Abstract
Inflammatory factors are elevated in animal and human subjects with hypertension and renal injury. We hypothesized that inflammation contributes to hypertension-induced renal injury by impairing autoregulation and microvascular reactivity to P2X1 receptor activation. Studies were conducted in vitro using the blood-perfused juxtamedullary nephron preparation. Rats receiving ANG II (60 ng/min) infusion were treated with the anti-inflammatory agent pentosan polysulfate (PPS) for 14 days. The magnitude and progression of hypertension were similar in ANG II and ANG II+PPS-treated rats (169 ± 5 vs. 172 ± 2 mmHg). Afferent arterioles from control rats exhibited normal autoregulatory behavior with diameter decreasing from 18.4 ± 1.6 to 11.4 ± 1.7 μm when perfusion pressure was increased from 70 to 160 mmHg. In contrast, pressure-mediated vasoconstriction was markedly attenuated in ANG II-treated rats, and diameter remained essentially unchanged over the range of perfusion pressures. However, ANG II-treated rats receiving PPS exhibited normal autoregulatory behavior compared with ANG II alone rats. Arteriolar reactivity to ATP and β,γ-methylene ATP was significantly reduced in ANG II hypertensive rats compared with controls. Interestingly, PPS treatment preserved normal reactivity to P2 and P2X1 receptor agonists despite the persistent hypertension. The maximal vasoconstriction was 79 ± 3 and 81 ± 2% of the control diameter for ATP and β,γ-methylene ATP, respectively, similar to responses in control rats. PPS treatment significantly reduced α-smooth muscle actin staining in afferent arterioles and plasma transforming growth factor-β1 concentration in ANG II-treated rats. In conclusion, PPS normalizes autoregulation without altering ANG II-induced hypertension, suggesting that inflammatory processes reduce P2X1 receptor reactivity and thereby impair autoregulatory behavior in ANG II hypertensive rats.
Keywords: P2X1 purinoceptor, afferent arteriole, pentosan polysulfate
renal autoregulation is an essential mechanism for maintaining glomerular capillary pressure and renal blood flow relatively constant during fluctuations in arterial pressure (34). This physiological control mechanism is primarily executed by adjusting afferent arteriolar resistance. Autoregulatory resistance adjustments manifest the combined influences of the myogenic response and tubuloglomerular feedback. Under hypertensive circumstances, loss of autoregulation can lead to inappropriate transmission of elevated arterial pressure to the glomerulus and result in elevated glomerular capillary hydrostatic pressure and ultimately glomerular injury (4, 18). Experimental evidence shows that the efficiency of the renal autoregulatory response is reduced in hypertensive animal models such as ANG II-infused, DOCA-salt, Dahl salt-sensitive, and two kidney-one clip Goldblatt hypertensive rats (17, 24, 26, 41, 54). The mechanisms responsible for hypertension-induced impairment of autoregulatory responsiveness remain uncertain.
Extracellular ATP activates a variety of ionotropic P2X receptors and metabotropic G protein-coupled P2Y receptors (1, 37). Evidence indicates that P2X1 receptors are highly expressed in afferent arterioles (8, 54) and that they are critically important in mediating autoregulation and in regulating afferent arteriolar function (20–23, 25, 35, 36). Autoregulatory behavior and afferent arteriolar reactivity to P2X1 receptor stimulation are markedly impaired in ANG II-induced hypertensive rats while the response to the P2Y2 receptor agonist UTP was unaltered (54). These data suggest a potentially important mechanism whereby reduced P2X1-mediated vasoconstriction of afferent arterioles accounts for the impairment of autoregulation and the promotion of renal injury progression.
Growing evidence suggests that inflammatory factors play a crucial role in the development of cardiovascular disease and renal injury in hypertensive subjects (14, 30, 46). Acute exposure to transforming growth factor-β (TGF-β) attenuates pressure-mediated afferent arteriolar vasoconstriction in normal Sprague-Dawley rats (49). Anti-inflammatory treatment, which did not modify the elevation of blood pressure, prevents progressive renal injury in several hypertensive animal models (5, 10, 11). Thus inflammatory factors may contribute to hypertension-induced renal injury by impairing vasomotor tone of afferent arterioles and reducing autoregulatory capability in ANG II-infused hypertensive rats.
Pentosan polysulfate (PPS) is a semisynthetic glycosaminoglycan (GAG) (31). Several studies suggest that PPS or heparin-derived GAG treatment reduces vascular injury by inhibiting smooth muscle cell proliferation, extracellular matrix accumulation, and prevents renal injury in models of arteriosclerosis, diabetes, and hypertension (5, 7, 16, 50). Therefore, the first purpose of this study is to test whether treatment with the nonspecific anti-inflammatory agent PPS would prevent the decline in renal autoregulatory behavior in ANG II-infused hypertensive rats. The second purpose of this study is to test whether normalization of autoregulatory behavior by PPS is associated with restoration of afferent arteriolar reactivity to P2X1 receptor activation. Our study reveals that PPS treatment not only preserves normal afferent arteriolar autoregulatory behavior but also preserves afferent arteriolar vasoconstriction to both ATP and β,γ-methylene ATP (β,γ-mATP) despite persistent hypertension. This functional observation is consistent with the findings that increased α-smooth muscle actin (α-SMA) staining in afferent arterioles and elevated plasma TGF-β1 concentration in ANG II-infused rats were significantly reduced by PPS treatment.
METHODS
Animals
Male Sprague-Dawley rats (total 167 rats, Charles River Breeding Laboratories, Raleigh, NC), weighing between 225 and 250 g, were used in all experiments. Rats were fed a standard chow (Harlan Teklad, Madison, WI) and had free access to water before all experiments. All animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Committee on Animal Use for Research and Education at the Medical College of Georgia.
