Abstract
The morphology of interstitial cells of Cajal (ICC) in the circular muscle layer of the cynomolgus monkey internal anal sphincter (IAS) and rectum and their relationship to sympathetic and nitrergic nerves were compared by dual-labeling immunohistochemistry. Contractile studies confirmed that nitrergic nerves participate in neural inhibition in both regions whereas sympathetic nerves serve as excitatory motor nerves only in the IAS. Muscle bundles extended from myenteric to submucosal edge in rectum but in the IAS bundles were further divided into “minibundles” each surrounded by connective tissue. Dual labeling of KIT and smooth muscle myosin revealed KIT-positive stellate-shaped ICC (ICC-IAS) within each minibundle. In the rectum intramuscular ICC (ICC-IM) were spindle shaped whereas stellate-shaped ICC were located at the myenteric surface (ICC-MY). ICC were absent from both the myenteric and submucosal surfaces of the IAS. Nitrergic nerves (identified with anti-neuronal nitric oxide synthase antibodies or NADPH diaphorase activity) and sympathetic nerves (identified with anti-tyrosine hydroxylase antibody) each formed a plexus at the myenteric surface of the rectum but not the IAS. Intramuscular neuronal nitric oxide synthase- and tyrosine hydroxylase-positive fibers were present in both regions but were only closely associated with ICC-IM in rectum. Minimal association was also noted between ICC-IAS and cells expressing the nonspecific neuronal marker PGP9.5. In conclusion, the morphology of rectal ICC-IM and ICC-MY is similar to that described elsewhere in the gastrointestinal tract whereas ICC-IAS are unique. The distribution of stellate-shaped ICC-IAS throughout the musculature and their absence from both the myenteric and submucosal surfaces suggest that ICC-IAS may serve as pacemaker cells in this muscle whereas their limited relationship to nerves suggests that they are not involved in neuromuscular transmission. Additionally, the presence of numerous minibundles, each containing both ICC-IAS and nerves, suggests that this muscle functions as a multiunit type muscle.
Keywords: gastrointestinal, enteric, motility, internal anal sphincter, rectum
the internal anal sphincter (IAS) is a thickening of the circular muscle (CM) layer at the distal terminus of the gastrointestinal tract. Basal anal pressure is high, aiding in the maintenance of fecal continence. In contrast, pressure in the adjacent rectum is lower, allowing this region to serve as a final site of storage prior to defecation (2). These functional differences are accompanied by significant differences in the contractile behavior of isolated strips of muscle from the IAS and rectum, i.e., the IAS is considered to be predominantly a “tonic” muscle whereas rectal muscles contract predominantly in a phasic manner (6, 37, 42). Excitatory motor innervation to the IAS and rectum also differ, i.e., in the IAS it is sympathetic whereas in rectum it is cholinergic/tachykinergic (3, 56). In contrast, nitrergic nerves participate in inhibitory motor innervation in both regions (39, 52).
Interstitial cells of Cajal (ICC) are specialized cells in the gastrointestinal (GI) tract that participate in the control of motor activity. These cells can be visualized with immunohistochemical techniques using antibodies against KIT, a receptor tyrosine kinase that is robustly expressed in ICC but not nerves or smooth muscle cells (SMC) (36). Several distinct populations of ICC exist in the GI tract. One population serves as pacemaker cells and generates slow waves (22, 46), and a second has been proposed to participate in neuromuscular transmission (63). Although ICC have been identified with immunohistochemical techniques in the human (17, 43), mouse (10), and dog (20) IAS, few details are available regarding their morphology and distribution, and their role in the control of motor activity is largely unknown.
Pacemaker type ICC are typically stellate shaped in morphology and confined to either the myenteric plexus region (i.e., ICC-MY) or the submucosal plexus region (ICC-SM). Some ICC also extend from plexus regions into the muscularis along septal structures (ICC-SEP; Refs. 32, 60). Pacemaker ICC are coupled to one other and to the adjacent SMC via low-resistance gap junctions (8). Rhythmic currents generated by ICC conduct into adjacent SMC, giving rise to slow waves and phasic contractile activity (22, 46). In addition, currents generated by ICC have been proposed to underlie tone generation in some smooth muscles [e.g., urethra (35)]. The present study compares the morphology and distribution of ICC in the tone-generating IAS to those of the phasically active rectum.
ICC proposed as participants in neuromuscular transmission are located within the musculature. These spindle-shaped cells run parallel to SMC in the longitudinal and CM layers. There is evidence that intramuscular ICC (ICC-IM) participate in excitatory (i.e., cholinergic) neuromuscular transmission in GI muscles (54, 62, 63). However, the excitatory motor innervation in the IAS is predominantly sympathetic (3, 56). At present there are no studies that have examined the role of ICC in sympathetic neuromuscular transmission in the GI tract. The present study therefore compares the distribution of sympathetic nerves in the IAS and rectum and their relationship to ICC.
ICC-IM have also been proposed to participate in nitrergic transmission (for review see Ref. 62) although there is still controversy regarding this conclusion (for review see Refs. 16, 22). The role of ICC in nitrergic transmission in the IAS has been investigated in the W/Wv KIT-deficient mouse. These mice have reduced Kit expression since one Kit allele (W) is nonfunctional and the other allele (Wv) has reduced function (38). Consequently ICC are absent from some regions of the GI tract (25) and greatly reduced in others including the IAS (10). Studies of the W/Wv KIT-deficient mouse IAS suggest that ICC are not required for nitrergic transmission in this region (10, 55). The present study establishes a functional role for nitrergic nerves in the monkey IAS and rectum and examines whether there are differences in the morphological relationship between ICC and nitrergic nerves between the IAS and rectum.
The model used for these studies is the cynomolgus monkey (Macaca facicularis facicularis). Macaca facicularis diverges at the nucleotide level from the related species Macaca mulatta by only 0.4% (40), and this latter species shares ∼93% gene sequence identity with humans (15). Thus studies of the cynomolgus monkey IAS may provide valuable new insight into how motility in the human IAS is controlled. Our results reveal a number of important differences in the morphology and distribution of ICC in the IAS vs. rectum and in the relationship of these cells to nerves. Whereas the morphology of ICC-MY and ICC-IM in the rectum is similar to that of ICC described elsewhere in the GI tract, IAS-ICC possess several unique morphological features that are likely to contribute to the unique functional role of this terminal portion of the GI tract.
MATERIALS AND METHODS
Tissue Preparation
Cynomolgus monkey tissues were obtained from Charles River Preclinical Services (Sparks, NV). The protocol for euthanizing monkeys was approved by their Institutional Animal Care and Use Committee assuring compliance with the United States Department of Agriculture, Public Health Service Office of Laboratory Animal Welfare Policy and the Animal Welfare act (Charles River Laboratories, Preclinical Services, Sparks, NV). Monkeys of either sex (32 monkeys, 2.5–7 yr of age) were initially sedated with ketamine (10 mg/kg), then administered 0.7 ml Beuthanasia-D solution (pentobarbital sodium and phenytoin sodium) followed by exsanguination. The rectoanal region was removed and placed in a screw-capped container with cold Krebs bicarbonate solution (KRBS) of the following composition (in mM): 118.5 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11.0 dextrose. The sample was then transported on ice within 30 min to the University of Nevada, Reno, where all adhering skeletal muscle and mucosa were removed after pinning in a dissecting dish. IAS muscles were further prepared by removing the longitudinal muscle (LM) layer whereas both LM and CM layers were retained in rectal segments. The LM in the sphincter region is not a discrete layer but rather divided into bundles embedded in connective tissue and glands. Thus orientation of the CM was best achieved by removal of this overlying structure. In contrast, the muscle layers of the rectum were much thinner and compact and orientation of CM strips was best achieved by retaining the LM layer. Either experiments were undertaken on freshly isolated muscles or muscles were stored overnight in KRBS at (4°C).
Immunohistochemistry
Thick muscle sections.
Muscle bundles of IAS and rectum (100 μm) were created by cutting either parallel or perpendicular to CM bundles with fine iris scissors. Sections were then pinned flat in a dissection dish and fixed for 30 min either with ice cold acetone or paraformaldehyde. Tissues were then washed in 0.1 M phosphate buffer solution overnight at 4°C. To reduce nonspecific antibody binding, tissues were incubated in bovine serum albumin (BSA; 1% wt/vol; Sigma, St. Louis, MO) for 1 h at 20°C. To achieve greater penetration during labeling, sequential incubations of the tissue preparations with primary antibodies [in combinations of anti-KIT; anti-tyrosine hydroxylase; anti-neuronal nitric oxide synthase (nNOS); anti-protein gene product 9.5; anti-smooth muscle myosin] were carried out with use of Triton-X 100 (0.5%; Sigma). Incubation with the first primary antibody was carried out for 48 h at 4°C. Following 3–4 h of washing with PBS, tissues were incubated with the second primary antibody for 48 h. A list of the primary antibodies used and their reactivity, source, and dilutions used in the study is found in Table 1. Tissues were washed with rotation for 12 h before detecting immunoreactivity with secondary antibodies (Molecular Probes, Eugene, OR). The secondary antibodies used are listed in Table 2. Incubations with secondary antibodies were performed sequentially, each for 1 h in the dark at 20°C, with washing for 1 h between antibodies.
