Abstract
The purpose of this study was to investigate whether luminal leptin alters ion transport properties of the intestinal epithelium under acute inflammatory conditions. Monolayers of human intestinal T84 epithelial cells and a rat model of chemotherapy-induced enterocolitis were used. Cells were treated with leptin and mounted in Ussing chambers to measure basal and secretagogue-induced changes in transepithelial short-circuit current (Isc). Furthermore, the role of MAPK and phosphatidylinositol 3-kinase (PI3K) signaling pathways in mediating responses to leptin was investigated. Acute colitis in Sprague-Dawley rats was induced by intraperitoneal injection of 40 mg/kg methotrexate. Leptin (100 ng/ml) induced a time-dependent increase in basal Isc in T84 intestinal epithelial cells (P < 0.01). Moreover, pretreatment of T84 cells with leptin for up to 1 h significantly potentiated carbachol- and forskolin-induced increases in Isc. Pretreatment with an inhibitor of MAPK abolished the effect of leptin on basal, carbachol- and forskolin-induced chloride secretion (P < 0.05). However, the PI3K inhibitor, wortmannin, only blunted the effect of leptin on forskolin-induced increases in Isc. Furthermore, leptin treatment evoked both ERK1/2 and Akt1 phosphorylation in T84 cells. In the rat model, luminal leptin induced significant increases in Isc across segments of proximal and, to a lesser extent, distal colon (P < 0.05). We conclude that luminal leptin is likely an intestinal chloride secretagogue, particularly when present at elevated concentrations and/or in the setting of inflammation. Our findings may provide a mechanistic explanation, at least in part, for the clinical condition of secretory diarrhea both in hyperleptinemic obese patients and in patients with chemotherapy-induced intestinal inflammation.
Keywords: intestinal ion transport
obesity is increasingly recognized as a risk factor for a number of benign and malignant gastrointestinal conditions (28). Obesity is also associated with functional disorders of the intestine, manifested clinically as a wide range of symptoms such as diarrhea, nausea, vomiting, and constipation (17, 31). In particular, a recent report from a general population sample of young adults revealed a significant univariate association between body mass index (BMI) and diarrhea that was characterized by a higher prevalence of diarrhea among obese and overweight individuals, compared with normal-weight participants (42). Furthermore, mesenteric adipose tissue has been implicated in a wide range of gastrointestinal disorders, including fatty liver, gastrointestinal cancers, acute pancreatitis, Crohn's disease, and even functional bowel disorders (11, 28). Finally, although obesity does not seem to increase the risk of inflammatory bowel disease, it is associated with increased disease severity and anorectal complications (9).
Obesity is also associated with hyperleptinemia (33). Leptin, the obesity gene cloned in 1994, is produced mainly by adipocytes and is the key hormone involved in central regulation of body weight (23). In addition to adipocytes, several other organs such as the stomach, skeletal muscle, placenta, and brain are able to synthesize leptin (34). In particular, the gastric mucosa has been shown to secrete leptin in both an exocrine and endocrine fashion, implying a possible physiological role (2). Immunohistochemistry revealed that chief cells of the fundic mucosa contain leptin in their secretory granules (39). Upon stimulation by nervous or hormonal factors, leptin is released into the gastric juice linked to a protein of high molecular weight, and this complex is translocated in its intact form to the small intestine. Moreover, at least a portion of the stomach-derived leptin escapes proteolysis, suggesting that leptin could reach the distal part of the intestine in an active form. It could therefore initiate biological processes that control functions of the intestinal tract, such as absorption and secretion (35). In keeping with this, leptin receptors, including the functional long isoforms (Ob-Rb), are expressed on the brush borders and basolateral membranes of human enterocytes, rat colonocytes, and cultured colonic epithelial cells, such as the T84 cell line (1, 6, 18).
In the normal colon, luminal leptin is in the low nanomolar range. However, in inflammatory states, leptin staining in colonic epithelial cells was detected and luminal leptin concentrations increased significantly (∼10 times greater than in noninflamed samples) (13). In addition, recent studies have demonstrated that the leptin concentration in colonic lavage fluids from patients with mild to severe inflammatory bowel disease (IBD) is >15-fold higher than that seen in normal subjects (38). Although it is likely that plasma leptin could more readily cross a leaky epithelium and thus be present in the intestinal lumen, it is thought that inflamed colonic epithelial cells additionally secrete leptin into the intestinal lumen, which in turn may act on these cells in an autocrine fashion (38).
The results outlined above form part of a growing body of evidence for a pathophysiological role of intraluminal leptin during states of obesity and intestinal inflammation. In obese individuals (BMI >30 kg/m2) plasma leptin levels can be as high as 100 ng/ml, which is more than 20-fold higher than plasma levels in individuals of normal weight (10). Several studies have additionally indicated that leptin may be involved in the acute stress response to severe illness and surgery (26, 45). Of note, other neurohormonal and inflammatory mediators and cytokines associated with states of intestinal inflammation and systemic stress have been shown to alter critical hallmark functions of the intestinal epithelium, such as ion transport (24). However, studies of the effect of luminal leptin on intestinal ion transport are limited. Therefore, the aim of the present study was to determine whether luminal leptin affects the ion transport properties of the intestinal epithelium under physiological, hyperleptinemic, and/or acute intestinal inflammatory conditions.
