Abstract
Although some studies show that the upper esophageal sphincter (UES) contracts during transient lower esophageal sphincter relaxation (TLESR), others show that it relaxes. We hypothesized that the posture of the subject and constituents of gastroesophageal reflux (GER) may determine the type of UES response during the TLESR. High-resolution manometry and esophageal pH/impedance recording were performed in 10 healthy volunteers in the right recumbent (1 h) and upright (1 h) positions following the ingestion of a 1,000-Kcal meal. The UES pressure response during TLESR and constituents of GER (liquid, air, and pH) were determined. 109 TLESRs (58 upright and 51 recumbent) were analyzed. The majority of TLESRs were associated with GER (91% upright and 88% recumbent) events. UES relaxation was the predominant response during upright position (81% of TLESRs), and it was characteristically associated with presence of air in the reflux (92%). On the other hand, UES contraction was the predominant response during recumbent position (82% of TLESRs), and it was mainly associated with liquid reflux (71%). The rate of esophageal pressure increase (dP/dt) during the GER, but not the pH, had major influence on the type of UES response during TLESR. The dP/dt during air reflux (127 ± 39 mmHg/s) was significantly higher than liquid reflux (31 ± 6 mmHg/s, P < 0.0001). We concluded that the nature of UES response during TLESR, relaxation or contraction, is related to the posture and the constituents of GER. We propose that the rapid rate of esophageal pressure increase associated with air reflux determines the UES relaxation response to GER.
Keywords: gastroesophageal reflux, high-resolution manometry, impedance
transient relaxation of the lower esophageal sphincter (TLESR) is the major mechanism of gastroesophageal reflux (GER) in normal subjects and patients with GER disease (4, 5, 8). The function of upper esophageal sphincter (UES) in response to simulated or spontaneous GER has been studied extensively (3, 6, 12, 15–17, 21, 22, 24). Earlier studies reported UES contraction response to esophageal distension with a balloon (3, 6), acid infusion into the esophagus (1, 7, 23), spontaneous GER, and esophageal common cavities (a marker of GER) (21). However, above observations were challenged by the reports of no UES response (11, 22) during induced or spontaneous acid exposure of the esophagus, or even UES relaxation (15) during TLESR. Another study reported UES contraction during more than 90% of the GER episodes in normal subjects and patients with GER disease (21).
A close review of the literature revealed that studies that report UES contraction response during TLESR were mostly performed with the subject in the supine position and implied a protective role of the UES against reflux (7, 21). On the other hand, studies that show UES relaxation during GER/distension were mainly conducted with the subjects in the upright position, proposing a venting function of the UES during esophageal pressurization (12, 15).
Several investigators have observed that rapid distension of the esophagus, with the use of either balloon or air injection, induces UES relaxation and that a slow distension induces UES contraction (3, 6, 7, 12, 17, 19). Although the association of rapidity of esophageal distension (dP/dt) with UES relaxation during simulated and spontaneous reflux events (12, 15) has been shown previously, it is not clear whether type of UES response is related with the subject's posture or constituents of reflux. We used high-resolution manometry (HRM) and impedance/pH to determine the effects of posture, nature of GER constituents (liquid, air, and pH), and rapidity of esophageal pressure changes caused by GER on the UES response during TLESR.
MATERIALS AND METHODS
Study protocol.
We enrolled 10 healthy subjects (19–56 yr, 4 males). The Human Investigation Committee of University of California, San Diego approved the study protocol, and each subject signed an informed written consent before enrollment in the study. Subjects fasted 6 h, and, following local lidocaine application, a catheter assembly, consisting of a 4.2-mm diameter solid-state HRM catheter with 36 circumferential pressure sensors, spaced 1 cm apart (Sierra Scientific, Los Angeles, CA), and a 1.5-mm diameter Comfortec multi-luminal impedance (MII)-pH probe (Sandhill Scientific, Highlands Ranch, CO), was placed into the esophagus through one nostril. The catheter was positioned such that at least three pressure sensors were in the stomach, and esophageal impedance was recorded at 3, 5, 7, 9, 15, and 17 cm and pH at 5 cm above the upper border of LES. Subjects were given a standard 1,000-Kcal meal and placed in the recumbent right decubitus position for 1 h, following which subjects changed their position to an upright sitting position, and data were recorded for an additional hour. All pressures were recorded on the Sierra Scientific System at a 35-Hz frequency and measured in reference to atmospheric pressure. All impedance measurements were recorded on a Sandhill Scientific System at 30-Hz frequency. Manometry and pH-impedance data were synchronized using hand-inserted bookmarks and a time encoder (Thalaner Electronics, Ann Arbor, MI) to temporally align the physiological signals with an accuracy of one-hundredth of a second.
