Abstract
The vacuolar H+-ATPase (V-ATPase) in type A kidney intercalated cells is a major contributor to acid excretion in the collecting duct. The mechanisms of V-ATPase-trafficking regulation in kidney intercalated cells have not been well-characterized. In developmentally related epididymal clear cells, we showed previously that PKA, acting downstream of soluble adenylyl cyclase (sAC), induces V-ATPase apical membrane accumulation. These PKA-mediated effects were inhibited by activators of the metabolic sensor AMP-activated kinase (AMPK) in clear cells. Here, we examined the regulation of V-ATPase subcellular localization in intercalated cells by PKA and AMPK in rat kidney tissue slices ex vivo. Immunofluorescence labeling of kidney slices revealed that the PKA activator N6-monobutyryl cAMP (6-MB-cAMP) induced V-ATPase apical membrane accumulation in collecting duct intercalated cells, whereas the V-ATPase had a more cytosolic distribution when incubated in Ringer buffer alone for 30 min. V-ATPase accumulated at the apical membrane in intercalated cells in kidney slices incubated in Ringer buffer for 75 min, an effect that was prevented by treatment with PKA inhibitor (mPKI). The V-ATPase distribution was cytosolic in intercalated cells treated with the carbonic anhydrase inhibitor acetazolamide or the sAC inhibitor KH7, effects that were overridden by 6-MB-cAMP. Preincubation of kidney slices with an AMPK activator blocked V-ATPase apical membrane accumulation induced by 6-MB-cAMP, suggesting that AMPK antagonizes cAMP/PKA effects on V-ATPase distribution. Taken together, our results suggest that in intercalated cells V-ATPase subcellular localization and therefore its activity may be coupled to acid-base status via PKA, and metabolic status via AMPK.
Keywords: metabolism, acid secretion, soluble adenylyl cyclase, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
luminal acid secretion in the kidney collecting duct is essential for the maintenance of total body acid-base homeostasis (reviewed in Refs. 29, 30, 60). Several transporters participate in this process, including Na+/H+ exchangers and proton pumps, such as the vacuolar H+-ATPase (V-ATPase) (18, 48). This proton-pumping complex is ubiquitously expressed in cells where it mediates acidification of several intracellular compartments. Proton secretion against an electrochemical gradient via the V-ATPase requires ATP consumption. In addition, these V-ATPase complexes are expressed at a high level at the apical plasma membrane in certain specialized epithelial proton-secreting cells, such as collecting duct type A intercalated cells, which secrete protons into the urine, and epididymal clear cells, which maintain an acidic luminal pH necessary for sperm maturation (9, 11, 20, 21, 69). In the mammalian kidney collecting duct, their presence is essential for the normal maintenance of acid-base homeostasis. Patients with mutations that significantly reduce the activity of the V-ATPase suffer from renal tubular acidosis, with severe metabolic acidosis leading to bone and kidney sequelae, such as nephrolithiasis (38, 59). When expressed in epididymal clear cells of the male reproductive tract, which are developmentally related to intercalated cells, V-ATPases acidify the lumen and thus contribute to sperm maturation. Therefore, the study of V-ATPase regulation is essential for understanding proton secretion under physiological and pathological conditions in the urogenital tract (51).
Acid secretion in epithelial cells such as kidney intercalated cells and epididymal clear cells is actively regulated by environmental cues (23, 51, 68). We and others showed that in clear cells, V-ATPase accumulation at the apical membrane is regulated by alkaline luminal pH, increased luminal [HCO3−], intracellular HCO3−, the HCO3−-activated soluble adenylyl cyclase (sAC), cAMP/PKA, phospholipase C, and the actin cytoskeleton (1, 50, 70). In addition, phosphatidylinositol 3-kinase, aldolase, phosphofructokinase, the microtubular network, and angiotensin II regulate the V-ATPase in a variety of cellular systems (6, 54–56, 58, 61). We also recently showed that activation of AMP-activated protein kinase (AMPK) prevents PKA-mediated V-ATPase apical accumulation in clear cells (26).
AMPK is a ubiquitous kinase that responds to metabolic and other cellular stresses and regulates a variety of cellular processes, including metabolic pathways, cell growth, inflammation, transcription, translation, as well as membrane transport (25, 33). In association with metabolic stress, slight drops in cellular ATP levels lead to substantial increases in AMP concentration and in the AMP:ATP ratio (31), which stimulate AMPK activity (62). AMPK acts to preserve ATP stores under conditions of metabolic depletion by inhibiting the activities of several important ATP-consuming biosynthetic enzymes and activating ATP-generating metabolic pathways (32). We previously showed that AMPK inhibits various transport proteins, including the CFTR Cl− channel, the epithelial Na+ channel, and the V-ATPase in epididymal clear cells (14, 26, 28). Regulation of transport proteins by AMPK appears to be a critical facet of its role as a regulator of cellular energy balance, as membrane transport comprises a substantial portion of total cellular energy expenditure in actively transporting epithelia (25). Indeed, ischemia in the kidney produces a rapid and robust AMPK activation, which is more pronounced in cortical tubules and may protect the renal tubular epithelial cells from injury (46). As we recently found that AMPK inhibits PKA-dependent trafficking of the V-ATPase from a subapical to apical membrane distribution in epididymal clear cells (26), in this study we sought to determine whether a similar effect occurs in the kidney, which is an organ that is potentially more susceptible to acute ischemic injury.
