Abstract
Glutathione peroxidase-3 (Gpx3), also known as plasma or extracellular glutathione peroxidase, is a selenoprotein secreted primarily by kidney proximal convoluted tubule cells. In this study Gpx3−/− mice have been produced and immunocytochemical techniques have been developed to investigate Gpx3 metabolism. Gpx3−/− mice maintained the same whole-body content and urinary excretion of selenium as did Gpx3+/+ mice. They tolerated selenium deficiency without observable ill effects. The simultaneous knockout of Gpx3 and selenoprotein P revealed that these two selenoproteins account for >97% of plasma selenium. Immunocytochemistry experiments demonstrated that Gpx3 binds selectively, both in vivo and in vitro, to basement membranes of renal cortical proximal and distal convoluted tubules. Based on calculations using selenium content, the kidney pool of Gpx3 is over twice as large as the plasma pool. These data indicate that Gpx3 does not serve in the regulation of selenium metabolism. The specific binding of a large pool of Gpx3 to basement membranes in the kidney cortex strongly suggests a need for glutathione peroxidase activity in the cortical peritubular space.
Keywords: selenoproteins, selenium metabolism, Gpx3 knockout mouse, Gpx3 localization
glutathione peroxidase-3 (Gpx3), a selenoprotein, is the extracellular member of a family of enzymes and is often referred to as plasma glutathione peroxidase (23). Although several tissues express Gpx3 mRNA, the major source of plasma Gpx3 is the kidney. Chronic renal failure reduces plasma glutathione peroxidase activity sharply, and kidney transplant restores the activity (1). In situ hybridization experiments have shown that Gpx3 is produced in kidney proximal convoluted tubule (PCT) cells (1, 25).
Sepp1 is another plasma selenoprotein, and it has been studied extensively (3). Experiments using mice with deletion of Sepp1 have demonstrated that it participates in the regulation of whole-body selenium (5) and in transport of selenium from the liver to the brain and the testis via a receptor-mediated mechanism (6, 18). In addition, Sepp1 filtered by the glomerulus is taken up by PCT cells via a receptor-mediated mechanism, presumably providing selenium for the synthesis of Gpx3 by those cells (17). This links Gpx3 and Sepp1 in the metabolism of selenium by the kidney.
To characterize Gpx3 physiology and to assess the role of Gpx3 in selenium metabolism, we have produced mice with Gpx3 deleted (Gpx3−/− mice) and have developed robust immunocytochemistry techniques for detection of Gpx3 in the kidney. Using them, we have determined that a large pool of Gpx3 binds to basement membranes of renal cortical tubule cells in a specific manner and that Gpx3 does not play a role in selenium homeostasis.
EXPERIMENTAL PROCEDURES
Animal husbandry.
Adult male Gpx3−/− mice were produced at Stanford University (Palo Alto, CA; as described in supplemental material, which is available on the journal web site) and transferred to the Vanderbilt University animal care facility. Those mice were bred with female C57BL/6 mice. The heterozygous offspring were used to establish separate Gpx3−/− and Gpx3+/+ colonies. At weaning, male mice were identified and fed pelleted Torula yeast-based diets (Harlan-Teklad, Madison, WI) deficient in selenium or supplemented with selenium as sodium selenite (10). The mice had free access to food and water. Some of the mice used for the immunocytochemical staining experiments were fed Purina rat chow 5001. The light cycle in the animal room was 14 h light and 10 h dark.
For blood and tissue collection in the metabolism experiments, mice were anesthetized with isoflurane, and blood was removed from the inferior vena cava with a syringe and needle. The blood was treated with disodium EDTA (1 mg/ml) to prevent clotting, and plasma was separated by centrifugation at 16,000 g for 2 min in a microcentrifuge. Liver, kidney, testis, and brain were removed and quickly frozen in liquid nitrogen. Tissues and plasma were stored at −80°C until assayed. For immunocytochemical staining experiments, mice were euthanized with CO2 and their kidneys were used immediately. The Vanderbilt University Institutional Animal Care and Use Committee approved all procedures.
Production of Sepp1−/−/Gpx3−/− double knockout mice.
Sepp1−/− female mice (9) congenic with C57BL/6 mice were mated with Gpx3−/− male mice. Their progeny were mated with one another. Male and female progeny that were homozygous for Gpx3 and heterozygous for Sepp1 were identified by PCR genotyping (Table 1) and mated to produce Sepp1−/−/Gpx3−/− mice for experiments. The Sepp1−/− mice are available from The Jackson Laboratory with the JAX Stock No. 008201 (http://jaxmice.jax.org/query).
Table 1.
PCR primers used for genotyping
| Gene | Primer Designation | Genotype Direction | Sequence (5′-> 3′) | PCR Product (Primer Pair) |
|---|---|---|---|---|
| Gpx3 | 57U | Wild-type forward | TCTCTCCCTGCTGGCAATCAT | 400 bp (57U/59N) |
| G3AS | Wild-type reverse | CAAGAGACGGGCTCATGC | 600 bp (57U/G3AS) | |
| 59N | Neo insert reverse | CGAGACTAGTGAGACGTGCTACTT | ||
| Sepp1 | SEPS14 | Wild-type forward | GCCATCAGGGCTCAGTGCAG | 500 bp (SEPS14/NeoS2) |
| SEPA600 | Wild-type reverse | GGATGCCTGCAGAAGACACAAGTA | 600 bp (SEPS14/SEPA600) | |
| NeoS2 | Neo insert reverse | GGTGTTGGGTCGTTTGTTCGGATCG | ||
| Gpx1 | OPE542 | Wild-type forward | GTTTCCCGTGCAATCAGTTCG | 500 bp (OPE542/OPE543) |
| OPE541 | Wild-type reverse | TCGGACGTACCCTTGAGGGAAT | 300 bp (OPE541/OPE542) | |
| OPE543 | Knockout reverse | CATTTGTCACGTCCTGCAC |
Production of Gpx1−/−/Gpx3−/− double knockout mice.
Gpx1−/− mice (11) were kindly provided by Dr. Y. S. Ho (Wayne State University, Detroit, MI). Gpx3−/− female mice were mated with Gpx1−/− male mice. Female and male mice heterozygous for both Gpx3 and Gpx1 were mated, and their progeny were genotyped by PCR for Gpx3 and Gpx1 (Table 1). Male and female progeny with deletion of both Gpx3 and Gpx1 were bred with one another to establish the double knockout colony.
