Abstract
Recently published epidemiological and outcome analysis studies have brought to our attention the important role played by acute kidney injury (AKI) in the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD). AKI accelerates progression in patients with CKD; conversely, CKD predisposes patients to AKI. This research gives credence to older, well-thought-out wisdom that recovery from AKI is often not complete and is marked by residual structural damage. It also mirrors older experimental observations showing that unilateral nephrectomy, a surrogate for loss of nephrons by disease, compromises structural recovery and worsens tubulointerstitial fibrosis after ischemic AKI. Moreover, review of a substantial body of work on the relationships among reduced renal mass, hypertension, and pathology associated with these conditions suggests that impaired myogenic autoregulation of blood flow in the setting of hypertension, the arteriolosclerosis that results, and associated recurrent ischemic AKI in microscopic foci play important roles in the development of progressively increasing tubulointerstitial fibrosis. How nutrition, an additional factor that profoundly affects renal disease progression, influences these events needs reevaluation in light of information on the effects of calories vs. protein and animal vs. vegetable protein on injury and progression. Considerations based on published and emerging data suggest that a pathology that develops in regenerating tubules after AKI characterized by failure of differentiation and persistently high signaling activity is the proximate cause that drives downstream events in the interstitium: inflammation, capillary rarefaction, and fibroblast proliferation. In light of this information, we advance a comprehensive hypothesis regarding the pathophysiology of AKI as it relates to the progression of kidney disease. We discuss the implications of this pathophysiology for developing efficient therapeutic strategies to delay progression and avert ESRD.
Keywords: end-stage renal disease, tubule regeneration, tubule atrophy, kidney fibrosis, signaling
despite the vast body of effort devoted to investigation of the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD), a full understanding of the underlying mechanisms has remained elusive. Progression is characterized by a continuously advancing and irreversible erosion of kidney structure due to loss of nephrons and replacement by scar tissue. It involves common pathological themes, arteriolosclerosis, glomerulosclerosis, and tubulointerstitial fibrosis (16, 38, 62, 78, 107, 109, 135). Hypertension, diabetes mellitus, caloric nutritional overload, and aging are antecedent or coincident processes that can initiate injury independently and/or potentiate ongoing damage during CKD by perturbing renal hemodynamics or imposing functional and metabolic loads on kidneys, thereby accelerating the progression of CKD initiated by other diseases of immune, infective, or genetic origin (2, 3, 11, 12, 16, 31, 43, 55, 78, 113). Multifarious structural, functional, and signaling abnormalities beset diverse kidney cell types during progression. Implicit in this complex pathology is our inability to distinguish proximal events that rise above the “guilt by association” criterion and drive entire pathological processes, from downstream mechanisms that produce unrelated secondary effects or cause disease events of limited clinical portent. Recent clinical studies have brought to light important links that connect acute kidney injury (AKI), CKD, and progression to ESRD (34, 66, 67, 70, 80, 93, 118, 150, 154, 155). Although they are based on epidemiological and outcome analysis data, these reports alert us to the possibility that mutually reinforcing interactions between AKI and CKD can profoundly alter kidney pathophysiology in ways that make more severe the pathological process that leads to ESRD. Realizing that the AKI-CKD connection is of far-reaching clinical significance, we summarize in this review the prior experimental work that supports this connection, reconsider and expand some older observations on the contribution of hypertension to acute tubulointerstitial injury and progression in models of renal mass reduction, and emphasize the adaptations that tubules undergo following disease-associated loss of renal mass which may make them vulnerable to acute injury. We discuss the possibility that tubulointerstitial fibrosis following AKI in the setting of reduced renal mass is caused by incomplete repair of regenerating tubules. We present a hypothesis evolving from older and emerging data suggesting that persistently high signaling in regenerating tubule epithelium that fails to differentiate gives rise to paracrine activity that causes inflammation and fibrosis. Summarized in Figs. 1 and 2, this paradigm provides a framework for new experimental directions and discussion to elucidate the pathogenesis of progression in CKD.
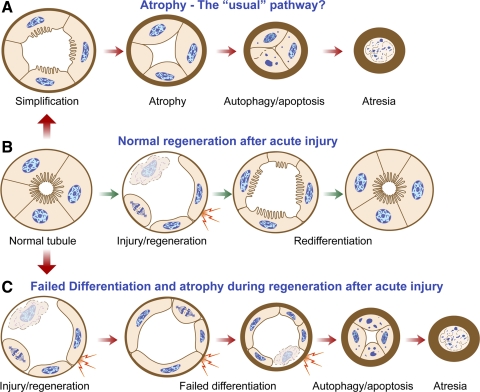
Fig. 1.
Schematic diagram to illustrate how tubules regenerating after acute injury may fail to differentiate and exhibit profibrotic paracrine activity before they become atrophic. A: the “usual” pathway of tubule atrophy conceptualized from morphological studies of diseased kidneys involves simplification of epithelial structure progressing to autophagy and apoptosis accompanied by marked thickening of tubule basement membranes. Toward the end, tubules become atrectic and become enveloped by thick basement membranes or disappear altogether. B: normal regeneration of tubules after AKI with death of epithelium involves initial dedifferentiation, migration, and proliferation of surviving cells, followed by redifferentiation and full restoration of normal structure. Unlike in the usual pathway (A), tubule cells that survive acute kidney injury (AKI) follow a somewhat different route to become atrophic (C). Following dedifferentiation, migration, and proliferation, they fail to redifferentiate. After an indefinite period of time during which they are in a state of “failed differentiation,” these abnormal tubules proceed to develop thick basement membranes and undergo atrophy by autophagy and apoptosis just as in the usual pathway and disappear by atresia. Hyperactive epithelial paracrine signaling (shown as lightning bolts) in proliferating cells during regeneration becomes suppressed again if tubules redifferentiate normally (B) but persists in tubules with a regenerative failed differentiation phenotype (C) for an indefinite period of time before atrophy takes place. This paracrine signaling gives rise to inflammation and fibrosis, as depicted in Fig. 2.
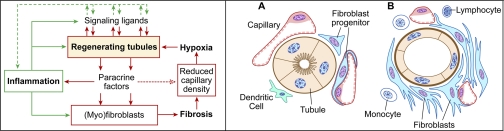
Fig. 2.
Left: hypothetical scheme for relationships between signaling activity in regenerating tubule cells and downstream signaling events in the interstitium: inflammation, fibroblast proliferation, and capillary rarefaction. Although epithelial signaling with ensuing paracrine activity is the proximate “trigger” in this scheme, subsequent interactions between inflammation, fibroblasts, capillary endothelium, and tubule epithelium become a self-reinforcing “vicious cycle” that makes the separation of early signals and downstream events difficult. Right: pathology of tubulointerstitial fibrosis. Operation of the complex interactive signaling network depicted on the left leads to transformation of the normal tubulointerstitium (A) to a state of tubulointerstitial fibrosis: undifferentiated/atrophic tubules, proliferation of fibroblast progenitors, decreased capillary density, and inflammation (B).
Progression to ESRD Occurs by Processes Unrelated to the Original Pathology that Initiates CKD
Renal function was reported to decline linearly over time in patients with CKD (103, 132). In light of experiments on the “ nephrectomy” model of CKD in rats, these clinical data led to the premise that progression to ESRD occurs by processes unrelated to the illness that caused CKD (29, 30, 64, 120). In the experimental paradigm, removal of one kidney is combined with ablation of two-thirds of the other kidney to simulate disease-associated loss of renal parenchyma. The “remnant kidney” develops compensatory adaptations (increased blood flow and glomerular hyperfiltration) that maintain function at increased levels per nephron and lead to hypertrophy of glomeruli and tubules. Nevertheless, as a consequence of these adaptations, renal structure and function deteriorate steadily, reaching an end-stage within several months. These kidneys exhibit arteriolosclerosis, glomerulosclerosis, and tubulointerstitial fibrosis, just as in human disease (1, 30, 57, 64, 104, 140, 147, 148). Similar findings were reported for other species (27, 28, 39). Impaired myogenic autoregulation of renal blood flow and barotrauma due to direct transmission of arterial pressure to arterioles and glomeruli give rise to arteriolosclerosis and glomerulosclerosis in remnant kidneys (16, 17, 19, 20, 54, 57–59). As a result, the view has been that worsening glomerulosclerosis, decreased peritubular capillary perfusion, and/or misdirected filtration cause tubulointerstitial pathology in the renal ablation model, and by inference, in human CKD progression (62, 87, 107, 135). However, other factors may predominate.
Tubulointerstitial Fibrosis is the Predominant Pathology that Reflects Progression
Both in human CKD and in the remnant kidney, tubulointerstitial fibrosis is disproportionately more severe than glomerulosclerosis and appears to be the self-reinforcing pathophysiology that ensures final denouement in ESRD. Thus progression very often occurs in human CKD without a primacy for continuing glomerular injury (62, 109, 130, 134, 143). Moreover, tubulointerstitial fibrosis can develop before glomerulosclerosis after nephrectomy (85) (Fig. 3). What could be the reason for these counterintuitive observations?
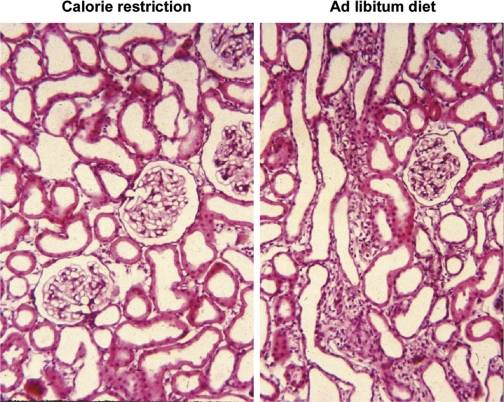
Fig. 3.
Tubulointerstitial fibrosis precedes glomerulosclerosis in the remnant kidney after nephrectomy and is prevented by caloric restriction. Paraffin sections of remnant kidneys of rats 4 wk after nephrectomy by the infarction method, perfusion fixed with formaldehyde, and stained with hematoxylin and eosin. Rats were fed a 21% casein-based diet ad libitum (right) or pair fed a 35% casein-based diet at 60% of total food weight consumed by the ad libitum group, so that both groups consumed equal amounts of protein. The ad libitum group, but not the calorie-restricted group will develop end-stage renal disease after 5 mo (148). Original magnification: ×200. Unpublished archival material from Ref. 85.
