Abstract
Gastroesophageal reflux disease (GERD) is one of the most common problems in clinical practice today. It is widely believed that functional and structural abnormalities of the gastroesophageal junction as well as an abnormal exposure to gastroduodenal contents are the main contributors to its pathogenesis. Novel findings of the inflammatory process in GERD suggest a far more complex process involving multifaceted inflammatory mechanisms. This review summarizes knowledge about the expression of inflammatory mediators in GERD and their potential cellular sources and provides an integrated concept of disease pathogenesis. In addition we evaluate the contribution of inflammatory mediators to well-known complications of GERD, namely motility abnormalities, fibrosis, and carcinogenesis. Novel findings regarding the pathophysiology of esophageal inflammation should enhance our understanding of GERD and its complications and provide new treatment insights.
Keywords: cytokines, motility, stricture, stenosis
The Clinical Problem
Gastroesophageal reflux disease (GERD) is one of the most common problems encountered in clinical practice today (31). The pathophysiology of GERD is complex, involving diverse factors such as gastric acid secretion, dysfunction of the antireflux barrier, gastric emptying disturbances, and abnormalities in esophageal defense mechanisms. How these different factors cause GERD is incompletely understood, but they all share one common initiating event: increased exposure of the esophageal squamous epithelium to gastric contents, namely acid, pepsin, trypsin, and bile acids (22). Mucosal injury, characterized by a nonspecific inflammatory infiltrate surrounding the acid damaged epithelial cells, may occur in the setting of pathological reflux. This leads to the endoscopic findings of mucosal breaks, strictures, columnar metaplasia (Barrett's esophagus), and adenocarcinoma (52, 80). Several publications have summarized our current understanding of the pathophysiology of GERD (89, 90), but work on mechanisms of disease has focused primarily on damage to the tight junctions and loss of epithelial integrity in response to acid (89, 90). Surprisingly little information is available about esophageal inflammation in GERD even though changes at the molecular level occur prior to macroscopic or even microscopic signs of inflammation. A subgroup of patients, most notably nonerosive reflux disease, responds less readily to conventional therapies with proton pump inhibitors (PPIs) (30), and GERD is a chronic relapsing condition with the potential for severe long-term complications.
The goal of this review is to summarize our current understanding of the origin and role of inflammatory mediators in GERD with an emphasis on their impact on three major GERD-related complications: motility disturbances, fibrosis, and carcinogenesis.
Inflammatory Mediators in the Inflamed Esophageal Mucosa
Inflammation in any organ involves a highly complex environment that is rich in biological mediators. These include vasoactive amines and peptides, complement components, lipid mediators, proteolytic enzymes, cytokines, growth factors, and chemokines, which act in an autocrine, paracrine, or endocrine fashion (Tables 1 and 2).
Table 1.
Inflammatory mediators present in GERD
| Mediators | References |
|---|---|
| Cytokines and chemokines | |
| Proinflammatory | |
| IL-1 | 9, 11, 13, 36, 46, 103 |
| IL-6 | 9, 11, 12, 103 |
| IL-8 | 36, 53, 54, 55, 56, 63, 83, 138 |
| Immunoregulatory | |
| IL-4 | 33, 36 |
| IL-10 | 33, 36 |
| PAF | 11, 14, 16 |
| Reactive oxygen species | 14, 70, 80, 84, 116, 130, 132 |
GERD, gastroesophageal reflux disease; PAF, platelet-activating factor.
Table 2.
Cellular sources of inflammatory mediators in GERD
Cytokines and chemokines.
Cytokines and chemokines are small peptide molecules synthesized and released by nearly all cell types present in the human body. They play a key role as communicators between cells modulating a wide variety of functions. Cytokines are known to induce, amplify, perpetuate, and terminate inflammation (105). Extensive studies exist on cytokine profiles of various chronic inflammatory disorders, such as rheumatoid arthritis, psoriasis, multiple sclerosis, or inflammatory bowel disease (76, 93, 105, 109). Surprisingly, in GERD the nature of the tissue response is still poorly defined and data on the expression profile of cytokines in the mucosa are limited.
Most studies in GERD have focused on a small group of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and IL-8. Even though their presence is not specific to the esophagus, the functional changes they induce might be distinct, since specific complications can be triggered through different mechanisms in different anatomic locations. Thus, even though the proinflammatory mediators present in the esophagus are not different from those associated with inflammation in other organs, the functional changes they produce in the esophagus may be characteristic for this organ.
IL-8 has been extensively studied in GERD and is expressed in high amounts in the affected mucosa of GERD patients (36, 55, 56, 83, 138). IL-8 is a powerful chemoattractant and activator of leukocytes and other nonimmune cells. IL-8 levels in the esophagus correlate and increase with both endoscopic and histological disease severity (54, 56, 138). In a Japanese study of patients with nonerosive reflux disease (NERD) with minimal mucosal involvement, as determined by endoscopy, IL-8 mRNA levels were increased compared with NERD patients with no mucosal involvement and with controls (63). After successful treatment with PPIs or Nissen fundoplication, IL-8 levels declined (55, 83, 138). High mucosal IL-8 levels predicted an increased relapse rate for GERD within 3 years, indicating a potential prognostic role for this cytokine (53).
IL-1 and IL-6 are additional cytokines relevant to the pathophysiology of GERD, and both are key mediators in the control of inflammatory responses. The IL-1 family consists of different forms. IL-1α and IL-1β both exert identical proinflammatory biological effects. A soluble IL-1 receptor antagonist (IL-1ra) acts as a negative regulator (21). IL-1 is a potent activator of many cell types and its expression is increased in inflammatory diseases of the bowel, skin, and other organs (62, 73, 117). IL-1β is increased in the esophageal mucosa during reflux induced inflammation, in animal models as well as in humans. It is produced upon contact of the esophageal mucosa with acid (11, 13, 36, 46, 103). IL-1β may be present in severe GERD cases only, and its expression is restricted to the lower third of the esophagus (103). Increased levels of IL-1β are found in the mucosa and muscle layers of the esophagus in animal models of esophageal inflammation as well as in GERD patients (9, 11, 103). IL-6 may precede and indirectly induce the formation of IL-1β (11).
Other cytokines have been investigated, namely tumor necrosis factor-α (TNF), IL-10 and IL-4. Hamaguchi et al. (46) detected elevated TNF mRNA levels in a rat esophagitis model, a finding in contrast to data derived from a cat esophagitis model (9), as well as from human biopsies (103). IL-10 and IL-4 are considered anti-inflammatory or immunoregulatory cytokines that exert an important function in the control of inflammation. They inhibit release of proinflammatory cytokines (105, 134). IL-10 and IL-4 mRNA were not increased in the mucosa of GERD patients vs. controls (36).
Platelet-activating factor.
Platelet-activating factor (PAF) is a potent proinflammatory phospholipid and chemoattractant, particularly for eosinophils (129). It enhances eosinophil adherence to vascular endothelial cells (65). PAF activates immune as well as nonimmune cells and induces release of itself and other inflammatory mediators, including reactive oxygen species (ROS) (114, 140). PAF is produced by and released from the esophageal mucosa after acid exposure (11) and it is increased in the circular muscle layer of feline esophagitis model and in chronic esophagitis in humans (14, 16).
Reactive oxygen species.