Changes in V-ATPase subcellular localization have been associated with the regulation of proton secretion in some epithelial cells, such as in the turtle bladder (60). The role of direct phosphorylation of the V-ATPase subunits in proton secretion in mammalian epithelial cells has not been well-characterized (47). In insect cells, phosphorylation of the C subunit by PKA contributes to the apical assembly and activity of the salivary gland V-ATPase (15, 67). In addition, the A subunit is a putative target for phosphorylation by AMPK in the mammalian brain (49). We previously showed that the V-ATPase A subunit in the V1 or cytoplasmic domain of this complex is phosphorylated by PKA in vitro and in the intact cellular environment in HEK-293 cells, suggesting that the direct A subunit phosphorylation by PKA could induce trafficking of the V-ATPase complex to the apical membrane from subapical pools (26). AMPK can also phosphorylate the V-ATPase A subunit in vitro and in HEK-293 cells. Moreover, activators of this kinase prevented the PKA-mediated apical accumulation of V-ATPase in epididymal clear cells, consistent with a general antagonistic relationship between AMPK and PKA observed in other settings (16, 28, 37, 40). In this study, we present evidence for the first time that there is coregulation of V-ATPase intracellular trafficking by PKA and AMPK in kidney intercalated cells.
MATERIALS AND METHODS
Reagents and chemicals.
All chemical compounds used in the studies presented here were purchased from Sigma or Fisher Scientific unless otherwise stated. The specific, cell-permeant sAC inhibitor KH7 was a generous gift of Drs. J. Buck and L. Levin (Dept. of Pharmacology, Weill Cornell Medical College) (35). 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) was purchased from Toronto Research Chemicals. The cell-permeant PKA-specific activator N6-monobutyryl-cAMP (6-MB-cAMP) was obtained from Biomol.
Kidney tissue slice preparation.
All animal experiments were approved by the Institutional Animal Care and Utilization Committee of the University of Pittsburgh, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (65 mg/kg). The rats were perfused via the left ventricle with Ringer solution (pH 7.4) containing (in mM) 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 d-glucose, in 5% CO2 and air, at 37°C using a modification of a previously described protocol used by our group and others (4–6, 66). Both kidneys were removed from the rat at the time of the perfusion, cut into 4- to 5-mm-thick transverse segments using a razor blade, and quickly placed in Ringer solution (pH 7.4) at 37°C equilibrated with 5% CO2-95% air. These larger slices were further sectioned into 0.5-mm slices as described (6). The thin slices obtained with a Stadie-Riggs slicer (Thomas Scientific) were first incubated at 37°C for 10 min in equilibrated Ringer buffer, to allow for their equilibration in the buffer, as previously described (4). These slices were then rapidly transferred to fresh vials containing Ringer buffer with or without various drugs. Slices incubated in the presence of agonists or antagonists were paired with control slices incubated in Ringer buffer alone for the same time period before simultaneous fixation of the tissues by immersion in periodate-lysine containing 4% paraformaldehyde, followed by washes, quenching, and cryoprotection in 30% sucrose as described (8, 26).
Immunofluorescence labeling of kidney tissue slices.
The fixed and cryoprotected kidney tissue slices were embedded in Tissue-Tek and sectioned (4-μm thick) using a Reicher Frigocut cryostat as previously described (4, 26). The cryosections were adhered to slides, rehydrated in PBS for 30 min, and treated with 1% SDS for antigen retrieval (12). Immunostaining of the sections was performed as described (26) using a previously characterized V-ATPase E subunit antibody (raised in chicken at 1:5,000 dilution, GenWay) for 75 min at room temperature. All antibody dilutions were performed in DAKO background-reducing reagent (DAKO). Sections were then washed twice for 5 min in high-salt PBS (2.7% NaCl) and once in PBS. The secondary antibody incubation for 60 min was performed with secondary antibodies raised in donkey (Jackson Immunologicals). The slides were again washed as described above. Slides were mounted on coverslips with Vectashield (Vector Labs). Sections were examined using a Leica laser-scanning microscope and imported into either Metamorph (Molecular Devices) for analysis or Adobe Photoshop for presentation.
Quantification of V-ATPase apical membrane accumulation.