MATERIALS AND METHODS
Materials.
Human and rat recombinant leptin were purchased from Sigma (St. Louis, MO). Upstate Biotechnology blocking reagent for Western blotting (skim milk), rainbow-colored protein molecular weight markers ECL Plus (Amersham Pharmacia Biotech, Piscataway, NJ), phospho-p44/42 MAPK antibody (ERK1/2) (rabbit polyclonal IgG1; Cell Signaling Technology, Beverly, MA), total ERK and GAPDH antibodies, as well as phospho-Akt 1/PKB-α and total Akt antibodies (rabbit polyclonal or monoclonal IgG; New England Biolaboratories, Frankfurt, Germany), carbachol (CCh) and forskolin (FSK) (Sigma), the MEK inhibitor PD98059 (PD), and the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin (EMD Biosciences, San Diego, CA) were obtained from the sources indicated.
Cell culture.
The human colonic epithelial cell line, T84, was cultured in DMEM/Ham's F-12 medium (50:50 mix) (Mediatech-Cellgro, Herndon, VA), supplemented with 5% (vol/vol) newborn calf serum (HyClone, Logan, UT). For Ussing chamber/voltage clamp studies, ∼5 × 105 cells were seeded onto 12-mm Millicell-HA Transwells (Millipore, Bedford, MA). For experiments involving Western blotting, ∼106 cells were seeded onto 30-mm Millicell-HA Transwells. Cells seeded onto Millicell filters were cultured for 10–15 days at 37°C in an atmosphere of 95% O2-5% CO2 before use. In cells pretreated with leptin, the hormone was added in regular T84 medium for various times before the experiment as indicated.
Measurement of epithelial ion transport.
T84 cell monolayers, prepared as above, were mounted in modified Ussing chambers (aperture = 0.6 cm2), and both sides of the monolayer were bathed with 5 ml of Ringer's solution with the following composition (in mM): 140 Na+, 5.2 K+, 1.2 Ca2+, 0.8 Mg2+, 119.8 Cl−, 25 HCO3−, 2.4 H2PO4−, and 10 glucose. The medium was warmed to 37°C by a circulating water jacket and gently mixed and oxygenated with 95%O2-5% CO2. Spontaneous tissue potential difference was short circuited continuously via an automatic voltage clamp (WP Instruments, New Haven, CT) and Ag-AgCl2 electrodes, except for brief (2–5 s) intervals at each time point when the open circuit potential difference was recorded. Short-circuit current and transepithelial resistance were determined at 20-s intervals by application of rectangular current (I) pulses during continuous voltage monitoring. Instrument calibration was performed before each experiment using a filter/ring unit without cells. All comparative studies used matched pairs of monolayers seeded at the same time and studied concurrently. Previous studies have shown that short-circuit current (Isc) values in this system are wholly reflective of net chloride transport (16). After a 15-min equilibration period, chloride secretion was elicited using well-characterized secretagogues. cAMP-dependent chloride secretion was induced by FSK (10 μM to mucosal and basolateral sides); Ca2+-dependent secretion was induced by the muscarinic agonist, CCh (100 μM added to the basolateral side).
Animal model of acute chemotherapy-induced intestinal inflammation.
In vivo studies were performed with male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing ∼230–280 g. They received standard pellet chow and water ad libitum. All studies received the approval of the UCSD Committee on Investigations using Animal Subjects. Acute colitis was induced by intraperitoneal injection of 40 mg/kg methotrexate (MTX) essentially as described by Ermens et al. (21). Animals were assessed for clinical signs of colitis, i.e., body weight, stool consistency, and fecal blood using Hemoccult testing as described previously by Cooper et al. (15). No weight loss was counted as 0 points, weight loss of 1–5% as 1 point, 5–10% as 2 points, 10–20% as 3 points, and 20% as 4 points. For stool consistency, 0 points were given for well-formed pellets, 2 points for pasty and semiformed stools that did not stick to the anus, and 4 points for liquid stools that did stick to the anus. Bleeding was scored as 0 points for no blood by hemoccult testing, 2 points for positive hemoccult, and 4 points for gross bleeding. These scores were added and divided by 3, forming a total clinical score that ranged from 0.0 (healthy) to 4.0 (maximal activity of colitis). A control group of animals received an intraperitoneal injection of an equal volume of PBS. On day 4 of MTX-induced colitis, rats were euthanized by halothane anesthesia, followed by cervical dislocation. The colon was removed and opened along the mesenteric border. Five-centimeter segments of proximal colon (starting ∼1 cm distal to the ileocecal junction) and distal colon (starting ∼1 cm distal to the left colic flexure) were stripped of their smooth muscle layers and cut into smaller sections that were then mounted on specially designed Ussing chamber inserts with a window area of 0.5 cm2. Both sides of the tissue segments were bathed with 10 ml of Ringer's solution as described above. The Ringer's solution was maintained at 37°C, pH 7.4, and was gassed with 95% O2-5% CO2. Tissues were allowed to equilibrate for a period of 20 min, at which point baseline PD, short-circuit current (Isc), and tissue conductance were measured. Thereafter, recombinant rat leptin (100 ng/ml) or an equal volume of vehicle (PBS) was added to the apical side, and changes in Isc were recorded. The studies were performed in a paired fashion so that leptin-treated tissues could be compared with control tissues from the same animal.