Antibody Production, Purification of Gpx3, and Western Blot Detection
Polyclonal antibodies were raised in two rabbits (8096 and 8099) by Rockland (Gilbertsville, PA). The antigen was the synthetic peptide C-SYMRRQAALSARGK, which represents the C terminus of mouse Gpx3. Purified antibodies were obtained by affinity column chromatography of antiserum from the terminal bleeds. The affinity column was prepared with the synthetic peptide and Aminolink Plus coupling gel according to the manufacturer's instructions (Pierce Chemical, Rockford, IL).
Affinity-purified antibody preparation 8096 was used for Western blot analyses and immunocytochemistry experiments. Gpx3 was immunoaffinity purified from mouse plasma using affinity-purified antibody preparation 8099 coupled to AminoLink Plus resin according to the manufacturer's instructions (Pierce Biotechnology). The plasma was applied to the column in PBS, and the column was washed with 2–3 volumes of PBS. Elution was accomplished using 0.1 M glycine, pH 2.5.
For some of the Western blots, proteins were separated on SDS-PAGE gels made with 10% Protogel (National Diagnostics, Atlanta, GA) and transferred to nitrocellulose using a semidry electroblotter (Apollo Instrumentation by CLP, San Diego, CA). The nitrocellulose membrane was blocked with 5% nonfat dry milk in TBST1 (0.5 M NaCl, 20 mM Tris, 0.05% Tween 20, pH 7.0) for 1 h at 37°C. Antibody 8096 was used for Western blot detection at 3.4 μg/ml in 5% nonfat dry milk in TBST1. The membrane was incubated with antibody for 2 h at room temperature and then washed three times for 20 min each with TBST1. Then, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit whole-molecule IgG (Sigma, St. Louis, MO) for 1 h at room temperature and washed three times for 20 min each with TBST1. Gpx3 protein was detected by chemiluminescence (Western Lightning, PerkinElmer, Boston, MA).
For other Western blots, SDS-PAGE was performed using 10% Bis-Tris NuPAGE Novex acrylamide gels (Invitrogen, Carlsbad, CA). Polypeptides were stained with Coomassie blue or transferred to nitrocellulose membranes for Western blot analysis. Blots were rinsed in PBS (150 mM NaCl, 10 mM sodium phosphate, pH 7.4) and blocked in Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE). Blots were then incubated in 8096 antibody at 0.27 μg/ml in Odyssey blocking buffer containing 0.1% Tween 20; parallel control blots substituted equivalent levels of nonimmune rabbit IgG. Blots were then washed at least four times with PBS containing 0.1% Tween (PBST) and incubated in Alexa 680-conjugated goat anti-rabbit IgG secondary antibody (Invitrogen) diluted 1:5,000 in PBST. The blots were then washed with PBST followed by PBS and imaged using an Odyssey infrared imager.
75Se labeling experiments.
Ten or fifteen μCi 75Se-selenite (University of Missouri Research Reactor Facility, Columbia, MO) with specific activity of 800–2,200 Ci/g selenium was administered by gavage or by intraperitoneal injection to fasted mice. The mice were housed individually in metabolic cages for 24 h with food and water available, and urine was collected. After the 24-h urine collection, blood was removed and plasma was separated for SDS-PAGE and autoradiography. 75Se was determined in urine (PerkinElmer model 1480 Wizard 3” gamma counter, Shelton, CT).
Separation of kidney centrifugal fractions.
Ten percent homogenates of kidneys from Gpx3−/− and Gpx3+/+ mice were made in PBS using a Polytron homogenizer. After an aliquot was taken, the homogenates were centrifuged at 600 g for 10 min. The supernatants were removed and saved. The pellets were washed three times by resuspension in PBS and centrifugation. Then, they were resuspended in 0.5 ml PBS and assayed for selenium.
Biochemical assays.
Ten percent tissue homogenates were prepared in 0.1 M potassium phosphate, pH 7.5, using a Polytron homogenizer. They were centrifuged at 16,000 g for 30 min at 4°C, and the supernatants were used for enzyme assays.
Glutathione peroxidase activity was measured in plasma and tissue supernatants as previously described using 0.25 mM H2O2 as a substrate (16) and Sepp1 was measured by the ELISA method reported previously (8).
Selenium was determined in plasma and tissues by the method of Koh and Benson (14) as modified by Sheehan and Gao (21). Protein concentration was determined using bicinchoninic acid (BCA) reagent (Pierce Chemical).
Immunofluorescence microscopy.
Mouse kidneys were fixed for 1 h at 4°C with 4% formaldehyde in 0.1 M sodium phosphate, pH 7.4, rinsed in phosphate buffer, and infiltrated overnight at 4°C in phosphate buffer containing 20% sucrose. Tissue was then immersed in Optimal Cutting Temperature Compound (OCT; Fisher Scientific, Atlanta GA), frozen in liquid nitrogen, and stored at −70°C until sectioning. Cryosections were rinsed with TBST2 (20 mM Tris·HCl, pH 8.0, 150 mM sodium chloride, 0.05% Tween 20, 0.025% sodium azide) and blocked with TBST2 containing 1% BSA and 0.1% glycine for 1 h. Sections were then incubated with antibody 8096 at 0.7 μg/ml in blocking solution for 1 h at room temperature. Other primary antibodies included rabbit affinity-purified anti-collagen IV (catalog no. 600–402-206, Rockland Immunochemicals) and affinity-purified rabbit anti-laminin (catalog no. L9393, Sigma). Control sections were incubated with equivalent levels of nonimmune rabbit IgG. Sections were then washed three times for 5 min each in TBST2 and incubated for 1 h in affinity-purified Cy3-goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted in blocking solution. Hoechst 33258, to stain DNA, and Alexa 488-phalloidin, to stain F-actin, (Invitrogen) were added to the secondary antibody solution. Slides were washed three times for 5 min each with TBST2 and mounted in 50% glycerol in TBS. Specimens were examined by phase-contrast and epifluorescence microscopy, and digital images of experimental and control samples were obtained using identical photographic settings. Each experiment was carried out with at least three mice, and the images shown are representative of all replicates.
Tissue overlay analysis of Gpx3 binding.
