Abstract
The expression of acid-sensing ion channel (ASIC) isoforms, ASIC1, ASIC2a, and ASIC3, was examined in the urinary bladder after cyclophosphamide (CYP)-induced cystitis of varying duration (4 h, 48 h, and chronic). Immunohistochemical, Western blot, and quantitative PCR approaches were used to evaluate channel expression and effects of CYP-induced cystitis in whole urinary bladder and split-bladder preparations from control (no inflammation) and CYP-treated rats. Quantitative PCR demonstrated significant (P ≤ 0.01) increases in ASIC2a and ASIC3 transcripts with CYP-induced cystitis (48 h and chronic) in the urothelium but no changes (e.g., ASIC3) or modest changes (e.g., ASIC2a) in detrusor smooth muscle. ASIC1 mRNA expression in the urothelium or detrusor was not affected by CYP-induced cystitis. Immunohistochemistry for ASIC2a and ASIC3 protein expression revealed significant (P ≤ 0.01) increases in ASIC immunoreactivity in the urothelium and suburothelial plexus with CYP-induced cystitis at all time points examined. Western blotting for ASIC2a and ASIC3 protein expression was complementary and revealed significant (P ≤ 0.01) increases in ASIC immunoreactivity. For the first time, these studies demonstrate that CYP-induced cystitis alters ASIC2a and ASIC3 expression in the urinary bladder; ASIC1 transcript expression is not altered by CYP-induced cystitis. Future studies are necessary to determine ASIC isoform contributions to micturition reflexes in control and inflamed urinary bladder.
Keywords: urothelium, suburothelial nerve plexus, acid-sensing ion channel isoform 1, acid-sensing ion channel isoform 2a, acid-sensing ion channel isoform 3, quantitative polymerase chain reaction
acid-sensing ion channels (ASICs), which belong to the amiloride-sensitive epithelial Na+ channel/degenerin family, are predominantly neuronal voltage-insensitive cationic channels activated by extracellular protons (42, 47, 48). ASICs are widely expressed in the central and peripheral nervous system (10, 29, 33), but nonneuronal ASIC expression has also been demonstrated in testis, pituitary gland, lung epithelial cells, bone, and urothelial cells (25, 26, 29). Functional ASICs result from the assembly of channels forming homomeric and heteromeric channels (36). ASICs are proposed as the channels that sense extracellular acidosis and contribute to nociception in diverse pathological conditions, including inflammation, ischemia, fractures, lesions, and postoperative states (10, 37, 44–46).
ASIC expression is influenced by a variety of proinflammatory mediators, including neurotrophins, serotonin, bradykinin, nitric oxide, and arachidonic acid, consistent with the suggestion that ASICs may contribute to peripheral sensitization of nociceptors and resulting nociception (32). Cyclophosphamide (CYP)-induced bladder inflammation is associated with alterations in neurochemical (38, 40), electrophysiological (49), organizational (41), and functional properties (18) of micturition pathways and altered referred somatic sensitivity (14–16). Chemical mediators (e.g., neurotrophins, cytokines, chemokines, and neuropeptides) produced in micturition reflex pathways with cystitis (2, 5, 30, 38–40) may underlie some of these changes. Among these mediators, many studies have demonstrated the involvement of nerve growth factor in altered bladder sensory function and the development of referred hyperalgesia in response to bladder inflammation. Recent studies have demonstrated ASIC1, ASIC2, and ASIC3 mRNA expression in control mouse urothelium (25) and functional ASIC-like channels in cultured rat urothelial cells (26). However, the expression and response of ASIC expression in the urinary bladder to CYP-induced cystitis have not been addressed.
The goal of this study was to determine and quantify ASIC1, ASIC2a, and ASIC3 transcripts and protein expression and effects of CYP-induced cystitis on ASIC expression in urinary bladder using immunohistochemistry, Western blot, and real-time quantitative PCR (qPCR) approaches. Results suggest that ASICs are expressed and expression of ASIC2a and ASIC3 is altered in the urinary bladder with CYP-induced cystitis. Functional roles of ASICs in normal and altered bladder function and referred somatic sensitivity with urinary bladder inflammation remain to be determined.
MATERIALS AND METHODS
Animals.
Adult female Wistar rats (200–225 g body wt; Charles River, St. Constant, PQ, Canada) were housed two per cage and maintained in standard laboratory conditions with free access to food and water. The University of Vermont Institutional Animal Care and Use Committee approved all animal use procedures (protocols 06-014 and 08-085).
Induction of CYP-induced cystitis.
Rats were anesthetized under isoflurane (2%), and acute cystitis was induced with a single injection of CYP (150 mg/kg ip) and rodents were used in studies at various time points (4 and 48 h) after treatment (8, 23, 24). Chronic CYP-induced cystitis was induced by administration of CYP (75 mg/kg ip) once every 3 days for 10 days (8, 23, 24). Control rodents received either saline injection or no treatment. Rats were euthanized using isoflurane (5%), and a thoracotomy was performed.
RNA extraction, reverse transcription, and PCR.