ANG II-Infused Hypertensive Rats
Hypertension was induced by subcutaneous implantation of an osmotic minipump (Durect, Cupertino, CA) filled with ANG II (60 ng/min, Phoenix Pharmaceuticals, Belmont, CA) into the dorsum of the rat, as previously described (24). Briefly, under anesthesia (2% isoflurane), the surgical area was shaved and washed with povidone-iodine solution and 70% ethanol. A 5-mm skin incision was made and, a subcutaneous pocket was formed for minipump implantation. The incision was closed with surgical clips. Systolic blood pressure was monitored in conscious animals by the tail-cuff method (IITC, Woodland Hills, CA) before and every 3 days after minipump implantation. The rats were divided into four groups: control (normotensive rats without any intervention), control+PPS, ANG II alone, and ANG II+PPS treatment. PPS-treated rats received PPS at 100 mg·kg−1·day−1 (ELMIRON, Ortho-McNeil Pharmaceutical, Raritan, NJ) in the drinking water. Daily dosing was calculated based on the water consumption measured over the previous 24 h. A 24-h urine collection was made on days 0, 6, and 12, and juxtamedullary nephron studies were performed on days 13 or 14 after minipump implantation.
An additional group of rats from either the ANG II-infused (n = 3) or ANG II+PPS (n = 4) groups were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) under pentobarbital sodium anesthesia (50 mg/kg body wt ip, Ovation Pharmaceuticals, Deerfield, IL) to exclude the possibility that PPS treatment did not induce differences in arterial pressure that might not be detected by tail-cuff monitoring procedures. The blood pressure was continuously monitored for 14 days.
In Vitro Blood-Perfused Juxtamedullary Nephron Technique
Renal autoregulatory and microvascular reactivity studies were performed using the in vitro blood-perfused juxtamedullary nephron technique, as described previously (13, 19). Two identically treated rats (kidney donor and an identically treated blood donor) were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg body wt) and prepared for videomicroscopy experiments. The right kidney from the kidney donor was cannulated via the superior mesenteric artery during continuous perfusion with Tyrode buffer containing 5.2% BSA (Calbiochem, La Jolla, CA) and l-amino acids. Blood was collected from the kidney donor into a heparinized syringe (500 IU) via a carotid artery catheter and processed with blood collected from the blood donor. The blood was centrifuged for 15 min at 4,000 rpm. The plasma was separated and filtered through a 0.2-μm filter (Nalgene, Thermo Fisher Scientific, Rochester, NY). The buffy coat was removed and discarded. The packed erythrocytes were washed, resuspended, and centrifuged two times at speeds of 1,300 (23 min) rpm and 4,000 (15 min) rpm, respectively. The washed erythrocytes were mixed with plasma to obtain a hematocrit of ∼33%. The reconstituted blood was filtered through 5-μm nylon mesh for kidney perfusion.
The right kidney was harvested and sectioned along the longitudinal axis, leaving the dorsal two-thirds of the kidney intact. The sectioned kidney was positioned on the perfusion chamber. The ends of the large arteries were tied with 10-0 nylon sutures to restore renal perfusion pressure. The glomeruli and microvessels were exposed by careful removal of connective tissue covering the inner cortical surface. After completion of the dissection procedures, the chamber, containing the prepared kidney, was moved to the stage of a Nikon Eclipse E600FN microscope (Nikon, Tokyo, Japan) connected to a Nikon water-immersion objective (×40). The perfusate was switched to the reconstituted blood from a sealed, pressurized reservoir continuously gassed with 95% O2-5% CO2. The kidney was superfused with 37°C Tyrode buffer containing 1% BSA. The image of the kidney was displayed on a video monitor and recorded on DVD for later analysis.
Perfusion pressure was monitored directly within the perfusion cannula via a polyethylene line connected to a pressure transducer (model TRN005, Kent Scientific, Torrington, CT) linked to a chart recorder (model 201, Cole-Parmer, Vernon Hills, IL). Afferent arteriolar inner diameters were measured at a single site, at 12-s intervals using a calibrated image-shearing monitor (model 908, Vista Electronics, Valencia, CA), and the diameter was calculated from the average of all diameter measurements obtained during the final 2 min of each treatment period.
Experimental Protocols
Autoregulatory behavior of afferent arterioles.
Autoregulatory behavior of afferent arterioles was assessed by measuring changes in arteriolar diameter in response to step-increases in perfusion pressure. Briefly, each experiment began with a 5-min control period at a perfusion pressure of 100 mmHg, and then the autoregulatory behavior of afferent arterioles was assessed by lowering perfusion pressure from 100 to 70 mmHg and then increasing perfusion pressure from 70 to 160 mmHg in 30-mmHg increments at 5-min intervals. Four groups were studied: control, control+PPS, ANG II, and ANG II+PPS groups (n = 7–8).
Afferent arteriolar response to endogenous P2 receptor ligand ATP.
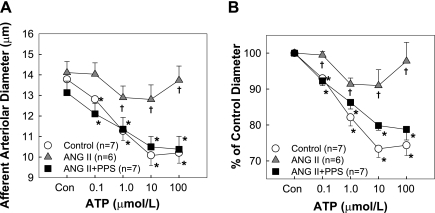
Since ATP is the important signaling molecule in regulating afferent arteriolar tone and in mediating renal autoregulatory behavior (3, 20, 21, 23, 25, 28, 35, 36, 40), the concentration-response relationship for ATP (Sigma, St. Louis, MO) was studied with afferent arterioles from control, ANG II, and ANG II+PPS groups (n = 6–7). As PPS treatment did not change afferent arteriolar autoregulatory behavior in normotensive control rats (Fig. 2), in this and the following β,γ-mATP studies afferent arterioles from control without PPS treatment were used as controls for both ANG II and ANG II+PPS groups. Briefly, after a 5-min control period, arterioles were exposed to increasing concentrations (0.1, 1.0, 10, and 100 μmol/l) of ATP via the superfusion solution. Concentrations were changed at 5-min intervals, while the perfusion pressure was maintained at 100 mmHg. Arteriolar diameter was continuously monitored before and during the ATP treatment period.