Kidneys from Gpx3−/− adult mice were immersed in OCT and frozen in liquid nitrogen. Cryosections were placed on Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA), air-dried for 20 min at 40°C, and used for tissue ligand-binding analysis (17). Tissue sections were blocked for 1 h in TBST2 containing 1% BSA and 0.1% glycine and incubated for 1 h in blocking solution containing 10 μg/ml Gpx3; control slides were incubated in blocking solution alone. Sections were then washed three times in TBST2 and immunostained with anti-Gpx3 and secondary antibody as described above. After staining, the slides were fixed with 4% formaldehyde in PBS for 15 min, washed in PBS, and then examined by epifluorescence and phase-contrast microscopy.
Preparation of kidney fractions for Gpx3-binding analysis.
Frozen or fresh kidneys were weighed and 5% homogenates were prepared in ice-cold HBS (150 mM NaCl, 10 mM HEPES, pH 7.4, 2 mM benzamidine, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) with a Duall homogenizer. In some experiments, the homogenizing buffer was modified and included high salt (1 M NaCl), disulfide reducing agent (20 mM DTT), or 0.25% Triton X-100 as specified in results. The homogenate was incubated for 30 min at 4°C, and an aliquot was removed for SDS-PAGE. The remainder of the homogenate was centrifuged at 14,000 g for 5 min. Supernatants and pellets were adjusted to the same volume, and equal aliquots were fractionated by SDS-PAGE.
To isolate basement membranes, 5% kidney homogenates were prepared using a Duall homogenizer in HBS containing 0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS). Homogenates were gently agitated at 4°C for 15 min and then centrifuged at 200 g for 2 min. The pellets were resuspended in homogenizing buffer, agitated again for 15 min at 4°C, and centrifuged at 200 g for 2 min. Then pellets were resuspended in homogenizing buffer and disrupted by sonication using two 5-s bursts at a medium power setting. The samples were then centrifuged at 200 g for 2 min, and the basement membrane-enriched pellets were washed twice by resuspension with homogenizing buffer and centrifugation as above.
Gpx3-binding assays.
Sedimentation assays were used to determine whether basement membranes of Gpx3−/− kidney possess Gpx3-binding activity. Basement membrane fractions were incubated with Gpx3 at 4°C for 1–2 h; controls consisted of basement membrane fractions without added Gpx3 or of Gpx3 alone. The samples were then centrifuged at 14,000 g for 3 min. Pellets and supernatants were recovered for assessment of Gpx3 content by Western blot analysis.
RESULTS
Production of Gpx3−/− mice.
Mice with the gene for Gpx3 deleted were produced by homologous recombination using a targeting vector produced by the strategy shown in Fig. S1. R1 embryonic stem cells positive for incorporation of the mutated DNA were selected and injected into C57BL blastocysts. Mice with germ-line transmission of the targeted allele were used to establish the Gpx3−/− mouse colony.
Gpx3−/− and Gpx3+/+ weanling mice were fed a selenium-deficient diet or a diet with selenium added at 0.25 mg/kg and observed for 4 wk. We were unable to distinguish the groups of mice from one another by observation. Neither deletion of Gpx3 nor selenium deficiency affected body weight (Table S1). Thus mice with Gpx3 deleted tolerated selenium deficiency.
Selenoproteins in plasma.
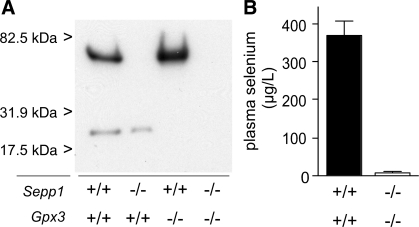
Figure 1A is an autoradiogram of an SDS-PAGE of plasma obtained from Sepp1+/+/Gpx3+/+ (C57BL/6), Sepp1−/−, Gpx3−/−, and Sepp1−/−/Gpx3−/− mice 24 h after injection of a tracer dose of 75SeO32−. Two bands of 75Se are visible in the lane loaded with Sepp1+/+/Gpx3+/+ plasma. The top band is absent from Sepp1−/− mouse plasma, and the bottom band is absent from Gpx3−/− mouse plasma. Neither band is detectable in Sepp1−/−/Gpx3−/− mouse plasma. These results confirm deletion of these two plasma selenoproteins in the respective mouse models and the production of a viable mutant with both extracellular selenoproteins deleted.
Fig. 1.
Effect of gene deletion on plasma selenoproteins and selenium. A: mice were injected with a tracer dose of 75SeO32−, and plasma was obtained 24 h later. The Gpx3−/− mouse had been fed a diet supplemented with 0.25 mg selenium/kg, and the other 3 mice had been fed a diet supplemented with 1 mg selenium/kg. Plasma samples (0.5 μl) from C57BL/6 (Sepp1+/+/Gpx3+/+), Sepp1−/−, Gpx3−/−, and Sepp1−/−/Gpx3−/− mice were subjected to SDS-PAGE on a 10% acrylamide gel, and the dried gel was exposed to Kodak XAR film for 3 days before being developed. B: plasma from C57BL/6 mice and Sepp1−/−/Gpx3−/− mice was obtained 4 wk after weaning. The C57BL/6 mice (n = 4) had been fed the diet supplemented with 0.25 mg selenium/kg, and the Sepp1−/−/Gpx3−/− mice (n = 6) had been fed the diet supplemented with 1.0 mg selenium/kg for 2 wk followed by the diet supplemented with 0.25 mg selenium/kg for 2 wk. The half-brackets indicate 1 SD, and the 2 values were significantly different (P < 0.001) by Student's t-test.
Figure 1B presents selenium concentrations of plasma from Sepp1+/+/Gpx3+/+ (C57BL/6) mice and from mice with both plasma selenoproteins deleted. The selenium concentration of the Sepp1−/−/Gpx3−/− mouse plasma was 2.4% of the C57BL/6 plasma selenium concentration and just above the detection limit (10 μg/l) of the assay method. The results shown in Fig. 1 indicate that Gpx3 and Sepp1 are the major selenoproteins in mouse plasma under basal conditions and that they account for all but a very small percentage of the plasma selenium. It should be noted that our experimental mice were fed selenium in the form of selenite. Plasma from animals or humans consuming diets containing selenomethionine, the major form of selenium in natural diets, has an additional selenium component because selenomethionine enters the methionine pool and is incorporated randomly into proteins at methionine positions (4).
Enzymes in plasma with glutathione peroxidase activity.