Pressure data analysis.
All TLESRs were identified using previously published criteria (9) adapted for the HRM. The LES and UES pressures were measured with electronic sleeve function of the Manoview system. We measured distal and proximal esophageal pressures as an average of two adjacent pressure sensors, 3–5 cm above LES and 3–5 cm below UES, respectively (15). All UES baseline measurements represent average pressure, just before TLESR, over at least five tidal volume respiratory cycles during the stable period when no pharyngeal, gastric, esophageal, UES, or LES contractions were present. The UES pressure during TLESR is presented as average and peak pressures. UES relaxation during TLESR was identified as a sudden drop of at least 10 mmHg in the UES pressure (15) and recorded only if it was not accompanied by primary peristalsis. Nadir pressure for UES relaxation, duration of nadir (duration over which pressure stays within 5 mmHg of proximal esophageal pressure), and total duration of relaxation (onset to return of pressure back to baseline) were recorded and compared with dry swallow-related UES relaxation measurements. The time of UES relaxation onset was recorded and compared with the onset of distal and proximal reflux events (if present). UES relaxation was considered complete if the nadir pressure was less than 5 mmHg of the proximal esophageal pressure. UES contraction was defined when the UES pressure increased by 10 mmHg or greater above the baseline pressure (15, 21). We only considered UES contraction related to reflux if it was not preceded or followed by UES relaxation (within 5 s). The peak and average UES contraction pressures over at least one respiratory cycle and the onset of UES contraction lag to the onset of TLESR were recorded.
We also identified upper and lower spatial borders of UES using isobaric contour function of Manoview software. Pressure greater than 5 mmHg above pharyngeal and esophageal pressures was used to define the upper and lower borders of UES, respectively. We determined the maximum length and position of the UES (its upper border) from the nostril at rest and during the TLESR (at the instant of maximum UES maximum length). The UES contraction related to primary or secondary peristaltic contraction at the end of TLESR was not taken into account for the above analysis. Pressure sensors in the HRM are spaced 1 cm apart from each other, and the data points between the sensors are linearly interpolated. Therefore, a reliable spatial resolution for axial measurements in the HRM is about 5 mm; all measurement differences of less than 5 mm were discarded.
To measure the rate of pressure change, the data were exported to an Excel program (Microsoft, Seattle, WA). Rate of pressure change (dP/dt) in the UES, proximal esophagus, and distal esophagus during GER was quantified, and the values were correlated with the contents of reflux and UES response. We also quantified the rate of pressure change during swallow-related UES relaxation, postrelaxation contraction, bolus pressure, and esophageal peristaltic contraction and compared these parameters with the similar parameters during TLESRs associated with esophageal pressure increase and UES response.
Impedance data analysis.
Impedance/pH recordings during TLESRs were examined for the evidence of GER by pH and impedance criteria using Bioview analysis software (Sandhill Scientific). Onset of liquid reflux was identified as a retrograde drop of impedance to 50% or less of the baseline value on at least two consecutive sensors, and air reflux was identified as a sharp rise in the impedance (>3 kΩ/s) over two consecutive sensors (18). Retrograde drop or rise of impedance at 3–5 and 15–17 cm above the LES was recorded as distal and proximal, liquid or air reflux, respectively. Both liquid and air reflux (mixed) episodes were identified when they had features of both air and liquid reflux.
Statistical methods.
Data are presented as means ± SD unless specified and compared using Student's t-test with unequal variances. We considered UES response during each TLESR as an independent event because it was clear that in all subjects it clearly correlated with constituents of refluxate rather than clustering similarly in each subject. Paired t-test was used when appropriate. Chi-square test was used to investigate the effects of posture and type of GER content on the UES response.