Immunoblotting.
Approximately 106 T84 cells were seeded onto 30-mm Millicell-HA Transwells for these studies and treated with recombinant human leptin (100 ng/ml) for various times. All incubations were stopped by washing (×3) with ice-cold PBS. Ice-cold lysis buffer was added (consisting of 1% Triton X-100, 1 mM NaVO4, 1 μg/ml leupeptin, 1 mg/ml pepstatin, 1 mg/ml antipain, 1 mM NaF, 1 mM EDTA, and 100 mg/ml PMSF in PBS), and the cells were incubated at 4°C for 30 min. Cells were then scraped into microcentrifuge tubes, centrifuged at 10,000 rpm for 10 min, and the supernatant retained. Aliquots were assayed from each sample to determine protein content, and samples were adjusted so that they contained equal amounts of protein. Samples were then mixed with gel loading buffer (50 mM Tris, pH 6.8, 2% SDS, 100 mM DTT, 0.2% bromphenol blue, 20% glycerol). The samples were boiled for 5 min and proteins separated by SDS-PAGE. Separated proteins were transferred onto a PVDF membrane (DuPont NEN, Boston, MA). The membrane was washed in 1% blocking buffer for 30 min, followed by incubation of the membrane with an appropriate dilution of primary antibody against pERK1/2 or pAkt in blocking buffer for 60 min. This was followed by washing (×3) in Tris-buffered saline with 1% Tween (TBST). Following washes, a horseradish peroxidase-conjugated secondary antibody was added to the membrane in 1% blocking buffer and allowed to incubate for an additional 30 min. This was followed by further washing (×3) in TBST. Immunoreactive proteins were detected using an enhanced chemiluminescence detection kit and exposure of the membrane to X-ray film. Quantification of protein phosphorylation was determined by densitometry using NIH Image software.
Statistical analysis.
Data are presented as the means ± SE. Statistical analysis was performed using SigmaPlot software v8.0 (SPSS, Chicago, IL). One-way ANOVA or, where appropriate, repeated-measures ANOVA with a Student-Newman-Keuls post hoc test, were performed on all data. A P value <0.05 was considered to be statistically significant.
RESULTS
Effect of apical leptin on basal chloride secretion in T84 cells.
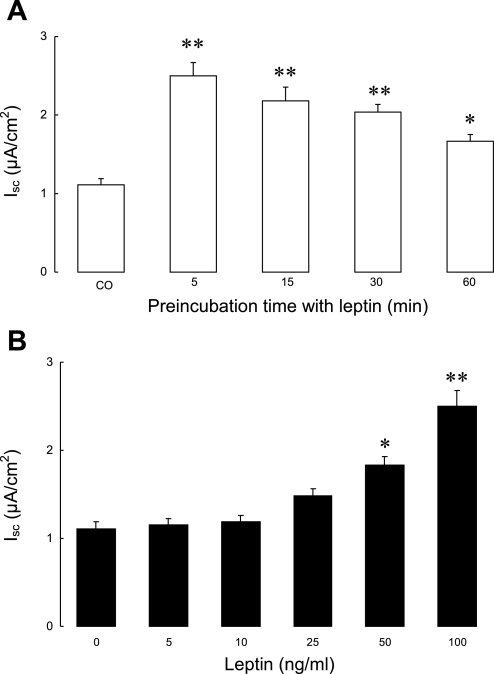
We first examined whether leptin alone could stimulate chloride secretion in T84 cells. Monolayers of T84 intestinal epithelial cells were treated with apically added leptin (100 ng/ml) and mounted in modified Ussing chambers, and any difference in basal Isc compared with untreated cells was noted. As shown in Fig. 1A, pretreatment with leptin more than doubled basal Isc when monolayers were studied after treatment with the peptide for 5 min (Isc: 2.5 μA/cm2 vs. control 1.1 μA/cm2; P < 0.01). The stimulatory effect of leptin pretreatment declined thereafter but persisted for at least 60 min. Furthermore, as shown in Fig. 1B, pretreatment of cells with increasing doses of leptin (5–100 ng/ml) for 5 min showed that the effects of leptin were concentration dependent.
Fig. 1.
Effect of leptin on basal short-circuit current (Isc) in T84 cells. A: T84 cell monolayers (n = 20/group) were pretreated with apically added leptin (100 ng/ml) for different times and were then mounted in modified Ussing chambers for measurement of basal Isc. B: T84 cell monolayers (n = 15/group) were pretreated with increasing doses of apically added leptin for 5 min and then mounted in modified Ussing chambers. Control cells (CO) received no treatment. Data are presented as means ± SE. Asterisks denote values that are statistically significantly different from the control (*P < 0.05; **P < 0.01 by ANOVA).
Effect of leptin on agonist-induced chloride secretion.