Table 1.
Primary antibodies
| Primary Antibody | Source | Mono- or Polyclonal | Host | Antigen | Specificity | Working Dilution |
|---|---|---|---|---|---|---|
| Anti-KIT (Ab-4; cocktail) | NeoMarkers, Fremont, CA | Mono | Mouse | CD117/KIT/SCF-receptor | Ab-4 cocktail is especially designed for sensitive detection of CD117/KIT | 1:100–200 |
| Anti-KIT (human CD117/KIT) | Dako USA, Carpinteria, CA | Poly | Rabbit | CD117/KIT | Labels peptide corresponding to amino acids 963 to 976 at the cytoplasmic COOH-terminus of KIT | 1:200 |
| Anti-tyrosine hydroxylase | Chemicon International, Temecula, CA | Poly | Rabbit | Tyrosine hydroxylase, the rate limiting step in the synthesis of catecholamines | Detects endogenous levels of total tyrosine hydroxylase | 1:1,000 |
| Anti-nNOS | Gift from Dr. Piers Emson, Molecular Science Group, Cambridge, UK | Poly | Sheep | nNOS | Detects nNOS in central and peripheral nitrergic neurons | 1:200 |
| Anti-protein gene product 9.5 | UltraClone Limited, Isle of Wight, UK | Poly | Rabbit | Protein gene product 9.5, an abundant neuron and neuroendocrine-cell specific protein | Labels neuronal cell bodies and axons in the central and peripheral nervous systems and small nerve fibers in peripheral tissues | 1:1,000 |
| Anti-smooth muscle myosin | Biomedical Technologies, Stoughton, MA | Poly | Rabbit | Smooth muscle myosin II heavy chain | Specifically labels the smooth muscle myosin II heavy chain without detectable cross-reactivity with either cardiac or skeletal muscle myosin | 1:200 |
nNOS, neuronal isoform of nitric oxide synthase.
Table 2.
Secondary antibodies
| Secondary Antibody | Source | Primary Antibody Reacted With | Working Dilution | Wavelength Used |
|---|---|---|---|---|
| Alexa-Fluor goat anti-mouse | Molecular Probes, Eugene, OR | Anti-KIT (Ab-4; cocktail) | 1:1,000 | 594 nm |
| Alexa-Fluor goat anti-rabbit | Molecular Probes | Anti-KIT (Human CD117/KIT) | 1:1,000 | 594 nm |
| Alexa-Fluor goat anti-rabbit | Molecular Probes | Anti-tyrosine hydroxylase; anti-protein gene product 9.5; anti-smooth muscle myosin | 1:1,000 | 488 nm |
| Alexa-Fluor donkey anti-sheep | Molecular Probes | Anti-nNOS | 1:1,000 | 488 nm |
Modified whole mount preparations.
A 1.5-cm-wide by 4-cm-long strip of the rectoanal region was isolated from distal IAS (anal verge) to proximal rectum (4 cm). The mucosa was removed, as well as the LM layer, with care taken to avoid damage to the myenteric plexus region. This procedure was facilitated by the wide connective tissue gap that separates longitudinal and CM layers in this region. Modified whole mount preparations were then either fixed with acetone and prepared for immunohistochemical labeling of KIT-positive cells or fixed with paraformaldehyde and prepared for labeling NADPH diaphorase activity.
Cryosections.
For immunohistochemical labeling of cryosections, tissues were fixed and washed as described for whole-mount specimens and then dehydrated in graded sucrose solutions (5, 10, 15%, 15 min each, 20% overnight) and embedded in Tissue Tek OTC compound (Sakura Finetek, Torrance, CA), before being frozen in liquid nitrogen. Sections were cut transverse to the CM layer at a thickness of 10–12 μm (thin cryosections) or 100 μm (thick cryosections) by use of a Leica CM 3050 cryostat (Leica Microsystems, Wetzlar, Germany). Tissues were preincubated with 1% BSA solution for 1 h at 20°C before sequential incubations with primary antisera in combination with 0.5% Triton-X 100 solution for 12–24 h at 4°C. Incubations with secondary antisera were carried out as described above for whole-mount preparations.
Controls.
To ensure specificity of labeling, control tissues were examined. These were prepared by omitting either primary or secondary antibodies from the incubation solutions. Single-labeled tissue specimens were also examined to confirm double-label experiment findings. For most ICC labeling the KIT antibody Ab-4 was used (including the images shown in figures). The specificity of this labeling was further examined by using a second anti-KIT antibody (i.e., DAKO CD117, see Table 1). The morphology and distribution of ICC and mast cells identified with DAKO was the same as that observed with Ab-4.
Imaging.
A Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Thornwood, NY) was used to examine both whole-mount specimens and cryosections. Micrographs of whole-mount specimens, generated via the confocal imaging system, are digital composites of Z-series of scans 0.25- to 1-μm optical sections through a depth of 0.5–35 μm. Final images were constructed by use of Zeiss LSM 5 Image Examiner Software, Adobe Photoshop CS2 Software, and CorelDRAW X3 Software. Immunoreactivity was detected with secondary antibodies conjugated with either Alexa Fluor 594 (KIT, red) or Alexa Fluor 488 (nerves, green) (Table 2).
Light Microscopy.
To assess overall morphology, 1-mm-wide cross sections of the rectoanal region were created. Muscles were fixed in ice-cold paraformaldehyde [4% wt/vol in 0.1 M phosphate buffer solution (PBS)] at 4°C overnight. After fixation, tissues were washed for 30 min in PBS, 0.01 M, pH 7.4. Tissues were then stained with 0.25% toluidine blue in 1% borax for 2 min and then washed overnight in 0.01 M PBS. To examine in more detail the relationship of muscle bundles to septal structures muscles were dehydrated and embedded in paraffin wax; 8-μm sections were stained with Masson's trichrome and examined with a Nikon Eclipse E800 microscope (Nikon). Photomicrographs were acquired via a Spot RT Slider CCD camera (Diagnostic Instruments) with proprietary software.
NADPH Diaphorase Histochemistry
Modified whole mount preparations were fixed in ice-cold paraformaldehyde [4% wt/vol in 0.1 M phosphate buffer solution] at 4°C for 2 h and washed in 0.01 M PBS as described above. Tissues were stained in a NADPH-diaphorase solution containing 1 mg/ml β-NADPH, 0.25 mg/ml nitroblue tetrazolium, and 0.5% Triton X-100 in 0.01 M PBS, for times ranging from 30–60 min at 4°C. Tissues were then washed in PBS with rotation before being pinned in a Sylgard-based dissection dish containing 0.01 M PBS for imaging. The above chemicals were purchased from Sigma-Aldrich (St. Louis). Specimens were imaged via a Nikon SMZ 800 inverted stereomicroscope (Nikon USA) and Diagnostic Instruments Spot Slider RT CCD camera.
Image Processing
Maximum or average intensity Z-projections were used to display confocal stacks in two dimensions. Three-dimensional surface renderings and anaglyphs were constructed by use of Volumetry G7mv (G. W. Hennig). A manual mask was applied to cells shown in Fig. 3, A and B, to isolate them from other cells in the stack. Red-cyan three-dimensional (3D) glasses are required to view anaglyphs (free glasses are available at http://www.rainbowsymphony.com/freestuff.html). In most images ICC are shown in red with the exception of Fig. 2 where KIT labeling has been converted to reverse grayscale via Adobe Photoshop. In Fig. 1 the contrast between muscle bundles and connective tissue in the IAS was enhanced by selecting all magenta pixels in the image and assigning them red with Photoshop (Adobe, Mountain View, CA).
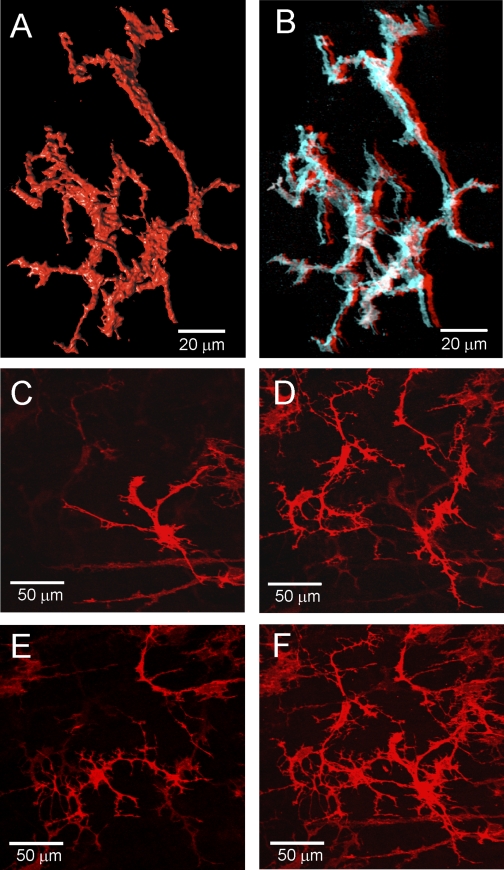
Fig. 3.