RESULTS
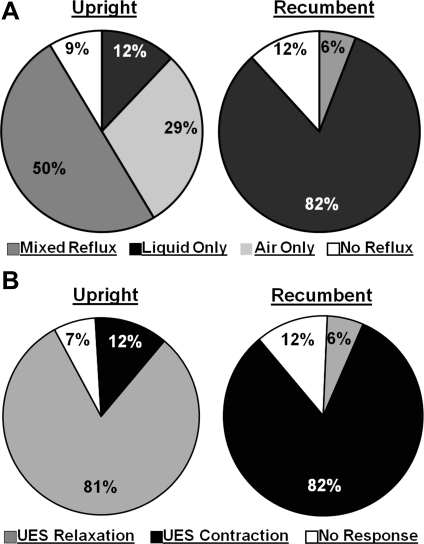
We recorded and analyzed 109 TLESRs (58 in the upright and 51 in the recumbent position) with a median of 11 (range 8–14) events per subject during the postprandial period. The mean duration of TLESR was 22.8 ± 5.1 s, and it was not different between the recumbent and upright position. We also scored 109 dry swallows (58 upright and 51 recumbent) with a median of 11 per subject (n = 10) to compare the features of swallow-related UES relaxation and TLESR-related UES relaxation along with the changes in the intraesophageal pressure. The majority of TLESRs were associated with GER (91% in upright and 88% in recumbent). The frequency of different types (based on constituents) of GER was considerably dependent on the posture of subject, as shown in the Fig. 1A. In the upright position, the GER during TLESR was mostly either of a mixed variety or pure air reflux. On the other hand, in the recumbent position the GER was constituted mostly of liquid type. The UES response during TLESR was dependent on the posture, which in turn determined the nature of reflux contents. UES relaxation was the main response in the upright position during TLESR. On the other hand, UES contraction was the major response in the recumbent position during TLESR, Fig. 1B.
Fig. 1.
A: frequency of types of reflux in upright and recumbent posture (P < 0.0001). B: frequency of upper esophageal sphincter (UES) response during transient lower esophageal sphincter relaxation (TLESR) in upright and recumbent posture (P < 0.0001).
UES response during TLESR in the upright position.
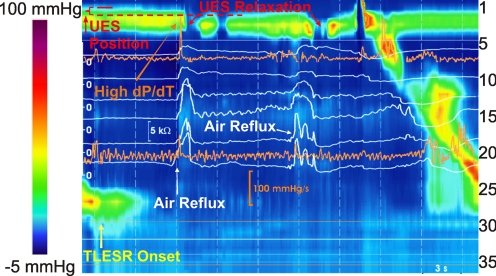
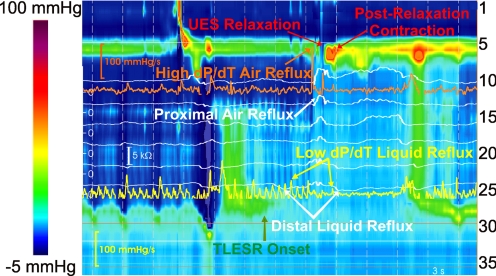
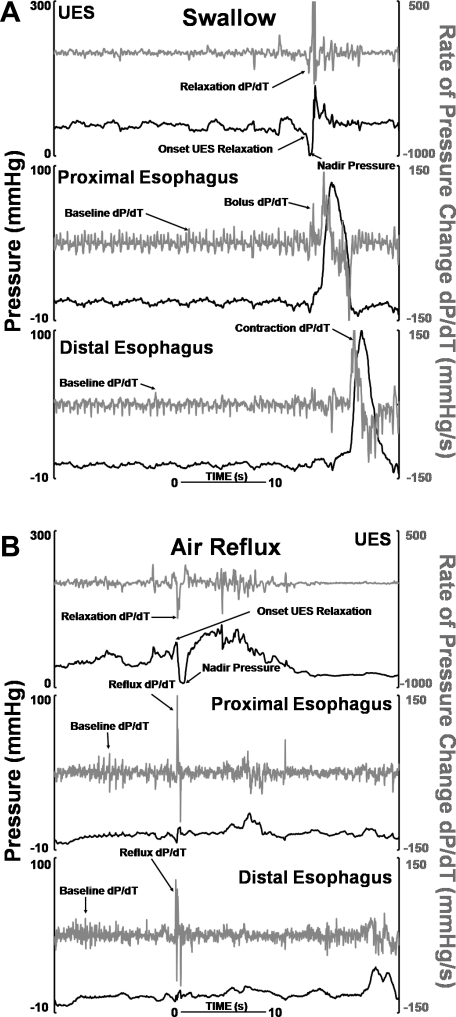
UES shows a dynamic and complex response during TLESR in the upright position. UES pressure during TLESR was not different from the UES pressure at baseline before TLESR. In the majority of TLESRs (47/58), the UES shows a relaxation response (62 times) that starts 9.9 ± 3.2 s after the onset of TLESR. Duration of UES relaxation during TLESR was 0.7 ± 0.4 s longer, and its nadir pressure was 8 ± 3 mmHg higher than swallow-related UES relaxation (Table 1). Eleven of the fifty-eight TLESRs were associated with more than one UES relaxation during the same TLESR (Fig. 2). Similar to swallow-related UES relaxation, the UES showed a postrelaxation contraction during TLESR with a peak pressure of 94 ± 38 mmHg (n = 49). Following air reflux-induced UES relaxation (Fig. 3), the UES length increased by 1.3 ± 0.5 cm (n = 45) and the UES moved caudal by 0.8 ± 0.3 cm (n = 27). These postrelaxation UES features were transient in nature and not present during all episodes. Characteristics of UES relaxation response during TLESR and comparison to the swallow-related UES relaxation are presented in Table 1.