V-ATPase accumulation at the apical membrane of collecting duct intercalated cells was quantified from images obtained by confocal microscopy and imported into Metamorph software. This quantification method for V-ATPase immunolabeling has been validated by comparison to measurements on electron micrographs in previous studies (1, 50). We carefully selected for analysis only cells in collecting duct tubules with open lumens and with significant apical pole V-ATPase-associated fluorescence (above the nucleus). Immunolabeled sections were inspected by at least two investigators blinded as to the experimental incubation conditions, and cells selected for quantification were reviewed in a blinded fashion by at least two investigators. Cells that displayed basolateral membrane immunolabeling were not considered for acquisition/analysis in this study. Some kidney slice experimental treatments (e.g., treatment with acetazolamide, KH7, or AICAR) caused a generalized cytoplasmic distribution of the V-ATPase in the majority of intercalated cells (see results). In all treated tissues, we still chose for analysis intercalated cells that were the most heavily apically labeled. This selection method would tend to minimize any measured differences under these conditions from those under conditions that promote apical V-ATPase accumulation (e.g., treatment with 6-MB-cAMP). The fact that significant differences in V-ATPase distributions were observed using these cell selection criteria underscores the robustness of the different treatment effects. In addition, the nucleus of the cell being quantified had to be visible as well as the nuclei of at least two of the cells flanking the cell under study. These visualization requirements ensured that all cells used in the quantification were in a consistent orientation within the epithelial layer under all conditions.
Mean pixel intensity of V-ATPase-associated fluorescence was measured for each selected cell for 1) a region of interest (ROI) at the apical border of the cell in contact with the tubular lumen selected wherever the immunolabeling was at its brightest for any given cell (apical ROI) and 2) an ROI of identical size and shape located in the cytoplasm immediately subjacent to the chosen apical ROI (cytoplasmic ROI), and above the nucleus, using methods validated in previous studies by the authors and others (4, 5). The shape and size of the ROI varied among cells and followed the contour of the apical staining (a range of 145–198 pixels per ROI across conditions). The cytoplasmic ROI was measured in an area immediately subjacent to the apical ROI, with a maximum separation of less than the width of the ROI (in the dimension that was perpendicular to the cell surface; Fig. 1, A and B, insets). At least two independent observers evaluated each cell image and ROI measurements used for quantification in a blinded fashion to ensure the accuracy of the findings. At least 30 cells per treatment from a total of at least 3 different kidney slice experiments were analyzed. For each treatment condition, this apical-to-cytoplasmic ratio was used as a measure of V-ATPase apical accumulation. This value was calculated for each cell and then a mean was obtained for each animal. The cellular V-ATPase apical accumulation obtained for each treatment was expressed as the means ± SE for each group.
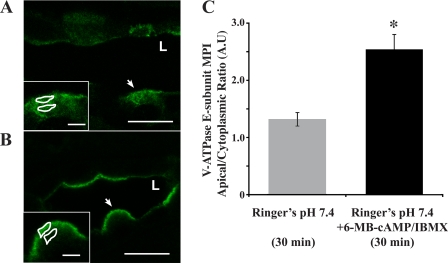
Fig. 1.
PKA agonists induce rapid apical accumulation of the V-ATPase in intercalated cells. Confocal images of V-ATPase E subunit immunofluorescence labeling in kidney slices incubated in Ringer buffer alone for 30 min display a cytoplasmic distribution for the V-ATPase (A; L indicates the position of the lumen of the collecting duct). Addition of 1 mM N6-monobutyryl cAMP (6-MB-cAMP) plus 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) induced an apical/luminal accumulation of the V-ATPase (B). Regions of interest (ROIs) were outlined for each cell at apical and cytoplasmic regions for quantification using previously described methods (4, 6, 66), as illustrated in A and B, insets (arrows indicate the cells depicted in the insets, where the scale bar represents 2.5 μm). C: quantification of the mean (±SE) V-ATPase-associated mean pixel intensity (MPI) apical/cytoplasmic ratio [arbitrary units (A.U.)] was used as a measure of apical V-ATPase accumulation, which was greater in the presence of PKA agonists. Data were obtained from at least 3 separate experiments measuring a total of at least 30 cells per condition (*P < 0.05 vs. Ringer pH 7.4, 30 min). Scale bar = 10 μm.
RESULTS
Carbonic anhydrase, cAMP, and PKA regulate V-ATPase subcellular localization in kidney intercalated cells.
We established that in epididymal clear cells, V-ATPase initially present in subapical vesicles accumulates at the apical membrane of these cells in response to alkaline luminal pH, carbonic anhydrase activity, HCO3−, and PKA activators (50). In addition, sAC inhibitors blocked the pH-mediated accumulation of V-ATPase at the apical membrane of clear cells (50). As in epididymal clear cells, collecting duct type A intercalated cells express V-ATPase abundantly at their apical plasma membrane (11). To investigate whether the sAC/cAMP/PKA pathway also regulates V-ATPase subcellular localization in intercalated cells, we performed ex vivo experiments in kidney tissue slices (4–6, 66). Kidney slices require incubation in a physiologic solution at pH 7.4, such as Ringer buffer containing HCO3− and gassed with 5% CO2-95% air at 37°C to ensure tissue viability (6).