Three-dimensional images of ICC in the monkey IAS. A: 3-dimensional (3D) surface reconstruction of a small cluster of ICC-IAS with characteristic branching processes. Image generated from 77 confocal slices (0.15 μm/slice) taken from a cross-sectional preparation (×100). Supplemental Fig. S4 shows rotation of these cells. B: anaglyph of cells shown in A. To view in 3D, red (left eye) and cyan (right eye) glasses are required (see materials and methods). Supplemental Fig. S5 shows anaglyph of a different cluster of cells. C–F: lower magnification images (×40) of ICC-IAS within confocal stack (27 slices, 0.25 μm/slice) from a transverse section. C–E: 3 different optical sections (each a 1.25-μm stack) selected from top (C), middle (D), and bottom (E) of stack. F: complete confocal stack (13.5 μm thick). A different transverse section taken at lower magnification is shown as an anaglyph in Supplemental Fig. S6 and a movie in Supplemental Video S7.
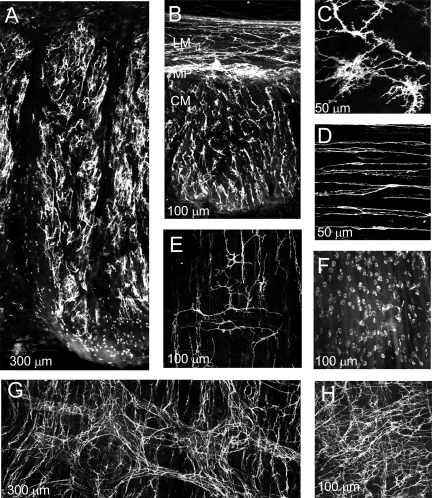
Fig. 2.
Immunohistochemical labeling of KIT-positive cells in the monkey rectoanal region. KIT-positive ICC (white) are seen distributed throughout the IAS (A) and rectum (B) in these lower magnification (×10) thick cross sections. B: both the longitudinal and circular muscle layers can be seen in this image. Labeling is most dense in the myenteric plexus (MP) region between muscle layers. Higher magnification images of transverse sections show stellate-shaped ICC-IAS in the IAS (C) and spindle-shaped intramuscular ICC in the rectum (D). E–H: images showing the distribution of KIT-positive cells at the myenteric and submucosal surfaces of the IAS and rectum in a modified whole mount preparation. Stellate-shaped ICC can be seen at both the submucosal (E, ICC-SM) and myenteric (G and H, ICC-MY) surface of the rectum whereas only small rounded mast cells were observed at the myenteric (F) and submucosal (A) surfaces of the IAS. Optical section thickness: A, 26 μm; B, 17 μm; C, 6 μm; D, 3.75 μm; E, 4 μm; F, 13 μm; G, 18 μm; H, 12 μm. Additional images from modified whole mounts can be seen in Supplemental Figs. S1 and S2.
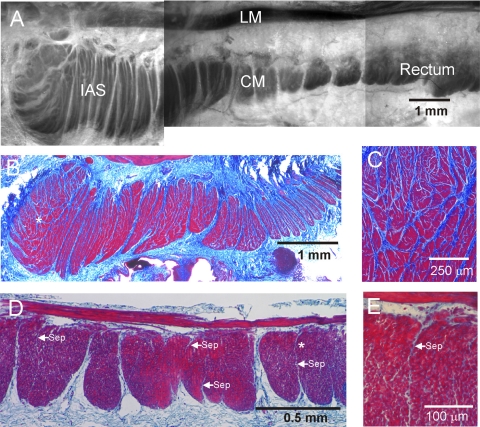
Fig. 1.
Morphological features of the monkey rectoanal region. A: composite image of a thick cross section from the rectoanal region; smooth muscle is contrasted with toluidine blue staining. The longitudinal (LM) and circular (CM) muscle layers can be seen separated by connective tissue. Prominent septal structures (white) are also apparent extending from the myenteric to the submucosal edge of the circular muscle separating this layer into bundles. The pinning procedure used for this image has exaggerated the gap between longitudinal and circular muscle layers. A more representative separation can be seen in D. B–E: thin (3 μm) cross sections of the internal anal sphincter (IAS) and rectum stained with Masson's trichrome to visualize both smooth muscle (red/purple) and connective tissue (blue). B: low magnification image of the IAS again showing prominent septal structures extending from myenteric (top) to submucosal (bottom) edge. Additional septal structures are apparent further separating the muscle into “minibundles.” C: higher magnification image of the region marked with an asterisk in B showing numerous minibundles (red) separated by connective tissue septa (blue). D: lower magnification image of rectum showing division of the circular muscle layer into compact bundles extending from the myenteric to the submucosal edge and separated by connective tissue septa. A few additional smaller septa can be seen penetrating for variable distances into muscle bundles (arrows labeled “sep”). The region marked with an asterisk in D is shown at higher magnification in E. Cells can be seen between longitudinal and circular muscle layers and these likely include both neurons and interstitial cells of Cajal (ICC) of the myenteric plexus.
The colocalization of nerves and ICC was assessed by determining the overlap of red (ICC) and green [nitric oxide synthase (NOS) or tyrosine hydroxylase (TH)] pixels throughout the stack. The pixels in each confocal slice that contained both red and green labels above a user-defined threshold intensity were then considered to represent areas of colocalization, and the percentage colocalization as well as the area of red and green pixels was calculated. The maximum density and percentage colocalization were calculated in a 1-μm region.
Apposition of nerves and ICC was also evaluated by a second method utilizing a minimum distance surface algorithm. Briefly, 3D surfaces were constructed around KIT and nerve structures and were smoothed by iteration of Laplacian smoothing (see Fig. 6, C and E, and Ref. 30), and the minimum distance from each ICC surface triangle to all nerve surface triangles was determined. Frequency histograms of the minimum distance (absolute) from ICC surfaces to nerve fiber surfaces were then constructed (see Fig. 6, D and F). The pixel dimension at ×63 was 0.2885 μm with a lower limit for determining separation distances on the order of 0.3–0.8 μm (see www.olympusconfocal.com/theory/resolutionintro.html).
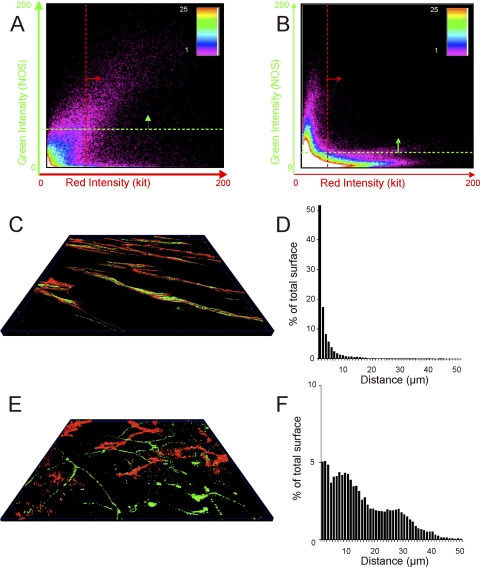
Fig. 6.
Colocalization and apposition of NOS-labeled and KIT-labeled structures in the IAS and rectum. A and B: plots showing the number of pixels (see spectrum scale bar) averaged throughout the stack at different intensities of red (x-axis) and green (y-axis) in rectum (A) and IAS (B). The quadrant above the green line and to the right of the red line indicates pixels considered to contain both KIT (red) and NOS (green) signals (i.e., sites of colocalization). Greater colocalization of signals is seen in rectum than in IAS. C and E: 3D surface reconstructions (see Ref. 30) of KIT and NOS labeling in IAS (C) and rectal (E) images. D and F: the distance between surface reconstructions was used to obtain the values plotted in histograms D (IAS) and F (rectum) that show the percent of total KIT and NOS surface triangles (y-axis) with minimum distances ranging from 1 to 50 μm (x-axis) in IAS (D) and rectum (F).
Contractile Experiments and Contraction Analysis
Muscle strips for contractile experiments (1.5 × 20 mm) were cut parallel to the CM and attached with suture thread to a stable mount and to a Gould strain gauge and immersed in tissue baths containing 3 ml of oxygenated KRBS maintained at 37°C. A initial stretch of 1 g was applied. After 15–30 min, active tone and phasic contractile activity developed. Nerves were stimulated with electrical field stimulation (EFS) by using a Grass S48 stimulator (1–20 Hz for 1 min, 12 V, 1.0 ms duration pulses). These stimulation parameters produced tetrodotoxin (TTX, 1 μM)-sensitive neural responses.