Rates of Functional Declines in CKD are not Necessarily Linear and May be Related to Superimposed Episodes of AKI
The concept that declines in renal function during progression are strictly linear has been questioned (138). Declines in glomerular filtration rate (GFR) are not necessarily constant (158), making it plausible that unsuspected pathologies may acutely accelerate progression. Recently published outcome analysis studies of patients with CKD and AKI have provided clues to pathological inputs that hasten progression. As summarized in recent editorials by Okusa et al. (118) and Waikar and Winkelmayer (154), these studies show an intertwined relationship between CKD and superimposed AKI. Preexisting kidney disease predisposes patients for AKI; risk for AKI is proportional to CKD stage (67, 70, 80, 108, 118, 122). Conversely, AKI episodes in patients with preexisting CKD markedly increase the ESRD burden by causing additional damage in compromised kidneys (66, 70, 80, 118). A role for acute injury in progression is further reinforced by clinical insights informing us that AKI may be followed by incomplete recovery with residual damage even in the absence of prior kidney disease (34, 42, 60, 65, 125, 141). As a corollary, the finding that progressive disease is more likely to occur after “acute on chronic” injury (i.e., AKI superimposed on CKD) than after AKI alone (80) is not surprising; intuitively, it seems reasonable that damage after AKI may add to or even synergize with preexisting pathology. These trends apply to pediatric populations as well (8). Interestingly, we now know that there were large increases in the incidence of AKI nationally between 1988 and 2002 accompanied by steady declines in mortality from the condition; analysis of these data suggests that the longer life spans accounted for concomitant increases in ESRD incidence (153), a finding that buttresses the concept that AKI contributes to the progression of preexisting renal disease.
The compelling inference from these investigations is that two injurious processes, one chronic and the other acute, may each “feed” on stress and damage caused by the other to make structural and functional deterioration worse, setting up a vicious cycle. Experimental support for self-reinforcing injury caused by acute insults superimposed on older damage was provided by Nath et al. (110). Myohemoglobinuric AKI that was reversible as a single incident was shown to cause tubulointerstitial fibrosis when it was episodically repeated (110). The effect of such acute on chronic injuries would be steady loss of functional renal parenchyma. Following this line of reasoning, it can be assumed that CKD patients who progress to ESRD after an AKI incident are likely to be those with preexisting loss of renal mass. Considered in the context of progressive damage that occurs in the remnant kidney, it is then reasonable to hypothesize that the essential pathophysiology that connects CKD, AKI, and ESRD is indeed the reduction of renal mass that occurs during CKD. Thus, in terms of understanding how reduced renal mass participates in the pathology that leads to ESRD, it is plausible that hypertrophic work-stressed surviving nephrons in CKD patients are more susceptible to undergo damage and/or less able to recover following a superimposed acute stress in the form of AKI.
By Increasing the Work Burden in Hypertrophic Nephrons, Progressively Increasing Deficits of Kidney Mass May Impose Unacceptably High Work-Related Stresses, Predisposing Tubules to Develop Acute Injuries or Recover Poorly from Them
Hyperfiltration caused by unilateral nephrectomy leads to tubule hypertrophy and adaptations such as increased epithelial transport (79). Single-nephron GFR increases more than twofold after subtotal nephrectomy, and this is accompanied by even larger increases in transport and O2 consumption. However, O2 consumption increases more than the elevated transport rates would require, mitochondrial respiration becomes uncoupled from ATP synthesis, and there is enhanced oxidant stress, particularly in animals eating casein-based high-protein diets (111, 139). It was suggested that work-related epithelial abnormalities and/or oxidant stress related to high protein intake in the setting of renal ablation contribute to development of tubulointerstitial fibrosis (111, 136, 137). Also, hypothetically, high rates of O2 consumption could reduce tissue oxygen tension and give rise to hypoxic signaling that perturbs epithelial responses during injury and repair. Furthermore, limitations on the extent to which tubule cells in remnant kidneys can proliferate rather than hypertrophy may impose otherwise avoidable stresses on work-burdened tubule epithelium. Progressive nephropathy was prevented in p21 null mice with nephrectomy, presumably because lack of this cdk inhibitor allowed more hyperplasia in remaining tubules (101). These effects are consistent with the theory that proliferative ability confers advantage to cells in organs subjected to enhanced workloads (such as the liver following subtotal hepatectomy) compared with nondividing cells that are forced to undergo hypertrophy and sustain more work-related stress per unit cell (such as the hypertrophied but failing myocardium) (5, 53). However, other effects of p21 on systemic or renal targets in this model also need to be studied. In particular, the effects of age on the relative contributions of hyperplasia and hypertrophy to adaptation in response to kidney mass reduction and the role played by p21 in these adaptations need investigation. Regardless, we would reiterate here that increased tubular workload has the potential to adversely impact disease progression, as shown by the ameliorative effects of interventions that mitigate hyperfiltration, the most effective of them being food restriction (1, 147, 148). Indeed, these protective effects include the prevention of tubulointerstitial fibrosis that develops in remnant kidneys at intervals after nephrectomy when glomerulosclerosis is little and insignificant or absent (85). As a corollary and in the context of clinical research that connects CKD and AKI, we would tender this hypothesis: that work-related stresses in tubules of hyperfiltering kidneys predispose them to develop acute injury and/or recover poorly from such injuries.
As in Aging Nephropathy, Intake of Calories Rather than Protein Governs Progression in Kidneys with Reduced Renal Mass
Nutrition profoundly affects the course of progressive renal disease and may therefore be expected to modify the severity of injury processes, acute as well as chronic. A discussion of nutrition as it affects injury during progression is relevant because an incomplete understanding of nutritional factors has led in the past to hastily designed expensive clinical trials that yielded inconclusive results. Moreover, there is disregard of published data showing that caloric intake rather than protein intake governs progression and that protein effects are entirely accountable by protein source. Caloric restriction prevents progressive disease in remnant kidneys. This effect is associated with decreased body mass, single-nephron GFR, and tubule workload. As a result, glomerular and tubular hypertrophy and tubulointerstitial fibrosis are mitigated and ESRD outcomes are averted relative to kidneys of animals fed ad libitum (1, 79, 85, 147, 148). In contrast, isocaloric protein restriction offers modest if any protection (148). These studies mirror the effects of nutrition on aging nephropathy (97–99).
Animal and Vegetable Proteins Affect Kidney Function and Disease Progression Differently: High Intake of Animal Protein is Nephrotoxic and May Potentiate Injury
The effects of protein restriction on aging nephropathy are modest (97, 98) and unique to protein source, i.e., the animal protein casein, since they were not seen with soy protein (71), an effect not attributable to isoflavones in soy meal (124). Similar observations were made in remnant kidneys (124, 157, 160, 161) and genetic models of kidney disease (115, 152). Reported protection by low-protein diets is likely due to reduced food (calorie) intake because animals on low-protein diets eat less (99, 148). These effects of protein quality have important implications. Vegetable proteins have little effect on renal hemodynamics and glomerular filtration unlike animal protein (86, 106), and both hypertension and hypertensive renal disease are ameliorated by vegetable protein diets relative to animal protein (100). Vegetable protein may also be protective against human kidney disease, including diabetic nephropathy (6). Most important is this consideration: consumed in equivalently high amounts, casein-fed animals develop nephropathy whereas soy protein-fed animals do not, suggesting that high-casein diets are nephrotoxic. Thus total food consumption as calories is the overriding factor that affects progression and high consumption of animal protein is nephrotoxic (112), considerations that highlight a need to reopen the issue of nutritional effects on kidney disease. Stress related to increased workloads caused by hyperfiltration is likely to be made worse by diets rich in calories and animal protein. Conceivably, these diet-induced stresses make work-burdened tubules more susceptible to injury.
Reduced Renal Mass Impairs Recovery from Experimental Ischemic AKI in Rats and Favors Development of Tubulointerstitial Fibrosis
Direct experimental evidence for the predisposition of kidneys with reduced renal mass to develop CKD and tubulointerstitial fibrosis following AKI was obtained well before the recently published clinical studies implicating such a relationship. Loss of tubule epithelium during AKI is followed by dedifferentiation, migration, and proliferation of survivor cells. Healthy regeneration after AKI should lead to recovery of normal structure with differentiated tubule epithelium. Tubule cells do proliferate vigorously after AKI. However, depending on the severity of damage, recovery of normal structure is frequently incomplete and kidneys may develop focal tubulointerstitial fibrosis (37, 41, 45), similar to observations made in kidneys after human AKI (42). This is likely related to a complex network of signaling and cytokine growth factor release involving a tubule epithelium-inflammatory cell-fibroblast axis. In support of the main premise that we develop in this commentary, ischemic AKI of a single kidney after uninephrectomy is followed by tubulointerstitial fibrosis more severe than in kidneys of rats subjected to ischemia with the other kidney left intact (9, 10). The impact of reduced renal mass on chronic pathology in solitary kidneys that suffer ischemic damage during transplantation was shown by the protection afforded through augmentation of kidney mass by transplanting two kidneys instead of one (95). Similar observations have emphasized the importance of available functional renal mass in preventing long-term allograft injury by nonimmunological mechanisms (94, 96). It is noteworthy that tubulointerstitial fibrosis that develops after ischemic AKI in the solitary kidney is indistinguishable from the pathology seen in CKD as documented by Pagtalunan, Forbes, and coworkers (44, 121) and subsequently by our work (48). Long after ischemia of the solitary kidney, there is histological evidence for CKD in the form of dilated and shrunken tubules, markedly thickened tubule basement membranes, interstitial fibrosis with increased numbers of fibroblasts, and diminished capillary density (48). Assuming that surgical reduction of renal mass by 50% is a reliable model to project what might happen in remaining nephrons following a comparable degree of tissue destruction by disease, these experimental findings in rats provide a basis to explain why AKI in human patients with CKD is followed by poor recovery and progression to ESRD.