Table 1.
Descriptive features of UES at rest and during TLESR
| Upright (N = 10) |
Recumbent (N = 10) |
|||
|---|---|---|---|---|
| NBaseline = 109 NTLESR = 109 NSwallow = 109 | Baseline | TLESR | Baseline | TLESR |
| UES length, cm | 3.4 ± 0.6 | 4.5 ± 0.8* (n = 45) | 3.4 ± 0.6 | 4.6 ± 1* (n = 35) |
| UES position to baseline, cm | N/A | 0.8 ± 0.5 (n = 25) | N/A | N/A |
| Average UES pressure, mmHg | 45 ± 17 | N/A | 46 ± 20 | 70 ± 30* (n = 45) |
| Peak UES pressure, mmHg | 54 ± 18 | N/A | 65 ± 35 | 122 ± 63* (n = 45) |
| Swallow | TLESR | Swallow | TLESR | |
| UES relaxation nadir pressure, mmHg | −4 ± 2 | 4 ± 2* (n = 47) | −3 ± 3 | N/A |
| UES relaxation duration, s | 0.7 ± 0.3 | 1.4 ± 0.6* (n = 62) | 0.6 ± 0.2 | N/A |
| UES relaxation nadir duration, s | 0.3 ± 0.1 | 0.7 ± 0.3* (n = 47) | 0.3 ± 0.1 | N/A |
| dP/dt of UES relaxation, mmHg/s | 596 ± 195 | 361 ± 127* (n = 62) | 605 ± 278 | N/A |
| dP/dt of UES contraction, mmHg/s | 1601 ± 557 | N/A | 1380 ± 345 | 266 ± 160* (n = 42) |
Applicable values are means ± SD.
P < 0.01 compared with the respective baseline or swallow-related value. UES, upper esophageal sphincter; TLESR, transient lower esophageal sphincter relaxation.
Fig. 2.
Color plot of esophageal pressures representing UES relaxation during upright TLESR. Impedance recordings are superimposed as white tracings, and dP/dt in proximal and distal esophagus are presented as orange tracings.
Fig. 3.
Color plot of esophageal pressures representing dP/dt in proximal and distal esophagus during a mixed (liquid and air) reflux episode in upright TLESR. Impedance recordings are superimposed as white tracings, and dP/dt tracings in proximal and distal esophagus are presented as orange and yellow tracings, respectively.
Air reflux has a characteristic sharp rise in the impedance tracing that allows one to identify its onset, as well as if there are multiple episodes of air reflux during the same TLESR (18). Analysis of impedance tracing revealed that the majority of reflux episodes contained a component of air reflux (33 pure air and 27 mixed, as seen in Figs. 2 and 3, respectively) in the upright posture (Table 2). All air reflux episodes were followed by a UES relaxation response. Looking at it another way, impedance-detected air reflux preceded 57/62 (92%) of the UES relaxation episodes by less than 1 s. UES relaxation response occurred after 0.5 ± 0.4 s (n = 49) and 0.2 ± 0.3 s (n = 54) after the onset of distal and proximal esophageal air reflux episodes, respectively. UES relaxations in the upright position were complete 47/62 (76%) times. UES contraction response (different form the postrelaxation contraction) was seen only during 7/58 upright TLESRs, and 3/7 times it was associated with liquid reflux event. Only two TLESR episodes elicited both UES contraction and relaxation response. Further interrogation of the relationship between UES response with the pH of the reflux content failed to show that acidity played a significant role in the UES response in the upright position (Table 3). The lack of effect of acidity on the UES response is evidenced by a relatively even distribution of acid and weak acid reflux during GER events. Air reflux episodes that did not extend to the proximal esophagus were few and not enough to determine whether there were differences between proximal and distal GER events on the UES response. Details of the UES response and reflux events in the upright posture are presented in Table 3.
