Abstract
The ultrasensitive energy sensor AMP-activated protein kinase (AMPK) orchestrates the regulation of energy-generating and energy-consuming pathways. AMPK is highly expressed in the kidney where it is reported to be involved in a variety of physiological and pathological processes including ion transport, podocyte function, and diabetic renal hypertrophy. Sodium transport is the major energy-consuming process in the kidney, and AMPK has been proposed to contribute to the coupling of ion transport with cellular energy metabolism. Specifically, AMPK has been identified as a regulator of several ion transporters of significance in renal physiology, including the cystic fibrosis transmembrane conductance regulator (CFTR), the epithelial sodium channel (ENaC), the Na+-K+-2Cl− cotransporter (NKCC), and the vacuolar H+-ATPase (V-ATPase). Identified regulators of AMPK in the kidney include dietary salt, diabetes, adiponectin, and ischemia. Activation of AMPK in response to adiponectin is described in podocytes, where it reduces albuminuria, and in tubular cells, where it reduces glycogen accumulation. Reduced AMPK activity in the diabetic kidney is associated with renal accumulation of triglyceride and glycogen and the pathogenesis of diabetic renal hypertrophy. Acute renal ischemia causes a rapid and powerful activation of AMPK, but the functional significance of this observation remains unclear. Despite the recent advances, there remain significant gaps in the present understanding of both the upstream regulating pathways and the downstream substrates for AMPK in the kidney. A more complete understanding of the AMPK pathway in the kidney offers potential for improved therapies for several renal diseases including diabetic nephropathy, polycystic kidney disease, and ischemia-reperfusion injury.
Keywords: CFTR, ENaC, NKCC, adiponectin
amp-activated protein kinase (AMPK) is a ubiquitously expressed heterotrimeric kinase that acts as an ultrasensitive cellular energy sensor (119). During energy stress as the level of ATP begins to fall, there is a marked increase in the cellular concentrations of AMP (45). This increase in AMP leads to activation of AMPK via multiple mechanisms (43). Once activated, AMPK acts to restore energy homeostasis by phosphorylating multiple substrates that act both to stimulate energy production and minimize energy consumption. Over the past decade, the biology and biochemistry of the AMPK pathway have been intensively studied in various organs such as the liver, skeletal muscle, and heart. Although AMPK is abundantly expressed in the kidney (29, 118), an understanding of its role in renal physiology and disease is less developed than in other organs. In recent years, however, interest regarding AMPK in the kidney has intensified, with studies describing roles for AMPK in multiple aspects of renal physiology and disease including ion transport (16), podocyte function (113), renal hypertrophy (69), ischemia (95), inflammation (101), diabetes (15, 36, 69), and polycystic kidney disease (122). AMPK is also potentially an important regulator energy metabolism in the kidney. This review gives an overview of AMPK biology and summarizes current knowledge of the functions and regulation of AMPK in the kidney.
Structure and Biology of AMPK
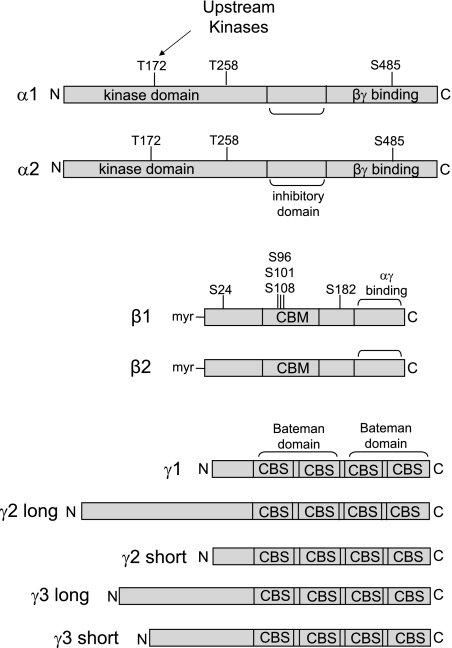
AMPK is a heterotrimer consisting of a catalytic α-subunit and regulatory β- and γ-subunits (98, 118). Each of these subunits exists as multiple isoforms (α1, α2, β1, β2, γ1, γ2, γ3), thereby giving rise to 12 possible heterotrimer combinations (118), with splice variants adding further to the possible diversity (68, 82) (Fig. 1). That the genes encoding the α-, β-, and γ-subunits are each highly conserved in all eukaryotic species (123) is indicative of the fundamental role AMPK plays in cell biology. There do not appear to be major differences in the substrate specificities between the α1- and α2-catalytic subunits, although in some cell types the α2-catalytic subunit has a preferential nuclear localization (107). The β-subunits (β1, β2) have conserved central and C-terminal domains and a more variable N terminus. The C terminus of the β-subunit is a subunit-binding domain that is essential for the formation of the αβγ AMPK heterotrimer (52) (Fig. 1). In the middle region of the β-subunit is a carbohydrate-binding module (CBM) that binds glycogen and contributes to the regulation of AMPK activity (49, 103). The activation of AMPK by AMP is explained by its binding to the γ-subunit (1). The C termini of the γ-subunits consist of four tandem cystathione-β-synthase (CBS) domains (111). Two CBS domains combine to form to a nucleotide-binding structure termed a Bateman domain (111) (Fig. 1). Structural analysis has demonstrated that each γ-subunit actually has 3 AMP-binding sites (135). Two of the sites can reversibly bind either AMP or Mg2+-ATP and account for the regulation of the kinase, whereas the third site binds AMP irreversibly (135). The major difference between the three γ-subunit isoforms (γ1, γ2, γ3) is the length of their N-terminal extensions, the functions of which remain poorly defined (123).
Fig. 1.
Structural features of AMP-activated protein kinase (AMPK) subunit isoforms. Known phosphorylation sites are shown. The midsection of the β-subunit contains a carbohydrate binding module (CBM). Regulation of AMPK by AMP is explained by the binding of AMP to the Bateman domains of the γ-subunit. Each Bateman domain consists of 2 cystathione-β-synthase (CBS) domains. Various phosphorylated residues in the α- and β-subunits are shown, including the Thr-172 site, which must be phosphorylated by upstream kinases for AMPK to have catalytic activity.
The activity of AMPK is exquisitely sensitive to cellular energy stress, which is detected as a rising concentration of AMP and an increase in the AMP/ATP ratio. As AMP levels rise, resulting in AMP binding to the Bateman domain of the regulatory γ-subunit (111), AMPK activity is increased by three mechanisms (56). First, there is a direct allosteric effect. Second, AMP binding allows the catalytic loop of the α-subunit to be phosphorylated at residue Thr172 by one of at least three potential upstream AMPK kinases (131, 132, 136). Phosphorylation at αThr172 is essential for activation of AMPK and increases its activity by ∼100-fold (47). Moreover, there is a ∼1,000-fold activation of AMPK by the combined effects of upstream kinases and saturating concentrations of AMP (121). The third mechanism is that AMP binding inhibits dephosphorylation of αThr172 by protein phosphatases, such as protein phosphatase 2C-α (109). In fact, recent evidence suggests that inhibition of dephosphorylation by phosphatases may be the major mechanism by which AMP binding causes increased phosphorylation of αThr172 (109, 121). These three effects of AMP make the system very sensitive to small increases in AMP concentration. All three effects are also antagonized by high concentrations of ATP. Because all eukaryotic cells express very active adenylate kinase, which maintains the reaction (2ADP ↔ AMP + ATP) close to equilibrium at all times, the cellular AMP:ATP ratio varies approximately as the square of the ADP:ATP ratio (45), making it a very sensitive indicator of cellular energy status. Of note, the half-maximal concentration of AMP required to activate AMPK is <2 μM (121), consistent with idea that AMPK has tonic cellular activity in the absence of metabolic stress. Indeed, our recent data suggest that tonic phosphorylation of CFTR by AMPK in bronchial epithelial cells plays an important role in preventing channel activation in the absence of PKA stimulation (59).