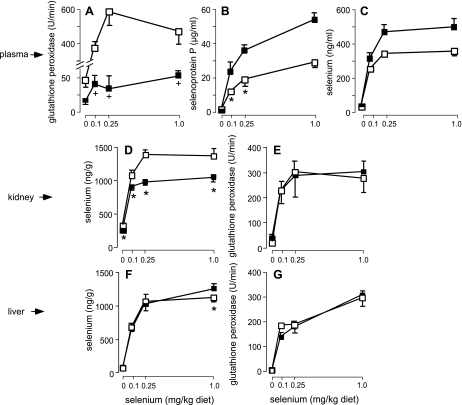
Deletion of Gpx3 decreased plasma glutathione peroxidase activity to ∼9% of that in Gpx3+/+ mice (Fig. 2A), indicating that a source of the activity in addition to Gpx3 was present. This minor glutathione peroxidase is selenium dependent because feeding a selenium-deficient diet to Gpx3−/− mice caused a further decrease in plasma glutathione peroxidase activity (Fig. 2A). Thus there are at least two selenium-dependent glutathione peroxidases in mouse plasma samples.
Fig. 2.
Plasma (A–C) and tissue (D–G) selenium markers in Gpx3−/− (■) and Gpx3+/+ (☐) mice fed selenium-deficient and selenium-supplemented diets. Values are means, n = 5, and the half-brackets indicate 1 SD. The Gpx3−/− values in A that are marked with crosses are different from the selenium-deficient value (P < 0.05) but not from one another by ANOVA. Pairs marked by asterisks in panels B–G are significantly different (P < 0.05) by Student's t-test.
Gpx1, the major intracellular glutathione peroxidase, was evaluated as the potential source of the non-Gpx3 plasma glutathione peroxidase activity. Mice with deletion of both Gpx1 and Gpx3 (Gpx1−/−/Gpx3−/− mice) appeared healthy and tolerated being fed a selenium-deficient diet without observed effect. Plasma glutathione peroxidase activity in Gpx1−/−/Gpx3−/− mice was lower than that in Gpx3−/− mice and was unaffected by the selenium status of the mice (Table 2). That selenium deficiency had been produced was demonstrated by a sharp decrease in plasma Sepp1 concentration (Table 2). Thus Gpx1 contributes to the glutathione peroxidase activity of the plasma samples we assayed.
Table 2.
Distribution of plasma glutathione peroxidase activity between Gpx3 and Gpx1*
| Dietary Selenium Supplementation | Gpx1−/−/Gpx3−/− Mice†‡ | Gpx3−/− Mice† |
|---|---|---|
| None | 13 ± 1 (8) | 39 ± 8 (9)§ |
| 0.25 mg/kg | 15 ± 5 (8) | 70 ± 18 (5)§ |
Plasma glutathione peroxidase activity of Gpx1+/+/Gpx3+/+ mice fed the selenium-replete diet was 580 ± 94 (5).
Values are means ± SD (n) expressed as μmol NADPH oxidized·ml−1·min−1. Mice were fed the experimental diets 4-5 wk beginning at weaning.
Sepp1 concentrations were 2.9 ± 0.4 μg/ml, n = 8, in the selenium-deficient group and 62 ± 8 μg/ml, n = 8, in the group supplemented with 0.25 mg selenium/kg diet.
Pairs sharing this superscript are different (P < 0.05) from one another by Student's t-test.
An experiment was carried out to assess the possibility that Gpx1 leaked from formed elements of the blood during storage at 4°C. Table S2 shows that glutathione peroxidase activity did not increase between 5 and 120 min after removal of blood from Gpx3−/− mice. This demonstrates that glutathione peroxidase did not leak from the formed elements of blood during storage.
The results of these experiments indicate that Gpx3 and Gpx1 account for the selenium-dependent glutathione peroxidase activity in plasma samples and confirm that virtually all the plasma glutathione peroxidase activity is selenium dependent. Moreover, calculations using the data presented in Table 2 indicate that Gpx3 accounted for 91% of the selenium-dependent glutathione peroxidase activity in plasma samples and that Gpx1 accounted for 9%.
Deletion of Gpx3 affects plasma Sepp1. It caused plasma Sepp1 concentration and, consequently, selenium concentration to rise (Fig. 2, B and C). The cause of this increase in Sepp1 concentration is not apparent.
Gpx3 genotype effect on selenium levels in kidney, liver, brain, testis, and the whole mouse.
At a supplemental level of 0.25 mg selenium/kg diet, 29% of kidney selenium was dependent on the presence of Gpx3 (Fig. 2D). However, kidney supernatant glutathione peroxidase activity was not affected by deletion of Gpx3 (Fig. 2E), indicating that kidney Gpx3-related selenium is not present as the soluble enzyme. Liver selenium concentration and cytosolic glutathione peroxidase activity were not affected by Gpx3 deletion (Fig. 2, F and G). Thus the kidney, the principal tissue of Gpx3 secretion, contains a large pool of selenium that is dependent on Gpx3. The liver does not contain such a selenium pool.
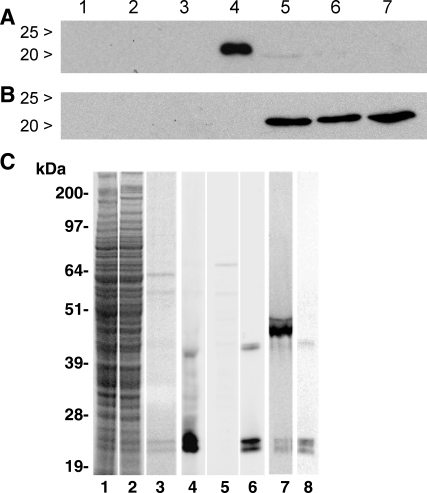
Kidney homogenate fractions from mice fed a diet with a supplemental level of 0.25 mg selenium/kg were assayed for selenium. Deletion of Gpx3 reduced the selenium content of the 600-g pellet but not that of the other centrifugal fractions (Table 3). Western blots of the 600-g pellet and its supernatant (Fig. 3, A and B) showed that Gpx3 was present in the pellet but not in the supernatant. These results indicate that over one-fourth of kidney selenium is in the form of Gpx3 bound to material that sediments at 600 g.
Table 3.