Incrementally Progressive Tubulointerstitial Fibrosis May Result from Multiple Episodes of Subclinical AKI in Microscopic Foci Caused by Hypertensive Arteriosclerosis in the Setting of Reduced Renal Mass
Clinical experience informs us that episodes of overt AKI cannot account for progression in all CKD patients who develop ESRD. In a significant number, the clinical course is unmarked by acute illness. Therefore, other explanations must be sought to explain the more indolent patterns of progression. In this respect, the importance of hypertensive nephrosclerosis has been emphasized (62). Studies using the nephrectomy model have shown how and in what context hypertension injures the kidney and accelerates CKD progression. Five-sixths nephrectomy is accomplished by removal of one kidney and either surgical excision of both poles of the other kidney or renal infarction produced by ligation of two of three branches of its main artery. Hemodynamic adaptations, hyperfiltration, and kidney hypertrophy occur equally after both procedures. In the “infarction model,” renin-angiotensin-dependent hypertension develops whereas after surgical excision, the animals remain normotensive for long periods (56, 59). Remarkably, autoregulation of blood flow, a myogenic arteriolar response to perfusion pressure, is comparably impaired in both models, with consequences to the evolution of kidney pathology (21, 22, 56). Measured by radiotelemetry and averaged over a 6-wk period, systemic arterial pressures were elevated after renal ablation produced by infarction but not by surgical excision (56). Although functional parameters and hypertrophy indices were similar in both groups, significant glomerulosclerosis developed only in the hypertensive group (56). These findings are explained by unregulated transmission of elevated arterial pressure through a microvasculature with impaired autoregulation (54). Autoregulation was preserved in the renal microvasculature of spontaneously hypertensive rats (SHR) with comparable increases in systemic arterial pressure (4). Interestingly, renal ablation in hypertensive (SHR) rats resulted in autoregulatory impairment and development of medial degeneration and proliferation in arteriolar walls, within days of surgery (19). Because SHR do not develop vascular or glomerular lesions until late in life despite significant hypertension, these findings underscore the role played by unregulated transmission of hydrostatic pressure through arterioles incapable of autoregulatory contraction in the pathogenesis of hypertensive arteriolosclerosis. Accordingly, correction of hypertension prevented the development of vascular lesions despite renal ablation in SHR (19). We have reassessed renal pathology in additional groups of normotensive rats with renal ablation produced by surgical excision, hypertensive rats with renal ablation produced by infarction, and in conventional SHR or stroke-prone (SP)-SHR without renal ablation. Systolic pressures in both hypertensive groups were comparable and averaged 180–210 mmHg over a 6- to 8-wk period by radio telemetry. The findings illustrate the paramount role played by hypertensive arteriolosclerosis in the development of tubulointerstitial pathology in contexts where renal mass is reduced (Fig. 4). Only hypertensive rats with renal ablation by infarction showed significant tubulointerstitial fibrosis (Fig. 4A); interlobular and afferent glomerular arterioles in these kidneys showed alterations ranging in severity from benign arteriolosclerosis to severe proliferative lesions and medial degeneration (Fig. 4D). Nonhypertensive rats with renal ablation produced by surgical excision showed equivalent tubular and glomerular hypertrophy as in the infarction group but no tubulointerstitial fibrosis or arteriolosclerosis (Fig. 4, B and E). Remarkably, SHR or SP-SHR without renal ablation did not show tubulointerstitial fibrosis (Fig. 4C). Renal arterioles in these hypertensive rats did show more nuclei in the medial layer of their walls suggestive of smooth muscle proliferation, but arteriolosclerosis was rare or absent, despite comparably elevated blood pressures (Fig. 4F). As referenced above, these rats will develop florid arteriolosclerosis within days after renal ablation (19). These data suggest that neither reduced renal mass nor hypertension is sufficient by itself to induce significant arteriolosclerosis or give rise to tubulointerstitial fibrosis. However, when present together, they induce both pathologies, owing to the permissive role of impaired autoregulation. This would be predicted by studies showing that arterioles contract irregularly in hypertension with a “sausage string” appearance of alternating short constricted zones and longer segments of dilatation. Dilated regions with higher hydrostatic pressures (49) are pathologically permeable to macromolecules and develop arteriolosclerosis (49–51, 72), an assessment that applies to the renal vasculature also (63, 162). Analogously, autoregulatory contraction protects arterioles and impaired autoregulation allows abnormal dilation that causes arteriolar damage and arteriolosclerosis (20). Because hypertension in the setting of failed autoregulation causes occlusive arteriolosclerosis in the renal ablation model, it is plausible that the resulting ischemia or hypoxia could give rise to focal kidney injury. Conceivably, focal ischemia could be induced in ablated kidneys even in the absence of severe arteriolosclerosis. Systemic blood pressures fluctuate abnormally in the setting of decreased renal mass and impaired autoregulation (57, 58), and systemic arterial pressures in the normotensive as well as hypertensive range can be transmitted through a nonresponsive vasculature to produce glomerular sclerosis (18, 57, 58). Conversely, downward fluctuations in the same setting of failed autoregulation may unduly decrease blood flow and cause ischemia or hypoxia, particularly in kidney microenvironments supplied by sclerotic arterioles. It is possible that this tenuous situation is made worse by vasospasm. Because arterioles with impaired myogenic responses remain responsive to vasoactive hormones, episodes of sustained vasoconstriction induced by overactivity of the sympathetic system or renin-angiotensin axis could work in conjunction with occlusive arteriolosclerosis and blood pressure lability to cause tissue ischemia (76–78). Indeed, kidneys from hypertensive rats with renal ablation frequently showed tubules with a “regenerative” phenotype, suggesting that epithelial cells were proliferating in response to acute injury. Tubules in these areas showed crowding of undifferentiated epithelial cells with hyperchromatic and pleomorphic nuclei (Fig. 4, G and H). That ongoing acute injury occurs in these foci was shown by the presence of apoptotic cells (Fig. 4H). In contrast, both hypertrophied tubules of kidneys in nonhypertensive rats with renal ablation induced by surgical excision and tubules of kidneys from hypertensive rats without renal ablation were devoid of such lesions, maintained their integrity, and displayed a fully differentiated phenotype (Figs. 4, I and J). These findings raise the interesting possibility that “piecemeal” AKI in microscopically small ischemic foci contributes to disease progression in the hypertensive nephrectomy model. Interestingly, tubules affected in this manner were located within well-defined zones of interstitial fibrosis that were more often than not separated by and demarcated sharply from adjoining areas of hypertrophic kidney with little or no injury. This topological characteristic of tubulointerstitial fibrotic lesions suggests a vascular distribution; i.e., that they resulted from ischemia or hypoxia caused by pathology at the level of arterioles.
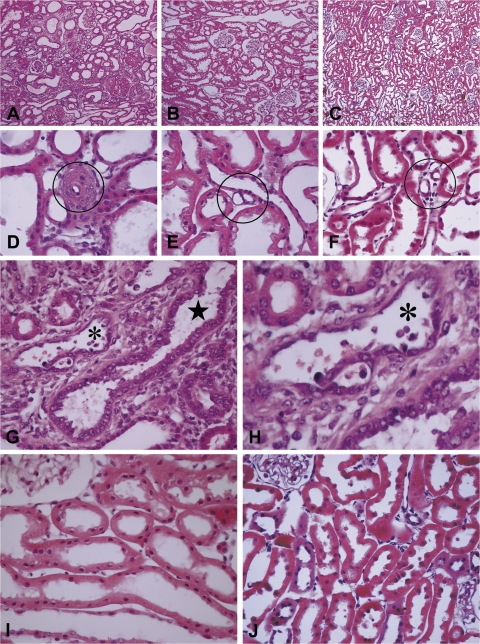
Fig. 4.
Sections of hypertrophied kidneys from rats subjected to nephrectomy by right nephrectomy and either ligation of 2 of 3 branches of the left renal artery (A, D, G, and H) or surgical excision of both poles of the left kidney (B, E, and I) and sections of intact kidneys of spontaneously hypertensive stroke prone (SHR-SP) rat (C, F, and J). Kidneys were obtained ∼8 wk after nephrectomy or after a comparable period of observation from SHR-SP rats. For the rats from which these sections were obtained, the averaged systolic blood pressures measured by radio telemetry over a 4-wk period preceding death were (in mmHg) nephrectomy (infarction) 214; nephrectomy (excision) 133; and SHR-SP 214. At death, kidneys were perfused with Karnovsky's fixative. Paraffin-embedded sections were stained with hematoxylin and eosin. A–C: micrographs to show presence or absence of tubulointerstitial fibrosis. D–F: micrographs to show presence or absence of occlusive arteriolosclerosis (circled). G–J: micrographs to show presence or absence of tubules with regenerative epithelium (star) and acute injury with apoptotic cells (asterisk). Original magnifications: ×100 (A–C); ×600 (D–F); ×400 (G, I, and J). H: magnified view of tubule marked with small asterisk in G. Unpublished archival material from Bidani-Griffin laboratories was evaluated by M. Venkatachalam. This material is related to but not derived from published work that examined the effects of hypertension alone, renal ablation alone or renal ablation with hypertension on the development of glomerular pathology (19, 56, 59).
Interstitial Inflammation After AKI is Likely to be Downstream of Primary Tubule Pathology and Driven by Tubule-Located Processes
How does AKI affect the progression of CKD? It is common knowledge among nephrologists that AKI is sometimes followed by incomplete recovery (34, 37, 42, 60, 65, 125, 141) and that tubulointerstitial fibrosis is the pathological counterpart of residual functional loss (37, 41, 42, 45). By far, the universally suspected culprit in the causation of interstitial fibrosis after AKI is inflammation. AKI is attended by inflammation characterized by neutrophil cell exudation in its acute phase and a monocytic-lymphocytic infiltrate in later stages as tubules regenerate (25, 26, 37, 73, 83, 133). The early neutrophilic response seen after ischemia (25, 26, 37) is a passing phase that may contribute to injury (83). On the other hand, delayed mononuclear cell infiltration correlates better with pathology during the established and recovery stages of the disease and is also a major factor involved in repair, regeneration, and tissue remodeling (25, 26, 32, 37, 83). Monocytes potentiate injury after ischemia as well as promote fibroblast proliferation and fibrosis; consequently, inflammation-related mechanisms of fibrosis have become an important target for research to develop antifibrotic drugs (32). But what are the proximate stimuli that lead to the phlogistic response and fibrosis? Intuitively, it would seem that drug targeting to prevent or ameliorate proximal events that elicit inflammation might be more effective than anti-inflammatory therapy itself. Endothelial activation and injury during reperfusion after ischemia is a potential trigger for inflammation. However, a mononuclear cell infiltrate is also invariably associated with selective tubule epithelial injury caused by nephrotoxins. For example, we have seen that proximal tubule selective ATP depletion and injury caused by the organic anion maleate (165) induces an interstitial inflammatory response with intense mononuclear cell infiltration indistinguishable from that caused by ischemia-reperfusion (Venkatachalam MA and Lan R, unpublished observations). Unlike ischemia-reperfusion injury, maleate nephrotoxicity occurs without a vascular perfusion defect (165), the pathophysiology that activates or injures endothelial cells. Perhaps the strongest case for tubule-specific effects in the causation of interstitial pathology was made by Fujigaki et al. (47), who investigated the relationships of interstitial myofibroblasts to tubule pathology after AKI induced by uranyl acetate. This study showed that myofibroblasts increased in number around tubules that were regenerating after acute injury caused by the nephrotoxin but not around immediately adjacent tubules that had not been damaged. Myofibroblasts were closely apposed to tubules with epithelial cells in DNA synthesis shown by [3H]thymidine uptake (47). These considerations suggest that tubule cells in addition to endothelial cells and perhaps more than endothelial cells are responsible for inflammation and fibrosis associated with tissue remodeling after AKI.