Total RNA was extracted from the whole urinary bladder of control and CYP-treated (4 h, 48 h, and chronic) rats (n = 5–7 each) with RNA/mRNA STAT-60 isolation reagent (Tel-Test, Friendswood, TX) and reverse-transcribed as described previously (8, 13, 23). Briefly, from 2 μg of total RNA, first-strand cDNA was synthesized using SuperScript reverse transcriptase and oligo(deoxythymidine) primers with the SuperScript II preamplification kit (Invitrogen, Carlsbad, CA). Amplification of cDNA was performed with AmpliTaq DNA polymerase (Applied Biosystems, Norwalk, CT) using oligonucleotide primers specific for rat ASIC. Amplification of cDNA was performed according to the following parameters: initial denaturation and enzyme activation at 94°C for 10 min, denaturation at 94°C for 30 s, annealing at primer-specific annealing temperature for 30 s, extension at 72°C for 45 s with 30–35 cycles, and final extension at 72°C for 5 min. PCR products were resolved by 1.6% agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. Routine controls included cDNA synthesis in the absence of either RNA or reverse transcriptase and amplification with omission of template or primers.
Split-bladder preparation and assessment of potential contamination of bladder layers.
ASIC mRNA and protein expression was determined in the urothelium + suburothelium and detrusor smooth muscle layers of the urinary bladder of rats with and without CYP treatment. The urothelium + suburothelium was dissected from the detrusor smooth muscle using fine forceps under a dissecting microscope as previously described (8, 23, 51). To confirm the specificity of our split-bladder preparations, urothelium + suburothelium and detrusor samples were examined for the presence of α-smooth muscle actin (Table 1) and uroplakin II (Table 1) by Western blotting or qPCR (8, 9). In urothelium + suburothelium layers, only uroplakin II was present. Conversely, in detrusor samples, only α-smooth muscle actin was present.
Table 1.
Antibody sources, dilutions, and applications
| Antibody | Dilution | Source |
|---|---|---|
| Primary | ||
| Rabbit anti-ASIC1 | 1:200 for WB | Alomone (Jerusalem, Israel) |
| Rabbit anti-ASIC2a | 1:200 for WB | Alomone |
| 1:5,000 for IHC | ||
| Rabbit anti-ASIC3 | 1:200 for WB | Alomone |
| Guinea pig anti-ASIC3 | 1:500 for IHC | Millipore (Billerica, MA) |
| Rabbit anti-actin | 1:1,500 | Santa Cruz Biotechnology (Santa Cruz, CA) |
| Mouse anti-PGP9.5 | 1:3,000 | AbD Serotec (Raleigh, NC) |
| Mouse anti-uroplakin II | 1:25 | American Research Products (Belmont, MA) |
| Secondary | ||
| Cy3 goat anti-rabbit | 1:500 | Jackson ImmunoResearch (Westgrove, PA) |
| Cy2 goat anti-mouse | 1:50 | Jackson ImmunoResearch |
| Cy3 donkey anti-guinea pig | 1:500 | Jackson ImmunoResearch |
| Cy2 donkey anti-mouse | 1:50 | Jackson ImmunoResearch |
| Goat anti-rabbit HRP | 1:5,000 | Jackson ImmunoResearch |
| Goat anti-mouse HRP | 1:300 | Jackson ImmunoResearch |
HRP, horseradish peroxidase; Cy, cyanine; ASIC, acid-sensing ion channel; WB, Western blotting; IHC, immunohistochemistry; PGP, protein gene product.
Real-time qRT-PCR.
Total RNA (n = 5–7 for each group) from the urothelium + suburothelium or detrusor layer was extracted using the RNA/mRNA STAT-60 isolation reagent (Tel-Test) as previously described (8, 13, 23). One microgram of RNA per sample was used to synthesize cDNA using SuperScript II reverse transcriptase and random hexamer primers with the SuperScript II preamplification system (Invitrogen) in a 20-μl final reaction volume.
The qPCR standards for all transcripts were prepared with the amplified ASIC and L32 cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Real-time qPCR was performed using SYBR Green I detection (8, 13, 23). cDNA templates, diluted fivefold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using SYBR Green I JumpStart. Taq ReadyMix (Sigma, St. Louis, MO) contains 5 mM MgCl2, 200 mM dATP, dGTP, dCTP, and dTTP, 0.64 U of Taq DNA polymerase, and each primer at 300 nM in a final 25-μl reaction volume. The primer sequences were as follows: ggccaacttccgtagcttca (ASIC1 upper), atgccctgctctgtcgtagaa (ASIC1 lower), tggacctcaaggagagccccagt (ASIC2a upper), tcgttggtggtaagcctggagaa (ASIC2a lower), tcagacatccgggtgtttg (ASIC3 upper), and cattgggtgtgaggcgtga (ASIC3 lower). The real-time qPCR was performed on a 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA) (13, 23) using the following standard conditions: 1) 94°C for 2 min and 2) amplification over 40 cycles at 94°C for 15 s and 63°C for 30 s.
The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis, which was accomplished by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using Sequence Detection Software version 1.3.1 (Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene L32. Control samples are set equal to 100%.
Western blotting for ASIC expression in urothelium + suburothelium or detrusor layers.