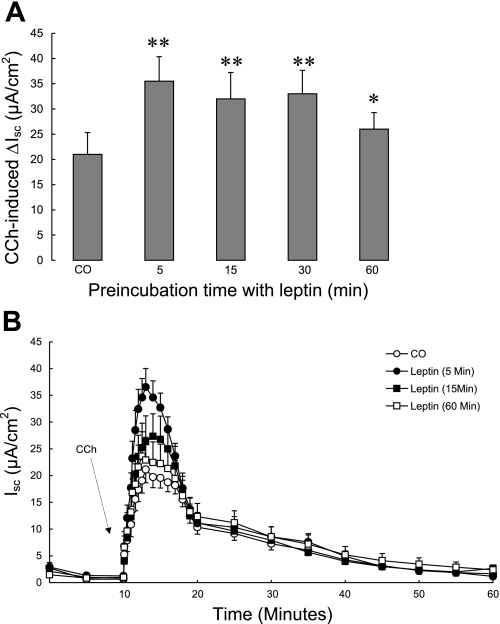
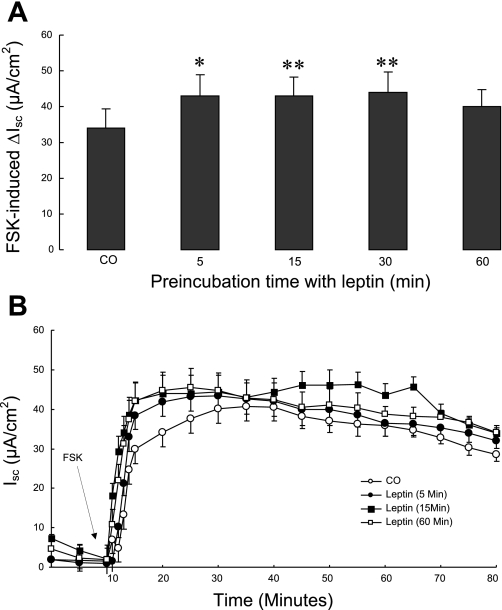
We next tested whether leptin was able to potentiate responses to known chloride secretagogues. T84 cell monolayers were treated with 100 ng/ml apical leptin for various times then mounted in modified Ussing chambers. After a 10-min period of equilibration, CCh was added basolaterally at a concentration of 100 μM. As shown in Fig. 2, A and B, pretreatment of T84 cells with leptin for up to 1 h significantly potentiated the subsequent CCh-induced increase in Isc, without altering the kinetics of the response (P < 0.01). Next, we tested the effect of leptin on chloride secretory responses to the cAMP-dependent agonist, FSK. T84 cell monolayers were treated with 100 ng/ml apical leptin for various times and then mounted in Ussing chambers. After a 10-min period of equilibration, FSK was added at a concentration of 10 μM to both the apical and basolateral sides. As shown in Fig. 3, A and B, pretreatment of T84 cells with leptin significantly increased FSK-induced ΔIsc, again without altering the kinetics of the response (P < 0.05).
Fig. 2.
Effect of leptin on carbachol (CCh)-induced chloride secretion across T84 cells. T84 cell monolayers (n = 15/group) were pretreated with apically added leptin (100 ng/ml) for different times and mounted in modified Ussing chambers, after which CCh (100 μM, basolateral) was added. Control cells received no pretreatment. A: chloride secretion was measured as changes in short-circuit current (ΔIsc). B: time course of changes in Isc after stimulation with CCh in T84 cells pretreated with leptin for different times. Data are presented as means ± SE. Asterisks denote values that are statistically significantly different from the control (*P < 0.05; **P < 0.01 by ANOVA).
Fig. 3.
Effect of leptin on forskolin (FSK)-induced chloride secretion across T84 cells. T84 cell monolayers (n = 15/group) were pretreated with apically added leptin (100 ng/ml) for different times and mounted in modified Ussing chambers, after which FSK (10 μM, apical and basolateral) was added. Control cells received no pretreatment. A: chloride secretion was measured as ΔIsc. B: time course of changes in Isc after stimulation with FSK in T84 cells pretreated with leptin for different times. Data are presented as means ± SE. Asterisks denote values that are statistically significantly different from the control (*P < 0.05; **P < 0.01 by ANOVA).
Signals involved in ion transport responses to leptin.
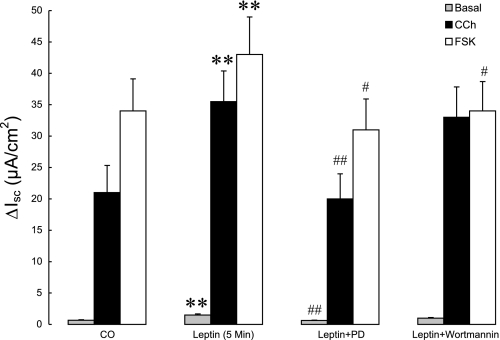
As shown in Fig. 4, the MEK inhibitor PD (40 μM) abolished the stimulatory effect of pretreatment with leptin (5 min) on basal Isc (P < 0.05). In contrast, the PI3K inhibitor wortmannin (100 nM) failed to reverse the effect of leptin pretreatment on basal Isc in T84 cells. Similarly, PD abolished the ability of leptin to potentiate CCh-induced increases in Isc (P < 0.01), whereas the PI3K inhibitor was without a significant effect (Fig. 4). In contrast, the ability of leptin to potentiate FSK-induced increases in chloride secretion was lost in the presence of either PD or wortmannin (Fig. 4). This could suggest a role for PI3K in leptin-induced chloride secretion that is specific for the cAMP pathway although we must also acknowledge the possibility of an independent effect of wortmannin on the response to FSK as we have reported previously for other cAMP-dependent secretagogues (16).