Capillary Rarefaction, an Important Factor that Drives a Final Common Pathway to ESRD According to the “Chronic Hypoxia Hypothesis,” May Occur Downstream of Tubule-Located Pathology
Considerable attention is devoted to endothelial signaling events that may be related to fibrosis. Capillary endothelium may be the site of signaling abnormalities initiated by ischemia or hypoxia; on the other hand, capillary rarefaction in fibrotic foci can give rise to tissue hypoxia or potentiate preexisting hypoxia (13, 14, 40, 90, 107, 145). Rarefaction of capillaries in foci of tubulointerstitial fibrosis in experimental as well as human CKD (13, 23, 33, 90, 116) is thought contribute to CKD progression by giving rise to signaling by hypoxia-inducible factors and other pathways that augment interstitial inflammation and fibrosis in a vicious cycle where capillary rarefaction, hypoxic signaling, and diverse hypoxia targets in the tubulointerstitium mutually reinforce each other in a final common pathway to ESRD. The prevailing view is that capillary rarefaction is caused by loss of angiogenic stimuli in foci of tubulointerstitial fibrosis. Indeed, treatment with angiogenic vascular endothelial growth factor (VEGF-121) was reported to ameliorate capillary rarefaction and fibrosis after ischemic injury (90). However, tubule epithelium is normally a source for VEGF which becomes decreased, and for the VEGF inhibitor ADAMTS-1 that becomes increased after ischemia (14). Moreover, tubule VEGF is decreased in human CKD together with increased hypoxic signaling (131), consistent with a scenario in which a primary tubule abnormality causes decreased production and secretion of VEGF and gives rise to capillary rarefaction. According to this scheme, interference at the level of deranged signaling in tubule cells could abort entire downstream events, capillary rarefaction as well as inflammation. We believe, therefore, that investigation of processes located in damaged and regenerating tubules that are likely to be the earliest triggers for downstream pathologies should be prioritized while research on inflammation, capillary rarefaction, and fibroblast proliferation continues.
Tubulointerstitial Fibrosis after AKI May be Caused by Amplified Epithelial Signaling Initiated Physiologically as an Early Repair Response but Persisting Pathologically in Regenerating Tubules that Fail to Differentiate
Experimental AKI is associated with enhanced production of a variety of peptides involved in repair, regeneration, and remodeling; many are also phlogistic and profibrotic (25, 26, 37, 73, 83, 129, 133). Injured and regenerating tubule cells in addition to endothelial or interstitial cells and subsequently recruited inflammatory cells can be the source of these molecules (25). Indeed, it is well known that tubule regeneration after AKI involves rapid and intense activation of several signaling pathways in injured and regenerating epithelial cells accompanied by the production and secretion of growth factors, cytokines, and sundry mediators of inflammation. These responses are protean and complex but appear to be integral components of an orchestrated repair response (24, 25, 37, 61, 133). Several of these bioactive molecules have autocrine as well as paracrine functions. They play important roles as autocrine factors in the epithelial dedifferentiation, migration, and proliferation required for tubule regeneration. As paracrine factors, they are likely to be chemotactic for circulating leucocytes either directly or indirectly by activating the endothelium in adjacent capillaries. Thus they could be the proximate factors that initiate inflammation before it becomes self-sustaining and gives rise to more acute injury in the short term or, by its effects on pericytes and perivascular fibroblasts (92), to interstitial fibrosis in the long term. In addition, tubule-derived factors could very well activate interstitial fibroblast progenitors, directly causing them to migrate and proliferate. These considerations lead to the hypothesis that hyperactive signaling and the production of diverse growth factors, cytokines, and other mediators in regenerating epithelium have adverse consequences, i.e., inflammation and fibrosis, in addition to their beneficial and required effects to make regeneration possible. Anti-inflammatory interventions generally decrease the severity of experimental AKI in its acute phase, and such interventions also ameliorate tubulointerstitial fibrosis (32). On the other hand, the signals that initiate inflammation are located in injured tubules. Therefore, it seems to us that interventions directed at critical signaling nexuses in activated epithelial cells might likely abort entire pathological processes and thereby reduce injury and fibrosis even more effectively than treatments focused on limited downstream events. However, many signaling pathways become simultaneously activated in regenerating epithelium, and it has been difficult to identify and characterize the critical ligands, receptors, and signaling intermediates that may be involved in the production and secretion of phlogistic and profibrotic peptides by tubules. Be that as it may, these observations lead to a fundamental question: assuming that regenerating epithelium is the site of proliferation-related and signaling-directed secretion of potentially injurious bioactive molecules during the acute and early recovery phase of AKI, how do these phenomena relate to the development and persistence of tubulointerstitial fibrosis, long after the tubular epithelium should have recovered? Answers to this vexing question must be sought in the physiology of the regenerative response and how it becomes pathologically perturbed during recovery.
Loss of tubule epithelium during AKI is followed by dedifferentiation, migration, and proliferation of survivor cells. Healthy regeneration after AKI should involve not only the cessation of cell division by contact inhibition but also redifferentiation. Tubule cells proliferate vigorously after AKI, but recovery of normal structure is frequently incomplete. Abnormalities that may beset the regenerating tubule and compromise structural recovery include perturbations of the cell cycle and failed differentiation.
Intriguing and important roles for perturbed cell cycle regulation in kidney tubules involving the cdk inhibitor p21 were revealed by several insightful studies (52, 127, 128). In the context of the immediate recovery period, induction of p21 has a protective role and allows proper recovery, and prevention of p21 induction worsens AKI and compromises recovery (127, 128, 169). However, it would appear that this poorly understood protein is Janus faced: in the context of reduced renal mass, it has an adverse impact on the eventual outcome of ESRD and genetic deletion of the protein is in fact protective (101). The reasons p21 is permissive for proper tubule regeneration after AKI but imposes growth arrest and hypertrophic stress on tubules in kidneys with reduced mass are not known. However, these curious differences are relevant to how tubule abnormalities may occur in the setting of AKI superimposed on decreased kidney mass. The discrepancies are in keeping with the multifaceted functional aspects of p21 and point out the need for further studies to explore whether abnormal regulation of this protein has a role to play as the regenerating tubule, unable to reconstitute normal structure, transitions toward the so called “atrophic” tubule present in foci of tubulointerstitial fibrosis.
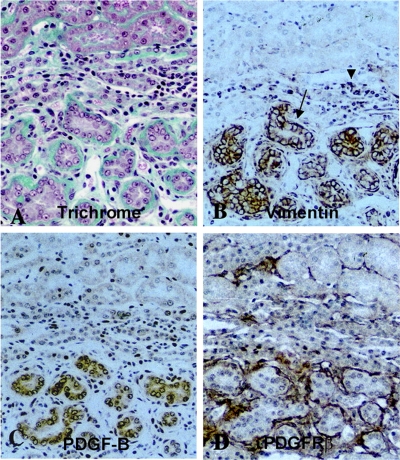
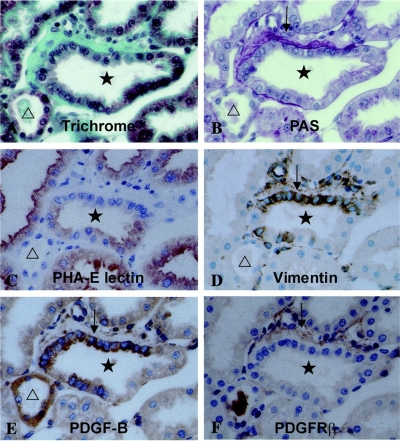
As may be predicted by the known effects of available renal mass on recovery from AKI (9, 10, 94–96), and as discussed below, tubule stress imposed by reduced renal mass may impair the regenerative process by compromising epithelial redifferentiation. Failed differentiation is likely to have several adverse consequences. An obvious and important consequence is impaired transport and homeostatic function. A more insidious and largely ignored consequence is the persistence of attributes that characterize the undifferentiated proliferating phenotype. A well-documented example of this abnormality was reported by Suzuki et al. (144). They induced experimental CKD with focal tubulointerstitial fibrosis by embolizing acrylic microspheres in afferent arterioles of rat glomeruli. Pathologically, these kidneys showed focal fibrotic zones with clusters of “atrophic” proximal tubules alternating with nonfibrotic areas containing normal tubules. Segments of atrophic tubules with undifferentiated epithelium that were surrounded by greatly thickened basement membranes and extracellular matrix shown by Trichrome staining (Fig. 5A) showed strongly increased immunohistochemical staining for platelet-derived growth factor B chain (PDGF-B) (Fig. 5C), whereas the adjacent expanded interstitium contained numerous cells with branching processes showing increased expression of PDGF receptor-β (PDGFR-β), the cognate receptor for PDGF-B (Fig. 5D). Tubules with PDGF-B immunoreactivity were also positive for vimentin (Fig. 5B), the intermediate filament protein that is normally not present in differentiated proximal tubule cells but is readily induced when they assume the regenerative phenotype after injury (156, 159). In contrast, better differentiated normal tubules did not show PDGF-B or vimentin staining (Fig. 5). This type of pathology was particularly well illustrated by images of “mosaic” tubules containing undifferentiated epithelium juxtaposed sharply with cells that reacted with the differentiation marker Phaseolus vulgaris agglutinin (PHA-E lectin) (Fig. 6C). In these tubules, undifferentiated epithelial cells expressing vimentin (Fig. 6D) and PDGF-B (Fig. 6E) were bordered by increased Trichrome staining fibrous tissue (Fig. 6A), thick reduplicated periodic acid-Schiff-positive basement membrane (Fig. 6B), and PDGFR-β-positive fibroblasts (Fig. 6F); in sharp contrast, within the same cross section of tubule, adjacent better differentiated epithelium did not express either PDGF-B or vimentin, and the bordering interstitium showed little if any basement membrane thickening, accumulation of extracellular matrix, or proliferation of PDGFR-β-reactive fibroblasts (Fig. 6). Of interest, PDGFR-β-positive interstitial cells were also reactive for vimentin (Fig. 6, D and F) and α-smooth muscle actin (144) (not shown), indicating that these cells were myofibroblasts. Almost identical patterns of expression of PDGF-B in vimentin-positive tubules and PDGFR-β in α-smooth muscle actin-containing interstitial cells were reported by Kimura et al. (82) to occur in foci of tubulointerstitial fibrosis caused by renal ablation. Kliem et al. (84) also found increases of PDGF-B and PDGFR-β in foci of tubulointerstitial fibrosis 2 and 4 wk after nephrectomy. The close spatial relationship between undifferentiated epithelium expressing the profibrotic peptide PDGF-B and interstitial fibroblasts expressing the cognate receptor PDGFR-β suggests the operation of a paracrine ligand-receptor couple that may have been responsible for fibroblast proliferation and collagen deposition in these CKD models. This inference is consistent with the observation that infusions of PDGF-B but not PDGF-A induce the proliferation of α-smooth muscle actin-positive fibroblasts and collagen deposition in the renal interstitium (146).