Whole urinary bladders (control, 4 h, and 48 h; n = 5–6) were homogenized separately in tissue protein extraction agent (a proprietary detergent in 25 mM bicine and 150 mM sodium chloride, pH 7.6; T-PER, Roche, Indianapolis, IN) containing a protease inhibitor mix (16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor, and 2 μg/ml pepstatin A; Sigma-Aldrich, St. Louis, MO), and aliquots were removed for protein assay as previously described (24). Samples (20 μg) were suspended in sample buffer for fractionation on gels and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and efficiency of transfer was evaluated. Membranes were blocked overnight in a solution of 5% milk and 3% bovine serum albumin in Tris-buffered saline with 0.1% Tween. For immunodetection, ASIC1, ASIC2a, and ASIC3 antibodies were used overnight at 4°C (Table 1). Washed membranes were incubated in species-specific secondary antibodies for 2 h at room temperature for enhanced chemiluminescence detection (Table 1). Blots were exposed to Biomax film (Kodak, Rochester, NY) and developed. The intensity of each band was analyzed, and background intensities were subtracted using Un-Scan It software (Silk Scientific, Orem, UT). Western blot analysis of actin (1:2,000 dilution; Cell Signaling Technology) in samples was used as a loading control. Immunoabsorptions with ASIC2a or ASIC3 peptide (5 μg/ml) and antisera in bladder sections resulted in no staining above background (data not shown). Immunoabsorptions with ASIC1 peptide (5–1,000 μg/ml) did not block putative ASIC1 expression (see results) in Western blot experiments. The inability to block ASIC1 expression in Western blot studies, together with no change in ASIC1 mRNA expression in the urothelium or detrusor with CYP-induced cystitis as demonstrated with qPCR (see results), resulted in our focus on ASIC2a and ASIC3 in subsequent immunohistochemical studies.
Immunohistochemical localization of ASICs in the urothelium.
The bladders from control and acute (4 h), intermediate (48 h), and chronic CYP-treated rats (n = 6 for each) were rapidly dissected, weighed, postfixed in 4% paraformaldehyde, and placed in ascending concentrations of sucrose (10–30%) in 0.1 M PBS for cryoprotection. Cryostat sections (20 μm) of urinary bladder were mounted on gelled (0.5%) microscope slides for on-slide processing as previously described (8, 9, 23, 24, 50). Briefly, sections were incubated with anti-ASIC2a or anti-ASIC3 (Table 1) in 1% goat or donkey serum and 0.1 M phosphate buffer overnight at room temperature. After they were washed (3 × 10 min) with 0.1 M PBS (pH 7.4), the tissues were incubated with species-specific secondary antibodies (Table 1) for 2 h at room temperature. The slides were washed (3 × 10 min) with PBS, and coverslips were applied with Citifluor (Citifluor, London, UK). Control sections incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed. Immunoabsorptions with ASIC2a or ASIC3 peptide (5 μg/ml) and antisera in bladder sections resulted in no staining above background (data not shown).
Visualization and semiquantitative analysis of ASIC immunoreactivity in urothelium.
The presence of ASIC-immunoreactive (ASIC-IR) staining in bladder sections was visualized and captured using an Olympus fluorescence photomicroscope as previously described (8, 9). The filter was set with an excitation range of 560–596 nm and an emission range of 610–655 nm to visualize Cy3. Meta Morph image analysis software (version 4.5r4, Universal Imaging, Downingtown, PA) was used (8, 9) for semiquantitative analysis of ASIC-IR in the urothelium. Briefly, each image was calibrated for specific pixel size using a predetermined calibration file. With the help of a free-hand drawing tool, the urothelium was chosen and measured in total pixel areas. A threshold encompassing an intensity range of 100–250 gray-scale values was applied to the region of interest in the least brightly stained condition first. The threshold was adjusted for each experimental series, with concomitantly processed negative controls used as a guide for setting background fluorescence. The same threshold was subsequently used for all images. ASIC-IR was considered to be positive only when it exceeded the established threshold. Percent ASIC expression above threshold in the total area selected was then calculated. ASIC-IR in the urothelium was consistent across all regions (dome, body, and neck) of the urinary bladder examined for a given experimental condition. Semiquantification of ASIC-IR in the urothelium was determined in the bladder neck region for consistency with the semiquantification performed on ASIC-IR in the suburothelial plexus (see below). Some bladder preparations were also examined, and optical sections were acquired using a Zeiss LSM 510 confocal scanning system attached to a Zeiss LSM 510 microscope with a plan Fluor ×40 oil objective as previously described (23).
Immunohistochemical localization of ASICs in the suburothelial nerve plexus in urinary bladder whole mounts.
The urinary bladder from control (n = 6) and experimental treatments (n = 6 each) was dissected and placed in Krebs solution. The bladder was cut open along the midline and pinned to a Sylgard-coated dish. Notches were made on one side of the bladder neck for orientation purposes and regional analyses of immunoreactivity. The bladder was incubated for 1.5 h at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid). Fine-tipped forceps and iris scissors were used to dissect the urothelium + suburothelium from the underlying detrusor smooth muscle with the aid of a dissecting microscope (9, 24). Urothelium + suburothelium and bladder musculature were processed for ASIC-IR (as described above). In some whole mounts processed for ASIC-IR, nerve fibers in the suburothelial nerve plexus were also stained with the pan-neuronal marker protein gene product (PGP) 9.5 (Table 1). After they were washed (3 × 10 min) with 0.1 M PBS (pH 7.4), the tissues were incubated with a cocktail of species-specific secondary antibodies (Table 1) for 2 h at room temperature. The whole mounts were washed and placed on microscope slides, and coverslips were applied as described above.
Assessment of immunohistochemical staining in urinary bladder regions.
Immunohistochemistry and subsequent semiquantification of ASIC-IR in bladder sections or whole-mount preparations were performed on control and experimental tissues simultaneously to reduce the incidence of staining variation that can occur between tissues processed on different days. Staining in experimental tissue was compared with that in experiment-matched negative controls. Urinary bladder sections or whole mounts exhibiting immunoreactivity that was greater than the background level in experiment-matched negative controls were considered positively stained.
Visualization and semiquantitative analysis of ASIC-IR in suburothelial nerve plexus.