Selenium concentration of Gpx3−/− and Gpx3+/+ kidney homogenate fractions
| Gpx3−/− Kidney | Gpx3+/+ Kidney | Gpx3−/− Kidney/Gpx3+/+ Kidney | |
|---|---|---|---|
| Homogenate (20%) | 6.7 ± 1.1* | 9.8 ± 1.0* | 0.68 |
| Pellet (600 g) | 4.8 ± 0.5† | 13 ± 0.8† | 0.37 |
| Supernatant (600 g) | 7.3 ± 0.8 | 6.7 ± 0.7 | 1.09 |
| Supernatant (100,000 g) | 10 ± 1.1 | 9.0 ± 0.8 | 1.11 |
Values are means ±SD expressed as ng selenium/mg protein; n = 4.
Pairs sharing a superscript are different (P < 0.05) from one another by Student's t-test.
Fig. 3.
Gpx3 in kidney centrifugal fractions. A: Western blot after SDS-PAGE of 600-g supernatants (40 μg protein/lane) of 10% homogenates of the kidneys of 3 Gpx3−/− (lanes 1–3) and 3 Gpx3+/+ (lanes 5–7) mice. Plasma (1 μl) from a Gpx3+/+ mouse is in lane 4 as a positive control. B: Western blot after SDS-PAGE of the 600-g pellets (40 μg protein/lane) of the same kidneys. In B, lane 4 did not contain a sample. C: assessments of Gpx3 after SDS-PAGE (NuPAGE gels) of kidney homogenates (Gpx3+/+ in lanes 1 and 4, Gpx3−/− in lanes 2 and 5; each lane is loaded with homogenate from 12.5 μg of kidney), 1 μg purified plasma Gpx3 (lanes 3 and 6), and 0.5 μl 75Se-labeled plasma (lanes 7 and 8). Lanes 1–3 were stained with Coomassie blue for protein loading. Lanes 4–6 and 8 were immunostained with anti-Gpx3. Lane 7 is an autoradiogram.
Selenium concentration was determined in selected tissues and the whole body of the mice fed 0.25 mg selenium/kg and used in the experiment presented in Fig. 2. Table 4 shows that the Gpx3 genotype did not significantly affect whole-body selenium concentration, and Fig. S2 shows that it did not affect urinary excretion of gavaged 75Se. Neither were liver, brain, testis, or muscle selenium levels significantly affected (Table 4). Taken together, the results presented in Table 4 and Fig. 2 indicate that kidney was the only solid tissue of those shown here to contain a statistically significant fraction of its selenium in the form of Gpx3.
Table 4.
Selenium concentration of tissues and whole body of Gpx3−/− and Gpx3+/+ mice fed a diet supplemented with 0.25 mg selenium/kg
| Gpx3−/− Mice | Gpx3+/+Mice | Gpx3−/−/Gpx3+/+ | |
|---|---|---|---|
| n | 5 | 5 | |
| Whole body | 223 ± 11 | 223 ± 29 | 1.00 |
| Muscle | 126 ± 7 | 127 ± 10 | 0.99 |
| Brain | 135 ± 19 | 138 ± 16 | 0.98 |
| Testis | 711 ± 85 | 749 ± 54 | 0.95 |
Values are means ± SD expressed as ng selenium/g. Genotype had no statistically significant effect on selenium concentration by Student's t-test.
These experiments identified two large pools of Gpx3, one in plasma and the other in kidney. Calculation of the amount of selenium in these two pools of Gpx3 allows comparison of their sizes. Earlier work had indicated that Gpx3 in plasma from mice fed a diet supplemented with 1 mg selenium/kg contained 66 ng selenium/ml (8). Mice fed the same diet in the present project (Fig. 2) weighed 20.6 ± 2.3 g; n = 10. Plasma is 4–5% of body weight in the mouse, so plasma Gpx3 in these mice can be calculated to contain up to 68 ng of selenium. Data from the mice that were used to construct Fig. 2 indicate that Gpx3 contained 150 ng of selenium in Gpx3+/+ kidneys. Thus the pool of Gpx3 selenium in the kidneys was more than twice the size of the pool of Gpx3 selenium in the plasma. This indicates that more than twice as much Gpx3 was present in kidney than in plasma.
Nature of Gpx3 binding in the kidney.
Homogenates of Gpx3+/+ and Gpx3−/− kidneys and Gpx3 purified from plasma were subjected to reducing SDS-PAGE (Fig. 3C). The electrophoresis system used in this experiment employed NuPAGE Novex acrylamide gels, whereas those used in earlier experiments (Figs. 1A and 3, A and B) were 10% gels made using Protogel. Lanes stained with Coomassie blue demonstrated equivalent loading of protein of the kidney homogenates and the absence of major protein contaminants in the purified Gpx3 (Fig. 3C, lanes 1–3). Western blotting of Gpx3+/+ kidney homogenate displayed two major bands that migrated at 22 and 24 kDa and a minor band that migrated at 42 kDa, all of which stained positively with anti-Gpx3 (Fig. 3C, lane 4). A homogenate of Gpx3−/− kidney displayed no specifically stained bands by Western blot analysis (Fig. 3C, lane 5). Two major bands that migrated at 22 and 24 kDa and a minor band of 42 kDa were also detected by Western blot analysis of purified plasma Gpx3 (Fig. 3C, lane 6). Control blots treated with nonimmune rabbit IgG had no stained bands (not shown). The absence of immunoreactive proteins in Gpx3−/− kidney homogenates confirms that the antibody is specific for Gpx3 and indicates that it does not cross-react with other glutathione peroxidase family members that are present in the kidney. The results shown in Fig. 3C demonstrate that two forms of Gpx3 that differ in migration on NuPAGE electrophoresis gels are present in the kidney and in plasma. The two forms were not resolved when electrophoresis gels made using Protogel were used (Figs. 1A and 3A). The 42-kDa band likely represents a dimer of Gpx3 subunits.
Autoradiograms of Western blots of 75Se-labeled mouse plasma revealed a major radiolabeled band that migrated at 50 kDa, the size of Sepp1, and minor labeled bands migrating at 42, 24, and 22 kDa (Fig. 3C, lane 7). When the blot was probed with anti-Gpx3, only bands at 42, 24, and 22 kDa stained (Fig. 3C, lane 8). This result indicates that both the 24- and the 22-kDa bands contain selenium and apparently represent Gpx3 isoforms.