Fig. 5.
Sections from kidneys of rats microembolized through the left renal artery with 20- to 30-μm-diameter acrylic microspheres and examined 4 wk later. Serial paraffin sections of methyl Carnoy's fixed tissue were stained with Masson's trichrome (A) or immunohistochemically stained for vimentin (B), PDGF-B (C), or PDGF receptor (PDGFR)-β (D). Original magnification ×200. Reproduced from Ref. 144 with kind permission from Dr. Akira Hishida, first department of Medicine, Hamamatsu University School of Medicine, Hamamatsu, Japan. Reproduced from Am J Pathol 158: 75–85, 2001; with permission from the American Society for Investigative Pathology.
Fig. 6.
Sections from kidneys of rats microembolized through the left renal artery with 20- to 30-μm-diameter acrylic microspheres and examined 4 wk later. Serial paraffin sections of methyl Carnoy's fixed tissue were stained with Masson's trichrome (A), periodic acid-Schiff (B), Phaseolus vulgaris agglutinin, a marker differentiated for proximal tubule cells (C), or immunohistochemically stained for vimentin (D), PDGF-B (E), and PDGFR-β (F). Original magnification ×400. All images show the same tubule cross section with adjacent interstitium in serial sections. Reproduced from Ref. 144 with kind permission from Dr. Akira Hishida, first department of Medicine, Hamamatsu University School of Medicine, Hamamatsu, Japan. Reproduced from Am J Pathol 158: 75–85, 2001; with permission from the American Society for Investigative Pathology.
That epithelial signaling activated acutely in response to injury may persist late during recovery at a time when it should become suppressed is supported by recent findings by de Borst et al. (36). They found that c-Jun NH2-terminal kinase (JNK) became activated in tubule cells early during reperfusion after ischemia as reported before (123) but that the activation persisted for an extended period, as late as 21 days following the insult. JNK activation was assessed by immunostaining for phospho-JNK and the phosphorylated form of its substrate c-Jun. The authors report that activation of the JNK pathway correlates with interstitial inflammation; however, inspection of the illustrations suggests that persistent JNK activation is also associated with tubulointerstitial fibrosis (36).
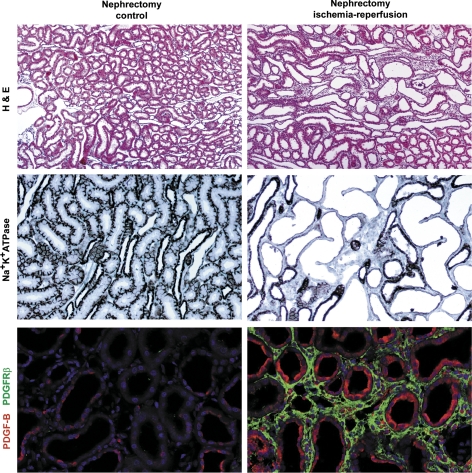
The concept that failed differentiation accounts for the development of an abnormal tubule phenotype in areas of tubulointerstitial fibrosis is supported by the findings of Geng et al. (48). In this study, ischemic AKI was induced by 45 min of ischemia of one kidney after removal of the other kidney to reduce renal mass by 50%. Despite recovery from azotemia 2 wk after reperfusion and despite histological evidence for vigorous proliferation, proximal tubules in areas that had been previously damaged failed to differentiate. In contrast to surrounding tubules that did recover normally, these abnormal tubules displayed greatly decreased expression of differentiation markers Na+-K+-ATPase, ksp-cadherin, and meprin (48). (Myo)fibroblasts had commenced proliferating in the interstitium adjacent to injured and regenerating tubules by 3 days of reperfusion, but, instead of regressing as might be expected for healthy repair, they continued to proliferate around the tubules that failed to differentiate. As a consequence, by 2 wk of reperfusion, foci of tubulointerstial fibrosis became established (Fig. 7, top right), and, in the same areas, tubule cells showed a flattened undifferentiated phenotype with greatly decreased or absent immunostaining for differentiation markers (shown for Na+-K+-ATPase in Fig. 7, middle right). As confirmation of the findings of Suzuki et al. (144), who used a microembolization model to produce CKD, foci of tubulointerstitial fibrosis 2 wk after ischemia-reperfusion (48) showed increased expression of PDGF-B in undifferentiated tubule epithelium and PDGFR-β in adjacent interstitial fibroblasts (Fig. 7, bottom right; Venkatachalam MA and Lan R, unpublished observations).
Fig. 7.
Sections of kidneys 14 days after right nephrectomy to remove 50% of renal mass and 45 min of ischemia of left kidney followed by reperfusion (right) or corresponding nonischemic nephrectomy controls (left). Paraffin sections of tissue perfusion fixed with periodic acid-lysine-paraformaldehyde were stained with hematoxylin and eosin (H&E; top) or immunohistochemically stained for the differentiation marker Na+-K+-ATPase (middle) or PDGF-B (red) and PDGFR-β (green; bottom). The images are from the outer stripe of the outer medulla, chief site of original ischemic damage. Top and middle: reproduced from Ref. 48 with permission from the American Society for Investigative Pathology. Bottom: unpublished archival tissue from Ref. 48. Original magnifications: ×100 (top); ×200 (middle and bottom).
We infer from the findings of Suzuki et al. (144) that increased PDGF-B in atrophic tubules of fibrotic foci represents the pathological persistence of a phenotypic alteration that tubules had undergone during an earlier physiological proliferative response to the needs of regeneration. If regenerating tubules had recovered physiologically and reconstituted their normal phenotype, PDGF-B should have returned to baseline levels. This would be predicted by the findings of Nakagawa et al. (105), who found that PDGF-B and PDGF receptors are expressed early in proximal tubules as autocrine signaling responses to ischemic AKI, but subside back to baseline levels by 1 wk of reperfusion. Our findings shown in Fig. 7 support the argument that failure to suppress PDGF-B expression in tubules after recovery should have been complete represents the persistence of undifferentiated epithelium that continues to display some aspects of the earlier proliferative phenotype. Taken to the next logical step, we propose that persistence of PDGF-B expression in undifferentiated epithelium in foci of tubulointerstitial fibrosis is only one aspect of a larger pathophysiology that often besets tubule cells late during regeneration. Stated broadly, failure by regenerating epithelium to differentiate normally is likely due to a broad spectrum of amplified signals that are physiologically initiated as early reparative responses but pathologically persist late during recovery and suppress differentiation.
What Proximate Signals Initiated by Tubule Epithelial Cells Give Rise to Production and Secretion of Inflammation Mediators and Profibrotic Peptides?
A confusing variety of signaling pathways in diverse cell types become activated during different stages of the evolution of tubulointerstitial fibrosis. This has been lucidly summarized by Schlondorff (135). As outlined in the previous section, the same is true for AKI, a disease with potential to cause tubulointerstitial fibrosis. Signaling alterations in AKI are of two broad categories. One involves the activation of signaling in injured or regenerating tubules. These signals result in the generation of both autocrine and paracrine factors. Some of these molecules have dual autocrine and paracrine functions. As indicated earlier, paracrine peptides derived from tubules could recruit and mobilize leukocytes as well as activate capillary endothelial cells and fibroblast progenitors. The other category includes a variety of cytokines, growth factors, and other mediators produced by inflammatory and immune cells, endothelial cells, and fibroblasts in a self-sustaining cycle of mutual interactions between these cell types as well as tubule cells. For this reason, once interstitial pathology is initiated, it becomes difficult to separate proximal and downstream signaling events, owing to the interactive nature of the pathophysiology. In this context, at least from the perspective of fibrosis that follows AKI, it is seems reasonable to postulate that early signaling events in tubule epithelium are likely to have a determining role since they are proximal both to the production of paracrine factors by tubules and to the subsequent and downstream pathophysiology in the interstitium. Thus, in a primary sense, fibrosis follows tubule damage and not the other way around, as emphasized by Kriz and LeHir (87) and more recently by Cook (35). There is a caveat to this line of reasoning, however. Both in overt ischemic AKI as well as in microscopically small foci of AKI in the remnant kidney caused by hypertensive arteriolosclerosis and/or blood pressure fluctuations (see discussion on Fig. 4), capillary endothelium can become damaged and activated independently of tubule signaling and give rise to inflammation in its own right. However, as emphasized earlier, interstitial inflammation and fibroblast proliferation are readily seen following selective injury of tubules also. These complexities notwithstanding, the considerations we have outlined lead us to believe that interventions directed at proximal signaling events in tubules have the potential to abort entire pathological processes that lead to tubulointerstitial fibrosis, in contrast to approaches that address downstream signaling events in leukocytes, immune cells, endothelial cells, or fibroblasts. This assumption is also predicated on our belief that regenerating tubules that fail to differentiate after episodic AKI and give rise to the so-called atrophic tubule are a fundamentally important aspect of pathology that drives fibrosis. Conjecturally, the atrophic phenotype evolves as actively proliferating tubule epithelium that fails to differentiate under conditions of stress and therefore is the site of active signaling that had been initiated earlier. These signals continue to drive the production and secretion of growth factors, cytokines, and other mediators, a regulated process that should have been suppressed otherwise as regenerating cells differentiate normally upon reaching contact inhibition. After an indefinite period of time, these abnormally signaling tubules are likely to finally become silent with respect to signaling, become truly atrophic, and even undergo atresia by autophagy and apoptosis, and, eventually, resorption (See Fig. 1). It must be emphasized at this point that there is a pressing need to characterize the phenotypic alterations that tubules undergo as they evolve to become atrophic. Tubule atrophy has been characterized morphologically in contexts where CKD has been initiated by a primary glomerular disease as well as in the microembolization rat model of tubulointerstitial fibrosis (46, 87). However, the information remains sketchy, particularly in settings of chronic injury caused by reduced renal mass and the residual damage that follows AKI. Moreover, the biochemical underpinnings, cell cycle alterations, and signaling contexts of the transition of regenerating cells to a state of failed differentiation and then on to a possibly senescent phenotype that we believe typifies residual pathology after AKI remain to be characterized.