Whole mounts from control (n = 6) and experimental (n = 6 each) groups were examined under an Olympus fluorescence photomicroscope as described above. Cy3 was visualized with a filter with an excitation range of 560–596 nm and an emission range of 610–655 nm. Cy2 was visualized with a filter with an excitation range of 470–490 nm and an emission range of 510–530 nm. Semiquantification of ASIC expression in the suburothelial nerve plexus was performed as previously described (8, 22) and modified from Brady et al. (6). Gray-scale images acquired in tiff format were imported into ImageJ (1), and images were thresholded. Images were acquired from the neck region of control and treated rats for suburothelial nerve plexus analyses. A rectangle of fixed dimension (500 × 500 pixels) was placed on the section according to a random selection of x and y coordinates. This process was repeated seven times for each image of the suburothelial nerve plexus. The average optical density of ASIC-IR in the suburothelial nerve plexus was then calculated. ASIC-IR in the suburothelial plexus was greatest in the bladder neck region. Semiquantification of ASIC-IR in the suburothelial plexus was determined in the bladder neck region for all animal groups.
Figure preparation.
Digital images were obtained using a charge-coupled device camera (MagnaFire SP, Optronics, Optical Analysis, Nashua, NH) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis). Exposure times were held constant when images were acquired from control and experimental animals processed and analyzed on the same day. Images were imported into Photoshop 7.0 (Adobe Systems, San Jose, CA), where groups of images were assembled and labeled.
Statistics.
Values are means ± SE. Data were compared using ANOVA. Percent data from image analysis were arcsin transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), Newman-Keuls post hoc test was used to compare experimental means.
RESULTS
ASIC transcript expression in urothelium or detrusor smooth muscle layers with CYP-induced cystitis.
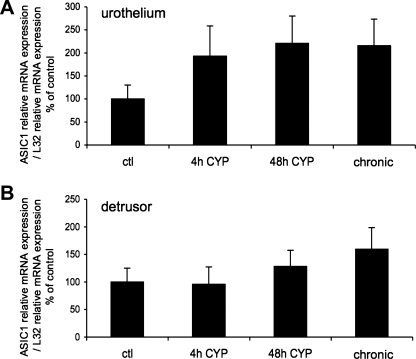
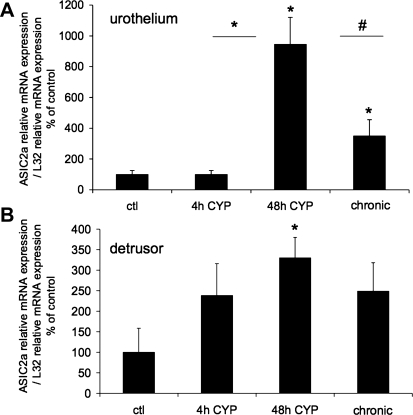
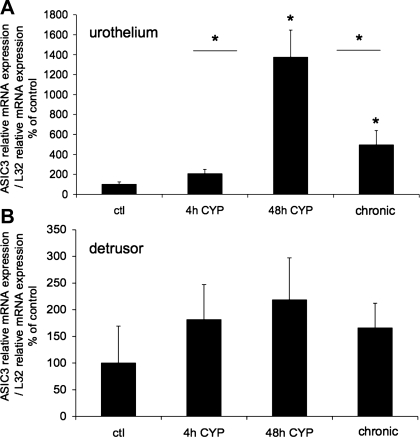
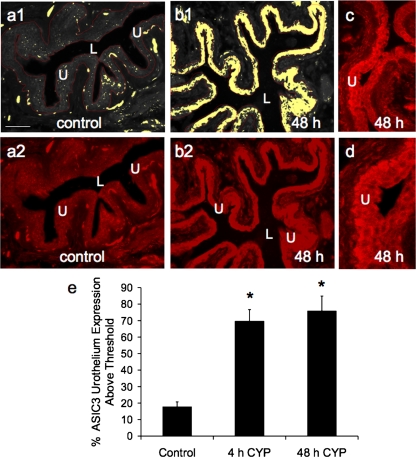
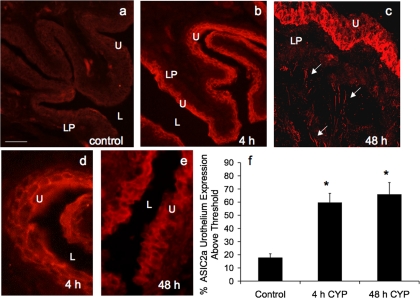
The effect of CYP-induced cystitis on ASIC1, ASIC2a, and ASIC3 transcript expression was examined by qPCR analyses (Figs. 1–3) in urothelium + suburothelium and detrusor smooth muscle layers. qPCR analyses demonstrated no differences in ASIC1 transcript expression with CYP-induced cystitis of any duration tested in urothelium + suburothelium or detrusor smooth muscle (Fig. 1; P = 0.64–0.76). qPCR analyses demonstrated a significant (P ≤ 0.01) increase in ASIC2a and ASIC3 mRNA in the urothelium after 48 h of CYP-induced cystitis compared with control, 4 h of CYP, and chronic CYP treatment (Figs. 2 and 3). ASIC2a and ASIC3 mRNA expression in the urothelium was also significantly (P ≤ 0.01) greater following chronic CYP-induced cystitis compared with control (Figs. 2A and 3A). No differences in ASIC3 mRNA expression in detrusor muscle were observed among the CYP-induced cystitis time points (Fig. 3B). ASIC2a mRNA expression in detrusor only increased with 48 h of CYP treatment (Fig. 2B).
Fig. 1.