To quantify spontaneous IAS contractions, phasic peaks and troughs were averaged during a 2-min time period by use of AcqKnowledge software (Biopac Systems). Phasic contractile amplitude was determined as average peak value minus average trough value. IAS tone was determined as the average of phasic troughs minus passive tension. In rectum, phasic contractions typically occur in complexes; thus phasic contractile amplitude was calculated as peak contraction during the complex minus tone between complexes. Rectal tone was determined as the value of tension between contractile complexes minus passive tension. Passive tension was determined at the end of the experiment by addition of 10 μM sodium nitroprusside and 1 μM nifedipine. Significant differences between groups were determined by one-way ANOVA followed by a post hoc Dunn's or Tukey test. Data are expressed as means ± SE and values were considered significantly different when P < 0.05.
Drugs
TTX, atropine sulfate, Nω-nitro-l-arginine (l-NNA), guanethidine, sodium nitroprusside, 2-(2-aminoethyl)pyridine, and nifedipine were all purchased from Sigma (St. Louis, MO).
RESULTS
Morphological Arrangement of Muscle Differs in the IAS and Rectum
The gross morphology of the rectoanal region was examined in cross sections (1 mm × 3 cm) stained with toluidine blue to provide contrast for smooth muscle visualization. LM (200–300 μm) was present throughout the rectoanal region and was separated from the CM layer by connective tissue. The rectal CM layer was ∼0.5 mm wide whereas the IAS (i.e., final 5–8 mm of GI tract) was ∼2 mm wide. Septal structures dividing adjacent bundles of muscle could be seen extending from the myenteric to submucosal edge of the CM layer at intervals of ∼0.5–0.7 mm in the rectum and ∼0.2–0.5 mm in the IAS (Fig. 1A). The composition of muscle bundles was further examined in paraffin-embedded cross sections (3–8 μm) of muscle stained with Masson's trichrome (Fig. 1, B–E). These sections revealed further subdivision of each IAS muscle bundle into “minibundles” of variable width (20–200 μm) separated from one another by connective tissue septa (20–50 μm wide, Fig. 1, B and C). In contrast, muscle bundles of the rectum generally spanned the entire CM layer and contained packed SMC. Figure 1D shows rectal muscle bundles separated from one another by large septa containing both connective tissue and a few cells (Fig. 1D). Additional thin (≤10 μm) septal structures were present penetrating variable distances into the interior of muscle bundles (see Fig. 1, D and E).
Distribution and Morphology of ICC in the IAS and Rectum Differ
A subpopulation of ICC has been shown to function as pacemaker cells that participate in the regulation of motor activity in the GI tract (22, 46). However, the motor activity of the IAS and that of the rectum differ substantially from one another (e.g., Ref. 37), suggesting that the populations of ICC within them may differ as well. Initial experiments were therefore undertaken to confirm that the differing patterns of motor activity reported for other species exist in the monkey IAS and rectum as well. This was followed by immunohistochemical studies to examine whether significant differences exist in the morphology and distribution of ICC between IAS and rectum.
When IAS and rectal muscle strips were immersed in isolated tissue baths, the IAS developed substantial tone along with superimposed phasic contractions whereas the rectum contracted predominantly in a phasic manner. Examples of these contractile patterns as well as mean values for tone and phasic contractile amplitudes can be seen in Supplemental Fig. S1.
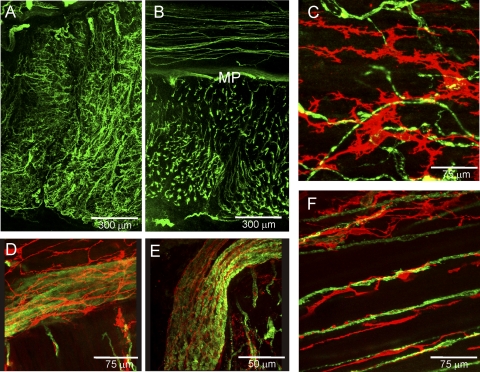
The morphology and distribution of ICC was examined in thick (100 μm) sections of IAS and rectum cut parallel (transverse) or perpendicular (cross section) to the long axis of circular SMC and labeled with anti-KIT antibody. Mast cells were distinguished from ICC on the basis of their distinctly different morphology. ICC were present throughout the rectoanal region, but their shape and distribution differed between regions. In the IAS, ICC were distributed throughout the muscularis (Fig. 2A) but they were absent at the myenteric and submucosal surfaces. These cells had a highly complex morphology including numerous branching processes as seen in the higher magnification image of Fig. 2C. In keeping with previous terminology (5, 9) we use the abbreviated term “stellate” to describe these cells, recognizing that their true morphology is in fact more complicated. Because ICC-IM in the IAS are distinct from ICC-IM described elsewhere in the GI tract we refer to them as “ICC-IAS.” In rectum, ICC were distributed throughout both the LM and CM layers. In addition, a plexus of myenteric ICC (ICC-MY) could be seen between muscle layers (Fig. 2B). Rectal ICC-IM were spindle shaped in morphology and oriented parallel to the long axis of SMC (Fig. 2D).
To further examine the distribution of ICC in plexus regions, modified whole mounts (1.5 cm × 4 cm, see materials and methods) were labeled with anti-KIT antibody. In rectum (2.5–4 cm proximal to the anal verge), a dense plexus of stellate-shaped ICC was present along the myenteric surface. Within this plexus, ICC-MY distribution varied from LM to CM surface, i.e., at the LM surface ICC-MY were organized into clusters and branches whereas at the CM surface they were preferentially oriented parallel to CM cells, forming denser bands at intervals of ∼50–150 μm (Fig. 2G). In some places ICC-MY were so numerous that these patterns were less apparent (Fig. 2H). ICC were also observed at the submucosal surface (ICC-SM, Fig. 2E), but their density was less than ICC-MY. In the distal direction ICC-MY and ICC-SM density decreased while the density of mast cells increased. In the IAS region (i.e., anal verge to 0.8 cm) ICC-MY were absent and mast cell density was ∼4–8/100 μm2 (Fig. 2F). Additional images from modified whole mounts can be seen in Supplemental Figs. S2 and S3.
Three dimensional characteristics of ICC-IAS.
The 3D characteristics of ICC-IAS were further examined by constructing surface renderings of cells generated from confocal stacks of cross-sectional preparations (Fig. 3, A and B). These renderings revealed ICC-IAS processes emanating from a central nuclear region in all three dimensions. Cell nuclei were apparent as a swollen region in the widest central region of the cell. Cell bodies were often elongated and contorted in appearance. The 3D features of these cells and their relationship to one another is also apparent in the anaglyph of Fig. 3B and by viewing the cell cluster in rotation as seen in Supplemental Fig. S4. A different cluster of ICC can also be viewed in Supplemental Fig. S5. To examine the overall distribution of ICC-IAS within the muscularis additional confocal stacks were collected from thick transverse sections at lower magnification. Three slices from such a stack are shown in Fig. 3, C–E and in Fig. 3F the entire stack is shown. Additional images of ICC-IAS in a different transverse section are shown as an anaglyph in Supplemental Fig. S6 and as a movie in Supplemental Video S7. These images again emphasize the complex branched nature of ICC-IAS that are present throughout the muscle thickness. Although clusters of ICC-IAS were often seen in close association to one another (e.g., Fig. 3, A and B) there were also gaps separating adjacent clusters suggesting that ICC-IAS may not form a continuous network within the muscle layer.
Since ICC-IAS were present throughout the muscle thickness, they are likely to be located within muscle bundles. To provide direct evidence for this assumption, additional experiments were undertaken in which SMC were labeled with anti-SM myosin antibody and ICC with anti-KIT antibody in thick (100 μm) cryostat sections. These dual-labeling experiments revealed consistent localization of ICC within muscle bundles as seen in Fig. 4, A and B.
Fig. 4.
Dual labeling of ICC-IAS and smooth muscle cells (SMC) with anti-KIT (red) and anti-SM myosin (green) antibodies. Thick (100 μm) cryostat cross sections of the IAS are shown. SMC were usually oriented vertically (A) but in some cases bundles fell to one side during mounting, allowing a transverse view of SMC and ICC within a 100-μm section of muscle (B). Optical section thickness: A, 3.5 μm; B, 5.75 μm.
Nitrergic Nerves Contribute to Inhibitory Motor Responses in Both the IAS and Rectum but the Relationship Between Nerves and ICC Differs
Nitrergic nerves contribute to inhibitory motor responses in both the human IAS and rectum (39, 52), and there is evidence that ICC-IM participate in nitrergic transmission (62). Initial experiments were therefore undertaken to confirm that nitrergic nerves functionally contribute to inhibitory motor innervation in both the monkey IAS and rectum. Dual-labeling immunohistochemical studies were also undertaken to determine whether the morphological relationship between nitrergic nerves and ICC differs between these two muscles.
For contractile experiments muscle strips were stimulated with EFS in the presence of atropine (1 μM). EFS (1 Hz, 1-min duration) caused inhibition of contraction in both the IAS and rectum and these responses were greatly diminished by the NOS inhibitor l-NNA (100 μM), indicating that nitrergic nerves significantly contribute to inhibitory motor responses in both regions (see Supplemental Fig. S8).