It has been shown previously that apical accumulation of the E subunit in epithelial proton-secreting cells is associated with active V-ATPase at the membrane, so we used immunolabeling of the E subunit to measure accumulation of the V-ATPase complex at the apical membrane (10, 44, 50, 60). As a control for each kidney tissue slice experiment, we fixed a slice after the initial 10-min incubation in Ringer buffer (4, 5), an incubation time that allowed for recovery from ischemia and to reduce the effects of hormones released during the perfusion on the tissue (4). This time 0 control was considered the V-ATPase baseline distribution at the start of longer 30- and 75-min incubations. Immunofluorescence staining of these slices revealed that at the initiation of the longer incubations, the V-ATPase was diffusely distributed in intercalated cells. The V-ATPase apical-to-cytoplasmic ratio at time 0 was 1.16 ± 0.10, a value that is not significantly different from the value for slices incubated in Ringer buffer for 30 min (1.32 ± 0.12). Intercalated cells in kidney slices incubated in Ringer buffer for only 30 min had a cytoplasmic V-ATPase distribution (Fig. 1A). However, when slices were incubated in the presence of both the PKA activator 6-MB-cAMP (1 mM) and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; 0.5 mM) for 30 min, the V-ATPase significantly accumulated at the apical membrane of intercalated cells (Fig. 1B; quantified in Fig. 1C). These findings are in agreement with previous reports that cAMP activates proton secretion in certain epithelial cell systems (19, 34, 36).
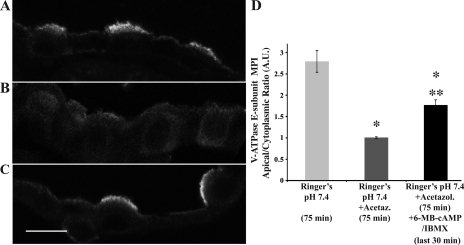
To evaluate the role of carbonic anhydrase and presumably intracellular HCO3−, slices were incubated for 75 min before fixation under different conditions. After 75 min in Ringer buffer alone, as found in kidneys fixed immediately after perfusion (11, 13), the large majority of intercalated cells in the collecting duct displayed significant V-ATPase E subunit accumulation at or near the apical membrane (V-ATPase apical/cytoplasmic accumulation of 2.79 ± 0.25 at 75 min in Ringer, compared with 1.32 ± 0.12 at 30 min in Ringer; Figs. 1 and 2, respectively). However, the apical V-ATPase accumulation in intercalated cells at 75 min was prevented by incubation of the kidney slices with acetazolamide (100 μM), a carbonic anhydrase inhibitor (Fig. 2B). This result suggests that the presence of intracellular HCO3− is required for V-ATPase apical membrane accumulation. When slices were incubated with acetazolamide for 45 min and then the PKA activator 6-MB-cAMP (100 μM) together with IBMX (0.5 mM) added for the last 30 min of the 75-min incubation, the V-ATPase distribution to the apical membrane of intercalated cells was partially restored (Fig. 2C), indicating that downstream PKA activation can override the cytoplasmic V-ATPase redistribution induced by acetazolamide (quantified in Fig. 2D). As almost all of the intercalated cells in slices incubated in acetazolamide revealed a diffuse intracellular V-ATPase distribution, the partial recovery of apical V-ATPase localization upon exposure to the PKA activator 6-MB-cAMP suggests that PKA activation induces apical targeting via exocytosis of the V-ATPase in intercalated cells. Overall, these results suggested to us that intracellular HCO3− and HCO3−-regulated sAC upstream of PKA could regulate V-ATPase trafficking to the apical membrane in kidney intercalated cells (50, 53).
Fig. 2.
Carbonic anhydrase and cAMP/PKA acutely regulate V-ATPase subcellular localization in kidney intercalated cells. Confocal images of immunofluorescence staining of fixed kidney slices using an antibody against the V-ATPase E subunit in collecting duct are shown. A: slices incubated in Ringer buffer for 75 min had V-ATPase accumulation at the apical membrane of intercalated cells. Unless otherwise stated, the apical/luminal membrane of the intercalated cells is facing upwards in the image. B: this accumulation was prevented by incubation with 100 μM acetazolamide, a carbonic anhydrase inhibitor, for the same period of time. C: additional presence of 6-MB-cAMP (100 μM + 0.5 mM IBMX) in the solution restored the V-ATPase to the apical membrane of intercalated cells in the presence of acetazolamide. D: graph summarizing the apical-to-cytoplasmic MPI of V-ATPase-associated fluorescence. Error bars are SE (*P < 0.05 with respect to control, Ringer pH 7.4, 75 min; **P < 0.05 with respect to Ringer + acetazolamide, 75 min). Scale bar = 10 μm.
sAC inhibitor KH7 prevents V-ATPase apical accumulation in intercalated cells.