Acid-sensing ion channel (ASIC) isoform 1 (ASIC1) mRNA expression in urothelium and detrusor in control rats and after 4 h (h), 48 h, and chronic (10 days) cyclophosphamide (CYP)-induced bladder inflammation (n = 5–7 for each group). A and B: summary histograms of relative expression of the ASIC1 transcript in urothelium and detrusor as a percentage of control and normalized to relative expression of the housekeeping gene L32 obtained from quantitative PCR. No differences in ASIC1 expression with CYP-induced cystitis of any duration compared with control in urothelium + suburothelium (A) or detrusor smooth muscle (B) were detected (P = 0.64–0.76).
Fig. 2.
ASIC2a mRNA expression in urothelium and detrusor in control rats and after 4 h, 48 h, and chronic (10 days) CYP-induced bladder inflammation (n = 5–7 for each group). A and B: summary histograms of relative expression of the ASIC2a transcript in urothelium and detrusor as a percentage of control and normalized to relative expression of the housekeeping gene L32 obtained from quantitative PCR. *P ≤ 0.01; #P ≤ 0.05 vs. control or as indicated between groups by horizontal lines.
Fig. 3.
ASIC3 mRNA expression in urothelium and detrusor in control rats and after 4 h, 48 h, and chronic (10 days) CYP-induced bladder inflammation (n = 5–7 for each group). A and B: summary histograms of relative expression of the ASIC3 transcript in urothelium and detrusor as a percentage of control and normalized to relative expression of the housekeeping gene L32 obtained from quantitative PCR. *P ≤ 0.01 vs. control or as indicated between groups by horizontal lines.
ASIC protein expression in urothelium or detrusor smooth muscle layers with CYP-induced cystitis.
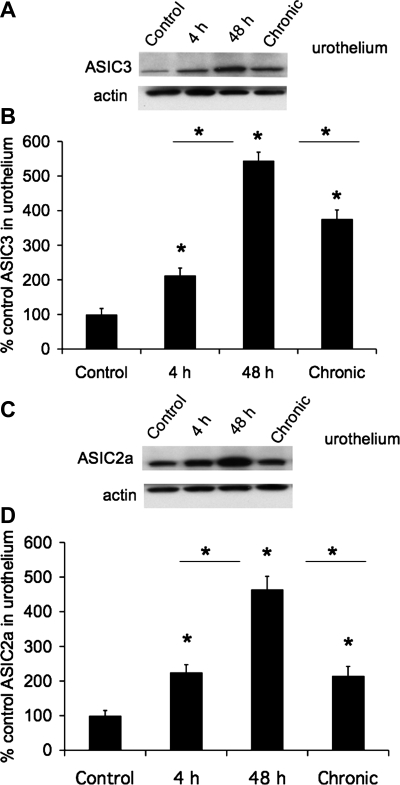
The effects of CYP-induced cystitis on ASIC1, ASIC2a, and ASIC3 protein were examined by Western blot techniques in urothelium + suburothelium (Fig. 4) and detrusor smooth muscle. Western blot analyses demonstrated that CYP-induced cystitis (4 h, 48 h, and chronic) significantly (P ≤ 0.01) increased ASIC2a (2.1- to 4.8-fold; Fig. 4, C and D) and ASIC3 (2- to 5.3-fold; Fig. 4, A and B) expression in the urothelium. ASIC2a (Fig. 4, C and D) and ASIC3 (Fig. 4, A and B) protein expression was significantly (P ≤ 0.01) greater with 48 h than with 4 h of CYP treatment or with chronic CYP treatment. No regulation of ASIC2a or ASIC3 protein expression was observed in detrusor smooth muscle with CYP-induced cystitis of any duration (data not shown; P > 0.05). ASIC1 protein expression in the urinary bladder (urothelium + suburothelium or detrusor) was not blocked by immunoabsorption approaches that used immunogen concentrations up to 200-fold greater than that required to block ASIC2a or ASIC3 protein expression with Western blot approaches.
Fig. 4.
Increased ASIC3 and ASIC2a protein expression in urothelium with CYP-induced cystitis (4 h, 48 h, and chronic). A: Western blot of urothelium (20 μg) for ASIC3 expression in control rats and those treated with CYP for 4 h, 48 h, and 10 days (chronic). Actin expression was used as a loading control. B: relative urothelium ASIC3 band density in all groups examined normalized to actin in the same samples presented as a percentage of control ASIC3 expression. C: Western blot of urothelium (20 μg) for ASIC2a expression in control rats and those treated with CYP for 4 h, 48 h, and 10 days (chronic). Actin expression was used as a loading control. D: relative urothelium ASIC2a band density in all groups examined normalized to actin in the same samples presented as a percentage of control ASIC2a expression. *P ≤ 0.01 vs. control or as indicated between groups by horizontal lines (n = 5–6 for each group).
Urothelium ASIC-IR in rats after induction of cystitis.