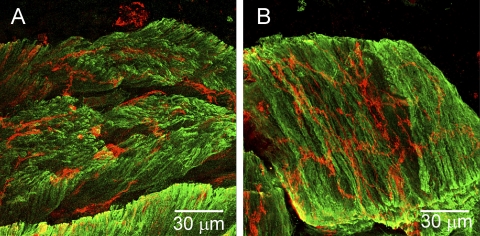
Nitrergic nerves in the rectoanal region were labeled with anti-nNOS antibodies. Varicose nNOS-positive fibers were present throughout the IAS musculature (Fig. 5A), but a neural plexus was not observed at either the myenteric or the submucosal edge of the muscle. Dual-labeling experiments revealed that the distribution of ICC-IAS and nitrergic nerves were largely independent of one another (Fig. 5B). In contrast to the IAS, a dense plexus of nNOS-positive fibers and cell bodies was present along the myenteric surface of the CM layer in rectum (Fig. 5C) and these nerves were surrounded by ICC-MY (Fig. 5, D and F). nNOS fibers running parallel to SMC and ICC-IM were also present in both the LM and CM layers (Fig. 5D). Higher magnification images revealed that nNOS-positive nerves were intimately associated with ICC-IM (Fig. 5E).
Fig. 5.
Dual labeling of ICC and nitrergic nerves with anti-KIT (red) and anti-neuronal nitric oxide synthase (nNOS; green) antibodies in the IAS (A and B) and rectum (C–F). A: distribution of nNOS-positive cells through the thickness of the IAS in a thick transverse section. B: dual labeling of a thick transverse section of IAS showing marked differences in the overall distribution of nNOS and KIT-positive cells. C and D: 2 images of the same thick cross section of rectal muscle. C: distribution of nNOS positive cells. D: dual image of nNOS and KIT-positive cells at low power (×20) reveals similar distribution of NOS fibers and ICC (yellow). E: high-magnification image of transverse section of circular muscle showing close association of KIT and nNOS-positive cells. F: high-magnification image of a myenteric ganglia containing nNOS-positive cell bodies surrounded by KIT-positive cells. Optical section thickness: A, 18 μm; B, 2 μm; C, 23 μm; D, 23 μm; E, 3.75 μm; F, 11 μm.
To compare the relationship of ICC to nerves in the IAS and rectum two methods were employed. The first measured the colocalization of green and red pixels in each confocal slice (see materials and methods for details). Examples of data generated with this technique are shown graphically in Fig. 6, A and B. In rectum a significant number of pixels contained both KIT and NOS signals (apparent as the points lying above the green line and to the right of the red line in Fig. 6A), indicating significant apposition of ICC with nerves. In contrast, the example shown in Fig. 6B for the IAS revealed few pixels containing both KIT and NOS signals, indicating that these cell types were not closely apposed to one another. The degree of apposition of NOS nerves to ICC was determined from 14 images. This analysis revealed significantly greater (P < 0.05) apposition of NOS nerves with ICC in rectum (38.4 ± 8.4%, n = 8) than in IAS (7.9 ± 2.6%, n = 6). Interestingly, although there was less apposition in the IAS than the rectum, the ratio of NOS to KIT densities in both preparations was close to 1.0 (1.1 ± 0.4 IAS; 1.1 ± 0.3 rectum) suggesting a similar volume of ICC and NOS nerves in both regions.
To further quantify the apposition of KIT to NOS-labeled fibers, a minimum distance surface algorithm was employed (see materials and methods). Figure 6, D and F, shows frequency histograms of the minimum distances (absolute) between ICC surfaces and NOS fiber surfaces calculated for a rectal (Fig. 6C) and an IAS (Fig. 6E) confocal stack. This analysis shows that in the rectal image examined, over 50% of ICC surfaces were positioned ≤1 μm distance from NOS fiber surfaces and almost all were ≤10 μm apart (Fig. 6D). In contrast, in the IAS section, only 5% of ICC surfaces were positioned ≤1 μm away from NOS fiber surfaces and the remainder were spread out over a wide range extending up to ∼50 μm distance (Fig. 6F).
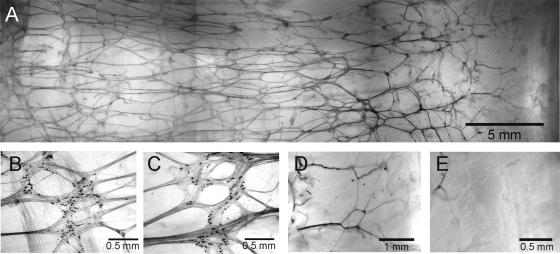
To further explore the distribution of nNOS nerves in plexus regions, we examined modified whole mount preparations labeled for NADPH diaphorase activity (see materials and methods). A plexus of NADPH diaphorase-positive nerves was apparent at the rectal myenteric surface (Fig. 7, A–C) but not at the submucosal surface. The density of neurons in the myenteric plexus declined in the distal direction ending approximately halfway through the IAS (Fig. 7, A, D, and E).
Fig. 7.
Distribution of NADPH diaphorase-positive enteric nerves along the myenteric surface of a modified whole mount preparation. A: composite image of the overall distribution of cells from the anal verge (AV, right edge) to the proximal rectum (left edge). B–D: higher magnification images of NADPH diaphorase positive cells located ∼3 cm (B), 2 cm (C), and 0.7 cm (D) from the AV. E: at 0.4 cm labeling is virtually absent.
Functional Role, Distribution, and Relationship of Sympathetic Nerves to ICC Differ Between the IAS and Rectum
Previous studies from a number of mammalian species suggest that excitatory motor innervation to the IAS is sympathetic (3, 56), whereas elsewhere in the GI tract sympathetic nerves serve a neuromodulatory role (29, 51). At present there are no studies that have examined the relationship of sympathetic nerves to ICC in the GI tract. Initial experiments were undertaken to confirm the differing functional roles of sympathetic nerves between the monkey IAS and rectum. Dual-labeling immunohistochemical studies were then undertaken to determine whether there are also differences in the morphological relationship of ICC to nerves between regions.
For contractile experiments muscle strips were stimulated with EFS in the presence of l-NNA (100 μM). In the IAS the contraction evoked with EFS (20 Hz, duration 1 min) was abolished by the sympathetic blocker guanethidine (10 μM), whereas in rectum nerve-evoked contraction was unchanged by guanethidine but was significant reduced by atropine (1 μM) (see Supplemental Fig. S9). These data suggest that sympathetic nerves serve an excitatory motor function in the monkey IAS but not in the rectum.
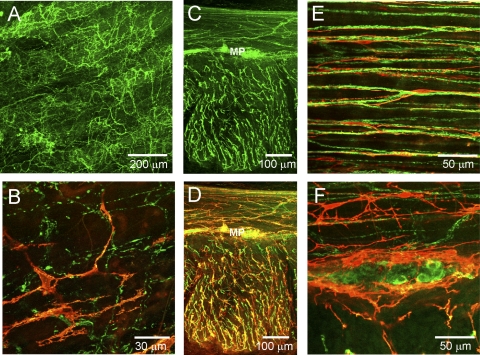
To examine the relationship of sympathetic nerves to ICC in the rectoanal region sympathetic nerves were labeled with antibodies raised against TH, an enzyme required for synthesis of norepinephrine. Varicose TH-positive fibers were identified throughout the musculature of the IAS but they were absent from the myenteric and submucosal regions (Fig. 8A). Dual-labeling experiments revealed that the distribution of ICC and TH-positive fibers in the IAS was largely independent of one another (Fig. 8, B and C), although occasional areas of overlap were observed. In the rectum, a dense plexus of TH-positive fibers surrounded by ICC-MY was observed at the myenteric surface of the CM layer (Fig. 8, D and E). TH-positive fibers were also present within the rectal muscle (Fig. 8D, arrows) but the density of these fibers was less than in the IAS as previously reported (14, 45). Higher magnification of intramuscular TH-positive fibers revealed that almost all were located in close proximity to ICC-IM (Fig. 8, F and G). Analysis of colocalization, apposition, and relative density (see Fig. 6) was also carried out for TH and KIT labeling in IAS and rectum. Like KIT/NOS, apposition of TH with KIT in rectum (23.3 ± 4.5%, n = 10) was significantly greater (P < 0.05) than in the IAS (7.8 ± 2.3, n = 8). However, in contrast to KIT/NOS, the ratio of TH to KIT densities in both the IAS and rectum was significantly less than 1.0 (0.4 ± 0.1 IAS vs. 0.2 ± 0.03 rectum), suggesting a greater density of NOS fibers than of TH fibers in both regions.
Fig. 8.
Dual labeling of ICC and sympathetic nerves with anti-KIT (red) and anti-tyrosine hydroxylase (TH, green) antibodies in the IAS (A–C) and rectum (D–G). A: distribution of TH-positive cells across the thickness of the IAS in a transverse cryosection. Dual labeling of ICC and TH-positive cells in cross section (B) and transverse sections (C) of the IAS. Note general lack of association between KIT-positive cells and TH-positive cells (B and C). D: dual labeling of rectal transverse section reveals a dense plexus of TH-positive cells within the myenteric plexus (MP) of the CM layer and with a few varicose fibers within the CM (arrows). ICC can be seen surrounding TH-positive cells in the plexus region (MP) and closely associated with TH-positive cells within the musculature. E: transverse section through a myenteric ganglia (MP) showing TH-positive cells along with underlying KIT-positive cells. F and G: higher magnification images of ICC-IM and TH-positive fibers showing intimate association. Optical section thickness: A, 14 μm; B, 6 μm; C, 11 μm; D, 6.5 μm; E, 8 μm; F, 8 μm; G, 4.5 μm.