The upstream AMPK kinase LKB1 was first identified as a tumor suppressor protein mutated in patients with Peutz-Jeghers syndrome, which is characterized by the development of benign hamartomatous polyps in the colon (53). LKB1 exists as a complex with two accessory subunits, termed STRAD and MO25 (46, 132). The LKB1 complex itself appears not to be activated by AMP, with the effect of the nucleotide making AMPK a better substrate for LKB1, while at the same time making it a worse substrate for protein phosphatases that dephosphorylate αThr172 (109). The calcium/calmodulin-dependent kinase kinases (CaMKKs) are also capable of activating AMPK by phosphorylation of αThr172 (50, 131). The in vivo evidence for regulation of AMPK by CaMKKs is strongest for the β-isoform (CaMKKβ) (50). Regulation of AMPK by CaMKKβ appears to be independent of the AMP/ATP ratio and is primarily regulated in response to changes in intracellular calcium concentration (50, 131). The tissue distribution of CaMKK appears more restricted than LKB1. CaMKKβ is expressed primarily in the brain but is also expressed in testis, thymus, and T cells (4). There is also evidence that in some cell types transforming growth factor (TGF)-β-activated kinase-1 (TAK1) can function as an upstream AMPK kinase (136).
In addition to being regulated at a single-cell level by the level of energy stress, AMPK can also be regulated by extracellular signals such as hormones and cytokines. For example, AMPK is activated in endothelial cells by ligands such as thrombin (116) and bradykinin (96) via a pathway that signals through the Ca2+/CaMKKβ pathway. Of particular interest is the regulation of AMPK by adipokines such as adiponectin (138) and leptin (93). The effect of these hormones on AMPK in diverse sites such as skeletal muscle and the hypothalamus is important in the regulation of whole body energy homeostasis (56). For example, inhibition of AMPK by leptin in the hypothalamus leads to a reduction in food intake (92), whereas activation of AMPK by leptin in skeletal muscle causes an increase in glucose uptake and fatty acid oxidation (93).
The best characterized pathways and substrates that are regulated by AMPK are those involved in energy metabolism. Detailed reviews of the substrates and pathways regulated by AMPK have been published elsewhere (44, 56, 58, 123). In general, AMPK acts to restore energy balance by stimulating pathways that lead to ATP synthesis and inhibiting pathways that lead to ATP consumption. For example, AMPK phosphorylates and inhibits both isoforms of acetyl-CoA carboxylase (ACC1 and ACC2), which is the rate-limiting enzyme in fatty acid synthesis (26). Phosphorylation of ACC by AMPK also stimulates fatty acid oxidation by increasing mitochondrial fatty acid uptake (26). AMPK also negatively regulates 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, which is the rate-limiting enzyme for cholesterol synthesis (21). Among the other diverse biological processes shown to be regulated by AMPK are cellular nutrient (glucose and fatty acid) uptake (51, 88), protein synthesis (12, 25, 48, 65), gene transcription (139), inflammation (101), ion transport (38), autophagy (76), cellular polarity (11), and nitric oxide synthesis (19).
Expression of AMPK in the Kidney
The pattern of AMPK subunit expression in the mammalian kidney has been described in the rat (15, 29). In the kidney, the α1-subunit is the predominant catalytic isoform, although the α2-subunit is also detectable (29). In the rat kidney, β2 is predominant (15, 29), although we have found β1 to be predominant in the mouse kidney (Power DA, unpublished observations). Both the γ1- and γ2-subunits are expressed at similar levels in the kidney, with the γ2-subunit appearing to exist as the short form (29). As would be expected, the muscle-specific γ3-isoform has never been detected in kidney tissue. While AMPK α1 is ubiquitously expressed throughout the kidney, immunostaining with an antibody specific for activated AMPK that is phosphorylated at αThr172 shows strongest staining at the apical surface of cortical thick ascending limbs and the macula densa (29, 95). Staining for αThr172 AMPK has also been detected on the basolateral surface of collecting ducts (29). A limitation of current knowledge of AMPK expression and its activity in the kidney is a lack of data regarding differences in expression between different cell populations within the kidney. This problem relates to the fact that most studies of AMPK expression have focused on expression in whole lysates or single cell types. Further characterizations with immunolocalization studies are required to determine expression differences that might exist in different cell populations, which could be important because there are known marked differences in the metabolic profile of different cell types in the kidney. For example, cells in the inner medulla, such as those of the thin descending and ascending limbs of the loop of Henle and the medullary collecting duct, have few mitochondria and depend predominantly on glycolytic metabolism (89). In contrast, tubular cells in the cortex, such as those in proximal tubules and the thick ascending limb of the loop of Henle, are rich in mitochondria and depend predominantly on oxidative metabolism, with fatty acids, ketone bodies, and lactate being the preferred metabolic substrates (7).
There is limited information concerning the expression of the upstream AMPK kinases LKB1 and CaMKKβ in the kidney. In general, LKB1 is widely expressed in most tissues, including significant expression in the kidney (24). The LKB1-associated proteins STRADα and MO25α are also expressed in the kidney (24). LKB1 exists as both long (LKB1L) and short (LKB1S) splice variants, with the more widely expressed LKB1L (50 kDa) protein being the isoform expressed in the kidney (24). Regarding the expression of CaMKKβ, a tissue distribution study using Western blot analysis of crude lysates from different tissues found that the highest level of expression was observed in the central nervous system and that CaMKKβ protein was not detected by this method in the kidney (60). Although this study was unable to demonstrate CaMKKβ expression in the kidney, it might be that the method of detection was not sufficient to detect low levels of CaMKKβ. Further study is required to determine the roles and distribution of LKB1, CaMKKβ, and other possible AMPK kinases in the kidney.
Regulation of Sodium and Ion Transport by AMPK
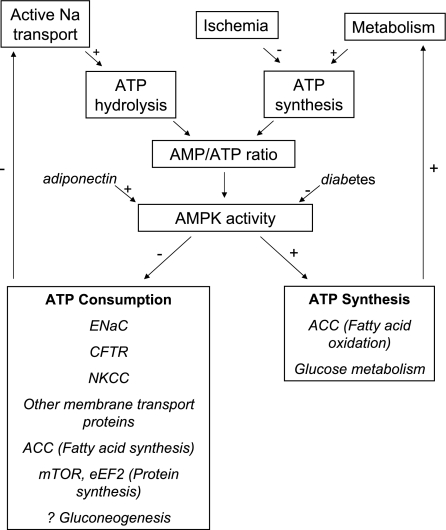
While the kidney constitutes <1% of body mass, renal oxygen consumption accounts for 7% of total body oxygen consumption, and renal blood flow constitutes 20–25% of total cardiac output (72, 80). The explanation for the kidney's large energy consumption is active tubular reabsorption of a large quantity of filtered sodium and other ions. In the 1960s, Whittam and colleagues (130) demonstrated that tubular sodium transport was closely coupled with tubular respiration, but the mechanisms linking ion transport with respiration remain incompletely defined. In recent years, AMPK has been identified as a regulator of various ion transport proteins (38). This has stimulated more general interest in the broader hypothesis that energy sensing by AMPK may be a physiologically relevant mechanism by which renal tubular cells maintain tight coupling between energy metabolism and tubular transport (16) (Fig. 2).