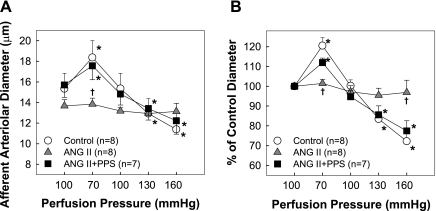
Fig. 2.
PPS preserves autoregulatory behavior of afferent arterioles. A: changes in afferent arteriolar diameter in response to alterations in renal perfusion pressure were measured in control (circle), chronic ANG II infusion only (60 ng/min, triangle), or ANG II+PPS (square) rats. B: data are expressed as percentage of the control diameter at 100 mmHg. Values are means ± SE. The pressure-mediated vasoconstriction was normal in control+PPS kidneys. These data are presented in results, but the plot of these data was removed from Fig. 2 for clarity. *P < 0.05 vs. control diameter in same group. †P < 0.05 vs. control kidneys at the same perfusion pressure.
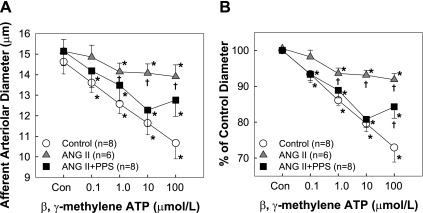
Afferent arteriolar response to a specific P2X1 agonist, β,γ-mATP.
Previous studies indicate a critical role for P2X1 receptors in mediating renal autoregulation (21, 23, 25). In this series, the afferent arteriolar concentration-response relationship to a nonhydrolysable P2X agonist, β,γ-mATP (Sigma), which is selective for P2X1 receptors and to a lesser degree, P2X3 receptors, was assessed in control, ANG II, and ANG II+PPS groups (n = 6–8). Arterioles were exposed to β,γ-mATP at concentrations of 0.1, 1.0, 10, and 100 μmol/l agonists at 5-min intervals, while the perfusion pressure was maintained at 100 mmHg. Arteriolar diameter was continuously monitored.
Plasma TGF-β1 Concentration Assays
Carotid arterial blood (3–4 ml) was collected in a tube containing 100 μl of 7.5% EDTA (Fisher Scientific, Fair Lawn, NJ) and centrifuged at 3,000 rpm at 4°C for 10 min. Plasma was aliquoted and stored at −80°C until assayed. Plasma samples were prepared for the measurement of total active TGF-β1 using a TGFβ1 Emax ImmunoAssay kit (Promega, Madison, WI) according to manufacturer's instructions.
Measurement of Urinary Albumin Excretion
For the evaluation of albumin excretion, rats were housed separately in metabolic cages that efficiently separate urine from food and feces. A 24-h urine collection was made on days 0, 6, and 12 of ANG II minipump implantation. Urinary albumin was measured by ELISA (Bethyl Laboratories, Montgomery, TX). Daily albumin excretion was calculated from the volume of urine collected from each rat over a 24-h period.
Kidney Histological Assessment
Kidneys were collected from control, ANG II, and ANG II+PPS groups for morphological evaluation. Briefly, the right kidney was flushed with physiological salt solution and then perfused with 10% formalin solution via the right renal artery. The kidney was cut longitudinally into three portions. The middle portion was immersed overnight in 10% formalin at 4°C and then transferred to 70% ethanol. The kidneys were prepared for paraffin embedding and were sectioned (3–4 μm) and placed on glass slides. Kidney sections were stained with hematoxylin and eosin, periodic acid-Schiff, picrosirius red, and immunolabeled for α-SMA, as described previously (45). The level of immunoreactivity for α-SMA in the afferent arteriole was scored as 0, none; 1, mild; 2, moderate; and 3, severe. Thirty randomly selected afferent arterioles were examined at ×400 magnification, and the mean score value was calculated in each sample. Sections were examined and scored by an observer who was blinded to the groups.
Statistical Analysis
All values are expressed as means ± SE. Within-group analysis was conducted using one-way analysis of variance for repeated measures combined with the Newman-Keuls multiple range test. Differences between groups, within each series, were determined using a Student-Newman-Keuls multiple range test. P values <0.05 were considered to indicate significant differences.
RESULTS
General Parameters
General physiological parameters are summarized in Table 1. All rats gained body weight during the 12-day treatment, but control rats gained more weight than the other two groups by day 12 (P < 0.05). ANG II (60 ng/min)-infused rats consumed less food by days 6 and 12 compared with day 0; however, water consumption and urine excretion were significantly increased in both ANG II-infused rats and ANG II-infused rats receiving PPS (100 mg·kg−1·day−1) treatment.
Table 1.
Effect of PPS on general parameters in ANG II-induced hypertensive rats
|
Day 0 (Baseline) |
Day 6 |
Day 12 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Control (n = 13) | ANG II (n = 12) | ANG II+PPS (n = 13) | Control (n = 13) | ANG II (n = 12) | ANG II+PPS (n = 13) | Control (n = 13) | ANG II (n = 12) | ANG II+PPS (n = 13) |
| Body wt, g | 253 ± 2 | 248 ± 4 | 249 ± 3 | 303 ± 2* | 295 ± 4* | 295 ± 5* | 342 ± 4* | 320 ± 5*† | 316 ± 5*† |
| Food intake, g/day | 23 ± 1 | 24 ± 1 | 20 ± 2 | 24 ± 1 | 20 ± 1* | 22 ± 2 | 25 ± 1 | 21 ± 1*† | 23 ± 1 |
| Water intake, ml/day | 33 ± 3 | 35 ± 3 | 40 ± 3 | 33 ± 4 | 43 ± 4*† | 49 ± 5*† | 38 ± 4* | 48 ± 4*† | 50 ± 6*† |
| Urine volume, ml/day | 15.5 ± 1.0 | 12.7 ± 1.0 | 15.7 ± 1.9 | 14.3 ± 1.3 | 25.0 ± 3.1*† | 33.2 ± 5.0* | 15.5 ± 1.3 | 31.9 ± 3.0*† | 36.5 ± 4.9*† |
Values are means ± SE. PPS, pentosan polysulfate.