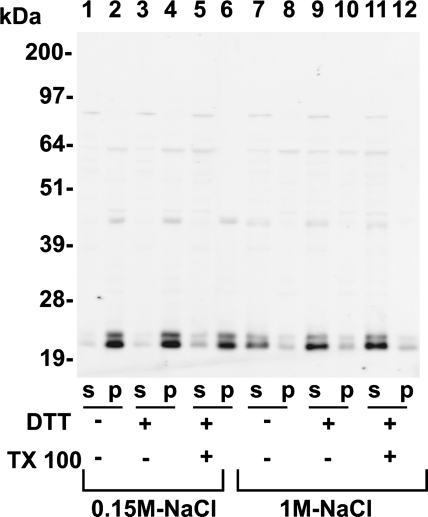
To investigate the mechanism of Gpx3 anchoring in the pellet material, homogenates were prepared in buffer containing 0.15 M NaCl or 1.0 M NaCl, with or without 20 mM DTT and 0.25% Triton X-100. The homogenates were centrifuged at 14,000 g for 5 min. Western blotting was used to assess the Gpx3 protein in the supernatant and pellet fractions (Fig. 4). In all homogenates prepared using 0.15 M NaCl, most of the Gpx3 remained in the particulate fraction (Fig. 4, lanes 1–6). In contrast, most of the Gpx3 was present in the supernatant fraction of homogenates prepared with 1.0 M NaCl (Fig. 4, lanes 7–12). These data indicate that Gpx3 binding to a particulate tissue component is not mediated by disulfide or selenenylsulfide cross-linking but by an alternative interaction, presumably of protein-protein nature.
Fig. 4.
Kidney homogenates from Gpx3+/+ mice fed a diet supplemented with 0.25 mg selenium/kg were prepared in either 0.15 M NaCl-containing (lanes 1–6) or 1 M NaCl-containing (lanes 7–12) homogenizing buffer. Samples were prepared in homogenizing buffer alone (lanes 1, 2, 7, and 8), homogenizing buffer containing 20 mM DTT (lanes 3, 4, 9, and 10), or homogenizing buffer containing 20 mM DTT and 0.25% Triton X-100 (lanes 5, 6, 11, and 12). Homogenates were centrifuged, and the supernatant (S) and pellet (P) fractions were analyzed for Gpx3 content by Western blot analysis. Gpx3 fractionated to the pellet (P) fraction in all 0.15 M NaCl homogenates. In contrast, Gpx3 was released to the supernatant (S) fraction in all homogenates prepared with 1 M NaCl. Each lane represents the P and S fractions from 62 μg of intact kidney.
Immunocytochemical localization of Gpx3.
The 600-g pellet contains nuclei, extracellular matrix, and connective tissue of the kidney. An earlier study had detected Gpx3 in the interstitial space at the basal pole of the proximal tubule cells (25). Because of that observation and the fact that Gpx3 is a secreted protein, a study of its association with kidney extracellular matrix was carried out.
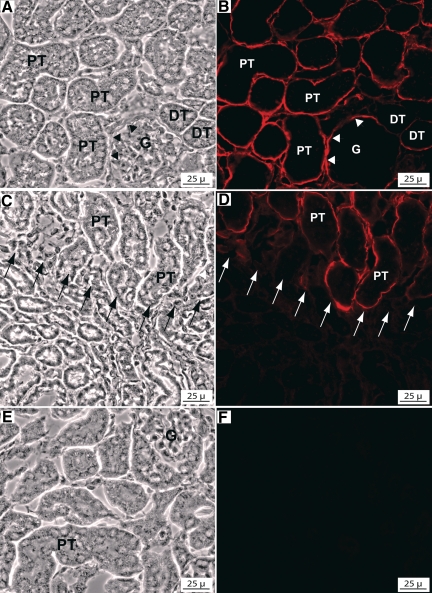
Immunofluorescence staining of Gpx3+/+ kidneys with anti-Gpx3 (8096) revealed staining in the cortex and outer zone of the medulla but no staining in the remainder of the medulla. In the cortex, intense staining localized to the perimeter of the proximal convoluted tubules and less intense staining was detected surrounding distal tubules and glomeruli (Fig. 5, A and B). Staining for Gpx3 terminated abruptly approximately at the junction of the cortex and medulla (Fig. 5, C and D). The staining appeared to extend to the termination of the descending thick segment of the proximal tubules (15).
Fig. 5.
Immunohistochemical localization of Gpx3 in the kidney. Matched phase-contrast and immunofluorescence images of anti-Gpx3-stained (red) cryosections of Gpx3+/+ and Gpx3−/− kidneys. A and B: outer cortex of a Gpx3+/+ kidney. Intense staining is detected at the perimeter of the proximal tubules (PT) and of the urinary pole (arrowheads) of the glomerulus (G). Less intense staining is detected at the perimeter of the distal tubules (DT). C and D: outer medulla of a Gpx3+/+ kidney. The Gpx3 staining terminates abruptly at the junction of the outer and inner stripes (arrows), where the PT terminate and transition into descending thin limbs. E and F: no Gpx3 expression is detected in the cortex of a Gpx3−/− kidney.
Intracellular Gpx3 was detected in PCTs (Fig. S3). This intracellular Gpx3 appeared to be in the Golgi region and was thus likely to be en route to secretion by these cells. No staining with anti-Gpx3 was detected in kidneys from Gpx3−/− mice (Fig. 5, E and F). These findings indicated that secreted Gpx3 binds to the extracellular matrix and, potentially, to the basement membrane of specific kidney tubules.
Tissue ligand-binding analysis localizes Gpx3-binding sites to the perimeter of kidney tubules in the cortex.
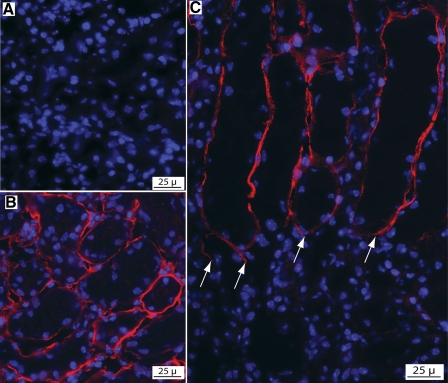
Cryosections of Gpx3−/− kidneys were used for tissue overlay (ligand-binding) analysis. Sections incubated only in blocking buffer before immunostaining with anti-Gpx3 showed no detectable staining (Fig. 6A). However, cryosections that had been incubated in blocking buffer containing purified Gpx3 before immunostaining with anti-Gpx3 displayed specific staining of the kidney tubule perimeter (Fig. 6, B and C). This demonstrates that Gpx3 binding sites are located at the perimeter of cortical kidney tubules. No binding was detected in the medulla, indicating that the binding sites are selectively localized to the cortex. These in vitro binding results are consistent with the in vivo binding shown in Fig. 5.