Table 2.
UES response and GER during upright and recumbent posture
| Upright Position |
Recumbent Position |
|||||
|---|---|---|---|---|---|---|
| NSubject = 10 | UES Relaxation | No Response | UES Contraction | UES Relaxation | No Response | UES Contraction |
| Air reflux | 33 | 0 | 0 | 0 | 0 | 0 |
| Mixed reflux | 27 | 0 | 2 | 3 | 0 | 2 |
| Liquid reflux | 1 | 3 | 3 | 0 | 5 | 37 |
| No reflux | 1 | 2 | 2 | 0 | 2 | 3 |
GER, gastroesophageal reflux.
Table 3.
UES response, acidity, and extent of reflux in upright and recumbent position during TLESR episodes associated with reflux
| Upright Position |
Recumbent Position |
|||||
|---|---|---|---|---|---|---|
| NSubject = 10 NTLESR = 99 | UES Relaxation | No Response | UES Contraction | UES Relaxation | No Response | UES Contraction |
| Acid | 24 | 1 | 3 | 3 | 1 | 30 |
| Weakly acid | 23 | 2 | 2 | 0 | 4 | 7 |
| Proximal | 44 | 1 | 3 | 2 | 1 | 22 |
| Distal | 3 | 2 | 2 | 1 | 4 | 15 |
UES response during TLESR in recumbent position.
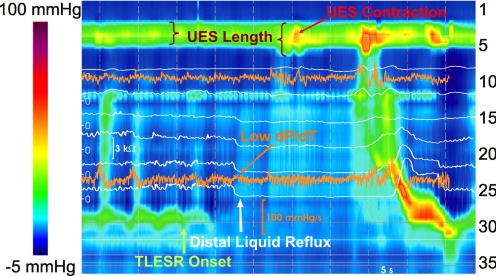
UES shows quite different features during TLESR in the recumbent position compared with the upright position. In a majority of TLESRs (42/51) the UES showed a contraction that occurred, either before the onset or during the period of TLESR. In (12/42) 29% of instances, the UES contractions occurred 0.4 ± 2.1 s before the onset of TLESR, and in (30/42) 71% it occurred 11.5 ± 3.2 s after the onset of TLESR (mid-TLESR, Fig. 4). During UES contraction response, peak UES pressure increased from baseline value of 65 ± 35 to 122 ± 63 mmHg (n = 42). UES maximum length during TLESR increased by 1.3 ± 0.5 cm (n = 35), and UES moved caudal only in a minority (6/51) of TLESRs. Increase in UES length only occurred when there was UES contraction. Characteristics of the UES response during TLESR compared with the swallow-related UES contraction and at rest are presented in Table 1.
Fig. 4.
Color plot of esophageal pressures representing UES contraction during recumbent TLESR. Impedance recordings are superimposed as white tracings, and dP/dt in proximal and distal esophagus are presented as orange tracings.
Liquid reflux has typical retrograde drop in the impedance tracing, and, in contrast to air reflux, repeated liquid reflux episodes during the same TLESR(18) cannot be identified. The latter is mainly because impedance measurements after liquid reflux remain low until the reflux is cleared by a peristaltic wave. We scored impedance recordings to determine the timing of UES contraction with respect to GER. In the recumbent posture, a total of 42 episodes of liquid reflux were detected (Table 2), of which 30 (71%) were followed by UES contraction. Liquid reflux preceded 25/30 (83%) of the mid-TLESR UES contractions by less than 3 s. Because 12 of the UES contractions occurred at the onset of TLESR, detection of further contraction on the top of an already hypertonic UES was difficult and further analysis was not done. Mid-TLESR, UES contraction response occurred 2 ± 1.3 s (n = 22) and 1 ± 1.5 s (n = 16) after the distal and proximal esophageal liquid reflux, respectively. A UES relaxation response was seen in only 3/51 recumbent TLESRs, and 3/3 times it was associated with an air component of the mixed reflux. UES relaxations in recumbent posture were all incomplete. Two TLESRs with mixed reflux showed both a relaxation and contraction response. We further interrogated the relationship between UES contraction responses, extent of the reflux (distal reflux only vs. distal and proximal reflux), and pH of the reflux (Table 3). However, the majority of the GER episodes were acidic and extended to the proximal esophagus, and therefore a valid comparison could not be performed.