Current understanding of epithelial signals that lead to the production and secretion of phlogistic and profibrotic paracrine factors by tubules is limited. We do know from a large body of published work that cultured tubule epithelial cells secrete potentially important cytokines, growth factors, and other bioactive molecules in quantities that we would not expect normal fully differentiated tubules to produce in vivo. The inference here is that proliferating epithelium in culture resembles the regenerative phenotype in vivo. Following damage in human and experimental AKI, surviving epithelial cells dedifferentiate, migrate and proliferate, analogous to the behavior of epithelial cells released from contact inhibition in culture. There is substantive as well as suggestive evidence for the activation and/or participation of a plethora of signaling pathways in tubules as they recover from AKI. Signals via Toll-like receptors (TLR), receptor tyrosine kinases, G protein-coupled receptors (GPCR), peroxisome proliferator-activated receptor-α (PPARα), and transforming growth factor-β (TGF-β) receptors are likely to participate in the pathophysiology of AKI through the NF-κB-, phosphatidylinositol 3-kinase-, MAPK-, and Smad-dependent pathways (7, 36, 48, 68, 69, 75, 81, 88, 89, 91, 102, 105, 114, 117, 119, 123, 129, 142, 163, 164, 166–168). Signals may be protective as well as injurious or restorative in their effects. Signaling that results in PPARα activation protects against the development of AKI (91), whereas induction of heme oxygenase-1 not only limits the extent of damage but mitigates inflammation and tubulointerstitial fibrosis (74, 151). In general, signaling through these multiple pathways is elevated early and subsides with recovery from AKI. It has been surmised that these confusingly numerous and diverse alterations regulate epithelial dedifferentiation, migration, and proliferation. Little information is available regarding how the signaling becomes activated. An understanding of how they relate to each other and coordinately orchestrate the regenerative process is lacking. Moreover, there is a paucity of knowledge with respect to how these signaling pathways control the production of paracrine molecules by tubules and complete lack of information on how injury-initiated signaling either becomes normally suppressed by differentiation or abnormally persists in cells that fail to differentiate. This is largely due to the multitude of activated intermediates in diverse pathways that need to be put in proper context and a formidably complex interacting signaling network that does not lend itself easily to systems analysis. Redundancy of signaling in addition to complexity accounts for the lack of clarity. Therefore, there is a need to develop newer approaches to look at signaling interactions in an integrated fashion.
Regardless of the confusion that surrounds injury-initiated signaling and in accordance with the central hypothetical theme under discussion, we would repeat that abnormally persistent signaling leading to continued production of paracrine outputs such as PDGF-B in recovering tubules with impaired differentiation (Figs. 5–7) is the likely root cause of tubulointerstitial fibrosis. PDGF-B is unlikely to be the only molecule in the gamut of tubule-derived factors that potentially cause interstitial inflammation and fibrosis. As referenced above, tubules can secrete a variety of cytokines, growth factors, and other mediators when appropriately stimulated. Little is known about the roles played by tubule-derived molecules in the pathogenesis of tubulointerstitial fibrosis. Even the persuasive case that we make for the PDGF-B/PDGFR-β couple is based on circumstantial evidence provided by static immunohistochemical images. Therefore, the strong case that we make for tubule-derived paracrine factors in tubulointerstitial fibrosis must remain hypothetical, albeit persuasive and deserving of future research.
Among signaling pathways in tubules that are potentially fibrogenic, the best case has been made for the activity of receptors for TGF-β, CSF-1, and EGF family of ligands (48, 102, 142, 149, 166). Little is known, however, regarding the injury-related mechanisms that activate these receptors. It is conceivable that GPCR activation by lysophosphatidic acid plays a role as demonstrated for fibrosis in obstructive uropathy (126). Even if this were the case, we must seek the very proximate activation mechanisms that trigger the possibly sequential and cascading increases in signaling activity along multiple pathways. For, as we know, AKI is rapidly followed by almost simultaneous activation of a bewilderingly large array of signaling pathways. Release of molecules such as ATP and damage-associated molecular patterns (DAMPs) (15) by injured epithelial cells and their binding to the cognate GPCRs and TLR/coreceptor complexes are likely to be among the earliest events that determine the activation of cells that survive. DAMP-mediated TLR signaling and cytokine release were shown to mediate graft dysfunction after kidney transplantation (88), but it was not determined whether such signaling bears any relationship to the activation of other signaling pathways. The sketchiness of our knowledge of basic facts in this critical and clinically important context emphasizes the need for intensive research using in vivo models of AKI that progress to tubulointerstitial fibrosis as well as in vitro models of regeneration in culture to characterize the signaling pathways that give rise to the production and secretion of paracrine molecules by tubule epithelial cells. Because the signaling networks are so complex and so many pathways become simultaneously activated in regenerating cells, it is going to be important to develop approaches to identify critical nexuses where these pathways may intersect so that we may identify the most effective molecular targets for pharmacological interventions to temper paracrine activity.
Summary and Conclusions
Carefully done outcome analysis and epidemiological studies published during the last few years have yielded remarkable insights into the relationships between the two most important clinical syndromes in nephrology practice: AKI and CKD (118). By mutually reinforcing the severity of the other, mechanisms yet to be characterized lead to the acceleration of disease progression. The AKI-CKD nexus underlies a pattern of progression that is not necessarily linear as it was originally thought but is punctuated by breakpoints of rapidly worsening disease status. Identification of the AKI-CKD nexus represents the single most important advance in understanding of the mechanisms of progression since hyperfiltration was shown to occur following renal ablation and chronic nephropathy was studied in this model by Morrison and colleagues (104, 140).
Animal studies have shown that reduced renal mass has a negative impact on repair and recovery following ischemic injury (9, 10, 94–96). Although AKI in itself can be followed by residual structural deficits, reduced renal mass impairs recovery to a greater extent and increases the severity of tubulointerstitial fibrosis that follows. These early observations provide experimental support for recently recognized interactions between AKI and CKD that adversely affect human kidney disease progression. Similarly to reductions of renal mass that obtain in CKD, diabetic- and aging-related pathology are also expected to predispose kidneys to develop acute injury. Diabetes and aging are not covered in this review because of the major scope of those fields, but some of the mechanisms being proposed here are undoubtedly relevant to the progression of CKD that occurs in those settings as well.
The impact of nutrition on kidney disease progression needs reevaluation. Caloric intake rather than protein consumption affects progression. Protein intake has little impact beyond the fact that animal protein adversely affects kidney disease progression compared with vegetable protein (99, 124). The animal protein casein used in experimental diet formulations has large effects on renal hemodynamics, hypertrophy, and kidney pathology, whereas isocaloric soy protein consumption has much less if any effects on these parameters. Consumed in large amounts, animal protein is nephrotoxic. Conceivably, these differences bear upon acute injury and repair in the setting of reduced renal mass. Therefore, the effects of protein source and quality on experimental renal disease need to be reexamined in light of studies showing that calories rather than protein have large effects on nephropathy and progression.
Development of tubulointerstitial fibrosis is governed by hypertension in models of nephropathy caused by renal ablation. In a nephrectomy model without hypertension, fibrosis does not develop or is markedly delayed. However, in a nephrectomy model with hypertension, occlusive arteriolosclerosis develops, accompanied by tubulointerstitial fibrosis. In fibrotic lesions, tubules are undifferentiated and display regenerative activity and focal apoptosis. Given that myogenic autoregulation of blood flow is impaired in both models, the findings imply that unregulated transmission of raised arterial hydrostatic pressure is responsible for arteriolosclerosis and consequent tubulointerstitial fibrosis. In contrast, SHR with intact kidneys that autoregulate normally do not develop arteriolosclerosis or tubulointerstitial fibrosis. Thus neither hypertension nor reduced renal mass is alone sufficient to cause rapid progression (16, 19, 20, 54, 56). The histopathology of progression in the hypertensive nephrectomy model suggests that recurring acute tubule damage in a multiplicity of microscopic foci contributes to disease progression even without overt clinical episodes of AKI. The nature of these focal lesions needs to be characterized as they develop acutely and then progress through stages of epithelial proliferation and impaired differentiation to become fibrotic scars.
Emerging evidence suggests that the proximate pathology that leads to tubulointerstitial fibrosis is “failed differentiation” in regenerating tubules, likely caused by stress related to reduced renal mass or the severity of the injury itself. Analogous to proliferating tubule epithelium, undifferentiated tubule cells in areas of tubulointerstitial fibrosis may be the seat of regenerative signals that are initiated as repair responses but persist pathologically and maintain phlogistic and profibrotic paracrine activity. Once fibrosis becomes established, it may progress further, owing to a vicious cycle of signaling and paracrine interactions between inflammatory cells, endothelial cells, fibroblasts, and epithelial cells that is made worse by capillary rarefaction and hypoxia. These concepts are pictorially summarized in Figs. 1 and 2. Much research is focused on these mechanisms and interventions aimed at monocytes, endothelial cells, and fibroblast progenitors. Tissue hypoxia that is made more intense by fibrosis is a particularly attractive target. Based on the data summarized in this review, there is also a pressing need to understand the most proximate pathology that leads to tubulointerstitial fibrosis, i.e., hyperactive signaling by the regenerating tubule that is trying to recover and fails to differentiate. We suggest that research in this area be targeted as a high priority because it is entirely plausible that interventions directed at critical signaling nexuses in undifferentiated tubules have the potential to abort entire downstream cascades of paracrine activity that cause inflammation and fibrosis.
GRANTS
Research by the authors was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK37139 (M. A. Venkatachalam), DK54472 (P. Saikumar), DK-61653 (K. A. Griffin), and DK-40426 (A. K. Bidani); the Veterans Affairs Administration (K. A. Griffin), and the Morrison Trust (P. Saikumar).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank our colleagues with interest in acute kidney injury and chronic kidney disease who patiently listened to our ideas and persuaded us to write this review.
