol 64: 1444–1451, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 271: 2255–2261, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 272: 28187–28190, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Volonte D, McTiernan CF, Drab M, Kasper M, Galbiati F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol 294: H392–H401, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Weigand LA, Myers AC, Meeker S, Undem BJ. Mast cell-cholinergic nerve interaction in mouse airways. J Physiol 587: 3355–3362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol 44: 423–450, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410: 207–212, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Cardell LO, Adner M. IL-1beta induces murine airway 5-HT2A receptor hyperresponsiveness via a non-transcriptional MAPK-dependent mechanism. Respir Res 8: 29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.