ROS are small molecules, including superoxide radical anions, singlet oxygen, and hydrogen peroxide (H2O2). ROS are normal by-products of oxygen metabolism. However, in inflammation, ROS levels can increase dramatically (66). ROS can induce their own production, cause lipid peroxidation of cellular molecules (58), and release intracellular calcium stores, and they may diffuse through cellular membranes to the nucleus, altering protein expression. The presence of increased levels of ROS results in a situation known as oxidative stress. The highly reactive species H2O2 is elevated in GERD (14). Antioxidants or radical scavengers that protect cells against ROS, such as reduced glutathione, superoxide dismutase, and catalase, are all depleted in esophagitis (70, 80, 84, 116, 130, 132). In addition, lipid peroxidation is increased, indirectly indicating the increased presence of ROS. These responses and the mucosal damage are inhibited in experimental esophagitis by administration of antioxidants or free radical scavengers (70, 80, 84, 116, 130, 132).
In summary, studies examining the inflammatory mediator profile in GERD show an inflammatory response with increased levels of proinflammatory cytokines such as IL-1β, IL-6, and IL-8, PAF, and ROS in the esophageal mucosa. These findings are further supported by increased activity of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) in the mucosa of GERD patients (55, 81). NF-κB is a ubiquitous transcription factor regulating proinflammatory responses. NF-κB can be activated by proinflammatory cytokines, and it can then mediate inflammatory mediator synthesis and secretion. To date the inflammatory infiltrate found in the esophageal mucosa of GERD patients appears to be nonspecific, because the same proinflammatory cytokines can also be found in other inflammatory disorders of the esophagus, like eosinophilic esophagitis (104) or esophageal Candida infection (64). However, it is possible that broader analytic approaches, such as microarray analysis, might reveal a distinct inflammatory profile for GERD.
Possible Sources of Inflammatory Mediators Present in GERD
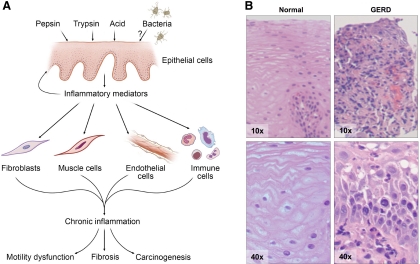
It is accepted that inflammatory responses are dominated by cells of the mucosal immune system. According to this view, activated immune cells, primarily represented by neutrophils, eosinophils, macrophages, and T cells, play the role of aggressors, that attack and destroy nearby cells, either directly through physical contact or indirectly through the release of soluble mediators (32). In this model the nonimmune cells, such as epithelial, endothelial, and mesenchymal cells, are passive bystanders, remaining quiescent until activated by the invading army of immune effector cells (32). However, this unidirectional concept of inflammation has been challenged by evidence showing that nonimmune cells play an active role in inflammation. Epithelial, endothelial, mesenchymal, and neural cells display a broad range of effector as well as regulatory functions, so that a multidirectional model of interactions between immune and nonimmune cells more closely resembles the real situation in the setting of inflammation (Table 2). A multidirectional model may also serve as an alternative and more appropriate means to explain the symptoms and structural changes seen in patients with GERD such as pain, motility disturbances, fibrosis and carcinogenesis (Fig. 1A). This model has been applied to several organ systems, including the gut in inflammatory bowel disease, but has not been extensively studied in GERD. Therefore, some of the discussion below refers to data from publications outside the esophagus but has potential implications for ongoing inflammation in the esophagus.
Fig. 1.
A: multidirectional model of interactions between immune and nonimmune cells in gastroesophageal reflux disease (GERD)-induced inflammation of the esophagus. This also explains the development of complications in patients with GERD such as motility disturbances, fibrosis, and carcinogenesis. B: hematoxylin and eosin staining of GERD affected vs. control mucosa. In GERD a nonspecific infiltrate of inflammatory immune cells can be seen. Magnification ×10 and ×40.
Epithelial cells.
The stratified squamous epithelium deserves special attention in the pathophysiological events leading to GERD. Twenty to 30 layers of keratinocytes provide a barrier between food, gastric contents, and the underlying subepithelial region. The esophageal epithelium is embryologically, morphologically, and functionally related to the skin epithelium, which is recognized as a major immunological organ (133). Esophageal keratinocytes most likely serve as the initiating cell type in esophageal inflammation (Fig. 1A). In addition to expressing activation and cell adhesion molecules, like HLA-DR and intercellular adhesion molecule-1 (ICAM-1) (51, 111), epithelial cells secrete a variety of proinflammatory cytokines affecting leukocyte recruitment and activity (59). Acid- and bile salt-activated keratinocytes cause significant increases in the chemotaxis of T cells and neutrophils (118). Immunohistochemical studies demonstrate an increase of IL-1β, IL-8, and IL-10 in epithelial cells of GERD patients (36). Components of the gastric juice induce IL-1β, IL-6 and IL-8 production by esophageal epithelial cells, with acidification further enhancing the IL-8 release, an effect mediated via NF-κB (103, 118, 137). IL-1β itself can induce IL-6 production (103). In addition acid induces PAF secretion by esophageal keratinocytes through vanilloid receptors (15).
Recently environmental factors, namely the esophageal micobiome, have been considered in the pathogenesis of GERD. The human body is colonized by a large number of microorganisms, and their relationship with one another can range from mutualism to pathogenicity (50, 108). In a variety of diseases, the best example being inflammatory bowel diseases, the microbiota appears to play a key pathogenic role (60, 108). Although the exposure and response of the esophageal epithelium to gastric contents has been extensively investigated, little attention has been given to the microbiome, its effect on the esophageal epithelial layer, and its potential changes in GERD (92). In a systematic analysis by Yang and coworkers (136) the distal esophageal microbiome was analyzed in 34 subjects and revealed a complex composition comparable to those in the mouth, stomach, vagina, or skin (1, 6, 37, 39). Based on the bacterial genotypes two distinct microbial clusters were identified. A correlation analysis with host phenotypes, namely GERD, Barrett's esophagus, and controls, revealed that almost all controls (11 of 12) located to one cluster that was predominated by streptococci, whereas most of the abnormal samples (13 of 22) were associated with the other cluster, predominated by gram-negative bacteria (136). The microbiome did not differ between GERD and Barrett's esophagus patients.
This finding is especially intriguing in lieu of the ability of bacteria and their products to activate epithelial cells and induce their secretion of proinflammatory cytokines (108). This therefore has the potential to contribute to the pathogenesis of GERD. The microbiome associated with GERD and Barrett's esophagus is predominated by gram-negative strains (136), and the effect of lipopolysaccharide, a major component of their cell wall, on epithelial cells is well established (108). The GERD-associated esophageal microbiome had the strongest link with GERD-related pathological changes (odds ratio > 15) compared with all known environmental factors (136). It is, however, unclear whether the esophageal microbiome is intrinsically stable in each individual or whether the host response to GERD might influence the composition of the esophageal microflora, giving bacteria a secondary pathogenic role. In addition, colonizing bacteria can have an important role in tissue homeostasis and therefore might present a counterregulatory anti-inflammatory mechanism (108). Proton pump inhibitors, a widely used therapy in GERD, have also been implicated in altering the bacterial flora, which can be a direct drug-induced effect or indirectly mediated via the changes in gastric pH (4, 40, 72, 121, 125, 128).
Mesenchymal cells.