REFERENCES
- 1.Abrams JR, Tapp DC, Venkatachalam MA. Dietary influence and pathologic changes. In: Progressive Nature of Renal Disease, edited by Mitch WE. New York: Churchill Livingstone, 1992, p. 133–148 [Google Scholar]
- 2.Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol 24: 46–53, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Abrass CK. Overview: obesity: what does it have to do with kidney disease? J Am Soc Nephrol 15: 2768–2772, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F305–F313, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Al-Awqati Q, Preisig PA. Size does matter: will knockout of p21(WAF1/CIP1) save the kidney by limiting compensatory renal growth? Proc Natl Acad Sci USA 96: 10551–10553, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JW, Blake JE, Turner J, Smith BM. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am J Clin Nutr 68: 1347S–1353S, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Andreucci M, Michael A, Kramers C, Park KM, Chen A, Matthaeus T, Alessandrini A, Haq S, Force T, Bonventre JV. Renal ischemia/reperfusion and ATP depletion/repletion in LLC-PK1 cells result in phosphorylation of FKHR and FKHRL1. Kidney Int 64: 1189–1198, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Azuma H, Nadeau K, Takada M, Mackenzie HS, Tilney NL. Cellular and molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation 64: 190–197, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Azuma H, Nadeau K, Takada M, Tilney NL. Initial ischemia/reperfusion injury influences late functional and structural changes in the kidney. Transplant Proc 29: 1528–1529, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Bakris GL, Ritz E. The message for World Kidney Day 2009: hypertension and kidney disease: a marriage that should be prevented. Clin J Am Soc Nephrol 4: 517–519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakumar P, Arora MK, Reddy J, Anand-Srivastava MB. Pathophysiology of diabetic nephropathy: involvement of multifaceted signalling mechanism. J Cardiovasc Pharmacol 54: 129–138, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens 11: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol Renal Fluid Electrolyte Physiol 265: F391–F398, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Bidani AK, Griffin KA, Plott W, Schwartz MM. Renal ablation acutely transforms ‘benign’ hypertension to ‘malignant’ nephrosclerosis in hypertensive rats. Hypertension 24: 309–316, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs. “dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1003–F1010, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Bohle A, von Gise H, Mackensen-Haen S, Stark-Jakob B. The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Functional interpretation of morphologic findings. Klin Wochenschr 59: 1043–1051, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 1: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Bourgoignie JJ, Gavellas G, Martinez E, Pardo V. Glomerular function and morphology after renal mass reduction in dogs. J Lab Clin Med 109: 380–388, 1987 [PubMed] [Google Scholar]
- 28.Bourgoignie JJ, Gavellas G, Sabnis SG, Antonovych TT. Effect of protein diets on the renal function of baboons (Papio hamadryas) with remnant kidneys: a 5-year follow-up. Am J Kidney Dis 23: 199–204, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Brenner BM, Anderson S. The Gordon Wilson lecture. Why kidneys fail: an unifying hypothesis. Trans Am Clin Climatol Assoc 98: 59–70, 1987 [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]
- 31.Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens 12: 273–282, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Castaño AP, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Duffield JS. Serum amyloid P inhibits fibrosis through FcgR-dependent monocyte-macrophage regulation in vivo. Sci Transl Med 1: 1–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim BK, Shim SI, Suki WN, Truong LD. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol 31: 1491–1497, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook HT. The origin of renal fibroblasts and progression of kidney disease. Am J Pathol 176: 22–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Borst MH, Prakash J, Sandovici M, Klok PA, Hamming I, Kok RJ, Navis G, van Goor H. c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J Pharmacol Exp Ther 331: 896–905, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol 17: 2964–2966, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Finco DR, Brown SA, Brown CA, Crowell WA, Cooper TA, Barsanti JA. Progression of chronic renal disease in the dog. J Vet Intern Med 13: 516–528, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Finn WF. Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ Res 46: 440–448, 1980 [DOI] [PubMed] [Google Scholar]
- 42.Finn WF. Recovery from acute renal failure. In: Acute Renal Failure (3rd ed.), edited by Lazarus J, Brenner BM. New York: Churchill Livingstone, 1993, p. 553–596 [Google Scholar]
- 43.Fioretto P, Caramori ML, Mauer M. The kidney in diabetes: dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia 51: 1347–1355, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int 57: 2375–2385, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Fox M. Progressive renal fibrosis following acute tubular necrosis: an experimental study. J Urol 97: 196–202, 1967 [DOI] [PubMed] [Google Scholar]
- 46.Fujigaki Y, Kimura M, Asano M, Suzuki T, Hishida A. Ultrastructure of tubular epithelial cells in response to microembolism-induced chronic ischemic injury in rats. Nephron Exp Nephrol 95: e144–e151, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Fujigaki Y, Muranaka Y, Sun D, Goto T, Zhou H, Sakakima M, Fukasawa H, Yonemura K, Yamamoto T, Hishida A. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch 446: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Geng H, Lan R, Wang G, Siddiqi AR, Naski MC, Brooks AI, Barnes JL, Saikumar P, Weinberg JM, Venkatachalam MA. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol 174: 1291–1308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giese J. Renin, angiotensin and hypertensive vascular damage: a review. Am J Med 55: 315–332, 1973 [DOI] [PubMed] [Google Scholar]
- 50.Goldby FS, Beilin LJ. How an acute rise in arterial pressure damages arterioles. Electron microscopic changes during angiotensin infusion. Cardiovasc Res 6: 569–584, 1972 [DOI] [PubMed] [Google Scholar]
- 51.Goldby FS, Beilin LJ. Relationship between arterial pressure and the permeability of arterioles to carbon particles in acute hypertension in the rat. Cardiovasc Res 6: 384–390, 1972 [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Michaca L, Farrugia G, Croatt AJ, Alam J, Nath KA. Heme: a determinant of life and death in renal tubular epithelial cells. Am J Physiol Renal Physiol 286: F370–F377, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Goss RJ. Hypertrophy versus hyperplasia. Science 153: 1615–1620, 1966 [DOI] [PubMed] [Google Scholar]
- 54.Griffin KA, Bidani AK. Hypertensive renal damage: insights from animal models and clinical relevance. Curr Hypertens Rep 6: 145–153, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol 294: F685–F696, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol 4: 2023–2031, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int 46: 1010–1018, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Griffin KA, Picken MM, Bidani AK. Blood pressure lability and glomerulosclerosis after normotensive 5/6 renal mass reduction in the rat. Kidney Int 65: 209–218, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Griffin KA, Picken MM, Churchill M, Churchill P, Bidani AK. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol 11: 497–506, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int 66: 523–527, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Hammerman M, Safirstein R, Harris R, Humes H. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol 279: F3–F11, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens 17: 266–270, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Hill GS, Heptinstall RH. Steroid-induced hypertension in the rat. A microangiographic and histologic study on the pathogenesis of hypertensive vascular and glomerular lesions. Am J Pathol 52: 1–40, 1968 [PMC free article] [PubMed] [Google Scholar]
- 64.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol Renal Fluid Electrolyte Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 65.Hsu CY. Linking the population epidemiology of acute renal failure, chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 16: 221–226, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol 4: 891–898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichimura T, Finch PW, Zhang G, Kan M, Stevens JL. Induction of FGF-7 after kidney damage: a possible paracrine mechanism for tubule repair. Am J Physiol Renal Fluid Electrolyte Physiol 271: F967–F976, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Ichimura T, Maier JA, Maciag T, Zhang G, Stevens JL. FGF-1 in normal and regenerating kidney: expression in mononuclear, interstitial, and regenerating epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 269: F653–F662, 1995 [DOI] [PubMed] [Google Scholar]
- 70.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol 43: B5–12, 1988 [DOI] [PubMed] [Google Scholar]
- 72.Jacobsen JC, Beierholm U, Mikkelsen R, Gustafsson F, Alstrom P, Holstein-Rathlou NH. “Sausage-string” appearance of arteries and arterioles can be caused by an instability of the blood vessel wall. Am J Physiol Regul Integr Comp Physiol 283: R1118–R1130, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41–50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep 11: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson RJ, Gordon KL, Suga S, Duijvestijn AM, Griffin K, Bidani A. Renal injury and salt-sensitive hypertension after exposure to catecholamines. Hypertension 34: 151–159, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens 18: 431–440, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sanchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol 16: 1909–1919, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Johnston JR, Brenner BM, Hebert SC. Uninephrectomy and dietary protein affect fluid absorption in rabbit proximal straight tubules. Am J Physiol Renal Fluid Electrolyte Physiol 253: F222–F233, 1987 [DOI] [PubMed] [Google Scholar]
- 80.Khosla N, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini E, Mehta RL. Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 4: 1914–1919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Kimura M, Asano M, Abe K, Miyazaki M, Suzuki T, Hishida A. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol Dial Transplant 20: 1559–1565, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kliem V, Johnson RJ, Alpers CE, Yoshimura A, Couser WG, Koch KM, Floege J. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int 49: 666–678, 1996 [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi S, Venkatachalam MA. Differential effects of calorie restriction on glomeruli and tubules of the remnant kidney. Kidney Int 42: 710–717, 1992 [DOI] [PubMed] [Google Scholar]
- 86.Kontessis P, Jones S, Dodds R, Trevisan R, Nosadini R, Fioretto P, Borsato M, Sacerdoti D, Viberti G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int 38: 136–144, 1990 [DOI] [PubMed] [Google Scholar]
- 87.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci USA 106: 3390–3395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int 76: 1049–1062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mackenzie HS, Azuma H, Rennke HG, Tilney NL, Brenner BM. Renal mass as a determinant of late allograft outcome: insights from experimental studies in rats. Kidney Int Suppl 52: S38–S42, 1995 [PubMed] [Google Scholar]