ICC-IAS Are Not Closely Aligned With Any Neurons in the IAS
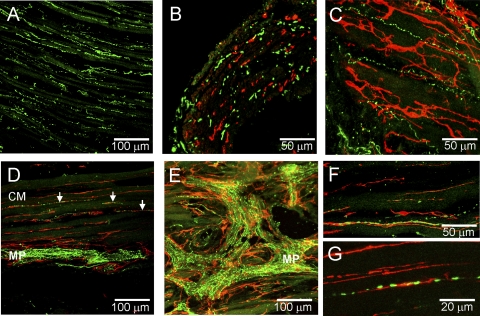
Dual-labeling studies of KIT with either TH or nNOS suggest little overlap in the distribution of nerves and ICC-IAS in the IAS. Additional studies were therefore undertaken to determine whether this lack of association is generally true for total nerve populations and ICC-IAS. Dual-labeling studies were therefore undertaken with the general neuronal marker PGP9.5 in combination with anti-KIT antibody. PGP9.5-positive fibers were densely distributed throughout the musculature of the IAS and rectum (Fig. 9, A and B). However, in agreement with the labeling studies of TH- and nNOS-positive cells, a plexus of nerves was only observed in the myenteric region of the rectum (Fig. 9B). Furthermore, dual-labeling studies did not reveal a special anatomical relationship between ICC-IAS and any PGP9.5-expressing population of cells in the IAS (Fig. 9C), whereas in the rectum ICC-IM were consistently associated with PGP9.5-positive fibers (Fig. 9F). An additional feature observed in these dual-labeling studies was that PGP9.5-positive nerve trunks in the rectum were often surrounded by stellate-shaped ICC that formed an anastomosing network of cells (Fig. 9, D and E).
Fig. 9.
Dual labeling of ICC and nerves with anti-KIT (red) and anti-PGP9.5 (green) antibodies in the IAS and rectum. Overall distribution of PGP9.5-positive cells in the IAS (A) and rectum (B). C–F: dual-labeling images from IAS and rectum. Very limited overlap of KIT and PGP9.5-positive cells is seen in the IAS (C). D and E: nerve trunks identified in the rectal myenteric region with PGP9.5 can be seen surrounded (D) and intertwined (E) with KIT-positive cells. Additional anaglyph images of ICC in nerve trunks are provided as supplemental data (see Supplemental Fig. S3). F: KIT-positive cells are closely aligned with PGP9.5-positive cells in the rectum. Optical section thickness: A, 32 μm; B, 25 μm; C, 2.5 μm; D, 5 μm; E, 7.5 μm; F, 9 μm.
DISCUSSION
ICC have been proposed to serve as either pacemaker cells or as participants in neuromuscular transmission in the gastrointestinal tract (22, 46, 63). However, the role of ICC in the control of motor activity in the IAS is still unclear. The present study compared the morphology and distribution of ICC in the monkey IAS to that of the rectum, an adjacent GI segment with distinctly different functional characteristics. Our studies identified a number of unique morphological features of ICC in the IAS that distinguish this region from the rectum as well as other more proximal parts of the GI tract. These unique features are discussed below.
Morphology of the IAS and Rectum Differ
In the large and small intestine the CM layer is divided into large compact muscle bundles that span the entire muscle layer [e.g., human jejunum (32); dog colon (60)]. This organization is also apparent in the monkey and canine rectum (present study and Ref. 20). In contrast, the smooth muscle of the monkey IAS is further subdivided into numerous “minibundles” that are surrounded by wide connective tissue septa. A similar organization of muscle is seen in the dog (20) and human (43) IAS. The arrangement of IAS muscle into minibundles has led us to speculate that this region functions in some respects like a “multiunit” type muscle (see also Ref. 37), a concept that is further discussed in the sections below.
ICC-IM in the IAS and Rectum Differ
ICC are present throughout the musculature of both the IAS and rectum but the morphology of these cells differs. Rectal ICC-IM are spindle shaped and run parallel to SMC as described for ICC-IM in other GI regions (22, 62). The ICC-IM proposed to participate in neuromuscular transmission are closely aligned with nerve fibers over much of their length, leading to a very similar distribution (54, 62, 63). ICC-IM in the monkey rectum are also closely associated with nerves, suggesting a possible role for rectal ICC-IM in neuromuscular transmission as well.
In contrast to rectum, the IAS exhibited stellate-shaped ICC (ICC-IAS) distributed throughout the musculature. This stellate-shaped morphology is typical of pacemaker cells (19, 27, 61) but has never before been described for ICC-IM in any species or region of the GI tract. Dual-labeling studies revealed little overlap in the distribution of ICC-IAS with either TH or nNOS-positive cells, suggesting that the primary role of ICC-IAS is not to function as intermediates in neuromuscular transmission. ICC-IAS also exhibited little overlap with PGP9.5-positive cells, making it unlikely that the primary role of these cells is to transmit sensory information to afferent neurons (10).
Slow waves are present in the cat (41) and dog IAS (37), and we have recently described slow waves in the monkey IAS (18). Thus we propose that ICC-IAS generate slow waves. In other GI regions, slow wave-generating ICC are usually confined to one or two plexus regions and activity is conducted to the remaining muscle (22, 33, 46). However, conduction of pacemaker potentials requires electrical coupling between ICC, between ICC and adjacent SMC, and between SMC (8). The septal structures that divide the IAS musculature into minibundles will tend to interrupt bundle to bundle communication, making it unlikely that slow waves from a single source can drive large regions of muscle. However, gap junctions have been shown to exist between ICC and SMC and between adjacent SMC within a minibundle in the canine IAS, suggesting that cells within a minibundle may be coupled (20). Taken together, we propose that each minibundle is regulated by its own set of pacemaker ICC, giving rise to a multiunit-type organization.
A unique feature of the IAS is the large amount of tone that is generated compared with other “phasic” GI muscles such as rectum (see Supplemental Fig. S1). Studies comparing the IAS and the “phasic” anococcygeus and rectum of rat suggest that IAS tone is due to greater expression and activation of proteins involved in myofilament sensitization (42). However, the present study of the monkey IAS and rectum reveal several unique anatomical features that distinguish the IAS from other “phasic” GI muscles. Of particular importance is the organization of this muscle into discrete minibundles and the presence of complex pacemaker-type ICC throughout the IAS musculature. Although pacemaker potentials are known to accompany phasic contractions in GI muscles such as colon (see Ref. 22, 46), these electrical events can also give rise to tone. For example, Ca2+ that enters with each slow wave can accumulate, keeping intracellular Ca2+ concentration above the threshold for contraction; this is reminiscent of the “partial tetanus” that occurs in skeletal muscle with repetitive firing of action potentials. In addition, asynchronous phasic activity in multiple independent motor units (i.e., minibundles) may sum to produce tone. Finally, at depolarized membrane potentials l-type Ca2+ channels can generate continuous Ca2+ entry via “window current” (see Refs. 13, 26, 31). Since membrane potential in the monkey IAS is more depolarized than rectum (see Refs. 18, 37) greater “window current” may also contribute to tone generation in this muscle. Thus several electromechanical coupling mechanisms, independent of differences in myofilament sensitivity, could account for the greater tone generated in the monkey IAS. Similar electromechanical-coupling pathways have been proposed to account for tone generation in the urethra, another visceral smooth muscle that contains spontaneously active ICC-like pacemaker cells (4, 48, 49).
ICC-MY in the IAS and Rectum Differ
The morphology and composition of the myenteric plexus region varied from proximal rectum to distal IAS. In the rectum, a dense plexus of stellate-shaped ICC was observed in the myenteric region (ICC-MY). Within the plexus there were also differences in the distribution of ICC-MY from LM to CM surface. ICC-MY along the LM surface were organized into clusters and branches, suggesting localization with ganglia and nerve trunks. Direct evidence for this association was obtained in dual-labeling studies that revealed ICC-MY surrounding ganglia and nerve trunks labeled with TH, nNOS, or PGP9.5 (see Figs. 8, D and E, 5E, and 9, D and E). A similar association between ICC and myenteric neurons has previously been described (e.g., Refs. 22, 27). It is unclear whether this structural relationship is linked to a functional relationship. ICC-MY at the CM surface were preferentially oriented parallel to the long axis of CM cells and formed denser bands at intervals of ∼50–150 μm. Septal structures were observed in cross sections of rectum penetrating variable distances into the muscle layer (see Fig. 1). The denser bands of ICC-MY observed in whole mounts are likely associated with these septal structures. ICC-SEP have previously been described (21, 32, 60), and functional studies of the human jejunum suggest that pacemaker activity originating in ICC-MY conducts rapidly into the musculature via Purkinje-like ICC-SEP (32).