Rate of pressure change (dP/dt) during TLESR.
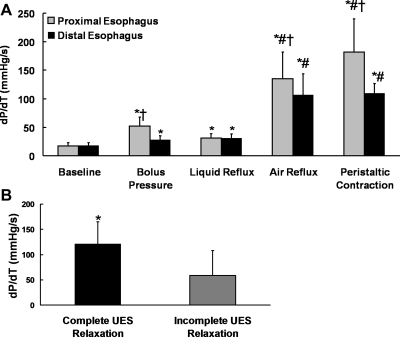
The rate of pressure change, dP/dt, shows fluctuations in the baseline period because of respiratory and cardiovascular effects (Fig. 5A). These were not different between the upright and recumbent position and the distal and proximal esophagus. However, during air-containing GER events, the change in dP/dt is distinctly fast (Fig. 5B). The esophageal dP/dt was significantly higher (P < 0.0001) during air reflux (127 ± 39 mmHg/s) compared with liquid reflux (31 ± 6 mmHg/s) episodes (Fig. 6A). The UES relaxation in the upright position occurred 0.1 ± 0.3 s (n = 52) and 0.4 ± 0.4 s (n = 48) after proximal and distal rapid pressure increase, respectively. The dP/dt of air reflux events that were followed by incomplete UES relaxation was significantly smaller (P < 0.001) than the dP/dt of air reflux episodes that caused complete UES relaxation (Fig. 6B). Two UES relaxation responses that were not associated with detectable air reflux by impedance were associated with a high distal/proximal esophageal dP/dt (82 mmHg/s). All UES relaxation episodes in the recumbent position were incomplete and had a significantly lower dP/dt compared with the upright air reflux episodes. The dP/dt during liquid reflux was at times distinguishable from the baseline fluctuations but was significantly lower than the air reflux.
Fig. 5.
Rate of pressure change during swallow (A) and air (B) reflux.
Fig. 6.
A: comparison of rate of pressure change dP/dt in different positions. *P = 0.01 compared with baseline, #P < 0.001 compared with baseline, liquid reflux, and bolus pressure, †P < 0.01 distal compared with proximal. B: comparison of reflux related to esophageal dP/dt during complete and incomplete UES relaxation, n = 47 for complete UES relaxation, n = 15 for incomplete relaxation. *P < 0.001.
DISCUSSION
In summary, our data show the following: 1) air reflux into the esophagus and associated UES relaxation during TLESR are characteristics of the subjects in the upright posture. On the other hand, UES contraction and liquid reflux occur during a majority of the TLESRs when the subjects are in the recumbent position. 2) UES relaxation is temporally correlated with the air reflux and represents a belch reflex. On the other hand, UES contraction with liquid reflux most likely represents an aerodigestive protective reflex in the recumbent posture. 3) The rapid rate of esophageal pressure increase (dP/dt) with air reflux compared with liquid reflux is likely to be the stimulus for UES relaxation.