Fig. 2.
Proposed role for AMPK in the kidney in coupling catabolic pathways requiring ATP hydrolysis (primarily sodium transport) with metabolic pathways leading to ATP synthesis (primarily fatty acid and glucose oxidation). +, Activating pathway; −, inhibitory pathway.
CFTR.
The first ion transport protein to be identified as a substrate of AMPK was the CFTR Cl− channel (42). While CFTR is most well known for its functions in respiratory and gastrointestinal epithelia, transcripts for its expression are also detected throughout the nephron and it participates in Cl− secretion in the distal tubule and the inner medullary collecting duct (117). In addition, fluid secretion by CFTR appears to have an important role in the pathogenesis of cyst development in autosomal dominant polycystic kidney disease (73). The relationship between AMPK and CFTR was initially identified by a yeast two-hybrid screen, which found that the AMPK α1-subunit interacted with the C-terminal cytoplasmic tail of CFTR (42). AMPK has been shown to inhibit CFTR channel activity in both Xenopus laevis oocytes and polarized bronchial and colonic epithelial cells (40–42, 127). The mechanism involves an inhibition of the CFTR channel open probability. AMPK phosphorylates CFTR predominantly at Ser768 in the CFTR regulatory (R) domain (59, 62). We and others have shown that phosphorylation of the R domain by AMPK inhibits activation of CFTR by PKA, and tonic AMPK activity may prevent CFTR activation in the absence of cAMP agonists (59). In respiratory epithelia, AMPK also interacts with nucleoside diphosphate kinase A (NDPK-A) (124), which has been proposed to bind to CFTR. AMPK and NDPK-A may have a cooperative role in the regulation of CFTR, although the detailed mechanisms are currently under investigation. A recent study in AMPK α1 −/− mice confirmed that the effect of AMPK on CFTR is physiologically relevant in vivo (63). In this study, CFTR activity was reduced by the AMPK activator phenformin and increased by the AMPK inhibitor compound C, but these effects were absent in the AMPK α1 −/− mice. Moreover, CFTR-dependent Cl− secretion was enhanced in the colon of AMPK α1 −/− mice, suggesting that tonic AMPK activity in the wild-type animals inhibits Cl− secretion in the absence of agonists (63). As yet, there are no studies specifically examining the physiological role of AMPK in the regulation of CFTR in the kidney. However, a recent study employing in vitro and in vivo mouse models suggests that AMPK activation using the drug metformin may be a useful therapeutic strategy to reduce cystogenesis in autosomal dominant polycystic kidney disease, through inhibition of CFTR-dependent fluid secretion into cysts and mammalian target of rapamycin (mTOR)-dependent growth and proliferation of cells lining the cysts (122).
Epithelial Na+ channel.
Na+ absorption by the epithelial Na+ channel (ENaC) takes place in the kidney, airways, and gastrointestinal tract. In the kidney, the primary location of Na+ reabsorption by ENaC is the connecting tubule and the cortical collecting duct (37). Na+ reabsorption by ENaC is a highly ATP-consuming process that uses the electrochemical driving force for luminal uptake of Na+ into the cell. Sodium ions that enter the cell through apical ENaC are pumped out on the basolateral side by the action of the Na+-K+-ATPase (104). Mechanisms regulating ENaC activity include synthesis, intracellular trafficking, membrane insertion and retrieval, proteolytic cleavage, and gating (9). The E3 ubiquitin-protein ligase Nedd4–2 is emerging as an important locus for the regulation of ENaC activity in response to various hormonal mediators and signaling pathways. Nedd4–2 interacts with the C terminus of ENaC to promote its internalization and degradation (114).
We have demonstrated that activation of AMPK inhibits ENaC activity in both the X. laevis oocyte expression system and in polarized mouse cortical collecting duct (mpkCCDc14) cells (16). In addition, the AMPK activators phenformin and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) inhibit ENaC transport in lung epithelial cells (133). In contrast to CFTR, however, AMPK does not appear to directly phosphorylate ENaC (16). The mechanism for AMPK inhibition of ENaC involves a decrease in the number of active channels at the plasma membrane (16). The inhibitory effect of AMPK on ENaC is dependent on an interaction between the ENaC β-subunit and Nedd4–2 (10). AMPK is able to phosphorylate Nedd4–2 both in vitro and in vivo, and activation of AMPK enhances the interaction between the ENaC β-subunit C terminus and Nedd4–2 (10). This presumably leads to increased Nedd4–2-dependent ENaC retrieval from the plasma membrane (10). It has also been reported that AMPK may inhibit the gating of ENaC in H441 lung epithelial cells through a phosphoinositide-dependent mechanism (81). In this way, activation of AMPK may limit cellular Na+ transport by ENaC under conditions of metabolic stress (Fig. 2).
A recent study has confirmed the role of AMPK in the regulation of ENaC in vivo (2). AMPK α1 −/− mice have enhanced expression and activity of ENaC in the kidney, colon, and airway epithelium (2). Amiloride-sensitive fractional Na+ excretion was enhanced in AMPK α1 −/− animals (2). The AMPKα1 −/− mice also had a larger rectal potential difference and enhanced amiloride-sensitive transport in the trachea (2). Also consistent with increased ENaC activity in the AMPK α1 −/− mice was increased urinary potassium excretion. Surprisingly, however, despite the increased renal ENaC expression, the AMPK α1 −/− mice were observed to have reduced arterial blood pressure (2), indicating that AMPK appears to have other effects on either renal or vascular function that override the effect that increased renal ENaC expression would be predicted to have on blood pressure.
Na+-K+-Cl− cotransporters.