In inflammation, mesenchymal cells including fibroblasts, myofibroblasts, and smooth muscle cells are traditionally considered purely structural, filling the space around other cells that are more functionally important in the inflammatory process. Several lines of evidence suggest a broader range of activity. Mesenchymal cells can produce proinflammatory cytokines, express cytokine receptors (45, 120), and physically interact with immune cells, such as T cells and eosinophils, that are also present in GERD (44, 126). In addition to secreting products of inflammation, mesenchymal cells are capable of modulating immune cell functions (110) and therefore directly influence the duration of the inflammatory process. In GERD, mesenchymal cells are an active source of IL-6 (103) and IL-8 (unpublished observations). Esophageal circular smooth muscle cells may also contribute to the pool of H2O2 as well as PAF (16).
Endothelial cells.
The human esophagus contains a rich microvascular bed within the submucosal stroma beneath the muscularis mucosae. In several organ systems including the intestine, endothelial cells actively contribute to inflammation by controlling the recruitment of leukocytes to the site of injury. Inflammation leads to an upregulation of adhesion molecules on endothelial cells and to increased binding of leukocytes (7). A key adhesion molecule expressed on endothelial cells is the mucosal addressin cell adhesion molecule (MAdCAM)-1 (29). Esophageal endothelial cells upregulate MAdCAM-1, ICAM-1, vascular cell adhesion molecule (VCAM)-1, and E-selectin after activation. Cytokines secreted by endothelial cells influence surrounding immune and nonimmune cells and vice versa. Esophageal endothelial cells are capable of secreting IL-8 (98).
Immune cells.
Inflammatory effector cells may secrete all of the currently known inflammatory mediators. Data on the type of immune cells present in GERD are derived from animal models as well as from immunostaining of human biopsy specimens. The cell infiltrate is nonspecific and classically proinflammatory in nature. Neutrophils, eosinophils, mast cells, Langerhans cells, and macrophages can all be found, along with cells of adaptive immunity such as T and B cells (42, 48). Immunohistochemical detection of cytokines is difficult, but ample data on the ability of immune cells to secrete cytokines are available from other organs. In GERD immunohistochemical staining shows expression of IL-1β, IL-8, TNF, and IL-10 (36, 46, 55). In addition, neutrophils, monocytes, and macrophages are a major source of ROS and PAF in inflammation.
Integrating the Present Knowledge: New Insights into GERD
Inflammation in the esophagus is not driven by immune cells alone. Epithelial cells are most likely the initiators of inflammation, since they are exposed to and react to gastric contents, such as pepsin, trypsin, acid, and gastric juice and potentially also to the esophageal microbiome. They actively secrete proinflammatory mediators, such as proinflammatory cytokines, ROS, and PAF, which increase the epithelial response and epithelial damage itself but also activate mesenchymal and endothelial cells. Their activation leads to upregulation of molecules and mediators that allow communication with immune cells and amplify the immune cell response. The response includes upregulation of adhesion molecules and secretion of additional cytokines and chemoattractants with direct proinflammatory activity, which then culminates in perpetuation of inflammation in a self-sustaining cycle (Fig. 1, A and B). The esophageal nonimmune cell compartment, activated by the esophageal epithelium, plays an important role, since under normal circumstances a minimal number of classical immune cells are present in the esophagus. Even in the initiating phases of GERD, this appears to be the case. This concept is supported by recent evidence showing that the inflammatory infiltrate in GERD starts in the submucosa and later progresses to the epithelium. Basal cell and papillary hyperplasia preceded the development of surface erosions (118). In fact the authors propose that injury to the epithelium is the result of the inflammatory immune cell response rather than its predecessor. Therefore the products secreted by immune cells are expected to contribute to later stages of esophageal inflammation. The recruitment and retention of classic immune cells (Fig. 1B) seem to be dependent on activation of endothelial cells and mesenchymal cells as well as the upregulation of adhesion molecules. Therefore, one can speculate that inhibition of endothelial and mesenchymal activation may decrease the progression of esophageal inflammation.
In summary, essentially all nonimmune cell types of the esophagus, such as resident epithelial, mesenchymal, and endothelial cells, actively contribute to the initiation and perpetuation of the inflammatory response. The inflammatory response in GERD is a result of nonimmune-nonimmune, nonimmune-immune, and immune-immune cell interactions. Inflammatory cytokines, PAF, and ROS are all involved in the pathogenesis of GERD. Once the inflammatory cascade with its inflammatory infiltrate described above fully unfolds, complications induced by proinflammatory mediators can occur, such as motility disturbances, fibrogenesis, and carcinogenesis.
Impact of Cytokines on Esophageal Motility Disturbances
One crucial component in the pathophysiology of GERD is the impairment of esophageal defense mechanisms due to defective motor function (99). Particularly after meals, reflux of gastric contents may occur as a result of transient relaxation of the lower esophageal sphincter (TLESR) unrelated to swallowing or to secondary peristalsis (18, 77). TLESR occurs in healthy individuals but is more frequent in patients with GERD (22, 49, 77). In the early stages of GERD, TLESR accounts for the largest portion of reflux episodes (18, 77). However, with progressive severity of the disease the proportion of TLESR unrelated reflux increases and impairment of the lower esophageal sphincter (LES) tone and an altered contraction of the esophageal circular body musculature become more important (18, 61). As motor function becomes impaired, the likelihood of further reflux episodes and of impaired acid clearance increases, aggravating the damage. The spiral of damage leading to further injury may contribute to permanent impairment of LES tone and of esophageal peristalsis. The underlying cause of this phenomenon has not been extensively explored.
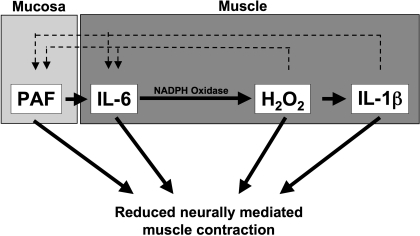
To test the direct effect of acid exposure on esophageal motility, Cheng and coworkers (12, 13) established an in vitro model of acute esophagitis, addressing the sequential activation of inflammatory events in the mucosa. A tubular segment of cat esophageal mucosa was removed and tied at both ends to form a mucosal sac that was filled with hydrochloric acid (HCl). The medium surrounding the tied sac (supernatant) was collected and used directly in cat muscle contraction assays. In the presence of the sac supernatant, neurally mediated contraction, induced by electrical field stimulation, was almost completely abolished. Muscle contraction induced by direct stimulation with acetylcholine (ACh) was not affected. This indicates that muscle function is not affected. In contrast mediators released by the esophageal mucosa in response to HCl affect the neurons that mediate muscle contraction, inhibiting neurotransmitter release and depressing contraction of esophageal circular muscle. When human mucosa is used, the HCl-filled mucosal sac releases PAF into the supernatant (11), and addition of a PAF receptor antagonist essentially abolished the supernatant-induced inhibition of neurally mediated circular muscle contraction. This indicates that PAF is the major mucosa-derived mediator that inhibits muscle contraction (11). But PAF can also activate the esophageal circular muscle to secrete IL-6 (Fig. 2) (11). The muscle-derived IL-6 then leads to enhanced production of H2O2, which sequentially induces the secretion of IL-1β (11). H2O2 as well as IL-1β can themselves induce PAF production (Fig. 2). This indicates the existence of a self-perpetuating cycle of inflammation and motility abnormalities.