P < 0.05 vs. day 0 in same group.
P < 0.05 vs. control during same periods.
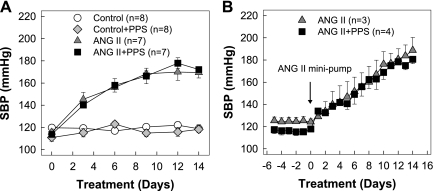
Effect of PPS Treatment on Systolic Blood Pressure
Blood pressure was monitored by tail cuff and telemetry and is depicted in Fig. 1, A and B, respectively. Systolic blood pressure started to increase after 3 days of ANG II infusion and reached a plateau of 168 ± 3 mmHg by day 9 in ANG II-infused rats (Fig. 1A). The magnitude and progress of blood pressure elevation, however, were unaffected by PPS treatment either in normotensive control or in ANG II-infused rats (Fig. 1A). Similar results were obtained when tail-cuff measurements were confirmed by telemetry (Fig. 1B).
Fig. 1.
Pentosan polysulfate (PPS) does not alter the progression of hypertension induced by chronic ANG II infusion. Systolic blood pressure (SBP) was measured by tail-cuff plethysmography (A) and telemetry (B) in control (circle), control+PPS (100 mg·kg−1·day−1, diamond), chronic ANG II infusion only (60 ng/min, triangles), or ANG II+PPS (squares) rats. PPS does not alter SBP in control or ANG II-infused rats. Values are means ± SE. Data presented from telemetry measurements reflect the average over a 24-h period.
Effect of PPS on Afferent Arteriolar Autoregulatory Behavior
The autoregulatory behavior of afferent arterioles was evaluated using the in vitro blood-perfused juxtamedullary nephron technique. As shown in Fig. 2A, basal arteriolar diameters were similar among all four groups and averaged 15.3 ± 1.5, 13.7 ± 0.7, 13.7 ± 0.6, and 15.7 ± 1.2 μm for control, control+PPS, ANG II, and ANG II+PPS groups, respectively. Arterioles from control kidneys exhibited normal autoregulatory behavior. After reducing perfusion pressure from 100 to 70 mmHg, the diameter increased by 21 ± 4% from 15.3 ± 1.5 to 18.4 ± 1.6 μm (P < 0.05). Increasing perfusion pressure reduced the diameter to 15.4 ± 1.5, 12.9 ± 1.5 and 11.4 ± 1.7 μm at perfusion pressure of 100, 130, and 160 mmHg, respectively (Fig. 2A), or 100 ± 3, 83 ± 4, and 72 ± 5% of the control diameter (Fig. 2B). PPS treatment alone did not change the autoregulatory profile in control rats. The diameter was 110 ± 3, 94 ± 2, 84 ± 1, and 74 ± 4% of control diameter at perfusion pressure of 65, 100, 130, and 160 mmHg, respectively (P < 0.05 vs. control). This autoregulatory response is nearly identical to the response from normal control kidneys. In contrast, the pressure-mediated vasoconstriction was significantly attenuated in ANG II-infused rats. Arteriolar diameter changed only slightly over the range of perfusion pressures studied (Fig. 2B), indicating marked impairment of autoregulatory behavior in ANG II-infused rats. In kidneys from PPS-treated ANG II hypertensive rats, however, autoregulatory behavior was well preserved, with pressure-mediated vasoconstriction being indistinguishable from responses observed in control rats. Arteriolar diameters averaged 112 ± 2, 95 ± 3, 86 ± 5, and 77 ± 5% of control at each perfusion pressure step (Fig. 2B).
Effect of PPS Treatment on Afferent Arteriolar Reactivity to ATP
Consistent with previous reports (20, 54), afferent arterioles from control kidneys exhibited concentration-dependent vasoconstriction to ATP (Fig. 3). Diameter decreased from 13.8 ± 0.6 to 12.8 ± 0.6, 11.3 ± 0.5, 10.1 ± 0.5, and 10.2 ± 0.5 μm (Fig. 3A) in response to increasing ATP concentrations. These changes reflect reductions in afferent diameter of 93 ± 1, 82 ± 2, 73 ± 2, and 74 ± 3% of the control diameter, respectively (Fig. 3B). In contrast, 2-wk ANG II infusion markedly blunted arteriolar vasoconstriction to ATP. The control diameter averaged 14.1 ± 0.5 μm (Fig. 3A) and declined to 99 ± 1, 91 ± 2, 91 ± 4, and 98 ± 5% of control in response to increasing ATP concentrations (Fig. 3B). Importantly, treatment with PPS preserved the ATP-mediated vasoconstriction in ANG II-infused rats. The diameter declined to 92 ± 1, 86 ± 2, 80 ± 1, and 79 ± 3% of control upon exposure to ATP over the same concentration range, and the response pattern was similar to responses from control rats. Since PPS treatment did not alter systolic blood pressure or autoregulatory behavior of afferent arterioles in normotensive control rats, this group was omitted from subsequent vascular reactivity studies.
Fig. 3.
Afferent arteriolar response to superfusion of ATP. A: changes in afferent arteriolar diameter in response to superfusion of ATP were assessed in control (circle), chronic ANG II infusion only (60 ng/min, triangle), or ANG II+PPS (100 mg·kg−1·day−1, square) rats. B: data are expressed as percentage of the control diameter at 100 mmHg. Values are means ± SE. *P < 0.05 vs. control diameter in same group. †P < 0.05 vs. control kidneys at the same perfusion pressure.