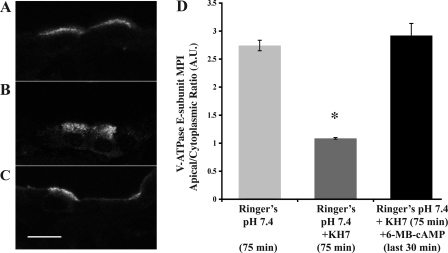
To directly test the role of sAC in V-ATPase trafficking to the apical membrane in cortical collecting duct intercalated cells, we incubated kidney slices in Ringer buffer in the presence or absence of the specific sAC inhibitor KH7 (40 μM; Fig. 3) (35). The apical accumulation of the V-ATPase observed after incubation in Ringer buffer for 75 min under control conditions (Fig. 3A) was prevented by the presence of KH7 (Fig. 3B). Addition of 6-MB-cAMP to the buffer during the last 30 min of the incubation induced apical V-ATPase accumulation in intercalated cells even in the presence of KH7 (Fig. 3C). Quantification of these images revealed that sAC inhibition of V-ATPase apical accumulation can be superseded by PKA activation (Fig. 3D). Thus, despite sAC inhibition, intercalated cells retained their capability to respond to PKA activators, suggesting that sAC acts upstream of PKA in the regulation of V-ATPase subcellular localization in kidney intercalated cells. In summary, these results underscore the similarities of V-ATPase regulation between clear and intercalated cells with respect to the sAC/cAMP/PKA pathway.
Fig. 3.
Soluble adenylyl cyclase (sAC) inhibitor KH7 prevents the HCO3−-mediated V-ATPase apical accumulation. Confocal images of V-ATPase E subunit immunofluorescence labeling in kidney slices incubated in Ringer buffer alone for 75 min (A) or in the presence of the sAC inhibitor KH7 (40 μM; B) or in the presence of KH7 with the addition of the PKA activator 6-MB-cAMP (1 mM) for the last 30 min (C). V-ATPase accumulation at the apical membrane of intercalated cells was prevented by KH7. Intercalated cells in tissue slices treated with the PKA activator 6-MB-cAMP had apical V-ATPase accumulation even in the presence of a sAC inhibitor. E: quantification of the mean (±SE) V-ATPase-associated MPI apical-to-cytoplasmic ratio under the different conditions (*P < 0.05 with respect to control, Ringer pH 7.4, 75 min; D). Scale bar = 10 μm.
PKA inhibitor blocks V-ATPase apical accumulation in kidney intercalated cells.
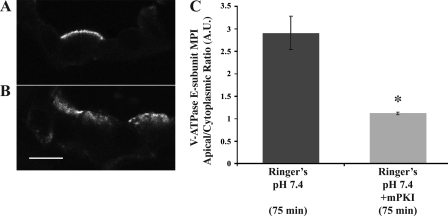
Compared with slices incubated in Ringer buffer for 75 min, slices incubated with the PKA inhibitor mPKI (10 μM) displayed a more diffuse cytoplasmic V-ATPase immunolabeling distribution (Fig. 4, A and B). Quantification of V-ATPase apical accumulation in confocal micrographs confirmed that PKA regulates the V-ATPase apical accumulation that occurs in kidney tissue when incubated in the HCO3−-containing Ringer buffer (Fig. 4C).
Fig. 4.
PKA inhibitor mPKI prevents apical V-ATPase accumulation in kidney intercalated cells. Confocal images of V-ATPase E subunit immunofluorescence labeling in kidney slices incubated either in Ringer buffer for 75 min (A) or in the presence of the specific PKA inhibitor mPKI (10 μM; B). Quantification of the mean (±SE) V-ATPase-associated MPI apical-to-cytoplasmic ratio under the different conditions demonstrates that mPKI prevented apical membrane accumulation (*P < 0.05 vs. Ringer buffer control, 75 min; C). Scale bar = 10 μm.
AMPK activator AICAR prevents PKA-mediated V-ATPase apical accumulation in intercalated cells.
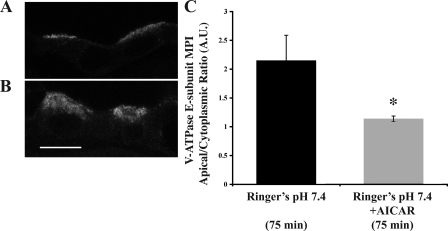
To test whether AMPK regulates V-ATPase apical accumulation in kidney intercalated cells, we treated kidney slices with the AMPK activator AICAR (Fig. 5). Immunofluorescence labeling of the V-ATPase E subunit in intercalated cells revealed that the apical V-ATPase accumulation apparent when slices are incubated in Ringer buffer for 75 min (Fig. 5A) was inhibited by AICAR (2 mM; Fig. 5B; quantitated in Fig. 5C). We also recently showed that AICAR inhibited alkaline buffer-mediated V-ATPase trafficking in epididymal clear cells (26).
Fig. 5.
AMP-activated kinase (AMPK) activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) prevents V-ATPase apical accumulation at 75 min. Confocal images illustrating cellular distribution changes of the V-ATPase E subunit in collecting duct cells of kidney slices treated with Ringer buffer (A) vs. Ringer buffer + 2 mM AICAR (B). C: quantification of the mean (±SE) V-ATPase-associated MPI apical-to-cytoplasmic ratio under the different conditions confirms a significant cytoplasmic redistribution of the V-ATPase in the presence of AICAR. *P < 0.05 vs. Ringer pH 7.4, 75 min. Scale bar = 10 μm.