Fig. 4.
Effects of inhibitors of MAPK and phosphatidylinositol 3-kinase (PI3K) pathways on leptin-induced increases in basal and stimulated chloride secretion. T84 cell monolayers (n = 10/group) were pretreated with either leptin alone (100 ng/ml, 5 min, apical addition) or with leptin plus the MEK inhibitor, PD98059 (PD, 40 μM) or the PI3K inhibitor, wortmannin (100 nM) and were mounted in modified Ussing chambers. After recording basal Isc values, chloride secretion was stimulated by CCh (100 μM, basolateral) or FSK (10 μM, apical and basolateral). Control cells received no pretreatment. Secretagogue-induced chloride secretion was measured as ΔIsc. Data are presented as means ± SE. Asterisks denote values that are statistically significantly different from the control (**P < 0.01 by ANOVA); pound signs denote values that are statistically significantly different from corresponding values obtained with leptin alone (#P <0.05; ##P < 0.01 by ANOVA).
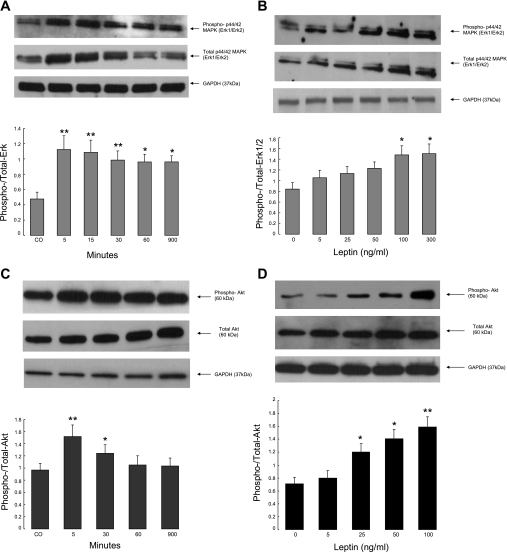
The previous experiments suggested a role for MAPK and, perhaps to a lesser extent, PI3K in the effects of leptin on basal and stimulated intestinal chloride secretion. To explore this further, T84 cells were treated with leptin, and phosphorylation of p42/44 MAPK (ERK1/2) and the downstream target of PI3K, Akt1, were assessed by Western blotting. As shown in Fig. 5, A–D, and as we have reported previously (8), leptin treatment evoked both ERK1/2 and Akt1 phosphorylation in T84 cells in a time- and dose-dependent manner. Responses were maximal after 5 min of leptin treatment and persisted for at least 1 and up to 15 h in the case of Akt1 or ERK phosphorylation, respectively.
Fig. 5.
A–B: leptin activates ERK1/2 isoforms of MAPK. T84 cell monolayers were cultured in serum-free media (DMEM/F12) for 24 h followed by exposure to recombinant human leptin. Leptin was added at 100 ng/ml for the times indicated (A) or at different doses for 5 min (B). Cellular extracts were fractioned by 12% SDS-PAGE, and Western immunoblotting was performed with a rabbit polyclonal anti-phospho-p44/42 MAPK and anti-total ERK as described in materials and methods. C–D: leptin activates Akt in T84 cells. Monolayers were cultured in serum-free media (DMEM/F12) for 24 h followed by exposure to human recombinant leptin at a concentration of 100 ng/ml for up to 15 h (C) or at different doses for 5 min (D). Cellular extracts were analyzed by Western immunoblotting with anti-phospho-Akt 1/PKB-α and anti-total Akt as described in materials and methods. Equal loading was also assessed using a rabbit monoclonal antibody to GAPDH. In all panels, representative blots are shown above with summary data from at least 3 similar experiments, analyzed by densitometry, below. These latter values are means ± SE, and those that differ significantly from controls are designated with asterisks (*P < 0.05; **P < 0.01 by ANOVA).
Leptin increases chloride secretion in chemotherapy-induced colitis.