Fig. 6.
Tissue overlay analysis of Gpx3−/− kidney demonstrating Gpx3 binding (red) to the perimeter of cortical tubules. A: control experiment showing an absence of Gpx3 staining in Gpx3−/− kidneys. B and C: cryosections of Gpx3−/− kidneys incubated with Gpx3 before immunostaining with anti-Gpx3. B: cortical tubules show specific staining at their perimeter. C: Gpx3 binding to the terminal portion of PT in the outer medulla where they abruptly terminate (arrows) and transition to the descending thin limbs. Medullary tubules located below this junction (arrows) show no Gpx3 binding.
Gpx3 is associated with basement membranes isolated from kidney.
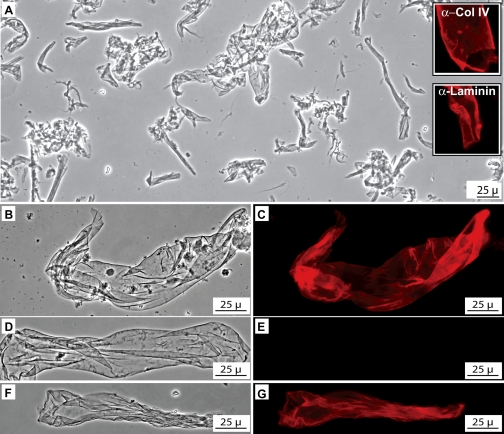
To prepare a basement membrane-enriched fraction, kidneys were homogenized in a CHAPS-containing buffer and centrifuged. Western blot analysis revealed that Gpx3 partitioned to the basement membrane-enriched fraction (not shown). Phase-contrast microscopy of the CHAPS-insoluble fraction revealed translucent sheetlike structures (Fig. 7A) that stained with antibodies to collagen IV and laminin (insets in Fig. 7A), confirming their identification as basement membranes. Most of the basement membranes prepared from Gpx3+/+ kidneys stained intensely with anti-Gpx3 antiserum (Fig. 7, B and C) whereas those from Gpx3−/− mice displayed no staining (Fig. 7, D and E). Tissue overlay analysis was then used to test the Gpx3-binding capability of the basement membranes from Gpx3−/− kidneys. Following exposure to Gpx3, most of the basement membranes stained intensely with anti-Gpx3 (Fig. 7, F and G). However, a minority of them, presumably those originating in the medulla, did not stain. The Gpx3-binding activity of the basement membrane fraction from Gpx3−/− kidneys was also confirmed using a centrifugation assay and Western blot analysis (Fig. S4). These data demonstrate that a population of kidney tubule basement membranes binds Gpx3.
Fig. 7.
Gpx3 in kidney basement membranes. A: phase-contrast photomicrograph of kidney basement membrane-enriched fraction showing phase lucent sheetlike structures. Insets show that these structures are intensely immunostained (red) by anti-collagen IV (α-Col IV) and anti-laminin (α-laminin). B and C: matched phase-contrast and fluorescence images of basement membrane from Gpx3+/+kidney immunostained with anti-Gpx3 (red). D and E: phase-contrast and fluorescence images of Gpx3−/− kidney basement membrane immunostained with anti-Gpx3. No Gpx3 is detected. F and G: phase-contrast and fluorescence images of Gpx3−/− kidney basement membrane that had been incubated with purified Gpx3 before immunostaining with anti-Gpx3. Gpx3 binding to the basement membrane is evident.
DISCUSSION
Knockout of both Gpx3 and Sepp1 revealed that these selenoproteins contain >97% of the selenium in plasma (Fig. 1). Based on studies with Sepp1−/− mice (8), the selenium in glutathione peroxidase can be estimated to be 21% of the total in plasma, leading to the conclusion that Sepp1 contains 76% of the plasma selenium. Excretory metabolites (13), putative transport forms (the viability of these double knockout mice implies the presence of an additional transport form of the element), and additional selenoproteins must all be accommodated in the <3% of plasma selenium remaining. Excretory and transport forms can be expected to transit the plasma compartment rapidly and thus not require high plasma concentrations for their function.
Table 2 shows that Gpx1 is present in plasma samples. This was an unexpected finding. The specific activity of Gpx1 is about four times that of Gpx3 when H2O2 is the reaction substrate (7). Since ∼9% of the plasma glutathione peroxidase activity was attributable to Gpx1, the amount of plasma selenium in Gpx1 can be estimated to be ∼2% of that in Gpx3. Plasma glutathione peroxidase accounts for 21% of plasma selenium, so Gpx1 will contain ∼0.4% of the selenium in plasma samples from selenium-replete mice. This low value demonstrates that the selenium in Gpx1 can be accommodated in the very low plasma selenium of the Sepp1−/−/Gpx3−/− mice (Fig. 1B) and explains why it was not detected in the Sepp1−/−/Gpx3−/− lane of the autoradiogram (Fig. 1A).
Gpx1 is the most abundant selenoprotein in the mouse and is intracellular. Thus it is not clear whether finding it in plasma samples at low levels indicates that it has an extracellular function or has simply been released from injured or dying cells. Assessing these possibilities will require additional work.
Two Gpx3 isoforms were identified in the mouse by Western blot analysis. Mouse Gpx3 is encoded by a single copy gene (20). Exon 1 of the mouse Gpx3 gene contains two potential ATG start sites separated by 66 nucleotides, but only the second of them has been proposed to initiate protein translation (20, 26). However, even if both start sites are utilized the two translation products would contain the same predicted signal peptide cleavage site and both should yield the same mature ∼23-kDa protein (2). Thus the two Gpx3 isoforms likely arise by posttranslational modification. Gpx3 might be glycosylated (22) and the mouse isoforms could be differentially glycosylated, although other posttranslational modifications remain a possibility. Gpx3 is a tetramer, and the identification of two isoforms in the mouse raises questions of whether they assemble into homo- or heterotetramers that could differ in function. Further work will be needed to characterize the isoforms and assess their significance.