With the advent of UES sleeve sensor in the early 1980s, it became possible to reliably record UES pressure over extended periods of time. Kahrilas et al. (12) conducted a comprehensive study with balloon and air distensions of the esophagus using the sleeve sensor and observed that rapid distension of esophagus, especially with air, evokes UES relaxation and that slow distension with balloon and fluid elicits UES contraction (12). Both relaxation and contractions were not blocked by mucosal anesthesia with lidocaine in the Kahrilas study. Shaker et al. (21) studied the UES response to spontaneous GER events in healthy subjects and patients with GER disease. They observed that the UES contracts in response to spontaneous GER consistently in both groups during more than 90% of the instances (21). GER in their study (and earlier studies) was detected when a common cavity pressure wave was seen on the manometry record. We hypothesized that the differences in the posture of the subjects during the study period may determine whether the UES relaxes or contracts in response to GER. Furthermore, our recent studies show that common cavity of the esophagus cannot be used as a reliable surrogate marker of the GER because it can be caused by longitudinal muscle contraction of the esophagus (20). Therefore, we used impedance measurements to record GER and at the same time determined whether the constituents of GER made a difference with regards to the UES response. We found that the differences in the UES response in the Kahrilas and Shaker studies can be reconciled on the basis of the posture of subject during the experiment. Impedance recording revealed that, in the upright posture, air reflux is the major constituent of GER and air reflux is associated with the UES relaxation. On the other hand, liquid reflux dominates during recumbent posture, and liquid reflux is associated with UES contraction response. During TLESRs associated with mixed GER episodes, we always saw UES relaxation, and it was difficult to know whether the UES contraction, if it occurred, was related to pre- or postrelaxation contraction vs. a response to liquid reflux (Fig. 3).
Why should air reflux cause UES relaxation and liquid reflux cause UES contraction? Several investigators have observed that the rapidity of esophageal distension is a major determinant of the type of UES response (12, 13, 15, 19). Accordingly, we performed detailed assessment of the rate of esophageal pressure increase (dP/dt) in association with the GER. We found that esophageal dP/dt in association with air reflux and UES relaxation response is significantly faster than liquid reflux and associated UES contraction. We also found that the esophageal dP/dt with air reflux episodes that were associated with incomplete UES relaxations was significantly slower than the ones associated with complete UES relaxations. Additionally, we found few episodes of UES relaxations that were associated with a fast dP/dt in the esophagus but without GER. The reason for fast dP/dt in the absence of reflux is not clear, but we suspect that impedance may have missed reflux detection or that another motor event in the esophagus, such as a strong longitudinal muscle contraction, may have resulted in an increase in esophageal pressure. Furthermore, a recent study (14) found that posture is an important determinant of UES relaxation evoked by air injection and UES contraction by water injection into the esophagus, further supporting our observations with spontaneous GER events. We observed that, for the same reflux episode, the dP/dt in the proximal esophagus was higher than the dP/dt in the distal esophagus. The latter may be related to the differences in the compliance of stretched skeletal muscle vs. contracted smooth muscles of the proximal and distal esophagus, respectively. Whether the difference between the faster dP/dt in the proximal vs. distal esophagus plays any role in the type of UES response is not clear from our study.
The hyoid bone and UES move in the ventrocranial direction with each swallow (10). On the other hand, during belch reflex the hyoid bone and UES may move caudally (10). Our HRM colored plots show that, with swallows, the UES indeed moves in the cranial direction. On the other hand, and in contrast to swallow, UES commonly moves in the caudal direction with air reflux-associated UES relaxation. Our recent studies show a unique pattern of longitudinal muscle contraction during TLESR (2). It begins before the onset of TLESR, gets stronger during TLESR, and ends at the termination of TLESR. We suspect that the caudal movement of the UES during TLESR is related to the strong contraction of the longitudinal muscles of the distal esophagus, which pulls the UES in the caudal direction. Another observation we made in our study is that the UES length increases with the increase in UES pressure, suggesting that either the distal pharyngeal or proximal esophageal muscle fibers get incorporated into the UES. Increase in length of UES during TLESR possibly adds to the strength of the UES and makes it a stronger barrier in the prevention of esophagopharyngeal reflux.
In summary, simultaneous HRM and impedance pH studies reveal that the differences in the UES response reported over last several decades can be explained on the basis of the effect of posture and the constituents of GER. Air reflux, in the upright position, causes a rapid increase in the esophageal pressure, which most likely induces UES relaxation. On the other hand, liquid reflux in the recumbent position causes a slow increase in esophageal pressure and UES contraction. Future studies may be needed to investigate whether there are differences in the effects of posture on the constituents of GER in normal subjects and patients with GER disease, which may form the pathophysiological basis of defective aeroprotective reflexes in patients with laryngeal and respiratory symptoms.
GRANTS
This study was funded by NIH Grant RO1-DK060733 to Dr. Ravinder K. Mittal.
DISCLOSURES
None of the authors have any conflict of interest to disclose.