Fig. 2.
Working model for the effect of inflammatory mediators on muscle contraction. Acid reflux in the esophagus causes formation of platelet-activating factor (PAF) by the esophageal mucosa. PAF is then released from the mucosa to activate the circular muscle, causing the sequential production of IL-6, H2O2, and IL-1β. H2O2 and IL-1β induce production of PAF and IL-6, all of which are known to depress neurogenic muscle contraction by inhibiting release of ACh.
IL-1β, IL-6, and H2O2 can independently alter neurogenic esophageal muscle contraction (Fig. 2) (9, 10, 16). HCl applied directly to the muscle did not change contraction, suggesting that inflammatory mediators are necessary for motility abnormalities.
In summary, mucosa and muscle derived proinflammatory mediators can induce their own secretion and influence muscle contraction in the esophagus (Fig. 2).
Impact of Inflammatory Mediators on Esophageal Fibrosis
Peptic fibrosis, defined as an excessive accumulation of mesenchymal cells and extracellular matrix (ECM) in the esophageal wall, used to be a common and potentially severe complication of GERD. Up to 70% of all benign esophageal strictures in the US were caused by reflux disease (74, 96). With the emergence of PPIs in the 1990s, the incidence of esophageal strictures decreased dramatically (26, 107). However, chronic inflammation invariably induces fibrogenesis in all organ systems and most likely also in the esophageal wall. In other words, even though the incidence of the most severe form of fibrosis, stricture formation and stenosis, might be reduced, fibrosis on a subclinical level may still affect the esophagus. Fibrosis may therefore alter esophageal motility and contribute to the symptom of dysphagia. This phenomenon can also be observed in other intestinal diseases such as the colon in ulcerative colitis, a disease commonly thought of as being nonfibrotic, despite substantial ECM accumulation in the colonic mucosa (104), or in eosinophilic esophagitis, a disease in which subepithelial fibrosis is considered a major cause of dysphagia (2, 3).
The pathophysiology of fibrosis in the esophagus is unclear (96, 101). It is believed that fibrosis develops after epithelial injury, causing proliferation and activation of resident fibroblasts and deposition of ECM, as a response to tissue damage caused by acid peptic injury. Restoration of normal tissue architecture occurs in transient, mild inflammation. In chronic inflammation the extent of damage may exceed the intrinsic regenerative capacity, resulting in scar tissue formation. This hypothesis is based on the association between severe, chronic esophageal inflammation with ulceration and the development of fibrosis (27). No data exist about the mechanisms of wound healing and fibrogenesis in GERD on a cellular or molecular level.
The main effector cell in fibrotic processes is the mesenchymal cell, responsible for synthesis of several ECM molecules, such as collagens and fibronectins. Mesenchymal cells can differentiate and dedifferentiate among three interrelated cell types: the fibroblast, the myofibroblast, and the smooth muscle cell (95). We will use the term “fibroblast” interchangeably for the first two cell types. TGF-β is the prototypical fibrogenic molecule found in all organs (102). Although three isotypes of TGF-β exist (TGF-β1, 2, and 3), the β1 isoform is considered to be the main driver of fibrogenesis. Essentially all cell types can produce TGF-β1, the major ones being macrophages and fibroblasts. Information is missing with respect to the presence and levels of TGF-β1 in reflux esophagitis but TGF-β1 is elevated in a wide variety of inflammatory processes and also in eosinophilic esophagitis, another esophageal disorder associated with inflammation and fibrosis (3, 104). TGF-β1, and other proinflammatory mediators such as IL-1β, IL-6 (43), or ROS (97), may activate fibroblasts to secrete enhanced amounts of ECM.
Local esophageal fibroblasts multiply in response to inflammatory signals, including IL-1β, IL-6, and PAF (43, 69, 106, 122, 124). Fibroblasts also proliferate in response to direct contact with inflammatory cells that are present in GERD, such as eosinophils, mast cells, and T cells (135). In addition to expanding in number, fibroblasts migrate toward a chemotactic gradient generated by inflammation but stop when inflammation subsides and the gradient disappears. Molecules inducing fibroblast migration can presumably also be found in the inflamed esophageal mucosa. Fibronectin is believed to be the most potent factor, but also platelet-derived growth factor (PDGF)-A, PDGF-B, insulin growth factor-I, and epithelial growth factor may contribute to fibroblast migration (71).
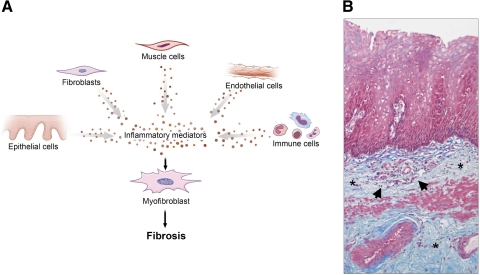
We conclude that chronic inflammation can drive fibrogenesis (Fig. 3, A and B). All cell types can contribute to activation of local mesenchymal cells. Certain inflammatory mediators are key components in that process and can be found or are presumably present also in the chronically inflamed esophageal mucosa of GERD patients.
Fig. 3.
A: working model for esophageal fibrosis. Chronic inflammation can drive fibrogenesis. This process involves essentially all cell types that can contribute to the activation of local mesenchymal cells. B: Masson trichrome staining of a peptic esophageal stricture. Collagen fibers are depicted in blue. Massive subepithelial and submucosal collagen accumulation (*) with neoangiogenesis and an inflammatory infiltrate (arrows) can be noted. Magnification ×10.
Effect of Inflammation on Carcinogenesis
The incidence of esophageal adenocarcinoma has increased almost sixfold over the past decades in the US and elsewhere in the Western world (20, 94, 113). Unless diagnosed at an early stage, the 5-year survival rate with locally advanced or metastatic disease is below 20% (28, 68). Adenocarcinoma typically arises from Barrett's esophagus and patients with Barrett's esophagus have a 30-fold increased risk of progressing to esophageal adenocarcinoma (67, 82).
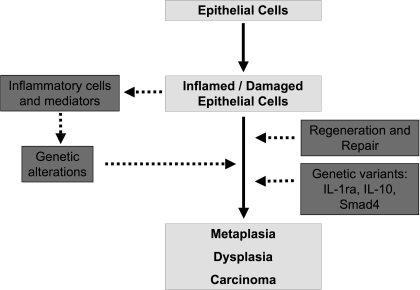
Inflammation appears to play an important role in carcinogenesis via at least two distinct mechanisms (Fig. 4): first the release of inflammatory mediators from immune cells, mainly ROS, and second enhanced reparative mechanisms of the esophageal epithelium. As explained above, inflammation in GERD induces oxidative stress. Oxidative stress in animal models leads to the development of adenocarcinoma (112), that may be mediated through damage to DNA, RNA, and lipids, specific gene alterations, genetic instability and aberrant DNA methylation (75, 127). These changes result in altered function of enzymes and proteins, such as activation of oncogene products (= carcinogenic) and/or inhibition of tumor suppressor proteins. Increased levels of ROS occur in the mucosa of GERD, Barrett's esophagus, and esophageal adenocarcinoma (85, 115). In metaplastic cells, ROS levels are elevated and antioxidant defenses are decreased (86, 131). Exposure of Barrett's esophagus and esophageal adenocarcinoma cell lines to components of the gastric refluxate further enhances formation of intracellular ROS, which subsequently cause DNA damage through induction of double-strand DNA breaks (17). Double-strand breaks can promote genomic instability, which can then lead to carcinogenesis (57, 112).