Effect of PPS on Afferent Arteriolar Reactivity to β,γ-mATP
The effect of PPS treatment on afferent arteriolar reactivity to P2X1 receptor stimulation in ANG II-infused rats is shown in Fig. 4. Similar to the responses observed with ATP, arterioles from control kidneys exhibited concentration-dependent vasoconstriction to β,γ-mATP. The maximal reduction in diameter was 73 ± 4% of control (Fig. 4B) from 14.6 ± 0.6 to 10.7 ± 0.8 μm in response to 100 μmol/l β,γ-mATP (Fig. 4A). In contrast, chronic ANG II infusion for 2-wk markedly attenuated β,γ-mATP-mediated vasoconstriction. PPS treatment, however, preserved afferent arteriolar constriction to β,γ-mATP in ANG II-infused rats (Fig. 4, A and B). Although the vasoconstriction to the highest concentration of β,γ-mATP (100 μmol/l) was not completely restored, the vasoconstriction to β,γ-mATP ranging from 0.1 to 10 μmol/l was essentially normal.
Fig. 4.
Afferent arteriolar response to superfusion of β,γ-methylene ATP (mATP). A: changes in afferent arteriolar diameter in response to superfusion of β,γ-mATP were assessed in control (circle), chronic ANG II infusion only (60 ng/min, triangle), or ANG II+PPS (100 mg·kg−1·day−1, square) rats. B: data are expressed as percentage of the control diameter at 100 mmHg. Values are means ± SE. *P < 0.05 vs. control diameter in same group. †P < 0.05 vs. control kidneys at the same perfusion pressure.
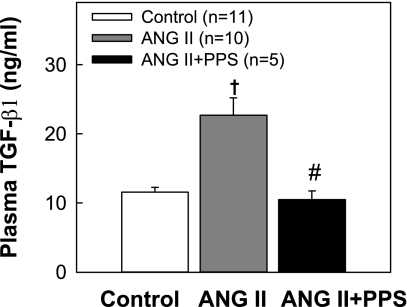
Effect of PPS on Plasma TGF-β1 Concentration
We also measured the concentration of total biologically active TGF-β1 in plasma to provide an index of inflammatory status in ANG II hypertensive rats with and without PPS treatment. As shown in Fig. 5, plasma TGF-β1 concentration increased significantly in rats receiving ANG II infusion alone (22.8 ± 2.5 vs. 11.6 ± 0.7 ng/ml in control, P < 0.05). In contrast, plasma samples taken from ANG II hypertensive rats also receiving PPS treatment exhibited TGF-β1 concentrations (10.5 ± 1.3 ng/ml) that were significantly lower than the ANG II alone group (P < 0.05) and indistinguishable from normotensive control rats (P > 0.05). These data suggest that PPS treatment suppresses inflammatory processes that include enhanced TGF-β1 expression in ANG II hypertensive rats and implicates increased TGF-β1 concentration in developing hypertensive renal microvascular injury.
Fig. 5.
Effect of PPS treatment on plasma transforming growth factor (TGF)-β1 concentration. The concentration of total biologically active TGF-β1 in plasma was measured by an ELISA. ANG II infusion significantly increased plasma TGF-β1 concentration (gray column). PPS (100 mg·kg−1·day−1, black column) treatment prevented elevation of plasma TGF-β1 concentration in ANG II rats, resulting in concentrations that were nearly identical to those of normotensive controls (white column). Values are expressed as means ± SE. †P < 0.05 vs. control rats. #P < 0.05 vs. ANG II rats.
Urinary Albumin Excretion
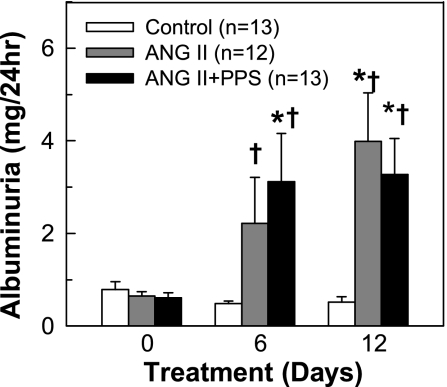
Indication of renal injury was assessed by measurement of 24-h urinary albumin excretion. As shown in Fig. 6, baseline albuminuria was similar in all groups. Chronic ANG II infusion led to modest increases in albuminuria from 0.7 ± 0.1 to 2.3 ± 1.1 mg/24 h by day 6 and 4.2 ± 1.0 mg/24 h by day 12 (P < 0.05 vs. control rats). PPS treatment slowed the progression of albumin excretion in ANG II rats. There was no further deterioration of albuminuria by day 12 (3.3 ± 0.8 mg/24 h) compared with day 6 (3.1 ± 1.0 mg/24 h).
Fig. 6.
Effect of PPS treatment on urinary albumin excretion. Urine (24 h) was collected on days 0, 6, and 12. Urinary albumin excretion increased progressively in ANG II-infused rats (60 ng/min, gray column) compared with control rats (white column). PPS (100 mg·kg−1·day−1, black column) treatment slowed the progression of albuminuria in ANG II rats, but it was still higher compared with control. Values are means ± SE. *P < 0.05 vs. day 0 in same group. †P < 0.05 vs. control rats in the same period of urine collection.