One objective of this study was to determine whether Gpx3 plays a role in selenium transport and homeostasis. Experiments using Gpx3−/− mice demonstrated that deletion of Gpx3 had no effect on selenium concentration in liver, brain, testis, muscle, or the whole body (Table 4) and no effect on its urinary excretion (Fig. S2). Deletion of Gpx3 did cause a decrease in kidney selenium concentration (Fig. 2), but that decrease was traced to a lack of Gpx3 in the kidney and not to a decreased supply of selenium to this organ (Fig. 2E). Thus no evidence was uncovered in this study for Gpx3 participating in selenium transport or homeostasis, but the study does demonstrate that a large amount of Gpx3 is present in the kidney. This latter observation led to our attempts to characterize kidney Gpx3 using immunocytochemistry.
The binding of Gpx3 to basement membranes adjacent to renal cortical epithelial cells was an unexpected finding (Fig. 5). This binding appears to be highly specific. For example, basement membranes in the renal medulla do not bind Gpx3 (Figs. 5 and 6), and preliminary experiments demonstrate selective binding of Gpx3 to basement membranes in some other tissues as well (Olson GE and Winfrey VP, unpublished observations). Tissue overlay experiments demonstrate that binding can take place in vitro in a pattern that is similar to binding in vivo (Figs. 6 and 7). Thus a restricted population of basement membranes has binding sites specific for Gpx3.
Binding of Gpx3 to basement membranes might be a clue to its function. Localization of Gpx3 by its binding to the basement membrane will increase glutathione peroxidase activity at that site. The tubule epithelial cells probably release GSH into this space, where it could serve as the reducing substrate for Gpx3. Thus binding of Gpx3 to basement membranes might be a method for increasing glutathione peroxidase activity at this extracellular location to regulate hydroperoxide levels. Another oxidant defense enzyme, extracellular superoxide dismutase, is also bound in the extracellular matrix (19), providing a precedent for this proposed function of Gpx3.
The amount of Gpx3 bound in the kidneys is more than twice the amount present in the plasma. This raises the possibility that Gpx3 bound to tubule cell basement membranes in the kidney cortex serves as a reservoir of Gpx3 that can be mobilized when needed to combat an oxidative challenge in another part of the body. In support of this possibility, it has been shown that hyperoxia and experimental colitis raise plasma glutathione peroxidase activity (12, 24). Finally, it is possible, but less likely, that Gpx3 contributes to the structure of the basement membrane to which it binds. Further work will be necessary to assess these possibilities.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK33231, R37 ES02497, P30 ES00267, and R01 DK82813. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1.Avissar N, Ornt DB, Yagil Y, Horowitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol Cell Physiol 266: C367–C375, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Burk RF, Hill KE. Selenoprotein P—expression, functions, and roles in mammals. Biochim Biophys Acta 1790: 1441–1447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors 14: 107–114, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Burk RF, Hill KE, Motley AK, Austin LM, Norsworthy BK. Deletion of selenoprotein P upregulates urinary selenium excretion and depresses whole-body selenium content. Biochim Biophys Acta 1760: 1789–1793, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, Winfrey VP, Austin LM. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci 27: 6207–6211, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esworthy RS, Chu FF, Geiger P, Girotti AW, Doroshow JH. Reactivity of plasma glutathione peroxidase with hydroperoxide substrates and glutathione. Arch Biochem Biophys 307: 29–34, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Hill KE, Zhou J, Austin LM, Motley AK, Ham AJ, Olson GE, Atkins JF, Gesteland RF, Burk RF. The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for supply of selenium to brain and testis but not for maintenance of whole-body selenium. J Biol Chem 282: 10972–10980, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF, Gesteland RF, Burk RF. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem 278: 13640–13646, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hill KE, Zhou J, McMahan WJ, Motley AK, Burk RF. Neurological dysfunction occurs in mice with targeted deletion of selenoprotein P gene. J Nutr 134: 157–161, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 272: 16644–16651, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Kim KK, Whitin JC, Sukhova NM, Cohen HJ. Increase in extracellular glutatione peroxidase in plasma and lungs of mice exposed to hyperoxia. Pediatr Res 46: 715–721, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y, Ogra Y, Ishiwata K, Takayama H, Aimi N, Suzuki KT. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc Natl Acad Sci USA 99: 15932–15936, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh TS, Benson TH. Critical re-appraisal of fluorometric method for determination of selenium in biological materials. J Assoc Off Anal Chem 66: 918–926, 1983 [PubMed] [Google Scholar]
- 15.Kriz W, Bankir L. A standard nomenclature for structures of the kidney. The Renal Commission of the International Union of Physiological Sciences (IUPS). Kidney Int 33: 1–7, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71: 952–958, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem 283: 6854–6860, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem 282: 12290–12297, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sandstrom J, Karlsson K, Edlund T, Marklund SL. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J 294: 853–857, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwaab V, Baud E, Ghyselinck N, Mattei MG, Dufaure JP, Drevet JR. Cloning of the mouse gene encoding plasma glutathione peroxidase: organization, sequence and chromosomal localization. Gene 167: 25–31, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Sheehan TMT, Gao M. Simplified fluorometric assay of total selenium in plasma and urine. Clin Chem 36: 2124–2126, 1990 [PubMed] [Google Scholar]
- 22.Takahashi K, Avissar N, Whitin J, Cohen H. Purification and characterization of human plasma glutathione peroxidase: a selenoglycoprotein distinct from the known cellular enzyme. Arch Biochem Biophys 256: 677–686, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Cohen HJ. Selenium-dependent glutathione peroxidase protein and activity: Immunological investigations on cellular and plasma enzymes. Blood 68: 640–645, 1986 [PubMed] [Google Scholar]
- 24.Tham DM, Whitin JC, Cohen HJ. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr Res 51: 641–646, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Whitin JC, Bhamre S, Tham DM, Cohen HJ. Extracellular glutathione peroxidase is secreted basolaterally by human renal proximal tubule cells. Am J Physiol Renal Physiol 283: F20–F28, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura S, Watanabe K, Suemizu H, Onozawa T, Mizoguchi J, Tsuda K, Hatta H, Moriuchi T. Tissue specific expression of the plasma glutathione peroxidase gene in rat kidney. J Biochem 109: 918–923, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.