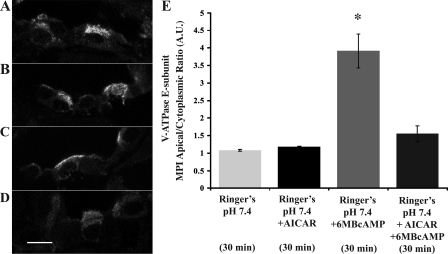
To evaluate the interregulation of PKA and AMPK on V-ATPase apical accumulation in intercalated cells, we incubated kidney slices for 30 min under the following conditions: Ringer buffer alone (Fig. 6A), or with either AICAR (2 mM; Fig. 6B), 6-MB-cAMP (1 mM; Fig. 6C), or both AICAR and 6-MB-cAMP (2 and 1 mM, respectively; Fig. 6D). Quantification of the immunofluorescence labeling revealed that after the 30-min incubation, the V-ATPase had a diffuse cytoplasmic distribution in all conditions except in the presence of the PKA activator 6-MB-cAMP alone (Fig. 6E). These results indicate that the AMPK activator AICAR is able to prevent PKA-mediated changes in subcellular localization of the V-ATPase in intercalated cells.
Fig. 6.
AMPK activator AICAR prevents PKA-mediated V-ATPase apical accumulation. Confocal images of kidney slices using an antibody against the V-ATPase E subunit in collecting duct cells. A: slices incubated in Ringer buffer for 30 min display a cytosolic V-ATPase distribution in intercalated cells. B: presence of AICAR (2 mM) in the buffer did not induce subcellular localization changes compared with the 30-min Ringer buffer control. C: presence of PKA activator 6-MB-cAMP (1 mM) during the 30-min incubation induced a redistribution of the V-ATPase to the apical membrane of intercalated cells. D: this PKA-mediated accumulation did not occur when AICAR was also present in the buffer. E: quantification of the mean (±SE) V-ATPase-associated MPI apical-to-cytoplasmic ratio under the different conditions reveals a significant inhibition by AICAR of PKA-mediated apical V-ATPase accumulation (*P < 0.05 vs. control, Ringer buffer pH 7.4, 30 min).
DISCUSSION
We hypothesized that AMPK activation in intercalated cells, as may occur with decreased blood flow during states of kidney hypoperfusion or metabolic stresses, may decrease V-ATPase accumulation at the apical membrane of intercalated cells and thereby reduce ATP consumption, overriding and antagonizing its PKA-dependent activation by acid-base signaling. The combined regulation of the V-ATPase by PKA and AMPK may afford the integration of proton secretory responses to both acid-base stimuli (via PKA) and to cellular metabolism and intracellular ATP concentration (via AMPK). This equilibrium in the regulation of V-ATPase activity could be protective to intercalated cells in periods of decreased kidney perfusion.
In this study, we showed using kidney slices in situ that collecting duct intercalated cells expressing the V-ATPase at their apical pole regulate V-ATPase trafficking to the apical membrane via the sAC/cAMP/PKA signaling pathway (26, 50, 52). In addition, the AMPK activator AICAR is able to prevent the PKA-mediated V-ATPase apical accumulation in intercalated cells. These results and our earlier results underscore the commonality in the mechanisms regulating apical V-ATPase trafficking in two different epithelial tissues of Wolffian duct origin (51).
Chronically, the V-ATPase in intercalated cells responds to bicarbonaturia over a few days by redistributing the V-ATPase and sAC to the apical pole (53). It has been postulated that this V-ATPase apical accumulation in response to lumen alkalinization is adaptive in allowing the maximal recovery of luminal HCO3−. The results of the present study also reflect acute changes (minutes) in the regulation of V-ATPase subcellular localization in intercalated cells in response to changes in intracellular [HCO3−]/carbonic anhydrase activity using the ex vivo kidney tissue slices method (4, 6, 66). The viability of these slices requires the use of a HCO3−-based buffer at pH 7.4, which limits the utility of this method to study the acute pH responsiveness of intercalated cells. However, this ex vivo system allowed us to identify, using pharmacological agents, that the same mechanisms that induce epididymal clear cell V-ATPase trafficking are involved in kidney intercalated cells.