Effect of PPS on α-SMA Staining in Afferent Arterioles
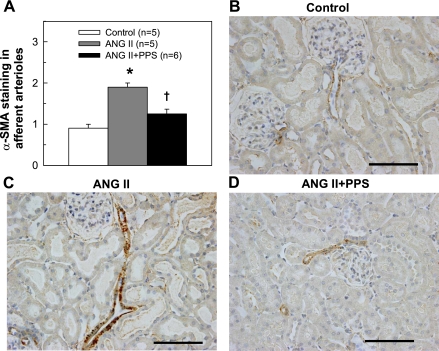
We did not find any significant histological changes in renal structure using conventional morphological assessment by light microscopy. Graded assessment of immunostaining for α-SMA, a marker of smooth muscle cell proliferation, in afferent arterioles revealed that the intensity and the areas staining positively for α-SMA (Fig. 7A) were more pronounced in ANG II-infused kidneys (1.9 ± 0.1) than in control kidneys (0.9 ± 0.1, P < 0.05). PPS treatment, however, significantly reduced α-SMA staining in ANG II-infused rats (1.3 ± 0.1, P < 0.05 vs. ANG II alone). Figure 7, B–D, illustrates the α-SMA staining (brown) in afferent arterioles from control, ANG II, and ANG II+PPS rats, respectively.
Fig. 7.
Score of α-smooth muscle actin (α-SMA) staining at afferent arterioles. Semiquantitative evaluation of α-SMA staining (A) revealed that immunostaining of α-SMA in afferent arterioles was significantly increased in ANG II-infused rats compared with that in control rats (P < 0.05), but markedly reduced by PPS (100 mg·kg−1·day−1) treatment to level similar to the control group (P > 0.05). Representative images (B–D) for immunostaining of α-SMA (brown) show strong arteriolar expression in ANG II-rats (C) in contrast to low expression in control (B) and ANG II+PPS (D) arterioles, respectively. Magnification ×40. Scale bars = 100 μm. Values are means ± SE. *P < 0.05 vs. control group. †P < 0.05 vs. ANG II group.
DISCUSSION
This study investigates the contribution of renal inflammation to the development of renal injury and impairment of afferent arteriolar autoregulation in ANG II-infused hypertensive rats. Earlier in vivo and in vitro studies showed that chronic ANG II-infusion led to impairment of renal autoregulation (24, 53, 54); however, the mechanism leading to this impairment is unclear. In the current study, we found that chronic ANG II-infused rats receiving a nonspecific anti-inflammatory agent, PPS, for 14 days exhibited normal afferent arteriolar autoregulatory behavior and normal arteriolar reactivity to both P2 and P2X1 receptor activation by ATP and β,γ-mATP, respectively, despite achieving an identical hypertension as ANG II-infused rats. These findings are strengthened by histological analysis, where PPS treatment significantly reduced cell proliferation on afferent arterioles, as assessed by immunostaining of α-SMA, in ANG II hypertensive rats. More importantly, PPS treatment prevented elevation of plasma TGF-β1 concentration in ANG II-treated rats. This suggests that inflammatory processes contribute to hypertension-induced renal microvascular dysfunction manifested as reduced responsiveness to P2X1 receptor activation and impaired pressure-mediated autoregulatory behavior.
Normal autoregulatory control is a critical renal microvascular function that minimizes the transmission of high blood pressure transients to the glomerulus, thus maintaining a stable renal blood flow and glomerular filtration rate and protecting the glomerulus from barotrauma. Growing evidence implicates a role for inflammatory factors in the development of cardiovascular disease, hypertension, and renal injury (14, 30, 46). We examined the effect of a nonspecific anti-inflammatory agent, PPS, on autoregulatory behavior of afferent arterioles in ANG II-infused hypertensive rats. Afferent arterioles from control rats showed normal autoregulatory behavior as demonstrated by robust pressure-mediated vasoconstriction, consistent with previous observations (24, 53, 54). In contrast, arterioles from rats receiving ANG II infusion for 2-wk exhibited impaired autoregulatory behavior. Increasing or decreasing perfusion pressure led to no significant changes in arteriolar diameter, indicating marked impairment of autoregulatory responsiveness. Importantly, ANG II hypertensive rats simultaneously receiving PPS exhibited normal autoregulatory behavior. Pressure-mediated afferent arteriolar responses in PPS-treated ANG II rats were similar to those of control rats despite enduring similar degrees of hypertension. These results suggest that inhibition of inflammatory cascades by PPS normalizes autoregulation in this ANG II-infused hypertensive model and indicates an important contribution of inflammatory factors in the development of renal microvascular injury manifested by impairment of renal autoregulation.
ATP is the endogenous ligand for P2 purinoceptor activation and is postulated as the major extracellular messenger molecule mediating afferent arteriolar autoregulatory function (18, 27). ATP functions via activation of two major P2 receptor families, P2X and P2Y. Members of both receptor families are present in renal vascular, glomerular, mesangial, and tubular epithelial cells (2, 8, 48, 51, 54). Previous studies demonstrate that P2X1 receptors are important in mediating renal autoregulatory behavior and regulating afferent arteriolar tone (3, 21–23, 28). β,γ-mATP is a useful agonist for differentiating P2X1 receptor activation from other P2X receptor isoforms such as P2X2 and P2X4 (29). β,γ-mATP is also resistant to degradation by ecto-nucleotidases, and its potential breakdown product, l-adenosine, is inactive at P1 receptors (29). Our experiments demonstrate that in addition to preserving normal autoregulatory behavior of afferent arterioles, PPS treatment also prevents blunting of afferent arteriolar vasoconstriction to P2X1 receptor activation in ANG II hypertensive rats. Arterioles from PPS-treated ANG II-infused rats show essentially normal vasoconstriction to both ATP and β,γ-mATP. Thus the preserved afferent arteriolar vasoconstriction to both ATP and β,γ-mATP with normalization of autoregulation by PPS treatment in this hypertensive animal model supports the theory that ATP plays an important role in regulating renal autoregulation. These studies suggest that inflammatory factors blunt afferent arteriolar reactivity to P2X1 receptor activation and hence impair renal autoregulation in ANG II-induced hypertensive kidneys. The mechanism responsible for the attenuation of P2X1 receptor activation in this ANG II hypertensive rat model, however, is unclear at present. The protein expression of P2X1 receptors examined by Western blotting was unchanged in preglomerular microvascular tissue in ANG II-treated rats, suggesting that attenuation of P2X1 receptor activation is not due to a downregulation of P2X1 receptor expression (54). We speculate that internalization of P2X1 receptors or disruption of lipid rafts, leading to redistribution of P2X1 receptors, might occur with hypertension and/or inflammation (6, 52) and that this disruption in P2X1 receptor localization may inhibit P2X1 receptor actions. Currently, this issue is under investigation but is beyond the scope of the present study.