On the basis of Western blot and qPCR data, we focused the immunostaining studies on the effects of CYP-induced cystitis (4 h and 48 h) on the urothelium. The expression of ASIC2a- and ASIC3-IR was absent or very weak in the urothelium of urinary bladder whole mounts or cryostat bladder sections from control rats (Figs. 5a and 6a), although some diffuse labeling was observed in the lamina propria in cryostat sections. With CYP treatment (4 h and 48 h), ASIC2a- and ASIC3-IR was intense in the urothelium, with ASIC2a- and ASIC3-IR being present in the lamina propria (Figs. 5, c–f, and Fig. 6, b–e). Z-stacks obtained with confocal microscopy revealed ASIC2a- and ASIC3-IR (data not shown) in nerve-like structures extending from the lamina propria to the detrusor muscle in cryostat sections (Fig. 6c). These nerve-like structures were examined in greater detail in whole-mount preparations of the suburothelial nerve plexus (see below). Semiquantitative analyses revealed significant (P ≤ 0.01) increases in ASIC2a- and ASIC3-IR in the urothelium 4 h and 48 h after CYP treatment (Figs. 5g and 6f). Sustained ASIC-IR was observed in the lamina propria with CYP treatment. No regional differences in ASIC2a- or ASIC3-IR in the urothelium of the dome, body, or neck region of the urinary bladder were observed in control or CYP-treated rats. ASIC2a- and ASIC3-IR were observed in all cell layers (apical, intermediate, and basal) of the urothelium (Figs. 5 and 6).
Fig. 5.
Semiquantitative analysis of ASIC3 immunoreactivity (IR) in the urothelium after CYP-induced cystitis. a1 and b1: gray-scale versions of ASIC3-IR in control urinary bladder and 48 h after CYP treatment, with urothelium (U) outlined in red. A threshold encompassing an intensity range of 100–250 gray-scale values was applied to the region of interest (a1 and b1); the same threshold was subsequently used for all images. Percent ASIC expression above threshold in the total area selected was then determined. Little ASIC3-IR (absence of yellow within the outlined region) is above threshold in control bladders (a1) compared with significant ASIC3-IR after 48 h of CYP treatment (b1, presence of yellow within U). a2 and b2: fluorescence images corresponding to gray-scale images (a1 and b1). c and d: higher-power images of ASIC3-IR in the urothelium after 48 h of CYP treatment. Calibration bar represents 50 μm. L, lumen. e: ASIC3 expression in the urothelium, with CYP treatment expressed as a percentage of control averaged for all bladders from all conditions examined (n = 6). *P ≤ 0.01.
Fig. 6.
ASIC2a-IR in cryostat sections of CYP-treated urothelium. CYP treatment [4 h (b and d) and 48 h (c and e)] significantly (P ≤ 0.05) increased the percentage of ASIC2a-IR in urothelium compared with control (a). c: confocal z-stack image demonstrating ASIC2a-IR in urothelium and diffuse ASIC2a in lamina propria (LP) and in putative nerve fibers (arrows). d and e: higher-power fluorescence images of ASIC2a-IR in urothelium after 4 h and 48 h of CYP-induced cystitis. For all images, exposure times were held constant, and all tissues were processed simultaneously. In rats treated with CYP for 4 and 48 h, ASIC2a expression was visible in urothelium (b–e), whereas control (a) urinary bladder showed little or no ASIC2a-IR. Calibration bar represents 50 μm in a–c and 25 μm in d and e. L, lumen. f: ASIC2a expression above threshold in urothelium of CYP-treated (4 h and 48 h) rats expressed as a percentage of control. Semiquantitative analyses were performed as described in Fig. 5 legend. CYP treatment (4 h and 48 h) significantly (P ≤ 0.01) increased ASIC2a-IR in urothelium (n = 6 for each group).
ASIC-IR in the suburothelial plexus with CYP-induced cystitis.
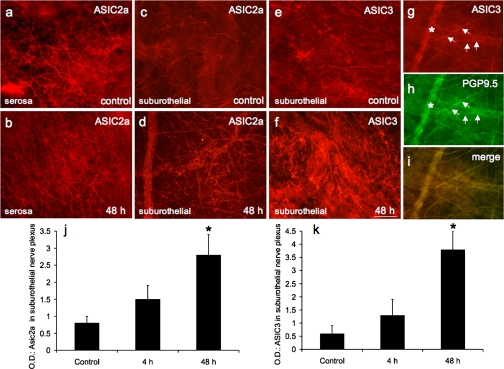
In whole-mount preparations, ASIC2a- and ASIC3-IR were faintly observed in the suburothelial nerve plexus throughout the control urinary bladder (Fig. 7, c and e). However, dense ASIC2a-IR (Fig. 7a) and ASIC3-IR (data not shown) were observed throughout the serosal surface of control urinary bladder. CYP treatment (48 h) increased the appearance of ASIC2a- and ASIC3-IR in the suburothelial plexus (Fig. 7, d and f). The density of the ASIC2a- and ASIC3-IR in suburothelial nerve fibers was greatest in the neck region with CYP treatment, and our analysis of CYP-induced effects was restricted to this region. A significant (P ≤ 0.01) increase (3.4- to 6.2-fold) in the density of the ASIC2a- and ASIC3-IR nerve fibers in the bladder neck region was observed with 48 h of CYP treatment (Fig. 7, j and k). Fine- and thicker-caliber ASIC2a- and ASIC3-IR neuronal fibers and neuronal fiber bundles were observed (Fig. 7, d and f) in the suburothelial plexus with CYP treatment. No changes in the ASIC2a- or ASIC3-IR in the suburothelial nerve plexus were observed with 4 h of CYP treatment (Fig. 7, j and k). ASIC3 and ASIC2a (data not shown) in the suburothelial nerve plexus also exhibited immunoreactivity for the pan-neuronal marker PGP9.5 (Fig. 7, g–i). In contrast to CYP regulation of ASIC2a and ASIC3 expression in the suburothelial nerve plexus, ASIC2a and ASIC3 expression in the serosal surface was not regulated by CYP-induced cystitis (Fig. 7b).
Fig. 7.