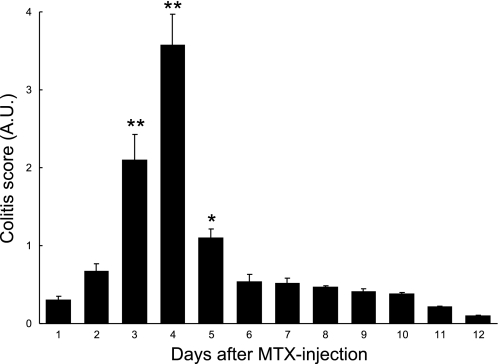
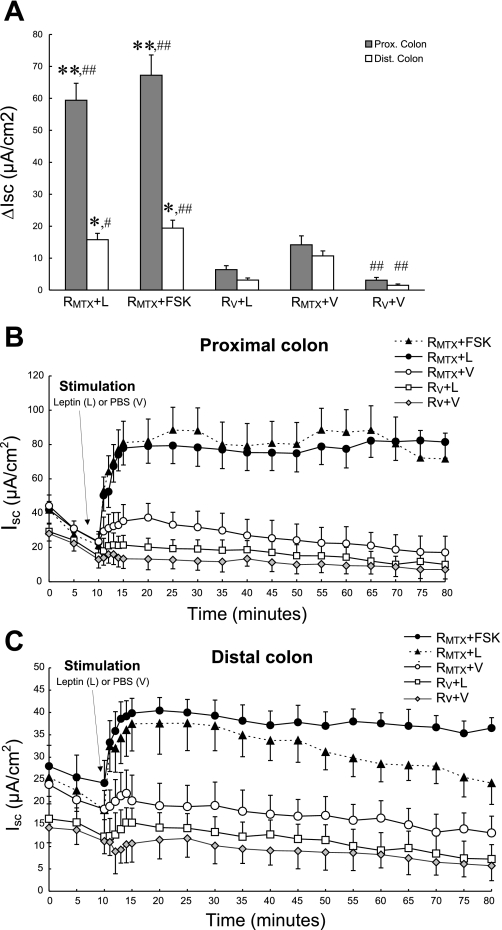
To evaluate further whether luminal leptin might participate in stimulating chloride secretion in the setting of acute intestinal inflammation, an in vivo model of MTX-induced colitis was used. MTX induced colitis 3–5 days after treatment as measured by a graded score of stool consistency, fecal blood using Hemoccult testing, and loss of body weight (Fig. 6). At the time of maximal colitis (4 days), segments of the distal and proximal colon were mounted in Ussing chambers as described. Baseline Isc values were increased in all MTX-treated groups compared with control animals, consistent with active inflammation and diarrhea (Fig. 7, A–C). After a 10-min period of equilibration, leptin (100 ng/ml or vehicle) was added to the luminal side, and changes in Isc were recorded. As shown in Fig. 7, A–C, luminal leptin (L) induced a robust increase in Isc (P < 0.05) across proximal colon segments of MTX-treated rats (RMTX + L; maximal Isc 78 μA/cm2), compared with vehicle-treated (V) noncolitic rats (RV + L; maximal Isc 37 μA/cm2) and with MTX-treated tissues exposed only to PBS as vehicle (RMTX + V; maximal Isc 21 μA/cm2) or with vehicle-treated tissues exposed only to PBS vehicle (RV + V; maximal Isc 16 μA/cm2). This effect of leptin was comparable in magnitude to the stimulatory effect of FSK, which was used here as a positive control (RMTX + FSK; maximal Isc 88 μA/cm2). A qualitatively similar response was seen in the distal colon, albeit of lower magnitude (Fig. 7, A–C). Moreover, the fact that only a weak response to leptin was observed in tissues from animals not receiving MTX (RV + L) indicates that the effect of leptin on net intestinal ion transport is strikingly potentiated in the setting of inflammation.
Fig. 6.
Acute colitis was induced by intraperitoneal injection of 40 mg/kg methotrexate (MTX) in rats as measured by a graded score that combined measures for stool consistency, the presence of fecal blood and loss of body weight, as described in materials and methods. Data are presented as means ± SE for 35 animals. Asterisks denote values that are statistically significantly different from those on treatment day 1 (*P < 0.05; **P < 0.01 by ANOVA).
Fig. 7.
A: leptin effect on net ion transport in colonic tissues isolated from rats subjected to an in vivo model of chemotherapy-induced colitis. Four days after induction of colitis in rats with MTX, segments of the distal (open bars) and proximal colon (shaded bars) were dissected free of mesenteric attachments, stripped of their muscular layers, and mounted in modified Ussing chambers. One control group of animals (n = 5) received an equal volume of PBS (RV), and the tissue was prepared similarly to the MTX group. After a 10-min period of equilibration, leptin (100 ng/ml) was added to MTX-treated tissues (RMTX + L) and to the tissues from vehicle-treated animals (RV + L). Additionally, some MTX-treated tissues were stimulated either with PBS (RMTX + V) or with FSK (RMTX + FSK) as negative and positive controls, respectively. Moreover, a group of tissues from vehicle-treated animals were treated with PBS (RV + V). All agents were added to the mucosal side, and changes in Isc were recorded (ΔIsc). B–C: time course of leptin-induced changes in Isc across proximal (B) and distal (C) colon in a rat model of chemotherapy-induced colitis (n = 10/group). The data correspond to the ΔIsc values shown in A. The data are presented as means ± SE for at least 5 rats in each category. Asterisks denote values that are statistically significantly different from vehicle-treated noncolitic rats (RV + L) (*P < 0.05; **P < 0.01 by ANOVA); pound signs denote values that are statistically significantly different from MTX-treated tissues exposed only to PBS as vehicle (RMTX + V) (#P <0.05; ##P < 0.01 by ANOVA).
DISCUSSION
Leptin, the product of the ob gene, is an adipose tissue-secreted peptide that signals the magnitude of fat stores to the brain and regulates energy homeostasis. Recent data suggest that leptin could also be considered a gastrointestinal hormone (14). Leptin mRNA and protein, as well as its receptor, have been found in the gastric epithelium in rats and humans (43). However, relatively little is known about the possible actions of leptin in the intestine. A regulatory role for leptin in the absorption of nutrients has been postulated by some authors (12, 19, 32, 35, 40). This led us to study the possible effect of leptin on intestinal ion transport, another important epithelial function.