An issue of intense research in kidney physiology is to identify how extracellular pH is sensed acutely by epithelial cells and translated into activation of transporters such as the V-ATPase (53). It is known that pH changes have been implicated in exocytosis of channels and organelle trafficking (24, 39, 43). Proton secretion by kidney epithelial cells can be modulated by CO2, acidic pHi (3), intracellular [Ca2+], and [HCO3−] (3, 22, 57, 65). In kidney intercalated cells, carbonic anhydrase is very abundant, as is sAC, which can become activated and generate cAMP upon increases of CO2 (17, 50). This phenomenon could be relevant in acute respiratory acidosis where CO2 levels rise quickly, as sAC has been proposed to serve as a CO2 sensor in some organisms (41, 45). We envision that in response to an acute intracellular increase of [CO2], via the equilibrium reaction CO2 + H2O ↔ H+ + HCO3− catalyzed by carbonic anhydrase (3, 60), the intercalated cell would generate cAMP via sAC, inducing trafficking of the V-ATPase to the apical membrane for rapid proton secretion. sAC-dependent CO2 sensing in respiratory acidosis would thus enhance V-ATPase-mediated renal acid excretion and HCO3− reclamation. We further envision that in acute ischemia, proton secretion in intercalated cells could be detrimental, as it requires ATP consumption and would thus further deplete energy stores of the cell at a time when perfusion and urine output are significantly decreased. Indeed, V-ATPase redistribution away from the apical membrane under conditions of renal ischemia has been well-described previously (6). AMPK, which is sensitive to minor cellular metabolic perturbations, should become activated in the kidney under these conditions and could account for the loss of V-ATPase localization at the apical membrane of intercalated cells. Our finding in this study that AMPK inhibits the PKA-mediated V-ATPase accumulation at the apical membrane of intercalated cells is consistent with this paradigm.
The finding that PKA and AMPK have opposing regulatory effects on V-ATPase subcellular localization in intercalated cells supports an emerging concept that PKA and AMPK may play mutually antagonistic roles on cellular transport processes. Specifically, we and others showed recently that AMPK directly phosphorylates CFTR, which inhibits PKA-dependent stimulation of channel gating and prevents the PKA-dependent activation of CFTR in the absence of cellular PKA agonists in epithelial cells (27, 40, 42). Conversely, the negative regulation of AMPK activity by PKA-dependent phosphorylation has also been the subject of recent studies. Witters and colleagues (37) first showed that AMPK activity is inhibited by multisite phosphorylation of the catalytic α-subunit in response to agents that elevate cellular cAMP. More recently, we showed that direct PKA-dependent phosphorylation of the AMPK α-subunit blocks the ability of LKB1 to activate AMPK in adipocytes and several cell lines (16). Thus, it is reasonable to propose that the net V-ATPase localization response likely depends on the relative strengths of the two opposing signals in response to different cellular stimuli.
The results of this study support our hypothesis that in kidney intercalated cells, V-ATPase subcellular localization is coupled to the sensing of acid-base status via PKA and to metabolic status via AMPK. The mechanisms of both PKA- and AMPK-dependent regulation of V-ATPase trafficking are currently unknown. Of note, we recently identified the A subunit in the cytoplasmic V1 sector of the V-ATPase as a potential direct phosphorylation target in an unbiased substrate screen for AMPK (64). We then confirmed that the A subunit is phosphorylated by both PKA and AMPK in vitro and in HEK-293 cells and found that AMPK also appeared to inhibit the PKA-dependent phosphorylation of this subunit in cells (26). PKA- and AMPK-dependent phosphorylation of the A subunit may play an important role in mediating the interaction between these two kinases with respect to the subcellular localization and/or activity of the V-ATPase complex in kidney and other proton-secreting epithelial cells. Further studies to explore these hypotheses are warranted.
GRANTS
This study was supported by the National Institutes of Health Grants P30-DK-079307 “Pittsburgh Kidney Research Center,” R01-DK-075048 (to K. R. Hallows) and K08-HD-045524 and R01-DK-084184 (to N. M. Pastor-Soler), the American Society of Nephrology Carl W. Gottschalk Research Scholar Award (to N. M. Pastor-Soler), the American Heart Association Grants 09GRNT2060539 (to N. M. Pastor-Soler) and 0825540D (to R. Alzamora), the Swiss National Science Foundation Grant 3100A0-11437/1, and the EU FP6 contract LSHM-CT-2004-005272 (EXGENESIS).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. J. Buck and L. Levin for helpful advice and for the gift of KH7. We also thank B. Miller for technical support.