Fig. 4.
Mechanisms of inflammation induced carcinogenesis in GERD. Genetic alterations and increased cellular regeneration link an inflamed and damaged epithelium to metaplasia, dysplasia, and carcinoma. IL-1ra, IL-1 receptor antagonist.
Enhanced epithelial regeneration may also be a driver of carcinogenesis. Somatic genetic errors, such as chromosomal nondisjunction events and DNA base mismatch repairs, are normal occurrences during cellular proliferation, but rates of these anomalies are low and therefore manageable. As an increase in epithelial cell proliferation in response to inflammation is accompanied by a rise in frequency of replication errors, increased cellular turnover will also contribute to the fixation and expansion of these changes in the cell population (57). Acid and bile exposure is known to affect growth control, differentiation, and apoptosis of esophageal epithelial cells (24, 34, 35, 119). Acid-induced H2O2 contributes to increased proliferation and decreased apoptosis in esophageal adenocarcinoma cells (38). In addition, other inflammatory mediators, such as IL-1, IL-6, or IL-8, are known to enhance epithelial turnover (47, 123, 139).
Interestingly, polymorphisms of the IL-1ra, a genotype that is linked to high levels of IL-1β in vivo, could be found three times more frequently in patients with Barrett's esophagus or esophageal adenocarcinoma compared with patients with erosive GERD only (81), emphasizing a potential role of IL-1β in more severe inflammation progressing to neoplastic or dysplastic complications through enhanced ROS and epithelial regeneration. However, this polymorphism exhibits a low overall frequency, being present in only 7.2% of Barrett's or esophageal adenocarcinoma patients vs. 2% of erosive GERD patients.
Taken together, inflammation, oxidative stress, and increased cellular turnover could work together to produce the events responsible for cellular transformation in the inflamed esophagus (Fig. 4). However, despite the emergence of PPIs and therefore enhanced control of GERD symptoms and inflammation, the incidence of esophageal adenocarcinoma is increasing. This is the case even though long-term inhibition of esophageal acid exposure by administration of PPIs in patients with Barrett's esophagus decreases proliferation of metaplastic cells (91) and reduced the incidence of dysplasia in Barrett's esophagus patients (25). In addition, only a small fraction of GERD patients develop Barrett's esophagus or esophageal adenocarcinoma whereas the majority remain unaffected. This indicates that the specific host responses toward esophageal reflux or inflammation may be an important determinant whether an individual will move along the neoplasia pathway or not.
Fitzgerald et al. (36) found a qualitatively and quantitatively different expression profile of cytokines in GERD vs. Barrett's esophagus. In GERD a proinflammatory cytokine profile was present, whereas in the Barrett's esophageal mucosa a relative increase in IL-10 and a highly significant increase in IL-4 were noted. All patients with Barrett's esophagus had a low expression of proinflammatory cytokines irrespective of PPI therapy (36). In addition in Barrett's esophagus vs. GERD an increased proportion of Th2 effector cells and the formation of isolated lymph follicles can be found (79). This led to the hypothesis that these two disease entities reflect distinct host responses to reflux disease, a notion that gained further support by the finding that a polymorphism associated with increased IL-10 levels in vivo was approximately twice as common in Barrett's esophagus and esophageal adenocarcinoma patients vs. esophagitis patients (81). In further studies, an inflammatory gradient within Barrett's segment was found, with the maximal extent of inflammation at the neosquamocolumnar junction, with an associated increase in IL-1β and IL-8 (33). Proinflammatory cytokines were induced by exposure of the Barrett's esophagus mucosa to acid and bile. In contrast to this, the distal part of the Barrett's esophagus segment was characterized by low-grade inflammation and high levels of IL-10, despite being maximally exposed to gastric contents. It seems likely that the specific immune microenvironment within the Barrett's metaplasia may be an important driver toward dysplasia and carcinoma (33).
TGF-β signaling is known to demonstrate tumor-suppressive activity by regulating differentiation and proliferation of epithelial cells (19). Gastrointestinal malignancies such as gastric or colon cancer frequently display inactivating mutations of the TGF-β cascade (8, 23, 78). This also holds true for a subgroup of esophageal adenocarcinoma cases (41). Responsiveness to TGF-β is reduced in the esophageal epithelium during all stages of the metaplasia-dysplasia-carcinoma sequence in Barrett's esophagus, owing to abnormalities at several levels of the TGF-β pathway, in particular alterations in the major TGF-β signaling molecule Smad 4 (87). Barrett's esophagus cell lines fail to mount an antiproliferative response when exposed to TGF-β (88). Although mutations directly in the Smad 4 gene are an infrequent event in esophageal adenocarcinoma (5), it was suggested that promoter methylation, which leads to inhibited transcription of Smad 4, could be a cause of decreased Smad 4 mRNA and subsequent protein expression (88).
In summary, inflammatory mediators appear to be drivers of dysplasia and carcinogenesis in esophageal inflammation through genetic alterations and enhanced cell turnover. In addition, the individual host response seems to be critical in rendering patient populations at risk for the development of esophageal adenocarcinoma. TGF-β exhibits distinct roles in cell proliferation and transformation. Knowledge about the specific inflammatory mechanisms present is crucial not only for developing anti-inflammatory therapies but also for controlling the rising incidence of Barrett's esophagus and esophageal adenocarcinoma.
Summary
It may be appropriate to consider a new conceptual paradigm for the pathogenesis of GERD and its complications. In addition to considering functional and structural abnormalities of the gastroesophageal junction as well as abnormal exposure to gastroduodenal contents, it may now be time to also focus on bacteria as epithelial cell activators, the cellular inflammatory processes involved in the pathogenesis of GERD and its complications. This approach may help in understanding why some patients develop GERD and its complications whereas others do not, despite similar exposure to acid and gastric contents. For instance it has been suggested that reflux esophagitis may develop as an immune-mediated injury rather than a caustic chemical injury (118). In addition, further work in this area may enhance our understanding of the reasons why only certain subgroups respond to therapy. Current therapy continues to focus on acid secretion, modulation of TLESR, and surgical approaches. The recent findings of rebound hypersecretion after PPI therapy (100) along with the well-recognized problems with antireflux surgery should further stimulate this line of investigation. Finally, enhanced control of inflammation could play a key role in the esophageal neoplasia pathway.
GRANTS
The authors acknowledge the support from the Deutsche Forschungsgemeinschaft, Germany, and the Crohn's and Colitis Foundation of America to F. Rieder, of the National Institutes of Health, Bethesda, MD, to P. Biancani and K. Harnett.
DISCLOSURES
The authors acknowledge the research grant support of Astra Zeneca and Takeda to G. W. Falk.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of J. Kanasz, Cleveland Clinic Foundation, in illustrating this manuscript. The authors also acknowledge the contributions of several other colleagues whose work could not be cited due to space limitations.