REFERENCES
- 1.Andreollo NA, Thompson DG, Kendall GP, McIntyre AS, Earlam RJ. Motor responses of the upper esophageal sphincter and body to intraluminal acid. Braz J Med Biol Res 22: 51–60, 1989 [PubMed] [Google Scholar]
- 2.Babaei A, Bhargava V, Korsapati H, Zheng WH, Mittal RK. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology 134: 1322–1331, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Creamer B, Schlegel J. Motor responses of the esophagus to distention. J Appl Physiol 10: 498–504, 1957 [DOI] [PubMed] [Google Scholar]
- 4.Dent J, Dodds WJ, Friedman RH, Sekiguchi T, Hogan WJ, Arndorfer RC, Petrie DJ. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest 65: 256–267, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 307: 1547–1552, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Enzmann DR, Harell GS, Zboralske FF. Upper esophageal responses to intraluminal distention in man. Gastroenterology 72: 1292–1298, 1977 [PubMed] [Google Scholar]
- 7.Gerhardt DC, Shuck TJ, Bordeaux RA, Winship DH. Human upper esophageal sphincter. Response to volume, osmotic, and acid stimuli. Gastroenterology 75: 268–274, 1978 [PubMed] [Google Scholar]
- 8.Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology 89: 779–784, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Holloway RH, Penagini R, Ireland AC. Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 268: G128–G133, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ. Upper esophageal sphincter function during antegrade and retrograde transit. Am J Med 103: 56S–60S, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Kahrilas PJ, Dodds WJ, Dent J, Haeberle B, Hogan WJ, Arndorfer RC. Effect of sleep, spontaneous gastroesophageal reflux, and a meal on upper esophageal sphincter pressure in normal human volunteers. Gastroenterology 92: 466–471, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Kahrilas PJ, Dodds WJ, Dent J, Wyman JB, Hogan WJ, Arndorfer RC. Upper esophageal sphincter function during belching. Gastroenterology 91: 133–140, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol 281: G1246–G1263, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Babaei A, Kuribayashi S, Surapaneni S, Shaker R. Mysteries of upper esophageal sphincter (UES) response to esophageal distension, effect of posture, volume and physical nature of distending agent. Gastroenterology 136: A526, 2009 [Google Scholar]
- 15.Pandolfino JE, Ghosh SK, Zhang Q, Han A, Kahrilas PJ. Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR); it is mainly about microburps. Neurogastroenterol Motil 19: 203–210, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Sabanathan S, Bryan AJ. The upper oesophageal sphincter and its response to gastrooesophageal reflux. J R Coll Surg Edinb 30: 167–172, 1985 [PubMed] [Google Scholar]
- 17.Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol Gastrointest Liver Physiol 262: G621–G628, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Sifrim D, Silny J, Holloway RH, Janssens JJ. Patterns of gas and liquid reflux during transient lower oesophageal sphincter relaxation: a study using intraluminal electrical impedance. Gut 44: 47–54, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczesniak MM, Fuentealba SE, Burnett A, Cook IJ. Differential relaxation and contractile responses of the human upper esophageal sphincter mediated by interplay of mucosal and deep mechanoreceptor activation. Am J Physiol Gastrointest Liver Physiol 294: G982–G988, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Tipnis NA, Liu J, Puckett JL, Mittal RK. Common cavity pressure during gastroesophageal reflux: reassessment using simultaneous pressure, impedance, and ultrasound imaging. Am J Physiol Gastrointest Liver Physiol 290: G1149–G1156, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Torrico S, Kern M, Aslam M, Narayanan S, Kannappan A, Ren J, Sui Z, Hofmann C, Shaker R. Upper esophageal sphincter function during gastroesophageal reflux events revisited. Am J Physiol Gastrointest Liver Physiol 279: G262–G267, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Vakil NB, Kahrilas PJ, Dodds WJ, Vanagunas A. Absence of an upper esophageal sphincter response to acid reflux. Am J Gastroenterol 84: 606–610, 1989 [PubMed] [Google Scholar]
- 23.Wallin L, Boesby S, Madsen T. The effect of HCl infusion in the lower part of the oesophagus on the pharyngo-oesophageal sphincter pressure in normal subjects. Scand J Gastroenterol 13: 821–826, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Winship DH. Upper esophageal sphincter: does it care about reflux? Gastroenterology 85: 470–472, 1983 [PubMed] [Google Scholar]