There are two distinct Na+-K+-Cl− cotransporter (NKCC) isoforms found in epithelia, designated NKCC1 and NKCC2. NKCC1 is widely expressed and plays fundamental roles in maintaining cell volume, mediating transepithelial ion fluxes in secretory tissues, and setting the appropriate intracellular ion concentrations for excitable cells to work (33). In contrast, NKCC2 expression is restricted to the thick ascending limb and the macula densa of the kidney (33). In recent years, phosphorylation of NKCC1 and NKCC2 has been identified as an important regulatory mechanism, although details of the specific phosphorylation sites and the regulating kinases are still being elucidated (33). Other kinases that have been identified as important in regulating NKCC1 and NKCC2 include WNK1, WNK3, and the Ste-20-related kinases OSR1 and SPAK (5). On the basis of the finding that activated AMPK was detectable on the apical membrane of the thick ascending limb and macula densa, we hypothesized that AMPK might regulate NKCC2 (29). Subsequently, we found that AMPK coimmunoprecipitated with the N-terminal cytoplasmic domain of NKCC2 and phosphorylated the N terminus of NKCC2 at position Ser126 in vitro (30). Furthermore, activation of AMPK in the macula densa cell line (MMDD1) resulted in an increase in NKCC2- Ser126 phosphorylation, suggesting that AMPK may phosphorylate NKCC2 in vivo (30). In addition, we recently found that AMPK is activated in MMDD1 cells in response to low salt, independently of osmolality (22). This effect of low salt in MMDD1 was not inhibited by bumetanide (22). Low salt-induced activation of AMPK in MMDD1 cells increased phosphorylation of both NKCC-Ser126 and ACC-Ser79 (22). When NKCC2-Ser126 was mutated to an alanine and expressed in X. laevis oocytes under isotonic conditions, cotransporter activity was markedly reduced, whereas under hypertonic conditions there was no change (30). Surprisingly, however, AMPK was not found to regulate NKCC2 transport in the X. laevis oocyte model (30). Possible explanations for this finding include endogenous AMPK expression by X. laevis oocytes causing constitutive phosphorylation, another unknown kinase that might target NKCC2-Ser126, or phosphorylation of NKCC2 by AMPK might require another protein to produce a modification of cotransporter activity.
Na+-K+-ATPase.
The basolateral Na+-K+-ATPase is the major active transport mechanism responsible for reabsorption of Na+ throughout the nephron. There is presently no direct evidence, however, for a role of AMPK in the regulation of Na+-K+-ATPase in the kidney. In contrast, there is evidence in H441 lung cells of regulation of Na+-K+-ATPase by AMPK. Specifically, Woollhead et al. (133, 134) found that AMPK activation with phenformin and AICAR inhibited ouabain-sensitive transepithelial sodium transport. Furthermore, Vadasz et al. (126) reported in alveolar epithelial cells that elevated Pco2 activated AMPK through CaMKKβ, leading to activation of PKCζ, thus promoting Na+-K+-ATPase endocytosis and reduced salt and water reabsorption. A preliminary report by Seo-Mayer and colleagues (112), however, suggested that preactivation of AMPK through metformin treatment attenuated the downregulation of basolateral membrane Na+-K+-ATPase expression following ischemia in Madin-Darby canine kidney (MDCK) cells. These results suggest that prior AMPK activation may enhance ischemic preconditioning in epithelial tissues and highlight that there may be differential effects of AMPK in epithelia depending on the setting and time course of activation (112).
Other ion transport proteins.
In addition to what is known about ion channels in the kidney, regulation of ion channels by AMPK has also been proposed in the heart for voltage-gated Na+ channels (78) and ATP-sensitive K+ (KATP) channels (120). Specifically, Light et al. (78) found that when the human cardiac Na+ channel hH1 and constitutively active AMPK were coexpressed in mammalian myocytes, the action potential duration was significantly prolonged. Sukhodub et al. (120) also described a role for AMPK regulation of KATP channels in a model of cardiac ischemic preconditioning. This study found that hearts overexpressing a dominant negative form of the α2-subunit of AMPK were more susceptible to ischemic injury. The cytoprotective effect of AMPK was not related to the effects of ischemic preconditioning on mitochondrial membrane potential but instead to its role in preconditioning-induced shortening of the sarcolemmal action potential via KATP channels (120). A recent study also found that the calcium-activated K+ channel KCa3.1, which is expressed at the basolateral membrane in a variety of epithelia, interacts with the AMPK γ1-subunit and is inhibited by AMPK in lung epithelial cells (61). In addition, we have shown recently that AMPK activation in the kidney-like epithelium of the epididymis inhibits PKA-induced apical membrane accumulation of the vacuolar H+-ATPase (V-ATPase) (39). Both kinases appear to directly phosphorylate one of the subunits of the V-ATPase in vitro and in cells. Very recent data also suggest a similar mode of V-ATPase regulation by AMPK in kidney collecting duct intercalated cells (34, 100). Another preliminary report suggests that the creatine transporter (SLC6A8), which is expressed at the apical membrane of proximal tubule cells and mediates reclamation of filtered creatine in the kidney (32), is inhibited by AMPK activation in mouse S3 proximal tubule cells (34) via effects on transporter plasma membrane expression (74). Finally, a preliminary report has demonstrated that the KCNQ1 potassium channel expressed in collecting duct principal cells is inhibited by AMPK, like ENaC, via a Nedd4–2-dependent mechanism (3).
Regulation of AMPK in the Kidney
Salt and water.
Regulation of renal AMPK activity in response to variation of salt intake and subsequent regulation of renal Na+ transport has been hypothesized to be a possible novel mechanism of Na+ homeostasis (29). Fraser et al. (29) have reported that AMPK activity was increased by 25% in rats receiving a high-salt diet, and this was confirmed by Western blotting for αThr172 phosphorylation. In addition, both low- and high-salt media activated AMPK in the macula densa cell line MMDD1 (22, 29). Activation of AMPK in MMDD1 cells by low salt occurred in the presence of either low Na+ or low Cl− and was unaffected by inhibition of NKCC2 with bumetanide (22). In addition, the antidiuretic hormone vasopressin has recently been reported to cause dephosphorylation of AMPK in MDCK clone 7 (MDCK-C7) cells (97). Since vasopressin is released from the posterior pituitary in response to water deprivation, this suggests another possible mechanism by which AMPK might contribute to fluid and electrolyte homeostasis. Taken together, the observations that AMPK activity can be regulated by both dietary salt intake and extracellular NaCl concentration and that AMPK is a regulator of various ion transport proteins suggest a physiological role for AMPK in the regulation of Na+ and electrolyte homeostasis and its relationship to energy metabolism.
Adiponectin.
An interesting study by Sharma et al. (113) identified a role for adiponectin and AMPK in the regulation of podocyte function and the pathogenesis of albuminuria. Adiponectin is a hormone produced by adipocytes, and the serum level is reduced in obesity. A low serum level of adiponectin in obesity is associated with albuminuria (113). Furthermore, adiponectin knockout mice exhibit increased albuminuria and fusion of podocyte foot processes (113). In cultured podocytes, adiponectin administration increased activity of AMPK, and both adiponectin and AMPK activation reduced podocyte permeability to albumin and podocyte dysfunction, as evidenced by zona occludens-1 translocation to the membrane (113). These effects seemed to be caused by reduction of oxidative stress, as adiponectin and AMPK activation both reduced protein levels of the NADPH oxidase 4 (Nox4) in podocytes.
Cammisotto et al. (14) further studied the role of AMPK and adiponectin in glomerular function. By electron microscopy and immunogold staining, the adiponectin receptor ADIPOR1 and the catalytic AMPK subunits α1 and α2 were localized in glomeruli at the plasma membrane of endothelial, mesangial, and podocyte cells, as well as on Bowman's capsule epithelial cells (14). Incubation of freshly isolated rat glomeruli with either adiponectin or AICAR led to the activation by phosphorylation of catalytic AMPK (14). This could suggest that activation of glomerular AMPK by adiponectin might play an important role in the control of oxidative stress and cell survival within the glomerulus.