REFERENCES
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: 5721–5732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am 29: 197–211, xiii–xiv, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 119: 206–212, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Altman KW, Chhaya V, Hammer ND, Pavlova S, Vesper BJ, Tao L, Radosevich JA. Effect of proton pump inhibitor pantoprazole on growth and morphology of oral Lactobacillus strains. Laryngoscope 118: 599–604, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Barrett MT, Schutte M, Kern SE, Reid BJ. Allelic loss and mutational analysis of the DPC4 gene in esophageal adenocarcinoma. Cancer Res 56: 4351–4353, 1996 [PubMed] [Google Scholar]
- 6.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA 103: 732–737, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 112: 1895–1907, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Blaker H, von Herbay A, Penzel R, Gross S, Otto HF. Genetics of adenocarcinomas of the small intestine: frequent deletions at chromosome 18q and mutations of the SMAD4 gene. Oncogene 21: 158–164, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol 287: G1131–G1139, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cao W, Harnett KM, Cheng L, Kirber MT, Behar J, Biancani P. H2O2: a mediator of esophagitis-induced damage to calcium-release mechanisms in cat lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 288: G1170–G1178, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Cao W, Behar J, Fiocchi C, Biancani P, Harnett KM. Acid-induced release of platelet-activating factor by human esophageal mucosa induces inflammatory mediators in circular smooth muscle. J Pharmacol Exp Ther 319: 117–126, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am J Physiol Gastrointest Liver Physiol 290: G1307–G1317, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. In vitro model of acute esophagitis in the cat. Am J Physiol Gastrointest Liver Physiol 289: G860–G869, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. Platelet-activating factor and prostaglandin E2 impair esophageal ACh release in experimental esophagitis. Am J Physiol Gastrointest Liver Physiol 289: G418–G428, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cheng L, de la Monte S, Ma J, Hong J, Tong M, Cao W, Behar J, Biancani P, Harnett KM. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 297: G135–G143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng L, Harnett KM, Cao W, Liu F, Behar J, Fiocchi C, Biancani P. Hydrogen peroxide reduces lower esophageal sphincter tone in human esophagitis. Gastroenterology 129: 1675–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Clemons NJ, McColl KE, Fitzgerald RC. Nitric oxide and acid induce double-strand DNA breaks in Barrett's esophagus carcinogenesis via distinct mechanisms. Gastroenterology 133: 1198–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Dent J, Holloway RH, Toouli J, Dodds WJ. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut 29: 1020–1028, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29: 117–129, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 83: 2049–2053, 1998 [PubMed] [Google Scholar]
- 21.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today 12: 404–410, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 307: 1547–1552, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Duval A, Reperant M, Compoint A, Seruca R, Ranzani GN, Iacopetta B, Hamelin R. Target gene mutation profile differs between gastrointestinal and endometrial tumors with mismatch repair deficiency. Cancer Res 62: 1609–1612, 2002 [PubMed] [Google Scholar]
- 24.Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, Holubec H, Sampliner RE, Guy N, Condon A, Bernstein C, Green SB, Prasad A, Garewal HS. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut 56: 763–771, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serag HB, Aguirre TV, Davis S, Kuebeler M, Bhattacharyya A, Sampliner RE. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett's esophagus. Am J Gastroenterol 99: 1877–1883, 2004 [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB, Lau M. Temporal trends in new and recurrent oesophageal strictures in a Medicare population. Aliment Pharmacol Ther 25: 1223–1229, 2007 [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 41: 594–599, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eloubeidi MA, Mason AC, Desmond RA, El-Serag H. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol 98: 1627–1633, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol 153: 517–528, 1994 [PubMed] [Google Scholar]
- 30.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol 41: 131–137, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fass R, Ofman JJ. Gastroesophageal reflux disease—should we adopt a new conceptual framework? Am J Gastroenterol 97: 1901–1909, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol Gastrointest Liver Physiol 273: G769–G775, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, Farthing MJ. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut 51: 316–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald RC, Omary MB, Triadafilopoulos G. Acid modulation of HT29 cell growth and differentiation. An in vitro model for Barrett's esophagus. J Cell Sci 110: 663–671, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus. An ex vivo proliferation and differentiation model. J Clin Invest 98: 2120–2128, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50: 451–459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353: 1899–1911, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem 281: 20368–20382, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA 104: 2927–2932, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia Rodriguez LA, Ruigomez A, Panes J. Use of acid-suppressing drugs and the risk of bacterial gastroenteritis. Clin Gastroenterol Hepatol 5: 1418–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Garrigue-Antar L, Souza RF, Vellucci VF, Meltzer SJ, Reiss M. Loss of transforming growth factor-beta type II receptor gene expression in primary human esophageal cancer. Lab Invest 75: 263–272, 1996 [PubMed] [Google Scholar]
- 42.Geboes K, Haot J, Mebis J, Desmet VJ. The histopathology of reflux esophagitis. Acta Chir Belg 83: 444–448, 1983 [PubMed] [Google Scholar]
- 43.Ghazizadeh M, Tosa M, Shimizu H, Hyakusoku H, Kawanami O. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol 127: 98–105, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol 116: 796–804, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Gruss HJ, Scott C, Rollins BJ, Brach MA, Herrmann F. Human fibroblasts express functional IL-2 receptors formed by the IL-2R alpha- and beta-chain subunits: association of IL-2 binding with secretion of the monocyte chemoattractant protein-1. J Immunol 157: 851–857, 1996 [PubMed] [Google Scholar]
- 46.Hamaguchi M, Fujiwara Y, Takashima T, Hayakawa T, Sasaki E, Shiba M, Watanabe T, Tominaga K, Oshitani N, Matsumoto T, Higuchi K, Arakawa T. Increased expression of cytokines and adhesion molecules in rat chronic esophagitis. Digestion 68: 189–197, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Quintero M, Kuri-Harcuch W, Gonzalez Robles A, Castro-Munozledo F. Interleukin-6 promotes human epidermal keratinocyte proliferation and keratin cytoskeleton reorganization in culture. Cell Tissue Res 325: 77–90, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Hetzel DJ, Dent J, Reed WD, Narielvala FM, Mackinnon M, McCarthy JH, Mitchell B, Beveridge BR, Laurence BH, Gibson GG, Grant AK, Shearman D, Whitehead R, Buckle PJ. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology 95: 903–912, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Holloway RH, Lyrenas E, Ireland A, Dent J. Effect of intraduodenal fat on lower oesophageal sphincter function and gastro-oesophageal reflux. Gut 40: 449–453, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 292: 1115–1118, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest 98: 572–583, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology 58: 163–174, 1970 [PubMed] [Google Scholar]