- 95.Mackenzie HS, Azuma H, Troy JL, Rennke HG, Tilney NL, Brenner BM. Augmenting kidney mass at transplantation abrogates chronic renal allograft injury in rats. Proc Assoc Am Physicians 108: 127–133, 1996 [PubMed] [Google Scholar]
- 96.Mackenzie HS, Tullius SG, Heemann UW, Azuma H, Rennke HG, Brenner BM, Tilney NL. Nephron supply is a major determinant of long-term renal allograft outcome in rats. J Clin Invest 94: 2148–2152, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats. II. Pathology. J Gerontol 40: 671–688, 1985 [DOI] [PubMed] [Google Scholar]
- 98.Masoro EJ, Iwasaki K, Gleiser CA, McMahan CA, Seo EJ, Yu BP. Dietary modulation of the progression of nephropathy in aging rats: an evaluation of the importance of protein. Am J Clin Nutr 49: 1217–1227, 1989 [DOI] [PubMed] [Google Scholar]
- 99.Masoro EJ, Yu BP. Diet and nephropathy. Lab Invest 60: 165–167, 1989 [PubMed] [Google Scholar]
- 100.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741, 2005 [DOI] [PubMed] [Google Scholar]
- 101.Megyesi J, Price PM, Tamayo E, Safirstein RL. The lack of a functional p21(WAF1/CIP1) gene ameliorates progression to chronic renal failure. Proc Natl Acad Sci USA 96: 10830–10835, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitch WE, Walser M, Buffington GA, Lemann J., Jr A simple method of estimating progression of chronic renal failure. Lancet 2: 1326–1328, 1976 [DOI] [PubMed] [Google Scholar]
- 104.Morrison AB, Howard RM. The functional capacity of hypertrophied nephrons. Effect of partial nephrectomy on the clearance of inulin and PAH in the rat. J Exp Med 123: 829–844, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakagawa T, Sasahara M, Haneda M, Kataoka H, Nakagawa H, Yagi M, Kikkawa R, Hazama F. Role of PDGF B-chain and PDGF receptors in rat tubular regeneration after acute injury. Am J Pathol 155: 1689–1699, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakamura H, Takasawa M, Kashara S, Tsuda A, Momotsu T, Ito S, Shibata A. Effects of acute protein loads of different sources on renal function of patients with diabetic nephropathy. Tohoku J Exp Med 159: 153–162, 1989 [DOI] [PubMed] [Google Scholar]
- 107.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 110.Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 111.Nath KA, Croatt AJ, Hostetter TH. Oxygen consumption and oxidant stress in surviving nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1354–F1362, 1990 [DOI] [PubMed] [Google Scholar]
- 112.Newburgh LH, Curtis AC. Production of renal injury in white rat by protein of diet; dependence of injury on duration of feeding, and on amount and kind of protein. Arch Intern Med 42: 801–821, 1928 [Google Scholar]
- 113.Nguyen S, Hsu CY. Excess weight as a risk factor for kidney failure. Curr Opin Nephrol Hypertens 16: 71–76, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Nony PA, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther 304: 905–912, 2003 [DOI] [PubMed] [Google Scholar]
- 115.Ogborn MR, Bankovic-Calic N, Shoesmith C, Buist R, Peeling J. Soy protein modification of rat polycystic kidney disease. Am J Physiol Renal Physiol 274: F541–F549, 1998 [DOI] [PubMed] [Google Scholar]
- 116.Ohashi R, Kitamura H, Yamanaka N. Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J Am Soc Nephrol 11: 47–56, 2000 [DOI] [PubMed] [Google Scholar]
- 117.Okusa MD. A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282: F10–F18, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okusa MD, Ye H, Huang L, Sigismund L, Macdonald T, Lynch KR. Selective blockade of lysophosphatidic acid LPA3 receptors reduces murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 285: F565–F574, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Olson JL, Hostetter TH, Rennke HG, Brenner BM, Venkatachalam MA. Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int 22: 112–126, 1982 [DOI] [PubMed] [Google Scholar]
- 121.Pagtalunan ME, Olson JL, Tilney NL, Meyer TW. Late consequences of acute ischemic injury to a solitary kidney. J Am Soc Nephrol 10: 366–373, 1999 [DOI] [PubMed] [Google Scholar]
- 122.Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 320: 143–149, 1989 [DOI] [PubMed] [Google Scholar]
- 123.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876, 2001 [DOI] [PubMed] [Google Scholar]
- 124.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int 73: 192–199, 2008 [DOI] [PubMed] [Google Scholar]
- 125.Ponte B, Felipe C, Muriel A, Tenorio MT, Liano F. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant 23: 3859–3866, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, Calise D, Chun J, Bascands JL, Saulnier-Blache JS, Schanstra JP. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol 18: 3110–3118, 2007 [DOI] [PubMed] [Google Scholar]
- 127.Price PM, Megyesi J, Safirstein RL. Cell cycle regulation: repair and regeneration in acute renal failure. Semin Nephrol 23: 449–459, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 [DOI] [PubMed] [Google Scholar]
- 131.Rudnicki M, Perco P, Enrich J, Eder S, Heininger D, Bernthaler A, Wiesinger M, Sarkozi R, Noppert SJ, Schramek H, Mayer B, Oberbauer R, Mayer G. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest 89: 337–346, 2009 [DOI] [PubMed] [Google Scholar]
- 132.Rutherford WE, Blondin J, Miller JP, Greenwalt AS, Vavra JD. Chronic progressive renal disease: rate of change of serum creatinine concentration. Kidney Int 11: 62–70, 1977 [DOI] [PubMed] [Google Scholar]
- 133.Safirstein RL. Acute renal failure: from renal physiology to the renal transcriptome. Kidney Int Suppl: S62–S66, 2004 [DOI] [PubMed] [Google Scholar]
- 134.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1: 631–641, 1970 [DOI] [PubMed] [Google Scholar]
- 135.Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int 74: 860–866, 2008 [DOI] [PubMed] [Google Scholar]
- 136.Schrier RW, Harris DC, Chan L, Shapiro JI, Caramelo C. Tubular hypermetabolism as a factor in the progression of chronic renal failure. Am J Kidney Dis 12: 243–249, 1988 [DOI] [PubMed] [Google Scholar]
- 137.Schrier RW, Shapiro JI, Chan L, Harris DC. Increased nephron oxygen consumption: potential role in progression of chronic renal disease. Am J Kidney Dis 23: 176–182, 1994 [DOI] [PubMed] [Google Scholar]
- 138.Shah BV, Levey AS. Spontaneous changes in the rate of decline in reciprocal serum creatinine: errors in predicting the progression of renal disease from extrapolation of the slope. J Am Soc Nephrol 2: 1186–1191, 1992 [DOI] [PubMed] [Google Scholar]
- 139.Shapiro JI, Elkins N, Reiss OK, Suleymanlar G, Jin H, Schrier RW, Chan L. Energy metabolism following reduction of renal mass. Kidney Int Suppl 45: S100–S105, 1994 [PubMed] [Google Scholar]
- 140.Shimamura T, Morrison AB. A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am J Pathol 79: 95–106, 1975 [PMC free article] [PubMed] [Google Scholar]
- 141.Snoeijs MG, van Heurn LE, Buurman WA. Biological modulation of renal ischemia-reperfusion injury. Curr Opin Organ Transplant In press [DOI] [PubMed] [Google Scholar]
- 142.Spurgeon KR, Donohoe DL, Basile DP. Transforming growth factor-beta in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568–F577, 2005 [DOI] [PubMed] [Google Scholar]
- 143.Striker GE, Schainuck LI, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum Pathol 1: 615–630, 1970 [DOI] [PubMed] [Google Scholar]
- 144.Suzuki T, Kimura M, Asano M, Fujigaki Y, Hishida A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol 158: 75–85, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr Opin Nephrol Hypertens 19: 43–50 [DOI] [PubMed] [Google Scholar]
- 146.Tang WW, Ulich TR, Lacey DL, Hill DC, Qi M, Kaufman SA, Van GY, Tarpley JE, Yee JS. Platelet-derived growth factor-BB induces renal tubulointerstitial myofibroblast formation and tubulointerstitial fibrosis. Am J Pathol 148: 1169–1180, 1996 [PMC free article] [PubMed] [Google Scholar]
- 147.Tapp DC, Kobayashi S, Fernandes G, Venkatachalam MA. Protein restriction or calorie restriction? A critical assessment of the influence of selective calorie restriction on the progression of experimental renal disease. Semin Nephrol 9: 343–353, 1989 [PubMed] [Google Scholar]
- 148.Tapp DC, Wortham WG, Addison JF, Hammonds DN, Barnes JL, Venkatachalam MA. Food restriction retards body growth and prevents end-stage renal pathology in remnant kidneys of rats regardless of protein intake. Lab Invest 60: 184–195, 1989 [PubMed] [Google Scholar]
- 149.Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thakar CV, Quate-Operacz M, Leonard AC, Eckman MH. Outcomes of hemodialysis patients in a long-term care hospital setting: a single-center study. Am J Kidney Dis 55: 300–306, 2009 [DOI] [PubMed] [Google Scholar]
- 151.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 18: 414–420, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Umezawa M, Hosokawa M, Kohno A, Ishikawa S, Kitagawa K, Takeda T. Dietary soybean protein compared with casein retards senescence in the senescence accelerated mouse. J Nutr 123: 1905–1912, 1993 [DOI] [PubMed] [Google Scholar]
- 153.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 154.Waikar SS, Winkelmayer WC. Chronic on acute renal failure: long-term implications of severe acute kidney injury. JAMA 302: 1227–1229, 2009 [DOI] [PubMed] [Google Scholar]
- 155.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Wallin A, Zhang G, Jones TW, Jaken S, Stevens JL. Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-l-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest 66: 474–484, 1992 [PubMed] [Google Scholar]
- 157.Walls J, Williams AJ. Influence of soya protein on the natural history of a remnant kidney model in the rat. Contrib Nephrol 60: 179–187, 1988 [DOI] [PubMed] [Google Scholar]
- 158.Walser M. Progression of chronic renal failure in man. Kidney Int 37: 1195–1210, 1990 [DOI] [PubMed] [Google Scholar]
- 159.Ward JM, Stevens JL, Konishi N, Kurata Y, Uno H, Diwan BA, Ohmori T. Vimentin metaplasia in renal cortical tubules of preneoplastic, neoplastic, aging, and regenerative lesions of rats and humans. Am J Pathol 141: 955–964, 1992 [PMC free article] [PubMed] [Google Scholar]
- 160.Williams AJ, Baker F, Walls J. Effect of varying quantity and quality of dietary protein intake in experimental renal disease in rats. Nephron 46: 83–90, 1987 [DOI] [PubMed] [Google Scholar]
- 161.Williams AJ, Walls J. Metabolic consequences of differing protein diets in experimental renal disease. Eur J Clin Invest 17: 117–122, 1987 [DOI] [PubMed] [Google Scholar]
- 162.Wilson SK, Heptinstall RH. Effects of acute, angiotensin-induced hypertension on intrarenal arteries in the rat. Kidney Int 25: 492–501, 1984 [DOI] [PubMed] [Google Scholar]
- 163.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 164.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294: F187–F197, 2008 [DOI] [PubMed] [Google Scholar]
- 166.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang G, Ichimura T, Maier JA, Maciag T, Stevens JL. A role for fibroblast growth factor type-1 in nephrogenic repair. Autocrine expression in rat kidney proximal tubule epithelial cells in vitro and in the regenerating epithelium following nephrotoxic damage by S-(1,1,2,2-tetrafluoroethyl)-l-cysteine in vivo. J Biol Chem 268: 11542–11547, 1993 [PubMed] [Google Scholar]
- 169.Zhou H, Fujigaki Y, Kato A, Miyaji T, Yasuda H, Tsuji T, Yamamoto T, Yonemura K, Hishida A. Inhibition of p21 modifies the response of cortical proximal tubules to cisplatin in rats. Am J Physiol Renal Physiol 291: F225–F235, 2006 [DOI] [PubMed] [Google Scholar]