A role for adiponectin and AMPK has also been identified by Cammisotto et al. (15) in the regulation of glycogen synthase in the distal tubule and thick ascending limb. This might be important in diabetic nephropathy, which is characterized by glycogen accumulation in distal tubular and thick ascending limb cells that eventually leads to apoptosis (8). ADIPOR1, catalytic AMPK subunits α1 and α2, and the regulatory glycogen-binding AMPK subunit β2 were detected in this study by Western blots of isolated distal tubules from the rat (15). While expression levels of ADIPOR1, AMPK α1, AMPK α2, and AMPK β2 were all increased in streptozotocin-treated diabetic rats, phosphorylated active AMPK (αThr172) levels were strongly decreased (15). In addition, immunohistochemistry revealed the presence of ADIPOR1 on the luminal portion of distal tubules and thick ascending limb cells. The AMPK subunits α1, α2, and β2 were also found in the same cells (15). In isolated distal tubules, adiponectin, acting through luminal ADIPOR1, was found to activate AMPK, which then caused inhibition of glycogen synthase, an effect that was inhibited with diabetes (15). Thus reduced AMPK activation with hyperglycemia may explain, at least in part, the accumulation of large tubular glycogen deposits in diabetic nephropathy.
Diabetes.
It has been reported that AMPK activity is reduced in the diabetic kidney (15, 36, 69). The mechanism for this observation is unclear, but Guo et al. (36) have correlated it in streptozotocin-induced type 1 diabetes to reduced adiponectin levels. The reduced AMPK activity in the diabetic kidney does not appear to be related to altered AMP or ATP levels (69). Lee et al. (69) have associated reduced AMPK activity in the diabetic kidney with diabetes-induced renal hypertrophy. Indeed, hyperglycemia may activate the mTOR pathway by the dual effects of Akt/protein kinase B activation and AMPK inhibition, thereby contributing to basement membrane thickening and mesangial matrix accumulation (77). Increased protein synthesis with reduced AMPK activity can also be explained by AMPK regulation of eukaryotic elongation factor 2 kinase (eEF2kinase) (12), which in turn regulates the elongation phase of mRNA translation by phosphorylation of eukaryotic elongation factor 2 (eEF2) (48, 70). In cultured glomerular epithelial cells, the AMPK activators metformin and AICAR increased AMPK phosphorylation, inhibited high-glucose stimulation of protein synthesis, and prevented high glucose-induced changes in phosphorylation of 4E binding protein 1 and eEF2 (69). In addition, expression of kinase-inactive AMPK further increased high glucose-induced protein synthesis. Furthermore, renal hypertrophy in rats with streptozotocin-induced type 1 diabetes was associated with reduced AMPK phosphorylation and increased mTOR activity. Reduced AMPK activity in the diabetic kidney has also been linked to increased triglyceride accumulation because of reduced inhibitory phosphorylation of acetyl-CoA carboxylase (36). The increased triglyceride accumulation in the diabetic kidney appears to increase expression of connective tissue growth factor, which is another mechanism that contributes to diabetes-induced renal hypertrophy (36). In addition, as described above, reduced AMPK activity in the diabetic kidney has been associated with increased renal tubular glycogen accumulation (15).
Another recent study by Lee et al. (70) also found that high glucose suppressed AMPK activity in cultured glomerular epithelial cells. In this study, hyperglycemia also increased the acetylation and reduced the activity of LKB1. Interestingly, the effects of hyperglycemia on both LKB1 and AMPK were reversed by addition of resveratrol, which is a polyphenol compound present in grapes and green tea that has been linked with increased longevity and amelioration of diabetes and the metabolic syndrome. The reduced acetylation of LKB1 in the presence of resveratrol was not dependent on silent information regulator 1 (SIRT1). The effect of resveratrol on AMPK also prevented the increased protein synthesis observed with hyperglycemia by correcting phosphorylation changes seen in eIF4E, eEF2, eEF2 kinase, and p70S6 kinase (70).
In addition to the effects of glycemia on AMPK in the kidney, both metformin and the thiazolidenediones (TZDs), which are currently used treatments for diabetes, activate AMPK independently of serum glucose (31). In diabetic rats, metformin increased renal AMPK phosphorylation, which then reversed mTOR activation and inhibited renal hypertrophy without affecting hyperglycemia (69). The TZD pioglitazone was observed to activate AMPK in a renal tubular cell line due to decreased mitochondrial membrane potential (125), but whether this observation contributes to the well-described effects of TZDs on kidney function is unknown (110).
Ischemia.
Given that AMPK is activated in response to an increase in cellular AMP/ATP ratio, it is not surprising that acute renal ischemia is a potent activator of AMPK. In fact, AMPK is activated within 1 min of the onset of acute ischemia, and by 5 min it is dramatically activated (95). By immunohistochemistry, the predominant site of AMPK activation in response to brief acute renal ischemia was found to be cortical tubules (95). The functional significance of AMPK activation in acute renal ischemia has, however, not yet been adequately studied. Specifically, it remains to be determined whether the net effect is beneficial, harmful, or neither. Lin et al. (79) have reported that combination therapy with the AMPK activator AICAR and the antioxidant N-acetylcysteine attenuates ischemia-reperfusion injury in a canine model of autologous renal transplantation, which suggests that AMPK preactivation might have a protective role in renal ischemia-reperfusion injury (79). This needs to be interpreted with caution, however, as the relative contributions of AICAR and N-acetylcysteine to this result are unclear. Also, AICAR can cause biological effects that are independent of its effects on AMPK. It is also unknown what the downstream targets are for AMPK in the ischemic kidney, and they could even differ from what has been described in other organs. For example, in contrast to the ischemic heart (19), in the ischemic kidney AMPK does not phosphorylate endothelial nitric oxide synthase (eNOS) (95). Furthermore, the upstream AMPK kinase(s) required for activation of AMPK in the ischemic kidney have not yet been identified.
In contrast to this paucity of knowledge of AMPK in the kidney, there is now a significant body of literature describing an important role for AMPK in response to acute myocardial ischemia (6, 17, 66, 84, 91, 105, 108, 137). In the heart, AMPK is activated rapidly during ischemia and in most studies the net effect of this activation appears to be beneficial (66, 84, 105). This view of a beneficial role of AMPK in myocardial ischemia has, however, been challenged by Lopaschuk's group (28), who have observed that in some situations activation of AMPK in myocardial ischemia is deleterious. The role of AMPK might also differ between ischemia and reperfusion. For example, in AMPK α2 knockout mice subject to no-flow or low-flow myocardial ischemia, there is a more rapid onset of left ventricular dysfunction but there is no difference in the recovery of contractile function after reperfusion (17, 140). It also appears that the functional effect of AMPK in response to organ ischemia may differ between different organs. For example, in contrast to its apparent protective role in myocardial ischemia, activation of AMPK is reported to be deleterious in acute stroke (75, 87). Thus it is reasonable to propose that the role and consequences of AMPK activation in ischemic injury could depend on the nature of the ischemic insult, both the timing and severity of AMPK activation, and the tissue involved.