Chloride secretion is the predominant driving force for fluid secretion in the intestine (7). To examine the effect of leptin on intestinal chloride transport, we mounted T84 human colonic epithelial cells in Ussing chambers and measured changes in short-circuit current (Isc). We observed that apical pretreatment of T84 cells with leptin at supraphysiological concentrations, which have been detected in obese patients, increased basal Isc, although lower, physiological concentrations of leptin had no appreciable effects (38). Supraphysiological concentrations of leptin also potentiated responses to other known chloride secretagogues, such as the calcium-dependent muscarinic agonist, CCh, and the cAMP-dependent agonist, FSK. Our data imply that levels of leptin observed in obese patients, as well as in the setting of intestinal inflammation, have at least the potential to trigger excessive fluid and electrolyte secretion under such conditions.
We hypothesized that specific signal transduction proteins might be involved in the prosecretory effects of leptin. Leptin receptors have the ability to activate components of intracellular signaling cascades including JAK/STAT, PI3K, and MAPK pathways (25, 41). Pretreatment of cells with the MEK inhibitor, PD, abolished the effect of leptin on both basal Isc and secretagogue-induced secretory responses in T84 cells. Similarly, although the PI3K inhibitor, wortmannin, had no significant effect on leptin-induced increases in basal Isc or responses to CCh, it apparently reversed the effect of leptin on FSK-stimulated secretory responses. These data were consistent with those from Western blot studies, which showed that leptin treatment evoked p42/44 MAPK (ERK1/2) and Akt1 phosphorylation in T84 cells with kinetics compatible with its effects on secretion. The differential involvement of PI3K, moreover, may relate to the fact that CCh is known not to activate this enzyme in T84 cells, whereas the secretory response to cAMP-dependent agonists appears to rely, at least in part, on PI3K activity (8, 20).
Several studies suggest that leptin and its receptors may be upregulated in inflammatory conditions (29, 30). We therefore hypothesized that leptin might additionally be involved in disrupting intestinal ion transport in the setting of acute intestinal inflammation. This hypothesis was tested in an in vivo model of chemotherapy-induced colitis, a condition accompanied by acute clinical, functional, and inflammatory changes in the intestinal epithelium. After induction of colitis in rats by MTX administration, apical leptin was able to induce a significant increase in Isc in proximal colonic segments, and to a lesser extent in the distal colon. Indeed, the secretagogue action of leptin in this setting was comparable to that of the positive control, FSK, and much greater than seen in T84 cells. Because luminal leptin is increased in the setting of acute colitis, the actions described may contribute to the pathogenesis of inflammatory diarrhea. Indeed, blood levels of leptin were elevated transiently during the early stages of trinitrobenzene sulfonic acid-mediated colitis in rats, with the increase correlated with the degree of inflammation and anorexia (4). Similarly, Sitaraman et al. (38) showed that leptin participates in the pathogenesis of enterotoxin-mediated intestinal secretion and inflammation, with inflamed colonic cells serving themselves as a source of luminal leptin. These authors also examined colonic lavage fluids from normal subjects and patients with ulcerative colitis and demonstrated that colonic lavage fluid contained more than 15-fold higher concentrations of leptin in mild to severe IBD than seen in controls (38). Others have shown that mice deficient in leptin (ob/ob mice) or the long isoform of its receptor (db/db mice) are resistant in a variety of experimental models of inflammation/autoimmunity (37), including intestinal inflammation induced by administration of dextran sulfate sodium or trinitrobenzene sulphonic acid (37). Resistance to Clostridium difficile toxin A-induced enteritis has also been demonstrated in ob/ob mice (36). On the other hand, data supporting a role for leptin in chronic IBD in humans are sparse and sometimes conflicting (3, 4, 22, 27, 44). Overall, further investigations will be needed to clarify fully the multiple possible roles of leptin in IBD.
In conclusion, we demonstrate that increased apical leptin concentrations can induce an ion transport response consistent with chloride, and thus fluid, secretion. It is conceivable that this corresponds to the clinical condition of diarrhea seen often in extremely obese patients or in patients with intestinal inflammation. The effects of leptin may be mediated, at least in part, by the activation of components of MAPK and PI3K signal transduction pathways. Our observations, together with recent studies showing participation of leptin in animal models of IBD, suggest that the use of specific leptin receptor antagonists could represent a novel therapeutic approach to the treatment of diarrheal symptoms seen in intestinal injury, IBD, or obesity, or following the administration of chemotherapeutic drugs during cancer therapy, particularly because leptin levels are positively correlated with colon cancer risk and may account for the association of such malignancy with obesity (25).
GRANTS
This work was supported by a grant to K. Barrett from the National Institutes of Health (DK28305) and by an award from the Shape-Up Settlement Fund to K. Barrett. M. Hoda was the recipient of the Erwin-Schroedinger Research Fellowship from the Austrian Science Foundation (Fonds zur Foerderung der wissenschaftlichen Forschung; FWF; Vienna, Austria), J2190-B08. S. Keely and D. McCole were supported by awards from the Crohn's and Colitis Foundation of America.
DISCLOSURES
The authors are not aware of financial conflicts with the subject matter or materials discussed in this article with any of the authors or any of their academic institutions or employers.
ACKNOWLEDGMENTS
We thank Glenda Wheeler for assistance with manuscript submission. K. Barrett is a member of the Biomedical Sciences Ph.D. Program, University of California, San Diego.