Isolated segments of monkey rectum contract predominantly in a phasic manner, as observed in other animal species (7, 28, 37). In the large and small intestine spontaneous phasic contractions are associated with slow waves generated by ICC (22, 46) and in the human and canine colon and the canine rectum (37, 44, 50) the predominant slow wave activity arises from ICC at the submucosal edge of the CM layer (ICC-SM). In contrast, in the human jejunum slow waves arise from ICC-MY (33). Since the density of ICC-MY in monkey rectum (Fig. 2, G and H) was much greater than ICC-SM (Fig. 2E), slow waves may also arise from ICC-MY in this muscle. However, additional studies are required before the functional role of either ICC-MY or ICC-SM in the monkey rectum can be confidently assigned.
In contrast to rectum, ICC were not present along the myenteric surface of the IAS. Instead, this region contained numerous mast cells (see Fig. 2, A and F). Mast cells are known to participate in allergic responses and in innate immune responses by releasing a wide range of substances including interleukins, prostaglandins, histamine, heparin, tryptase, platelet-activating factor, along with other factors (11). The role of mast cells in the vicinity of the IAS is not known but it may be related to the close proximity of this muscle to feces with high bacterial loads and possibly to greater potential for injury in this terminal portion of the GI tract. It is also possible that mast cells in this region serve a more physiological role. The role of mast cells in the IAS, in both health and disease, warrants further investigation.
Relationship Between ICC and Nitrergic Nerves Differs in the IAS and Rectum
The role of ICC-IM in nitrergic transmission is controversial, with a number of studies suggesting that they participate in nitrergic transmission (for review see Ref. 62), whereas others suggest that they do not (for review see Refs. 16, 22). The novel observation made in the present study is that although nitrergic nerves significantly contribute to inhibitory motor responses in both the IAS and rectum, the morphological relationship differs significantly between nitrergic nerves and ICC-IM, as discussed below.
Fibers and cell bodies positive for nNOS were identified in thick sections at the myenteric edge of the rectum but not the IAS. The morphology of this plexus was further examined in modified whole mount preparations, which revealed numerous ganglia containing cell bodies and connected by internodal strands (Fig. 7). A similar morphology has been described for the myenteric plexus of the large intestine (57, 64). The density of nerves in the myenteric plexus decreased in the distal direction ending approximately halfway through the IAS. Nonetheless, nNOS-positive fibers were still present within the IAS musculature. The decline of nerves in the myenteric plexus from rectum to IAS of mammals (including humans) was first noted in 1971 (1). Thus nerves involved in the rectoanal reflex originate in the rectum (41). These descending neurons are activated by rectal stretch and give rise to IAS relaxation (41, 53).
As noted previously, ICC-MY density also declines from rectum to IAS. Thus the myenteric surface of the IAS is largely devoid of both ICC and nerves. ICC and nerves are also absent from a 6- to 8-mm portion of the feline pylorus myenteric surface. In this case the absence of ICC-MY and nerves has been proposed as a means to separate antral and duodenal pacemaker functions (58).
Rectal intramuscular nitrergic nerves were closely aligned with ICC-IM. Quantitation of this relationship revealed that ∼38% of NOS fibers were colocalized with ICC cell surfaces (see Fig. 6A). Indeed, further analysis revealed that much of this apposition was ≤1 μm (see Fig. 6D). Given the constraints of confocal microscopy the lower limits of such determinations is on the order of 0.3–0.8 μm at ×63 (www.olympusconfocal.com/theory/resolutionintro.html) thus synaptic contacts (62) are not resolvable using this technique. Therefore, our results do not provide definitive evidence for transmission between nerves and ICC in the rectum but show a high degree of association between these cell types. A similar association has been reported in a number of other GI regions (58, 59, 62).
Despite the close association often observed between ICC-IM and nitrergic nerves, controversy still exists with regard to the role of ICC-IM in neurotransmission (see Ref. 16). For example recent studies of the W/Wv mutant mouse lower esophageal sphincter suggest that the reduction in nitrergic transmission observed in this region is due to a reduction in Ca2+-dependent signaling in SMC rather than to a loss of ICC-IM (65). This raises an important issue regarding the use of mutant models for evaluating the role of ICC-IM in neurotransmission since protein expression, cell expression patterns, and neural connections may all change developmentally in these models. For example, fibroblast-like cell (FLC) populations have been shown to increase near nitrergic nerves in the Ws/Ws mutant rat (12). There is evidence that FLC express guanylyl cyclase (23), making them additional candidates for nitrergic transmission. Further definitive insight into this issue therefore requires techniques or transgenic models that allow ICC-IM to be rapidly eliminated in the adult animal. Such approaches have not yet been developed. However, there is also evidence for ICC-IM as participants in nitrergic transmission derived from studies of wild-type animals, and these show that ICC-IM express the appropriate proteins for nitrergic transmission (i.e., guanylyl cyclase and PKG, Ref. 24) and respond to nerve stimulation with an increase in cGMP levels above that observed in SMC (for review see Ref. 62).
A possible explanation for the differences described between regions and animal models is that nitrergic transmission involves parallel pathways including ICC-IM, SMC, and possibly FLC and that the contribution of each cell type differs between regions. Certainly the present studies of the monkey IAS suggest that ICC-IAS are unlikely to be the sole effector cells of nitrergic transmission in this region since colocalization between ICC-IAS and nitrergic nerves is much less than that of the rectum (i.e., ∼8%). Further analysis of minimum distances revealed that distances between ICC and nerves were spread out over a wide range extending from 1 to 50 μm (see Fig. 6F). Thus in the IAS we propose that nitrergic transmission occurs largely independent of ICC-IAS and that both SMC as well as FLC are possible candidates for neurotransmission.
Relationship Between ICC and Sympathetic Nerves Differs in the IAS and Rectum
Sympathetic nerves innervating the rectoanal region arise predominantly from the inferior mesenteric ganglion and enter the GI tract at the colon via the lumbar colonic and hypogastric nerves (29). In this and previous studies we and others have shown that the predominant excitatory motor innervation to the IAS is sympathetic (3, 6, 56), whereas in the remainder of the intestine sympathetic nerves serve a neuromodulatory role (29, 51). The present study shows that these functional differences are also accompanied by differences in the distribution of sympathetic nerves between IAS and rectum and in the relationship of nerves to ICC.
Throughout most of the GI tract, sympathetic nerves modulate the activity of other enteric nerves. For example, norepinephrine inhibits cholinergic motor nerves via presynaptic inhibitory α2 adrenoceptors (51). In the monkey rectum, a dense plexus of varicose sympathetic nerves was present in the myenteric region as described for other intestinal regions (e.g., Ref. 47). These nerves likely participate in neuromodulation. Indeed, our contractile studies did not identify a role for these nerves in excitatory motor innervation in the rectum (see Supplemental Fig. S9). Interestingly, a smaller population of varicose sympathetic nerves was also present within the rectal musculature and these nerves were closely aligned with ICC-IM (∼23% colocalization was found between TH and KIT cell surfaces). The functional nature of this relationship requires further study.
Varicose sympathetic nerves were also present within the IAS musculature and their density exceeded that of rectum. The greater density of sympathetic nerves in IAS vs. rectum has been described for other animal species (14, 45). The distribution of sympathetic nerves in the IAS differed from that of the rectum in that a plexus of TH-positive nerves was absent from the myenteric edge of the muscle layer. Thus the distribution of sympathetic nerves in the IAS is commensurate with their role as excitatory motor neurons. Dual-labeling studies revealed little overlap in the distribution of sympathetic nerves and ICC-IAS (TH/KIT and NOS/KIT apposition in the IAS were both ∼8%), suggesting that sympathetic transmission occurs largely independent of ICC-IAS. In blood vessels, where excitatory motor innervation is sympathetic, specialized junctions have been identified between sympathetic varicosities and SMC (34). It is possible that sympathetic transmission occurs via a similar pathway in the IAS.
Our morphological data reveal striking differences in the relationship between ICC and nerves in the IAS vs. the rectum. These differences may reflect in part differences in the arrangement of SMC between the IAS and rectum. As discussed in earlier sections, the IAS is composed of numerous minibundles surrounded by connective tissue septa that will diminish electrical coupling between adjacent minibundles. The input resistance of SMC in a minibundle is therefore predicted to be greater than the input resistance of SMC in a large syncytium and a quanta of neurotransmitter would be predicted to cause a greater change in membrane potential in the minibundle. The arrangement of smooth muscle into minibundles in the IAS may therefore diminish the need for specialized junctions to achieve effective neurotransmission.