- 53.Isomoto H, Inoue K, Kohno S. Interleukin-8 levels in esophageal mucosa and long-term clinical outcome of patients with reflux esophagitis. Scand J Gastroenterol 42: 410–411, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Isomoto H, Nishi Y, Wang A, Takeshima F, Omagari K, Mizuta Y, Shikuwa S, Murata I, Kohno S. Mucosal concentrations of proinflammatory cytokines and chemokines at gastric cardia: implication of Helicobacter pylori infection and gastroesophageal reflux. Am J Gastroenterol 99: 1063–1068, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Isomoto H, Saenko VA, Kanazawa Y, Nishi Y, Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M, Mizuta Y, Murata I, Yamashita S, Kohno S. Enhanced expression of interleukin-8 and activation of nuclear factor kappa-B in endoscopy-negative gastroesophageal reflux disease. Am J Gastroenterol 99: 589–597, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol 98: 551–556, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 287: G7–G17, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Jack-Hays MG, Xie Z, Wang Y, Huang WH, Askari A. Activation of Na+/K+-ATPase by fatty acids, acylglycerols, and related amphiphiles: structure-activity relationship. Biochim Biophys Acta 1279: 43–48, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 95: 55–65, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, Onoue M, Matsuoka Y, Ohwaki M, Morotomi M. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res 61: 2395–2398, 2001 [PubMed] [Google Scholar]
- 61.Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology 94: 73–80, 1988 [DOI] [PubMed] [Google Scholar]
- 62.Kalliolias GD, Liossis SN. The future of the IL-1 receptor antagonist anakinra: from rheumatoid arthritis to adult-onset Still's disease and systemic-onset juvenile idiopathic arthritis. Expert Opin Investig Drugs 17: 349–359, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Kanazawa Y, Isomoto H, Wen CY, Wang AP, Saenko VA, Ohtsuru A, Takeshima F, Omagari K, Mizuta Y, Murata I, Yamashita S, Kohno S. Impact of endoscopically minimal involvement on IL-8 mRNA expression in esophageal mucosa of patients with non-erosive reflux disease. World J Gastroenterol 9: 2801–2804, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiehne K, Brunke G, Meyer D, Harder J, Herzig KH. Oesophageal defensin expression during Candida infection and reflux disease. Scand J Gastroenterol 40: 501–507, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Kimani G, Tonnesen MG, Henson PM. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J Immunol 140: 3161–3166, 1988 [PubMed] [Google Scholar]
- 66.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol 275: C1–C24, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340: 825–831, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics 1999. CA Cancer J Clin 49: 8–31, 31, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis 7: 226–236, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Kim HJ, Hahm KB. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res 480–481: 189–200, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Leeb SN, Vogl D, Grossmann J, Falk W, Scholmerich J, Rogler G, Gelbmann CM. Autocrine fibronectin-induced migration of human colonic fibroblasts. Am J Gastroenterol 99: 335–340, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 102: 2047–2056; quiz 2057, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease—enhanced production during active disease. Gut 31: 686–689, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marks RD, Shukla M. Diagnosis and management of peptic esophageal strictures. Gastroenterologist 4: 223–237, 1996 [PubMed] [Google Scholar]
- 75.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis 21: 361–370, 2000 [DOI] [PubMed] [Google Scholar]
- 76.McInnes IB, Liew FY. Cytokine networks—towards new therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol 1: 31–39, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology 95: 593–599, 1988 [DOI] [PubMed] [Google Scholar]
- 78.Miyaki M, Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, Utsunomiya J, Kuroki T, Mori T. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene 18: 3098–3103, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Moons LM, Kusters JG, Bultman E, Kuipers EJ, van Dekken H, Tra WM, Kleinjan A, Kwekkeboom J, van Vliet AH, Siersema PD. Barrett's oesophagus is characterized by a predominantly humoral inflammatory response. J Pathol 207: 269–276, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut 40: 175–181, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Riordan JM, Abdel-Latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Keeling PW, Kelleher D, Reynolds JV. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol 100: 1257–1264, 2005 [DOI] [PubMed] [Google Scholar]
- 82.O'Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, Sigman CC, Bertagnolli MM, Stratton SP, Lam S, Nelson WG, Meyskens FL, Alberts DS, Follen M, Rustgi AK, Papadimitrakopoulou V, Scardino PT, Gazdar AF, Wattenberg LW, Sporn MB, Sakr WA, Lippman SM, Von Hoff DD. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res 8: 314–346, 2002 [PubMed] [Google Scholar]
- 83.Oh DS, DeMeester SR, Vallbohmer D, Mori R, Kuramochi H, Hagen JA, Lipham J, Danenberg KD, Danenberg PV, Chandrasoma P, DeMeester TR. Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett's esophagus with antireflux surgery. Arch Surg 142: 554–559; discussion 559–560, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Hahm KB. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med 30: 905–915, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys 417: 3–11, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Olyaee M, Sontag S, Salman W, Schnell T, Mobarhan S, Eiznhamer D, Keshavarzian A. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut 37: 168–173, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Onwuegbusi BA, Aitchison A, Chin SF, Kranjac T, Mills I, Huang Y, Lao-Sirieix P, Caldas C, Fitzgerald RC. Impaired transforming growth factor beta signalling in Barrett's carcinogenesis due to frequent SMAD4 inactivation. Gut 55: 764–774, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Onwuegbusi BA, Rees JR, Lao-Sirieix P, Fitzgerald RC. Selective loss of TGFbeta Smad-dependent signalling prevents cell cycle arrest and promotes invasion in oesophageal adenocarcinoma cell lines. PLoS One 2: e177, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orlando RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol 42: 584–588, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Orlando RC. Reflux esophagitis: overview. Scand J Gastroenterol Suppl 210: 36–37, 1995 [DOI] [PubMed] [Google Scholar]
- 91.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett's esophagus and the effects of acid suppression. Gastroenterology 117: 327–335, 1999 [DOI] [PubMed] [Google Scholar]
- 92.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA 101: 4250–4255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, Osemlak P, Paszkowski T, Rolinski JM. Cytokines and anticytokines in psoriasis. Clin Chim Acta 394: 7–21, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97: 142–146, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Pregun I, Hritz I, Tulassay Z, Herszenyi L. Peptic esophageal stricture: medical treatment. Dig Dis 27: 31–37, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Qi S, den Hartog GJ, Bast A. Superoxide radicals increase transforming growth factor-beta1 and collagen release from human lung fibroblasts via cellular influx through chloride channels. Toxicol Appl Pharmacol 237: 111–118, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Rafiee P, Ogawa H, Heidemann J, Li MS, Aslam M, Lamirand TH, Fisher PJ, Graewin SJ, Dwinell MB, Johnson CP, Shaker R, Binion DG. Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation. Am J Physiol Gastrointest Liver Physiol 285: G1277–G1292, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Rai AM, Orlando RC. Gastroesophageal reflux disease. Curr Opin Gastroenterol 17: 359–365, 2001 [DOI] [PubMed] [Google Scholar]