In chronic kidney disease, inflammatory mediators are often elevated and the renin-angiotensin system is frequently activated (14, 30, 46). In addition to the pressor effect, ANG II also contributes significantly to the progression of vascular injury by stimulating cytokine activation and inflammatory mediators. This raises questions about whether high blood pressure induced by chronic ANG II infusion stimulates cytokine activation or ANG II directly stimulates the inflammatory cascade. Recent studies by Mori and colleagues (32, 33), using a chronic pressure servo-control technique in ANG II-infused or Dahl salt-sensitive models of hypertension, found that kidneys exposed to elevated arterial pressure developed more severe renal injury than kidneys exposed to normal renal arterial pressure. This observation suggests that chronic elevation of renal perfusion pressure specifically contributes to renal injury. Other studies show that mechanical stretch upregulates cytokine and adhesion molecule expression in mouse carotid arteries (43), indicating that hypertension may directly contribute to development of vascular injury. In the current study, we applied a nonspecific anti-inflammatory agent, PPS, to ANG II-infused hypertensive rats. The magnitude and progress of hypertensive development were similar in both ANG II and ANG II+PPS rats. However, impairment of autoregulation in ANG II-infused rats was entirely prevented by PPS, suggesting that hypertension may trigger inflammatory cascades, which promote diverse intracellular events leading to progressive renal microvascular injury. This is consistent with our previous observation that normal autoregulatory behavior of afferent arterioles is preserved simply by reducing systolic blood pressure with triple therapy (24). Furthermore, the observation that PPS treatment prevents plasma TGF-β1 concentration from rising, despite persistent ANG II-mediated hypertension, strongly supports the notion that inflammatory factors promote hypertension-induced renal injury in this model.
PPS is a semisynthetic GAG that is commonly used for treatment of patients with interstitial cystitis (38) and is also used in treating antithrombotic prophylaxis and inflammatory conditions (12, 31). However, little information is available with respect to the mechanism of action of PPS. The structural and chemical properties of PPS resemble GAG, which possesses a high negative charge density. Evidence indicates that this negative charge allows GAG to bind a variety of cytokines and growth factors including TGF-β (47), which may limit the expression or activity of cytokines and growth factors. The study by Ceol and colleagues (7) showed that administration of a modified heparin GAG preparation prevented overexpression of TGF-β1 mRNA in kidneys with diabetic nephropathy, suggesting that the renal-protective property of these agents might involve inhibition of the TGF-β cascade. This is also consistent with our current finding that elevation of plasma TGF-β1 concentration was significantly prevented by PPS treatment.
We also examined immunostaining of α-SMA intensity as a semiquantitative score to monitor vascular smooth muscle integrity in the afferent arterioles. We found that α-SMA staining in afferent arterioles was markedly increased in ANG II-infused rats, but this increase was significantly reduced by PPS treatment. This is consistent with earlier studies showing that PPS inhibited vascular smooth muscle cell proliferation and extracellular matrix formation (9, 39), probably through inhibition of protein kinase C activity (15). Overall, our functional findings combined with this evidence suggest that PPS acts through reductions of circulating TGF-β1 and of vascular smooth muscle cell proliferation and hence maintains the normal contractile phenotype in ANG II-infused hypertensive rats.
In the current study, PPS treatment for 2 wk did not show a significant reduction in albumin excretion, although it tended to slow the progression of albuminuria compared with ANG II alone-treated rats. These results indicate that glomerular injury still persists in PPS-treated ANG II hypertensive rats. However, a long-term treatment of PPS (4 wk) caused a significant reduction of albuminuria in nephrectomized hypertensive rats (5, 16). Additionally, it is known that under physiological conditions small amounts of albumin are filtered from glomeruli and reabsorbed in proximal tubular cells (42, 44). Therefore, the persistent albuminuria could reflect reduced proximal tubular reabsorption of albumin, which was not prevented by PPS treatment or a longer treatment with PPS is needed.
In conclusion, we showed that chronic ANG II infusion impairs afferent arteriolar autoregulation and blunts arteriolar vasoconstriction to ATP and β,γ-mATP. The impaired autoregulatory behavior of afferent arterioles and afferent arteriolar reactivity to P2X1 receptor activation are prevented by the nonspecific anti-inflammatory agent PPS. Furthermore, PPS treatment prevented elevation of plasma TGF-β1 concentration and afferent arteriolar smooth muscle cell proliferation in ANG II-infused rats. This study suggests that inflammatory processes contribute to hypertensive renal microvascular dysfunction manifested as blunted pressure-mediated vasoconstriction and reduced microvascular reactivity to P2X1 receptor activation in this hypertensive model. The results of this study provide compelling evidence that anti-inflammatory intervention with PPS confers significant renal protection against the decline of renal autoregulation and this is associated with improved reactivity to P2X1 receptor stimulation in ANG II-infused hypertensive rats.