Ischemia-associated downregulation of various epithelial transport proteins may help prevent the dissipation of transmembrane cellular ionic gradients that are important for normal cellular functioning and survival (38). Whereas ischemia activates AMPK in the kidney (95) and AMPK activation, like ischemia, induces the acute downregulation of various transport proteins (16, 30, 34, 42, 74), it is reasonable to propose that the ischemia-induced inhibition of epithelial transport is mediated by AMPK. As such, this acute inhibition of ion transport proteins by AMPK may represent an adaptive response by limiting the need for active transport via the sodium pump to maintain transmembrane ionic gradients. It is also possible that ischemia-induced activation of AMPK might help explain the increased fractional excretion of sodium that is observed in ischemic models of acute renal failure (115), although presently there is no direct evidence for this hypothesis.
Aging and oxidative stress.
Renal AMPK activity has also been reported to change with aging (54, 102). Jin et al. (54) found that in the kidneys of aged rats (24 mo) compared with young rats (2 mo) there was a nearly threefold increase in the expression of active phosphorylated AMPK (αThr172), despite the fact that total AMPK α1 expression in the older animals was reduced by ∼30%. In the young rats, administration of menadione, which is an inducer of oxidative stress, caused a twofold increase in the expression of active (αThr172 phosphorylated) AMPK, whereas in the aged rats no change in AMPK phosphorylation or expression with menadione was observed (54). Percy et al. (102) also found that renal phospho-AMPK (αThr172) expression was increased with aging in Wistar rats, but not in spontaneously hypertensive and Wistar-Kyoto rats. The explanation for the increased renal AMPK activity observed with aging in these studies is unclear, although Jin et al. (55) speculated that it could be related to the fact that in aged animals under various stresses there are decreased intracellular ATP levels from decreased cellular energy metabolism and accelerated depletion of ATP. Neither of these two studies was able to establish the role of increased AMPK activity in the aging kidney, and whether it contributes to reduced kidney function with age or is a protective response.
Inflammation.
Activation of AMPK has been described to have anti-inflammatory effects (57, 106, 141). For example, the AMPK activator AICAR inhibits TNF-α- and IL-1β-induced NF-κB reporter gene expression dose dependently in immune cells and inducible nitric oxide synthase and cyclooxygenase-2 (COX-2) expression in stimulated macrophages (106). In addition, activators of AMPK inhibit chemotaxis in the monocyte-like cell line U937 (57). The only study to date that specifically examines the role of AMPK in inflammation in the kidney is by Peairs at al. (101) in mesangial cells derived from the MRL/lpr mouse that develops a form of renal disease similar to lupus nephritis. This study found that activation of AMPK by AICAR profoundly inhibited lipopolysaccharide- and IFN-γ-stimulated production of the proinflammatory molecules nitric oxide synthase, COX-2, and IL-6. AICAR did not, however, cause NF-κB (p65) nuclear translocation in MRL/lpr mesangial cells (101). Further evidence for cross talk between the AMPK pathway and the immune system comes from the finding that macrophage migration-inhibitory factor (MIF) activates AMPK in the heart (91). While the kidney also has significant endogenous MIF expression (67), it is yet to be determined whether MIF can also regulate AMPK in the kidney. Further studies are required to examine the role of AMPK in inflammatory diseases of the kidney.
Endothelial function.
A growing body of evidence for a variety of important roles for AMPK in the regulation of endothelial cell function has recently been reviewed in detail (27). Activation of AMPK in endothelial cells has been reported in response to multiple and diverse stimuli, including shear stress (20), ATP depletion (95, 144), hormones such as adiponectin (18, 20, 99), bradykinin (96) and thrombin (116), the drugs metformin (143) and atorvastatin (20), and the free radical peroxynitrite (144). Activation of AMPK in endothelium has been implicated in the regulation of fatty acid oxidation (23, 96), nitric oxide production (94), inflammation (13), and angiogenesis (99). Overall, the effect of AMPK in endothelium has been proposed to be antiatherogenic and to potentially improve the endothelial dysfunction observed with diabetes and the metabolic syndrome (71, 85). It is important to acknowledge, however, that while Cammissoto et al. (14) have demonstrated AMPK expression in glomerular endothelial cells, to date the specific roles of endothelial AMPK in the kidney have not been defined.
Gluconeogenesis.
In addition to the more well-known role of the liver, the renal cortex is also a significant site of gluconeogenesis.. For example, it has been reported that in the postabsorptive phase renal glucose release approaches 20% of all glucose released in the circulation (90). The site of renal gluconeogenesis is the proximal tubule (35). While activation of AMPK has been clearly demonstrated to suppress gluconeogenesis in the liver (64), a role of AMPK in the regulation of renal gluconeogenesis has not been studied. In the liver, activation of AMPK promotes phosphorylation of the transducer of regulated CREB activity 2 (TORC2), which then reduces the ability of TORC2 to transactivate gluconeogenic genes such as phosphoenolpyruvate carboxykinase (64). This appears to be the mechanism by which AMPK-activating drugs such as metformin suppresses hepatic gluconeogenesis (142). Further studies are required to determine whether AMPK has a role in the regulation of renal gluconeogenesis and whether this contributes to the antidiabetic effects of AMPK activators.
Summary and Future Directions
While it has been known for over a decade that the metabolic sensor AMPK is abundantly expressed in the kidney (118), an understanding of its roles in the kidney is now beginning to emerge. AMPK is potentially an important regulator of energy metabolism in the kidney. For example, AMPK is a key regulator of lipid metabolism, and in the kidney fatty acids are an important energy source (7). Significant fatty acid and triglyceride synthesis occurs in the kidney (128). Glucose is also an important metabolic substrate for the kidney (129). AMPK is known to be an important regulator of multiple aspects of glucose metabolism, including glycolysis, glucose uptake, glycogen synthesis, and gluconeogenesis (43). Present knowledge of AMPK biology in the kidney is significantly less advanced than that for other organs such as muscle, adipose tissue, liver, and heart. For example, little is known about the roles of upstream AMPK kinases such as LKB1 and CaMKKβ in the regulation of AMPK in the kidney. It is apparent, however, that AMPK has a variety of important roles in the kidney in both physiology and disease. Regarding physiology, most interest has focused on the role of AMPK in the regulation of ion transport by transport proteins such as CFTR (42), ENaC (16), NKCC2 (30), and the V-ATPase (39). This work potentially throws fresh mechanistic light on the hypothesis of Whittam (130), who described the close coupling that exists between cellular ionic transport and respiration almost a half-century ago. Recent important studies showing a role for adiponectin in the regulation of AMPK in the kidney (14, 15, 113) suggest that systemic factors could be as important as local factors in regulating renal AMPK function. Surprisingly, even though the kidney is an important contributor to whole body glucose homeostasis (83), there are as yet no studies examining the role of AMPK in renal glucose metabolism. Regarding kidney diseases, an emerging area of interest is the role of AMPK in autosomal dominant polycystic kidney disease (86, 122). Other kidney diseases where AMPK is of increasing interest include acute renal ischemia (95) and diabetic nephropathy (15, 69). Further studies are required to advance our current understanding of the roles of AMPK in renal physiology and disease and, specifically, to identify the specific protein substrates targeted by AMPK in the kidney.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK075048 (to K. R. Hallows) and R01 DK084184 (to N. M. Pastor-Soler), American Heart Association Grant AHA 09GRNT2060539 (to N. M. Pastor-Soler), and project grant 434109 from the Australian National Health and Medical Research Association (to D. A. Power). N. M. Pastor-Soler is a recipient of the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Adams J, Chen ZP, Van Denderen BJ, Morton CJ, Parker MW, Witters LA, Stapleton D, Kemp BE. Intrasteric control of AMPK via the gamma1 subunit AMP allosteric regulatory site. Protein Sci 13: 155–165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almaca J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral MD, Kunzelmann K. AMPK controls epithelial Na+ channels through Nedd4–2 and causes an epithelial phenotype when mutated. Pflügers Arch 458: 713–721, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Alzamora R, Pastor-Soler NM, Smolak C, Hallows KR. AMP-activated kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4–2. FASEB J 23: 602–607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem 273: 31880–31889, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA 103: 10883–10888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res 100: 474–488, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Balaban RS, Mandel LJ. Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol Renal Fluid Electrolyte Physiol 254: F407–F416, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Bamri-Ezzine S, Ao ZJ, Londono I, Gingras D, Bendayan M. Apoptosis of tubular epithelial cells in glycogen nephrosis during diabetes. Lab Invest 83: 1069–1080, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4–2. J Biol Chem 281: 26159–26169, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Brenman JE. AMPK/LKB1 signaling in epithelial cell polarity and cell division. Cell Cycle 6: 2755–2759, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem 279: 12220–12231, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun 324: 1204–1209, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Cammisotto PG, Bendayan M. Adiponectin stimulates phosphorylation of AMP-activated protein kinase alpha in renal glomeruli. J Mol Histol 39: 579–584, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Cammisotto PG, Londono I, Gingras D, Bendayan M. Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol 294: F881–F889, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the α2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol 292: H3136–H3147, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 268: 45021–45026, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power D, Oritz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 104: 496–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J 9: 2439–2446, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook N, Fraser SA, Katerelos M, Katsis F, Gleich K, Mount PF, Steinberg GR, Levidiotis V, Kemp BE, Power DA. Low salt concentrations activate AMP-activated protein kinase in mouse macula densa cells. Am J Physiol Renal Physiol 296: F801–F809, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ Res 88: 1276–1282, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Denison FC, Hiscock NJ, Carling D, Woods A. Characterization of an alternative splice variant of LKB1. J Biol Chem 284: 67–76, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Dubbelhuis PF, Meijer AJ. Hepatic amino acid-dependent signaling is under the control of AMP-dependent protein kinase. FEBS Lett 521: 39–42, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP activated protein kinase. Eur J Biochem 262: 184–190, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105: 114–127, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5′-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am J Physiol Heart Circ Physiol 297: H313–H321, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp BE, Power DA. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+-K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405: 85–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277: 25226–25232, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Delgado M, Peral MJ, Cano M, Calonge ML, Ilundain AA. Creatine transport in brush-border membrane vesicles isolated from rat kidney cortex. J Am Soc Nephrol 12: 1819–1825, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Gimenez I. Molecular mechanisms and regulation of furosemide-sensitive Na-K-Cl cotransporters. Curr Opin Nephrol Hypertens 15: 517–523, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol. (First published February 10, 2010). doi:10.1152/ajprenal.00645.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guder WG, Ross BD. Enzyme distribution along the nephron. Kidney Int 26: 101–111, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Zhao Z. Effect of N-acetylcysteine on plasma adiponectin and renal adiponectin receptors in streptozotocin-induced diabetic rats. Eur J Pharmacol 558: 208–213, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frøkiær J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of α-, β-, and γ-ENaC in rat kidney. Am J Physiol Renal Physiol 280: F1093–F1106, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Hallows KR. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284: C1297–C1308, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 32, Suppl 4: S7–S12, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23: 1112–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem 270: 27186–27191, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol 12: 1419–1423, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol 13: 861–866, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280: 29060–29066, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Iglesias MA, Furler SM, Cooney GJ, Kraegen EW, Ye JM. AMP-activated protein kinase activation by AICAR increases both muscle fatty acid and glucose uptake in white muscle of insulin-resistant rats in vivo. Diabetes 53: 1649–1654, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Iseli TJ, Walter M, van Denderen BJ, Katsis F, Witters LA, Kemp BE, Michell BJ, Stapleton D. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270). J Biol Chem 280: 13395–13400, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev 89: 777–798, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Jin Q, Jhun BS, Lee SH, Lee J, Pi Y, Cho YH, Baik HH, Kang I. Differential regulation of phosphatidylinositol 3-kinase/Akt, mitogen-activated protein kinase, and AMP-activated protein kinase pathways during menadione-induced oxidative stress in the kidney of young and old rats. Biochem Biophys Res Commun 315: 555–561, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Joo HJ, Ma JY, Choo YG, Choi BK, Jung KY. Age-related alteration of intracellular ATP maintenance in the cell suspensions of mice cerebral cortex. Mech Ageing Dev 110: 1–12, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Kahn B, Alquier T, Carling D, Hardie D. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Kanellis J, Kandane RK, Etemadmoghadam D, Fraser SA, Mount PF, Levidiotis V, Kemp BE, Power DA. Activators of the energy sensing kinase AMPK inhibit random cell movement and chemotaxis in U937 cells. Immunol Cell Biol 84: 6–12, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31: 162–168, 2003 [DOI] [PubMed] [Google Scholar]
- 59.King JD, Jr, Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK, Hallows KR. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol 297: C94–C101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitani T, Okuno S, Fujisawa H. Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biochem 122: 243–250, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Klein H, Garneau L, Trinh NT, Prive A, Dionne F, Goupil E, Thuringer D, Parent L, Brochiero E, Sauve R. Inhibition of the KCa3.1 channels by AMP-activated protein kinase in human airway epithelial cells. Am J Physiol Cell Physiol 296: C285–C295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem 284: 5645–5653, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kongsuphol P, Hieke B, Ousingsawat J, Almaca J, Viollet B, Schreiber R, Kunzelmann K. Regulation of Cl− secretion by AMPK in vivo. Pflügers Arch 457: 1071–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437: 1109–1111, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Krause U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem 269: 3751–3759, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270: 17513–17520, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Lan HY. Role of macrophage migration inhibition factor in kidney disease. Nephron 109: e79–e83, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Lang T, Yu L, Tu Q, Jiang J, Chen Z, Xin Y, Liu G, Zhao S. Molecular cloning, genomic organization, and mapping of PRKAG2, a heart abundant gamma2 subunit of 5′-AMP-activated protein kinase, to human chromosome 7q36. Genomics 70: 258–263, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Lee MJ, Feliers D, Sataranatarajan K, Mariappan MM, Li M, Barnes JL, Choudhury GG, Kasinath BS. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal 22: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. α-Lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol 25: 2488–2494, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Lewy PR, Quintanilla A, Levin NW, Kessler RH. Renal energy metabolism and sodium reabsorption. Annu Rev Med 24: 365–384, 1973 [DOI] [PubMed] [Google Scholar]