ASIC2a and ASIC3 expression in nerve fibers in urinary bladder. Fluorescence photographs show ASIC2a- or ASIC3-IR nerve fibers of suburothelial nerve plexus in whole-mount preparations of urothelium in control (c and e) and CYP-treated (48 h) rats (d and f). ASIC2a expression was also present in presumptive nerve fibers on the serosal surface of urinary bladder (a), but expression was not regulated by CYP treatment (b). ASIC3-IR nerve fibers in suburothelial nerve plexus expressed the pan-neuronal marker protein gene product (PGP) 9.5. g: ASIC3 expression in nerve fibers (arrows) of suburothelial nerve plexus in CYP-treated (48 h) rats. h: whole-mount preparation in a stained for PGP9.5, with ASIC3-IR nerve fibers expressing PGP9.5 (arrows). i: merged image demonstrating overlap between ASIC and PGP immunostaining in suburothelial nerve plexus. j and k: optical density (OD) of ASIC2a and ASIC3 expression in suburothelial nerve plexus. CYP treatment (48 h) significantly (P ≤ 0.01) increased ASIC2a (d and j) and ASIC3 (f and k) expression in suburothelial nerve plexus. ∗ in g and h shows a larger-caliber nerve fiber out of the focal plane. Calibration bar represents 80 μm in a–i.
Additional ASIC2a- and ASIC3-IR structures in the urinary bladder.
ASIC2a- and ASIC3-IR were also associated with the vasculature of the urinary bladder, being present in the endothelium of control and inflamed urinary bladder (data not shown) in small- and larger-diameter vessels. CYP-induced cystitis did not appear to affect ASIC2a and ASIC3 expression associated with the vasculature. In inflamed urinary bladder, numerous inflammatory infiltrates were present as previously described (18, 22), and infiltrates also expressed ASIC2a- and ASIC3-IR (data not shown).
DISCUSSION
The present studies demonstrate several novel findings with respect to expression of ASIC1, ASIC2a, and ASIC3 in the urinary bladder in rodents and the effects of CYP-induced cystitis on ASIC expression. ASIC2a and ASIC3 mRNA and/or protein expression in the urothelium and suburothelial nerve plexus increased significantly with CYP-induced cystitis of varying duration. In contrast, ASIC1 mRNA expression in the urothelium or detrusor was not affected by CYP-induced cystitis. ASIC2a and ASIC3 protein expression in the whole urinary bladder is significantly increased with CYP-induced cystitis at all time points examined (4 h, 48 h, chronic), with the magnitude of the protein increase being significantly greater with 48 h than 4 h of CYP-induced cystitis or chronic CYP-induced cystitis. In contrast, ASIC2a and ASIC3 expression in the detrusor smooth muscle is largely unregulated by CYP-induced cystitis. Future studies will address the contribution of ASICs to functional plasticity of micturition reflexes after urinary bladder inflammation.
ASIC changes may be mediated, in part, by inflammatory changes in the urinary bladder. Among potential mediators of inflammation, neurotrophins (e.g., nerve growth factor) and proinflammatory cytokines have been implicated in the peripheral sensitization of nociceptors (11, 12, 28). ASICs are regulated by proinflammatory mediators, including neurotrophins, serotonin, bradykinin, nitric oxide, and arachidonic acid, consistent with the suggestion that ASICs may contribute to peripheral sensitization of nociceptors (7, 31, 32, 43). Extracellular acidosis correlates with pain sensations (20, 36), and ASICs have been proposed as the channels in sensory neurons (i.e., dorsal root ganglion neurons) and central nervous system neurons that sense extracellular acidosis in diverse conditions, including inflammation, ischemia, fractures, lesions, and postoperative states (10, 21, 44–46). ASICs are also major contributors to colonic mechanotransduction (17, 19), and ASIC3 has been evaluated as a potential target for visceral pain treatment (10, 17, 19). In the present study, we demonstrate increased expression of ASIC2a and ASIC3 in rat urothelium and suburothelial nerve plexus with CYP-induced cystitis. However, we do not address whether these changes are direct effects of CYP treatment or are secondary to inflammation. Because of ASIC regulation by numerous proinflammatory mediators (32), future studies involving specific inhibitors of inflammatory mediators are necessary to distinguish between direct and indirect effects of CYP-induced cystitis on ASIC expression in the urinary bladder.
In recent years, the functional contribution of the urothelial lining of the urinary bladder has advanced beyond the view that the urothelium is a passive barrier to the idea that is an active sensor with a potential signaling (i.e., sensory) role, especially in the context of urinary bladder inflammation (4). Urothelial cells express a number of receptors and ion channels similar to those found in sensory neurons (3, 4, 9, 23, 27, 34), and it was therefore not surprising to observe ASIC expression in the urothelium in control or CYP-treated rats, given the widespread distribution of ASICs in dorsal root ganglia. Previous studies in mouse (25) and cultured rat (26) urothelium demonstrated ASIC expression and activation leading to Ca2+ influx and generation of ionic currents. The present study extends these previous studies by characterizing transcript and protein expression in the urothelium and the suburothelial nerve plexus of the urinary bladder of two ASIC family members, ASIC3 and ASIC2a. Furthermore, CYP-induced bladder inflammation increased ASIC3 and ASIC2a transcript and protein expression in these same tissues. The qPCR data that demonstrate a robust increase in ASIC2a and ASIC3 mRNA in the urothelium with CYP-induced cystitis are likely to represent both urothelial and suburothelial elements, including the suburothelial nerve plexus. The immunohistochemical and Western blot studies of ASIC2a and ASIC3 expression in the urinary bladder with and without CYP-induced cystitis were consistent and complemented the qPCR data from the split-bladder preparations. ASIC3 and ASIC2a transcripts were detected in the detrusor smooth muscle; however, no changes (e.g., ASIC3) or a modest change (e.g., ASIC2a) in transcript expression compared with the urothelium were observed with CYP-induced cystitis. Increases in ASIC protein expression in the urothelium and suburothelial plexus with CYP-induced cystitis were observed at the earliest time point examined (4 h). In contrast, ASIC transcript expression in the urothelium was first significantly increased with 48 h of CYP-induced cystitis. Differences between detection of protein and transcript upregulation may reflect 1) translocation of existing protein to membrane surfaces, 2) altered protein degradation events, and/or 3) increased translation events that precede transcription events (35). Because of such discrepancies, we examine protein and mRNA expression to understand cell or tissue phenotypes (35).