In conclusion, this study has identified important differences in the morphology and distribution of ICC in the monkey IAS and rectum. The shape and distribution of ICC in the rectum is similar to that described for more proximal parts of the intestine and includes ICC populations at both the myenteric and submucosal plexuses as well as spindle-shaped ICC-IM closely aligned with nerves. In contrast, several unique morphological features were noted in the IAS, including 1) the division of the muscle layer into numerous minibundles separated by connective tissue septa, 2) the distribution of stellate-shaped ICC throughout the musculature, 3) the lack of ICC (and presence of mast cells) at both the myenteric and submucosal surfaces of the IAS, and 4) the general lack of association between ICC-IAS and nerve fibers. These features have led us to propose that the IAS functions as a multiunit-type muscle with each “unit” (i.e., minibundle) controlled by its own set of pacemaker cells (ICC-IAS) and nerves. These data also suggest that ICC-IAS do not play a central role in neuromuscular transmission in the IAS. To better understand the unique functional properties of the IAS it is important to take into account these unique morphological features.
GRANTS
Confocal images were obtained from a Morphology Core laboratory supported by NIH P01 DK41315 and NIH1 S10 RR16871 and processed with the aid of a core imaging laboratory supported by NIH COBRE P20 RR018751. Dr. Hennig also received support from NIH COBRE P20 RR018751.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Maria Durazo (Nevada State Health Laboratory) for processing Masson's trichrome images. We would also like to express our sincere appreciation to Charles River Laboratories, Preclinical Services for generous donation of monkey rectoanal samples.
REFERENCES
- 1.Baumgarten HG, Holstein AF, Stelzner F. [Differences in the innervation of the large intestine and the internal sphincter of the anus in mammals and humans]. Verh Anat Ges 66: 43–47, 1971 [PubMed] [Google Scholar]
- 2.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil 18: 507–519, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Brading AF, Ramalingam T. Mechanisms controlling normal defecation and the potential effects of spinal cord injury. Prog Brain Res 152: 345–358, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bridgewater M, MacNeil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol 150: 223–228, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res 290: 11–20, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil 19: 937–945, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cook TA, Brading AF, Mortensen NJ. Effects of nifedipine on anorectal smooth muscle in vitro. Dis Colon Rectum 42: 782–787, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol 550: 829–844, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol 173: 1385–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 10.De Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut 54: 1107–1113, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhadi A, Keshavarzian A, Fields JZ, Jakate S, Shaikh M, Banan A. Reduced immunostaining for c-kit receptors in mucosal mast cells in inflammatory bowel disease. J Gastroenterol Hepatol 22: 2338–2343, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Farre R, Wang XY, Vidal E, Domenech A, Pumarola M, Clave P, Huizinga JD, Jimenez M. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil 19: 484–496, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fleischmann BK, Murray RK, Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci USA 91: 11914–11918, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furness JB, Costa M. The ramifications of adrenergic nerve terminals in the rectum, anal sphincter and anal accessory muscles of the guinea-pig. Z Anat Entwicklungsgesch 140: 109–128, 1973 [DOI] [PubMed] [Google Scholar]
- 15.Gibbs RA, et al. (Rhesus Macaque Genome Sequencing and Analysis Consortium). Evolutionary and biomedical insights from the rhesus macaque genome. Science 316: 222–234, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 298: G10–G13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagger R, Gharaie S, Finlayson C, Kumar D. Distribution of the interstitial cells of Cajal in the human anorectum. J Auton Nerv Syst 73: 75–79, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Harvey N, McDonnell B, McKechnie M, Keef KD. Role of l-type calcium channels, membrane potential and nitric oxide in the control of myogenic activity in the primate internal anal sphincter (Abstract). Gastroenterology 134: A63, 2008 [Google Scholar]
- 19.Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol 556: 585–599, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiguchi K, Keef KD, Ward SM. Distribution of interstitial cells of Cajal in tunica muscularis of the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol 284: G756–G767, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol 537: 237–250, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology 137: 1548–1556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience 152: 437–448, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Iino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol Motil 21: 542–543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iino S, Horiguchi S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal in the gastrointestinal musculature of W mutant mice. Arch Histol Cytol 70: 163–173, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Imaizumi Y, Muraki K, Takeda M, Watanabe M. Measurement and simulation of noninactivating Ca current in smooth muscle cells. Am J Physiol Cell Physiol 256: C880–C885, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Jessen H, Thuneberg L. Interstitial cells of Cajal and Auerbach's plexus. A scanning electron microscopical study of guinea-pig small intestine. J Submicrosc Cytol Pathol 23: 195–212, 1991 [PubMed] [Google Scholar]
- 28.Kato K, Kito Y, Suzuki H. Mechanical and electrical responses modulated by excitation of inhibitory nerves during stimulation with high-potassium solutions in circular smooth muscle of the rabbit rectum. J Smooth Muscle Res 43: 229–246, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Krier J. Motor function of anorectum and pelvic floor musculature. In: Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. I, pt. 1, chapt. 27, p. 1025–1053 [Google Scholar]
- 30.Kwon JG, Hwang SJ, Hennig GW, Bayguinov Y, McCann C, Chen H, Rossi F, Besmer P, Sanders KM, Ward SM. Changes in the structure and function of ICC networks in ICC hyperplasia and gastrointestinal stromal tumors. Gastroenterology 136: 630–639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langton PD, Burke EP, Sanders KM. Participation of Ca currents in colonic electrical activity. Am J Physiol Cell Physiol 257: C451–C460, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology 133: 907–917, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology 132: 1852–1865, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Luff SE, McLachlan EM, Hirst GDS. An ultrastructural analysis of the sympathetic neuromuscular junctions on arterioles of the submucosa of the guinea pig ileum. J Comp Neurol 257: 578–594, 1987 [DOI] [PubMed] [Google Scholar]
- 35.McHale NG, Hollywood MA, Sergeant GP, Shafei M, Thornbury KT, Ward SM. Organization and function of ICC in the urinary tract. J Physiol 576: 689–694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 13: 205–220, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Mutafova-Yambolieva VN, O'Driscoll K, Farrelly A, Ward SM, Keef KD. Spatial localization and properties of pacemaker potentials in the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol 284: G748–G755, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J 9: 1805–1813, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut 34: 689–693, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osada N, Hashimoto K, Kameoka Y, Hirata M, Tanuma R, Uno Y, Inoue I, Hida M, Suzuki Y, Sugano S, Terao K, Kusuda J, Takahashi I. Large-scale analysis of Macaca fascicularis transcripts and inference of genetic divergence between M. fascicularis and M. mulatta. BMC Genomics 9: 90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papasova M. Sphincteric function. In: Handbook of Physiology. The Gastrointestinal System. Motility and Circulation Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. I, pt. 2, chapt. 26, p. 987–1023 [Google Scholar]
- 42.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Piotrowska AP, Solari V, Puri P. Distribution of interstitial cells of Cajal in the internal anal sphincter of patients with internal anal sphincter achalasia and Hirschsprung disease. Arch Pathol Lab Med 127: 1192–1195, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol 510: 309–320, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayner V. Characteristics of the internal anal sphincter and the rectum of the vervet monkey. J Physiol 286: 383–399, 383–399, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol 68: 307–343, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Scheuermann DW, Stach W. Fluorescence microscopic study of the architecture and structure of an adrenergic network in the plexus myentericus (Auerbach), plexus submucosus externus (Schabadasch) and plexus submucosus internus (Meissner) of the porcine small intestine. Acta Anat (Basel) 119: 49–59, 1984 [DOI] [PubMed] [Google Scholar]
- 48.Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol 526: 359–366, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Interstitial cells of Cajal in the urethra. J Cell Mol Med 10: 280–291, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol Cell Physiol 252: C215–C224, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. J Physiol 519: 539–550, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stebbing JF. Nitric oxide synthase neurones and neuromuscular behaviour of the anorectum. Ann R Coll Surg Engl 80: 137–145, 1998 [PMC free article] [PubMed] [Google Scholar]
- 53.Stebbing JF, Brading AF, Mortensen NJ. Nitric oxide and the rectoanal inhibitory reflex: retrograde neuronal tracing reveals a descending nitrergic rectoanal pathway in a guinea-pig model. Br J Surg 83: 493–498, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol 546: 751–763, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terauchi A, Kobayashi D, Mashimo H. Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 289: G291–G299, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Tichenor SD, Buxton IL, Johnson P, O'Driscoll K, Keef KD. Excitatory motor innervation in the canine rectoanal region: role of changing receptor populations. Br J Pharmacol 137: 1321–1329, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timmermans JP, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MH. Functional morphology of the enteric nervous system with special reference to large mammals. Eur J Morphol 30: 113–122, 1992 [PubMed] [Google Scholar]
- 58.Wang XY, Liu LW, Diamant NE, Huizinga JD. Unique distribution of interstitial cells of Cajal in the feline pylorus. Cell Tissue Res 329: 13–24, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Wang XY, Sanders KM, Ward SM. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res 302: 331–342, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. Am J Physiol Gastrointest Liver Physiol 259: G264–G273, 1990 [DOI] [PubMed] [Google Scholar]
- 61.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol 281: G602–G611, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol 576: 675–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil 16, Suppl 1: 112–117, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Ward SM, Xue C, Shuttleworth CW, Bredt DS, Snyder SH, Sanders KM. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol Gastrointest Liver Physiol 263: G277–G284, 1992 [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol 298: G14–G24, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.