- 100.Reimer C, Sondergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 137: 80–87, 87 e81, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Richter JE. Peptic strictures of the esophagus. Gastroenterol Clin North Am 28: 875–891, vi, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Rieder F, Brenmoehl J, Leeb S, Scholmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut 56: 130–139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rieder F, Cheng L, Harnett KM, Chak A, Cooper GS, Isenberg G, Ray M, Katz JA, Catanzaro A, O'Shea R, Post AB, Wong R, Sivak MV, McCormick T, Phillips M, West GA, Willis JE, Biancani P, Fiocchi C. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology 132: 154–165, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Rieder F, Nonevski I, Ouyang Z, West GA, Scaldaferri F, Goldblum J, Bonfield T, Falk G, Fiocchi C. Integrated pathways of fibrogenesis in eosinophilic esophagitis: active secretion of Th2 cytokines and TGF-β1, and binding of activated eosinophils promote collagen I and fibronectin production by human esophageal mesenchymal cells (Abstract). Gastroenterology 136: A–137, 2009 [Google Scholar]
- 105.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg 22: 382–389, 1998 [DOI] [PubMed] [Google Scholar]
- 106.Roth M, Nauck M, Yousefi S, Tamm M, Blaser K, Perruchoud AP, Simon HU. Platelet-activating factor exerts mitogenic activity and stimulates expression of interleukin 6 and interleukin 8 in human lung fibroblasts via binding to its functional receptor. J Exp Med 184: 191–201, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruigomez A, Garcia Rodriguez LA, Wallander MA, Johansson S, Eklund S. Esophageal stricture: incidence, treatment patterns, and recurrence rate. Am J Gastroenterol 101: 2685–2692, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2008 [DOI] [PubMed] [Google Scholar]
- 109.Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. ScientificWorldJournal 8: 1119–1147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scott S, Pandolfi F, Kurnick JT. Fibroblasts mediate T cell survival: a proposed mechanism for retention of primed T cells. J Exp Med 172: 1873–1876, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selby WS, Janossy G, Mason DY, Jewell DP. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol 53: 614–618, 1983 [PMC free article] [PubMed] [Google Scholar]
- 112.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 24: 353–362, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA 287: 1972–1981, 2002 [DOI] [PubMed] [Google Scholar]
- 114.Shute JK, Rimmer SJ, Akerman CL, Church MK, Holgate ST. Studies of cellular mechanisms for the generation of superoxide by guinea-pig eosinophils and its dissociation from granule peroxidase release. Biochem Pharmacol 40: 2013–2021, 1990 [DOI] [PubMed] [Google Scholar]
- 115.Si J, Behar J, Wands J, Beer DG, Lambeth D, Chin YE, Cao W. STAT5 mediates PAF-induced NADPH oxidase NOX5-S expression in Barrett's esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol 294: G174–G183, 2008 [DOI] [PubMed] [Google Scholar]
- 116.Sihvo EI, Salminen JT, Rantanen TK, Ramo OJ, Ahotupa M, Farkkila M, Auvinen MI, Salo JA. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer 102: 551–555, 2002 [DOI] [PubMed] [Google Scholar]
- 117.Sims J, Towne J, Blumberg H. 11 IL-1 family members in inflammatory skin disease. Ernst Schering Res Found Workshop 56: 187–191, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux may cause esophagitis through a cytokine-mediated mechanism, not by caustic (acid) injury. Gastroenterology 137: 1776–1784, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Souza RF, Shewmake K, Terada LS, Spechler SJ. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett's esophagus. Gastroenterology 122: 299–307, 2002 [DOI] [PubMed] [Google Scholar]
- 120.Strong SA, Pizarro TT, Klein JS, Cominelli F, Fiocchi C. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology 114: 1244–1256, 1998 [DOI] [PubMed] [Google Scholar]
- 121.Suzuki H, Miyazawa M, Nagahashi S, Sato M, Bessho M, Nagata H, Miura S, Ishii H. Rabeprazole treatment attenuated Helicobacter pylori-associated gastric mucosal lesion formation in Mongolian gerbils. J Gastroenterol Hepatol 18: 787–795, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Tabata C, Kubo H, Tabata R, Wada M, Sakuma K, Ichikawa M, Fujita S, Mio T, Mishima M. All-trans retinoic acid modulates radiation-induced proliferation of lung fibroblasts via IL-6/IL-6R system. Am J Physiol Lung Cell Mol Physiol 290: L597–L606, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Tateyama F, Yamabe H, Osawa H, Kaizuka M, Shirato K, Okumura K. Interleukin-1beta is an autocrine growth factor of rat glomerular epithelial cells in culture. Nephrol Dial Transplant 16: 1149–1155, 2001 [DOI] [PubMed] [Google Scholar]
- 124.Vesey DA, Cheung C, Cuttle L, Endre Z, Gobe G, Johnson DW. Interleukin-1beta stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-beta-dependent mechanism. J Lab Clin Med 140: 342–350, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Vesper BJ, Jawdi A, Altman KW, Haines GK, 3rd, Tao L, Radosevich JA. The effect of proton pump inhibitors on the human microbiota. Curr Drug Metab 10: 84–89, 2009 [DOI] [PubMed] [Google Scholar]
- 126.Vogel JD, West GA, Danese S, De La Motte C, Phillips MH, Strong SA, Willis J, Fiocchi C. CD40-mediated immune-nonimmune cell interactions induce mucosal fibroblast chemokines leading to T-cell transmigration. Gastroenterology 126: 63–80, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Wang D, Kreutzer DA, Essigmann JM. Mutagenicity and repair of oxidative DNA damage: insights from studies using defined lesions. Mutat Res 400: 99–115, 1998 [DOI] [PubMed] [Google Scholar]
- 128.Wang K, Lin HJ, Perng CL, Tseng GY, Yu KW, Chang FY, Lee SD. The effect of H2-receptor antagonist and proton pump inhibitor on microbial proliferation in the stomach. Hepatogastroenterology 51: 1540–1543, 2004 [PubMed] [Google Scholar]
- 129.Wardlaw AJ, Moqbel R, Cromwell O, Kay AB. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest 78: 1701–1706, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wetscher GJ, Hinder PR, Bagchi D, Perdikis G, Redmond EJ, Glaser K, Adrian TE, Hinder RA. Free radical scavengers prevent reflux esophagitis in rats. Dig Dis Sci 40: 1292–1296, 1995 [DOI] [PubMed] [Google Scholar]
- 131.Wetscher GJ, Hinder RA, Klingler P, Gadenstatter M, Perdikis G, Hinder PR. Reflux esophagitis in humans is a free radical event. Dis Esophagus 10: 29–32; discussion 33, 1997 [DOI] [PubMed] [Google Scholar]
- 132.Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, Adrian TE, Hinder RA. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci 40: 1297–1305, 1995 [DOI] [PubMed] [Google Scholar]
- 133.Williams IR, Kupper TS. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci 58: 1485–1507, 1996 [DOI] [PubMed] [Google Scholar]
- 134.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59: 1073–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 135.Xu X, Rivkind A, Pikarsky A, Pappo O, Bischoff SC, Levi-Schaffer F. Mast cells and eosinophils have a potential profibrogenic role in Crohn disease. Scand J Gastroenterol 39: 440–447, 2004 [DOI] [PubMed] [Google Scholar]
- 136.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137: 588–597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoshida N, Katada K, Handa O, Takagi T, Kokura S, Naito Y, Mukaida N, Soma T, Shimada Y, Yoshikawa T, Okanoue T. Interleukin-8 production via protease-activated receptor 2 in human esophageal epithelial cells. Int J Mol Med 19: 335–340, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Yoshida N, Uchiyama K, Kuroda M, Sakuma K, Kokura S, Ichikawa H, Naito Y, Takemura T, Yoshikawa T, Okanoue T. Interleukin-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand J Gastroenterol 39: 816–822, 2004 [DOI] [PubMed] [Google Scholar]
- 139.Zachrisson K, Neopikhanov V, Samali A, Uribe A. Interleukin-1, interleukin-8, tumour necrosis factor alpha and interferon gamma stimulate DNA synthesis but have no effect on apoptosis in small-intestinal cell lines. Eur J Gastroenterol Hepatol 13: 551–559, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Zoratti EM, Sedgwick JB, Vrtis RR, Busse WW. The effect of platelet-activating factor on the generation of superoxide anion in human eosinophils and neutrophils. J Allergy Clin Immunol 88: 749–758, 1991 [DOI] [PubMed] [Google Scholar]