- 73.Li H, Findlay IA, Sheppard DN. The relationship between cell proliferation, Cl− secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int 66: 1926–1938, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Li H, Thali R, Smolak C, Gong F, Alzamora R, Neumann D, Pastor-Soler NM, Hallows KR. Novel regulation of creatine transporter by AMP-activated kinase in kidney epithelial cells (Abstract). J Am Soc Nephrol 20: 281A, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38: 2992–2999, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9: 218–224, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol 20: 2493–2502, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation 107: 1962–1965, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Lin A, Sekhon C, Sekhon B, Smith A, Chavin K, Orak J, Singh I, Singh A. Attenuation of ischemia-reperfusion injury in a canine model of autologous renal transplantation. Transplantation 78: 654–659, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 39, Suppl 4: 10–21, 1990 [DOI] [PubMed] [Google Scholar]
- 81.Mace OJ, Woollhead AM, Baines DL. AICAR activates AMPK and alters PIP2 association with the epithelial sodium channel ENaC to inhibit Na+ transport in H441 lung epithelial cells. J Physiol 586: 4541–4557, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for γ3 in white skeletal muscle. Am J Physiol Endocrinol Metab 286: E194–E200, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 53: 875–883, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255, 2000 [DOI] [PubMed] [Google Scholar]
- 85.McCarty MF. AMPK activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med Hypotheses 64: 1211–1215, 2005 [DOI] [PubMed] [Google Scholar]
- 86.McCarty MF, Barroso-Aranda J, Contreras F. Activation of AMP-activated kinase as a strategy for managing autosomal dominant polycystic kidney disease. Med Hypotheses 73: 1008–1010, 2009 [DOI] [PubMed] [Google Scholar]
- 87.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280: 20493–20502, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273: E1107–E1112, 1997 [DOI] [PubMed] [Google Scholar]
- 89.Meury L, Noel J, Tejedor A, Senecal J, Gougoux A, Vinay P. Glucose metabolism in dog inner medullary collecting ducts. Ren Physiol Biochem 17: 246–266, 1994 [DOI] [PubMed] [Google Scholar]
- 90.Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab 282: E428–E434, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 451: 578–582, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Morrow VA, Foufelle F, Connell JMC, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric oxide synthesis in human aortic endothelial cells. J Biol Chem 278: 31629–31639, 2003 [DOI] [PubMed] [Google Scholar]
- 95.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Mount PF, Lane N, Venkatesan S, Steinberg GR, Fraser SA, Kemp BE, Power DA. Bradykinin stimulates endothelial cell fatty acid oxidation by CaMKK-dependent activation of AMPK. Atherosclerosis 200: 28–36, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Nofziger C, Kalsi K, West TA, Baines D, Blazer-Yost BL. Vasopressin regulates the phosphorylation state of AMP-activated protein kinase (AMPK) in MDCK-C7 cells. Cell Physiol Biochem 22: 487–496, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Oakhill JS, Scott JW, Kemp BE. Structure and function of AMP-activated protein kinase. Acta Physiol (Oxf) 196: 3–14, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304–1309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pastor-Soler NM, Alzamora R, Naveed S, Smolak C, Gong F, Hallows KR. Novel regulation of V-ATPase by PKA and AMPK in kidney intercalated cells. FASEB J 23: 602–613, 2009 [Google Scholar]
- 101.Peairs A, Radjavi A, Davis S, Li L, Ahmed A, Giri S, Reilly CM. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin Exp Immunol 156: 542–551, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Percy CJ, Brown L, Power DA, Johnson DW, Gobe GC. Obesity and hypertension have differing oxidant handling molecular pathways in age-related chronic kidney disease. Mech Ageing Dev 130: 129–138, 2009 [DOI] [PubMed] [Google Scholar]
- 103.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol 13: 867–871, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Rossier BC, Canessa CM, Schild L, Horisberger JD. Epithelial sodium channels. Curr Opin Nephrol Hypertens 3: 487–496, 1994 [DOI] [PubMed] [Google Scholar]
- 105.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 181: 8633–8641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J 334: 177–187, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog Lipid Res 42: 238–256, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int 70: 1223–1233, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113: 274–284, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seo-Mayer PW, Zhang L, Thulin G, Jeschke G, Kashgarian M, Caplan MJ. Effects of preactivation of AMP-activated protein kinase (AMPK) by metformin prior to energy depletion in renal epithelial cells are not mediated by ATP availability (Abstract). J Am Soc Nephrol 19: 176A, 2008 [Google Scholar]
- 113.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Spiegel DM, Wilson PD, Molitoris BA. Epithelial polarity following ischemia: a requirement for normal cell function. Am J Physiol Renal Fluid Electrolyte Physiol 256: F430–F436, 1989 [DOI] [PubMed] [Google Scholar]
- 116.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol 26: 5933–5945, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stanton BA. Cystic fibrosis transmembrane conductance regulator (CFTR) and renal function. Wien Klin Wochenschr 109: 457–464, 1997 [PubMed] [Google Scholar]
- 118.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271: 611–614, 1996 [DOI] [PubMed] [Google Scholar]
- 119.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanovic A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol 210: 224–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281: 32207–32216, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Takiar V, Nishio S, King JD, Jr, Hallows KR, Somlo S, Caplan MJ. Metformin activation of AMPK slows renal cystogenesis (Abstract). J Am Soc Nephrol 19: 26A, 2008 [Google Scholar]
- 123.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007 [DOI] [PubMed] [Google Scholar]
- 124.Treharne KJ, Best OG, Mehta A. The phosphorylation status of membrane-bound nucleoside diphosphate kinase in epithelia and the role of AMP. Mol Cell Biochem 329: 107–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turturro F, Oliver R, Friday E, 3rd, Nissim I, Welbourne T. Troglitazone and pioglitazone interactions via PPAR-γ-independent and -dependent pathways in regulating physiological responses in renal tubule-derived cell lines. Am J Physiol Cell Physiol 292: C1137–C1146, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest 118: 752–762, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walker J, Jijon HB, Churchill T, Kulka M, Madsen KL. Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol Gastrointest Liver Physiol 285: G850–G860, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, Scherzer P, Lewis L, Miyazaki-Anzai S, Levi M. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol 297: F1587–F1596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weidemann MJ, Krebs HA. The fuel of respiration of rat kidney cortex. Biochem J 112: 149–166, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Whittam R. Active cation transport as a pace-maker of respiration. Nature 191: 603–604, 1961 [DOI] [PubMed] [Google Scholar]
- 131.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. C(Ca2+)/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Woollhead AM, Scott JW, Hardie DG, Baines DL. Phenformin and 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J Physiol 566: 781–792, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Woollhead AM, Sivagnanasundaram J, Kalsi KK, Pucovsky V, Pellatt LJ, Scott JW, Mustard KJ, Hardie DG, Baines DL. Pharmacological activators of AMP-activated protein kinase have different effects on Na+ transport processes across human lung epithelial cells. Br J Pharmacol 151: 1204–1215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449: 496–500, 2007 [DOI] [PubMed] [Google Scholar]
- 136.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 103: 17378–17383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem 278: 28372–28377, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 139.Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T. Regulation of transcription by AMP-activated protein kinase. Phopsphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem 276: 38341–38344, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, Vanoverschelde JL, Bertrand L. Role of the α2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol 291: H2875–H2883, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou G, Sebhat IK, Zhang BB. AMPK activators—potential therapeutics for metabolic and other diseases. Acta Physiol (Oxf) 196: 175–190, 2009 [DOI] [PubMed] [Google Scholar]
- 143.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, IV, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo: role of mitochondrial reactive nitrogen species. J Biol Chem 279: 43940–43951, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem 278: 34003–34010, 2003 [DOI] [PubMed] [Google Scholar]