GRANTS
This study is supported by National Institutes of Health Grants DK44628, HL074167, and HL098135 (to E. W. Inscho) and HL60653 (to J. S. Pollock) and a postdoctoral fellowship (0825465E) from the Greater Southeast Affiliate of the American Heart Association (to Z. Guan).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Janet L. Hobbs for the preparation of kidney histological samples and Dr. David M. Pollock and Hiram Ocasio for telemetry implantation.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey MA, Turner CM, Hus-Citharel A, Marchetti J, Imbert-Teboul M, Milner P, Burnstock G, Unwin RJ. P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiol 96: 79–90, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bobadilla NA, Tack I, Tapia E, Sanchez-Lozada LG, Santamaria J, Jimenez F, Striker LJ, Striker GE, Herrera-Acosta J. Pentosan polysulfate prevents glomerular hypertension and structural injury despite persisting hypertension in 5/6 nephrectomy rats. J Am Soc Nephrol 12: 2080–2087, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Callera GE, Montezano AC, Yogi A, Tostes RC, Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens 16: 90–104, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ceol M, Gambaro G, Sauer U, Baggio B, Anglani F, Forino M, Facchin S, Bordin L, Weigert C, Nerlich A, Schleicher ED. Glycosaminoglycan therapy prevents TGF-beta1 overexpression and pathologic changes in renal tissue of long-term diabetic rats. J Am Soc Nephrol 11: 2324–2336, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford AP, Townsend-Nicholson A, Burnstock G. Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol Renal Physiol 274: F799–F804, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Elliot SJ, Striker LJ, Connor E, Stetler-Stevenson W, McQuinn WC, Blagg CR, Striker GE. Pentosan polysulfate decreases proliferation and extracellular matrix deposition by vascular smooth muscle cells isolated from failed hemodialysis access grafts. Clin Nephrol 54: 121–127, 2000 [PubMed] [Google Scholar]
- 10.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II-salt hypertension. Hypertension 50: 1069–1076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin Arthritis Rheum 28: 211–267, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep 10: 464–469, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Herbert JM, Maffrand JP. Effect of pentosan polysulphate, standard heparin and related compounds on protein kinase C activity. Biochim Biophys Acta 1091: 432–441, 1991 [PubMed] [Google Scholar]
- 16.Herrera-Acosta J, Tapia E, Sanchez-Lozada LG, Franco M, Striker LJ, Striker GE, Rodriguez IB. Restoration of glomerular haemodynamics and renal injury independent of arterial hypertension in rats with subtotal renal ablation. J Hypertens Suppl 20: S29–S35, 2002 [PubMed] [Google Scholar]
- 17.Imig JD, Passmore JC, Anderson GL, Jimenez AE. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med 121: 608–613, 1993 [PubMed] [Google Scholar]
- 18.Inscho EW. Mysteries of renal autoregulation. Hypertension 53: 299–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inscho EW, Carmines PK, Cook AK, Navar LG. Afferent arteriolar responsiveness to altered perfusion pressure in renal hypertension. Hypertension 15: 748–752, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Inscho EW, Carmines PK, Navar LG. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension 17: 1033–1037, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1077–F1085, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol 10, Suppl 11: S178–S183, 1999 [PubMed] [Google Scholar]
- 25.Inscho EW, Ohishi K, Navar LG. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. Am J Physiol Renal Fluid Electrolyte Physiol 263: F886–F893, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertension 30: 975–983, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Komlosi P, Fintha A, Bell PD. Renal cell-to-cell communication via extracellular ATP. Physiology (Bethesda) 20: 86–90, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286: F1054–F1058, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lambrecht G. Agonists and antagonists acting at P2X receptors: selectivity profiles and functional implications. Naunyn Schmiedebergs Arch Pharmacol 362: 340–350, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Luft FC. Angiotensin, inflammation, hypertension, and cardiovascular disease. Curr Hypertens Rep 3: 61–67, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Maffrand JP, Herbert JM, Bernat A, Defreyn G, Delebassee D, Savi P, Pinot JJ, Sampol J. Experimental and clinical pharmacology of pentosan polysulfate. Semin Thromb Hemost 17, Suppl 2: 186–198, 1991 [PubMed] [Google Scholar]
- 32.Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 19: 1472–1482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama A, Jackson KE, Majid DS, Rahman M, Navar LG. Renal interstitial fluid ATP responses to arterial pressure and tubuloglomerular feedback activation during calcium channel blockade. Am J Physiol Heart Circ Physiol 290: H772–H777, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656–662, 2000 [DOI] [PubMed] [Google Scholar]
- 37.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosanpolysulfate. J Urol 138: 513–516, 1987 [DOI] [PubMed] [Google Scholar]
- 39.Paul R, Herbert JM, Maffrand JP, Lansen J, Modat G, Pereillo JM, Gordon JL. Inhibition of vascular smooth muscle cell proliferation in culture by pentosan polysulphate and related compounds. Thromb Res 46: 793–801, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Ploth DW, Roy RN, Huang WC, Navar LG. Impaired renal blood flow and cortical pressure autoregulation in contralateral kidneys of Goldblatt hypertensive rats. Hypertension 3: 67–74, 1981 [DOI] [PubMed] [Google Scholar]
- 42.Pollock CA, Poronnik P. Albumin transport and processing by the proximal tubule: physiology and pathophysiology. Curr Opin Nephrol Hypertens 16: 359–364, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Riou S, Mees B, Esposito B, Merval R, Vilar J, Stengel D, Ninio E, van Haperen R, de Crom R, Tedgui A, Lehoux S. High pressure promotes monocyte adhesion to the vascular wall. Circ Res 100: 1226–1233, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 112: 375–384, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Schamhart DH, Kurth KH. Proteoglycans and glycosaminoglycans in tumor growth and migration: first experience with tumors of bladder and prostate origin. World J Urol 12: 55–61, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Striker GE, Lupia E, Elliot S, Zheng F, McQuinn C, Blagg C, Selim S, Vilar J, Striker LJ. Glomerulosclerosis, arteriosclerosis, and vascular graft stenosis: treatment with oral heparinoids. Kidney Int Suppl 63: S120–S123, 1997 [PubMed] [Google Scholar]
- 51.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem 280: 30705–30711, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension 46: 562–568, 2005 [DOI] [PubMed] [Google Scholar]