REFERENCES
- 1.Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4–2. J Biol Chem 281: 26159–26169, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Boron WF. Intracellular pH regulation in epithelial cells. Annu Rev Physiol 48: 377–388, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Breton S, Brown D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J Am Soc Nephrol 9: 155–166, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Breton S, Hammar K, Smith PJ, Brown D. Proton secretion in the male reproductive tract: involvement of Cl− independent HCO3− transport. Am J Physiol Cell Physiol 275: C1134–C1142, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Breton S, Nsumu NN, Galli T, Sabolic I, Smith PJ, Brown D. Tetanus toxin-mediated cleavage of cellubrevin inhibits proton secretion in the male reproductive tract. Am J Physiol Renal Physiol 278: F717–F725, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 2: 470–472, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199: 2345–2358, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Brown D, Sabolic I, Gluck S. Polarized targeting of V-ATPase in kidney epithelial cells. J Exp Biol 172: 231–243, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dames P, Zimmermann B, Schmidt R, Rein J, Voss M, Schewe B, Walz B, Baumann O. cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc Natl Acad Sci USA 103: 3926–3931, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Makela TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPK to promote efficient lypolysis. EMBO J 29: 469–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobyan DC, Magill LS, Friedman PA, Hebert SC, Bulger RE. Carbonic anhydrase histochemistry in rabbit and mouse kidneys. Anat Rec 204: 185–197, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gekle M, Golenhofen N, Oberleithner H, Silbernagl S. Rapid activation of Na+/H+ exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proc Natl Acad Sci USA 93: 10500–10504, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluck S, Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest 73: 1704–1710, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluck S, Caldwell J. Proton-translocating ATPase from bovine kidney medulla: partial purification and reconstitution. Am J Physiol Renal Fluid Electrolyte Physiol 254: F71–F79, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Gluck S, Cannon C, Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci USA 79: 4327–4331, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluck S, Nelson R. The role of the V-ATPase in renal epithelial H+ transport. J Exp Biol 172: 205–218, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 5: 533–545, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallows KR. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamm LL, Hering-Smith KS. Acid-base transport in the collecting duct. Semin Nephrol 13: 246–255, 1993 [PubMed] [Google Scholar]
- 30.Hamm LL, Nakhoul NL. Renal acidification. In: Brenner and Rector's The Kidney (8th ed.), edited by Brenner BM. Philadelphia, PA: Saunders Elsevier, 2008, p. 248–279 [Google Scholar]
- 31.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144: 5179–5183, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol 574: 7–15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Hays S, Kokko JP, Jacobson HR. Hormonal regulation of proton secretion in rabbit medullary collecting duct. J Clin Invest 78: 1279–1286, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9: 249–259, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson RL. Bafilomycin-sensitive acid secretion by mantle epithelium of the freshwater clam, Unio complanatus. Am J Physiol Regul Integr Comp Physiol 264: R946–R951, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281: 36662–36672, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Keyes SR, Rudnick G. Coupling of transmembrane proton gradients to platelet serotonin transport. J Biol Chem 257: 1172–1176, 1982 [PubMed] [Google Scholar]
- 40.King JD, Jr, Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK, Hallows KR. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol 297: C94–C101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, Levin LR, Buck J, Muhlschlegel FA. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15: 2021–2026, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem 284: 5645–5653, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambers TT, Oancea E, de Groot T, Topala CN, Hoenderop JG, Bindels RJ. Extracellular pH dynamically controls cell surface delivery of functional TRPV5 channels. Mol Cell Biol 27: 1486–1494, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl 33: S57–S63, 1991 [PubMed] [Google Scholar]
- 45.Mogensen EG, Janbon G, Chaloupka J, Steegborn C, Fu MS, Moyrand F, Klengel T, Pearson DS, Geeves MA, Buck J, Levin LR, Muhlschlegel FA. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell 5: 103–111, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Myers M, Forgac M. The coated vesicle vacuolar (H+)-ATPase associates with and is phosphorylated by the 50-kDa polypeptide of the clathrin assembly protein AP-2. J Biol Chem 268: 9184–9186, 1993 [PubMed] [Google Scholar]
- 48.Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79: 361–385, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Pan YX, Gu HH, Xu J, Dean GE. Saccharomyces cerevisiae expression of exogenous vacuolar ATPase subunits B. Biochim Biophys Acta 1151: 175–185, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology Bethesda 20: 417–428, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol 18: 2085–2093, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL. Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 25: 575–589, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz GJ, Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest 75: 1638–1644, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 135: 1108–1117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26: 71–75, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Steinmetz PR. Cellular organization of urinary acidification. Am J Physiol Renal Fluid Electrolyte Physiol 251: F173–F187, 1986 [DOI] [PubMed] [Google Scholar]
- 61.Su Y, Zhou A, Al-Lamki RS, Karet FE. The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans. J Biol Chem 278: 20013–20018, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281: 32207–32216, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Tuerk RD, Auchli Y, Thali RF, Scholz R, Wallimann T, Brunisholz RA, Neumann D. Tracking and quantification of 32P-labeled phosphopeptides in liquid chromatography matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem 390: 141–148, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Tuerk RD, Thali RF, Auchli Y, Rechsteiner H, Brunisholz RA, Schlattner U, Wallimann T, Neumann D. New candidate targets of AMP-activated protein kinase in murine brain revealed by a novel multidimensional substrate-screen for protein kinases. J Proteome Res 6: 3266–3277, 2007 [DOI] [PubMed] [Google Scholar]
- 65.van Adelsberg J, Al-Awqati Q. Regulation of cell pH by Ca2+-mediated exocytotic insertion of H+-ATPases. J Cell Biol 102: 1638–1645, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H+-ATPase by protein kinase A. J Biol Chem 282: 33735–33742, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Zeidel ML, Silva P, Seifter JL. Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells. Role of plasma membrane proton adenosine triphosphatase. J Clin Invest 77: 113–120, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 15: 15, 2002 [DOI] [PubMed] [Google Scholar]