REFERENCES
- 1.Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, Fujimoto K. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol 292: G923–G929, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature 394: 790–793, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Ballinger A, Kelly P, Hallyburton E, Besser R, Farthing M. Plasma leptin in chronic inflammatory bowel disease and HIV: implications for the pathogenesis of anorexia and weight loss. Clin Sci (Lond) 94: 479–483, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Barbier M, Cherbut C, Aube AC, Blottiere HM, Galmiche JP. Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut 43: 783–790, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, Cherbut C, Galmiche JP. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol 27: 987–991, 2003 [PubMed] [Google Scholar]
- 6.Barrenetxe J, Villaro AC, Guembe L, Pascual I, Munoz-Navas M, Barber A, Lostao MP. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut 50: 797–802, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem 279: 6271–6279, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr 21: 51–57, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Blum WF, Englaro P, Attanasio AM, Kiess W, Rascher W. Human and clinical perspectives on leptin. Proc Nutr Soc 57: 477–485, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 89: 2583–2589, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, Merlin D, Laburthe M, Lewin MJ, Roze C, Bado A. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest 108: 1483–1494, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277: 28182–28190, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem 53: 851–860, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993 [PubMed] [Google Scholar]
- 16.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 384–354, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado-Aros S, Locke GR, 3rd, Camilleri M, Talley NJ, Fett S, Zinsmeister AR, Melton LJ., 3rd Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol 99: 1801–1806, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Drew JE, Farquharson AJ, Padidar S, Duthie GG, Mercer JG, Arthur JR, Morrice PC, Barrera LN. Insulin, leptin, and adiponectin receptors in colon: regulation relative to differing body adiposity independent of diet and in response to dimethylhydrazine. Am J Physiol Gastrointest Liver Physiol 293: G682–G691, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes 54: 348–354, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ecay TW, Dickson JL, Conner TD. Wortmannin inhibition of forskolin-stimulated chloride secretion by T84 cells. Biochim Biophys Acta 1467: 54–64, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Ermens AA, Schoester M, Spijkers LJ, Lindemans J, Abels J. Toxicity of methotrexate in rats preexposed to nitrous oxide. Cancer Res 49: 6337–6341, 1989 [PubMed] [Google Scholar]
- 22.Franchimont D, Roland S, Gustot T, Quertinmont E, Toubouti Y, Gervy MC, Deviere J, Van Gossum A. Impact of infliximab on serum leptin levels in patients with Crohn's disease. J Clin Endocrinol Metab 90: 3510–3516, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Friedmann JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol 278: C212–C233, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Hoda MR, Keely SJ, Bertelsen LS, Junger W, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and anti-apoptotic factor for colon cancer cells via mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling pathways. Br J Surg 94: 346–354, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Hoda MR, El-Achkar H, Schmitz E, Scheffold Th, Vetter HO, De Simone R. Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass. Ann Thorac Surg 82: 2179–2186, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hoppin AG, Kaplan LM, Zurakowski D, Leichtner AM, Bousvaros A. Serum leptin in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 26: 500–505, 1998 [DOI] [PubMed] [Google Scholar]
- 28.John BJ, Irukulla S, Abulafi AM, Kumar D, Mendall MA. Systematic review: adipose tissue, obesity and gastrointestinal diseases. Aliment Pharmacol Ther 23: 1511–1523, 2006 [DOI] [PubMed] [Google Scholar]
- 29.La Cava A, Matarese G. The weight of leptin in immunity. Nat Immun 4: 371–378, 2004 [DOI] [PubMed] [Google Scholar]
- 30.La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med 82: 4–11, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Levy RL, Linde JA, Feld KA, Crowell MD, Jeffery RW. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol 3: 992–996, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Lostao MP, Urdaneta E, Martinez-Anso E, Barber A, Martinez JA. Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett 423: 302–306, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Maffei M, Halaas J, Ravussin E, Prately RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med 3: 1029–1033, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem 273: 26194–26201, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Mykoniatis A, Anton PM, Wlk M, Wang CC, Ungsunan L, Bluher S, Venihaki M, Simeonidis S, Zacks J, Zhao D, Sougioultzis S, Karalis K, Mantzoros C, Pothoulakis C. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 124: 683–691, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology 122: 2011–2025, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J 18: 696–698, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M, Lewin MJ. Leptin secretion and leptin receptor in the human stomach. Gut 47: 178–183, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobhani I, Buyse M, Goiot H, Weber N, Laigneau JP, Henin D, Soul JC, Bado A. Vagal stimulation rapidly increases leptin secretion in human stomach. Gastroenterology 122: 259–263, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Sweeney G. Leptin signalling. Cell Signal 14: 655–663, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Talley NJ, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol 99: 1807–1814, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia LA, Dembski M, Weng X. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Tuzun A, Uygun A, Yesilova Z, Ozel AM, Erdil A, Yaman H, Bagci S, Gulsen M, Karaeren N, Dagalp K. Leptin levels in the acute stage of ulcerative colitis. J Gastroenterol Hepatol 19: 429–432, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Tzanela M, Orfanos SE, Tsirantonaki M, Kotanidou A, Sotiropoulou CH, Christophoraki M, Vassiliadi D, Thalassinos NC, Roussos CH. Leptin alterations in the course of sepsis in humans. In Vivo 20: 565–570, 2006 [PubMed] [Google Scholar]