The present studies also point out several differences, as well as similarities, in ASIC distribution between rat and mouse urinary bladder. Using immunohistochemistry, Kobayashi et al. (25) reported the virtual absence of constitutive ASIC2- and ASIC3-IR in the urothelium of mouse bladder, with greater staining in detrusor smooth muscle and suburothelial regions, respectively. Consistent with these findings, ASIC2a and ASIC3 expression was minimal in the urothelium of control rats; however, expression of ASIC2a and ASIC3 was evident in the suburothelial plexus of control rats. Bladder inflammation induced by CYP was a potent regulator of both ASIC2a and ASIC3 expression in the urothelium and suburothelial nerve plexus. Using qPCR, Kobayashi et al. demonstrated that ASIC1 was the prominent isoform expressed in mouse urothelium. In addition, they reported greater ASIC1 protein expression in the urothelium than in the detrusor in the mouse (25). It was therefore surprising in the present studies that although ASIC1 transcripts were expressed in rat urinary bladder, expression was not affected in the urothelium or detrusor with CYP-induced cystitis. Kobayashi et al. also demonstrated sex differences in ASIC expression in mouse urinary bladder, with greater ASIC1 expression in male mice and greater ASIC2 expression in female mice. The documented sex differences in ASIC expression (25), as well as species differences (mouse vs. rat), could contribute to the differences observed in the present study that used only female rats. It is clear that the functional contributions of ASIC urothelial and suburothelial nerve expression to the micturition reflex function need to be determined under control and bladder-inflamed conditions in different species and sexes. Such studies are hampered by a lack of specific blockers for ASIC isoforms (29).
Conclusions.
These studies demonstrate mRNA and protein expression and increased expression of two ASIC isoforms (ASIC2a and ASIC3) in urothelium and suburothelial nerve plexus following urinary bladder inflammation in female rats. These initial observations are the foundation for future studies determining the functional contribution of ASICs to normal micturition reflexes as well as to altered micturition reflexes associated with urinary bladder inflammation.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-051369, DK-060481, and DK-065989. National Institutes of Health Grant P20 RR-16435 from the Centers of Biomedical Research Excellence Program of the National Center also supported the project for Research Resources.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical expertise and support provided by the Vermont Cancer Center DNA Analysis Facility.
REFERENCES
- 1.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
- 2.Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589–F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol 289: F489–F495, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol 45: 221–226, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT. Role for pituitary adenylate cyclase activating polypeptide (PACAP) in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290: R951–R962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int 93: 770–776, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F826–F836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 27: 3047–3055, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello CAD. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112: 321S–329S, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Dray A. Inflammatory mediators of pain. Br J Anaesth 75: 125–131, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept 109: 89–101, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Guerios SD, Wang ZY, Bjorling DE. Cyclophosphamide-induced peripheral hypersensitivity is mediated by nerve growth factor. Rep IUPS Satellite Meeting, San Diego, CA, 2005, p. 52 [Google Scholar]
- 15.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson AR, Aziz Q. Modulation of visceral nociceptive pathways. Curr Opin Pharmacol 7: 593–597, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol 500: 863–875, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett 208: 191–194, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab 21: 734–740, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Klinger MB, Girard B, Vizzard MA. p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi H, Yoshiyama M, Zakoji H, Takeda M, Araki I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: a possible involvement in irritative bladder symptoms. BJU Int 104: 1746–1751, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 291: R692–R703, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337: 362–367, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9: 5–13, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIlwrath SL, Hu J, Anirudhan G, Shin JB, Lewin GR. The sensory mechanotransduction ion channel ASIC2 (acid sensitive ion channel 2) is regulated by neurotrophin availability. Neuroscience 131: 499–511, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci 25: 9893–9901, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172: 2434–2439, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Pradet-Balade B, Boulme F, Beug H, Mullner EW, Garcia-Sanz JA. Translation control: bridging the gap between genomics and proteomics? Trends Biochem Sci 26: 225–229, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res 113: 143–151, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Staniland AA, McMahon SB. Mice lacking acid-sensing ion channels (ASIC) 1 or 2, but not ASIC3, show increased pain behaviour in the formalin test. Eur J Pain 13: 554–563, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420: 335–348, 2000 [PubMed] [Google Scholar]
- 41.Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res 844: 174–187, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs). Curr Drug Targets Inflamm Allergy 3: 71–79, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldmann R. Proton-gated cation channels—neuronal acid sensors in the central and peripheral nervous system. Adv Exp Med Biol 502: 293–304, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol 8: 418–424, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol 8: 25–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder following chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci 126–127: 380–389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zvarova K, Vizzard MA. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. J Comp Neurol 489: 501–517, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]