Abstract
Adipose tissue dysfunction, featured by insulin resistance and/or dysregulated adipokine production, plays a central role not only in disease initiation but also in the progression to nonalcoholic steatohepatitis and cirrhosis. Promising beneficial effects of betaine supplementation on nonalcoholic fatty liver disease (NAFLD) have been reported in both clinical investigations and experimental studies; however, data related to betaine therapy in NAFLD are still limited. In this study, we examined the effects of betaine supplementation on hepatic fat accumulation and injury in mice fed a high-fat diet and evaluated mechanisms underlying its hepatoprotective effects. Male C57BL/6 mice weighing 25 ± 0.5 (SE) g were divided into four groups (8 mice/group) and started on one of four treatments: control diet, control diet supplemented with betaine, high-fat diet, and high-fat diet supplemented with betaine. Betaine was supplemented in the drinking water at a concentration of 1% (wt/vol) (anhydrous). Our results showed that long-term high-fat feeding caused NAFLD in mice, which was manifested by excessive neutral fat accumulation in the liver and elevated plasma alanine aminotransferase levels. Betaine supplementation alleviated hepatic pathological changes, which were concomitant with attenuated insulin resistance as shown by improved homeostasis model assessment of basal insulin resistance values and glucose tolerance test, and corrected abnormal adipokine (adiponectin, resistin, and leptin) productions. Specifically, betaine supplementation enhanced insulin sensitivity in adipose tissue as shown by improved extracellular signal-regulated kinases 1/2 and protein kinase B activations. In adipocytes freshly isolated from mice fed a high-fat diet, pretreatment of betaine enhanced the insulin signaling pathway and improved adipokine productions. Further investigation using whole liver tissues revealed that betaine supplementation alleviated the high-fat diet-induced endoplasmic reticulum stress response in adipose tissue as shown by attenuated glucose-regulated protein 78/C/EBP homologous protein (CHOP) protein abundance and c-Jun NH2-terminal kinase activation. Our findings suggest that betaine might serve as a safe and efficacious therapeutic tool for NAFLD by improving adipose tissue function.
Keywords: betaine, fatty liver, insulin resistance, adipose tissue, endoplasmic reticulum stress, adipokine
nonalcoholic fatty liver disease (NAFLD) covers a spectrum of liver disease ranging from simple hepatic steatosis (accumulation of triglyceride inside hepatocytes) to nonalcoholic steatohepatitis (necrosis and inflammation), with some people ultimately progressing to fibrosis and cirrhosis and liver failure. Because of its high prevalence in conjunction with obesity, diabetes, and insulin resistance, NAFLD is being increasingly appreciated as a hepatic manifestation of the metabolic syndrome and represents a major cause of liver-related morbidity and mortality (3, 7, 14).
The pathogenesis of NAFLD has not yet been clearly defined; however, adipose tissue dysfunction, characterized by insulin resistance and dysregulated adipokine production, is considered to be the central mechanism involved in the development of steatosis and the transitional process to steatohepatitis in NAFLD (16, 35, 41). Fat accumulation in the liver results from an imbalance among the uptake, synthesis, export, and oxidation of fatty acids. Adipose tissue is the major depot in the body to release nonesterified free fatty acids (NEFAs). At normal circumstance, insulin inhibits NEFA release (lipolysis) from adipose tissues via suppressing hormone-sensitive lipase. As insulin resistance develops, insulin-mediated suppression of lipolysis is overwhelmed, and increased release of NEFAs in the portal circulation ensues. Excessive exposure and uptake of NEFA by the liver lead to hepatic steatosis, which conversely exacerbates the degree of insulin resistance and accelerates its subsequent transition to steatohepatitis and fibrosis (6, 40).
There is currently no Food and Drug Administration (FDA)-approved treatment for NAFLD or nonalcoholic steatohepatitis. Managing body weight is likely the best overall method in the treatment of the condition in both the metabolic syndrome and NAFLD; however, in practice, it is difficult to achieve and even more difficult to maintain for most patients. Because of the strong relationship between insulin resistance and NAFLD, pharmacological treatment using insulin sensitizers, such as thiazolidinediones (TDZs) and metformin, has been shown to be beneficial in the prevention or improvement in NAFLD (22, 26, 27, 34), although the varying degrees of side effects, specifically increases in body weight and body fat content (29, 36), of these agents were also reported; therefore, searching for the safe compound with high preventive and/or therapeutic efficacies is urgently needed.
Betaine (trimethylglycine) is naturally found in microorganisms, plants, and animals and is a significant component of many foods (19, 37, 43), including wheat, shellfish, spinach, and sugar beets. It is also a metabolite of choline and an essential biochemical component of the methionine-homocysteine cycle (8). Hepatoprotective effects of betaine were reported in a variety of experimental animal models of liver diseases, including alcoholic liver disease and bile acid-induced liver injury (4, 11, 15, 18), with different mechanisms involved. The therapeutic effects of betaine on NAFLD have also been investigated and reported in both clinical and experimental studies (1, 17, 21, 24, 28). Although the overall results are promising, data relating betaine to NAFLD are limited, and cellular and molecular mechanisms remain elusive because of the lack of detailed experimental investigations. Thus detailed studies are warranted before any recommendations regarding the use of betaine in NAFLD can be made.
The beneficial effects of betaine on an animal model of NAFLD were previously shown by our laboratory using a long-term high-carbohydrate (sucrose) diet-feeding mouse model (38). We found that betaine supplementation alleviated hepatic fat accumulation via direct activation of the hepatic AMP-activated protein kinase (AMPK) system and subsequent inhibition of the de novo lipogenesis pathway in the liver. At the same time, we also observed increased circulating adiponectin levels and improved plasma insulin and glucose levels by betaine supplementation, implying that betaine had the potential to improve adipose tissue function and insulin sensitivity. In this study, the experimental model of long-term (12-wk) high-fat diet feeding to mice was used. Our aims were: 1) to investigate the hepatoprotective effects of betaine in a well-established animal model of NAFLD; 2) to evaluate beneficial effects of betaine on adipose tissue function, i.e., insulin sensitivity and adipokine production; and 3) to identify the underlying mechanisms involved.
MATERIALS AND METHODS
Animal model and experimental protocol.
Male C57BL/6 mice (8 wk) weighing 25 ± 0.5 (SD) g were obtained from the Jackson Laboratory (Bar Harbor, ME). The mice were housed in the animal quarters at the University of Louisville Research Resources Center, and the studies were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care. Initially, all mice were housed in conventional conditions and fed standard diet and water ad libitum at the animal facility (Research Resource Facility) for 1 wk before experiments began. Thereafter, the mice were divided into four groups (n = 8/group) and started on one of four treatments: control diet (Con), control diet supplemented with betaine (BT), high-fat diet (HF), and high-fat diet supplemented with betaine (HB). Both control (D12450B) and high-fat diet (D12451) were obtained from ResearchDiets (New Brunswick, NJ). The detailed diet composition was shown in the formula table (Table 1). Betaine was supplemented in the drinking water at a concentration of 1% (wt/vol) (anhydrous; Sigma, St. Louis, MO) and started simultaneously with high-fat diet feeding. All animals had access to diet and water ad libitum. Mice were maintained on the treatments for 12 wk before being killed. At the end of the experiment, the mice were anesthetized with Avertin (300 mg/kg body wt) after 4 h fasting, and plasma, liver, and epididymal fat pad samples were harvested for assays.
Table 1.
Diet formula
| Diet | Control, kcal/100 kcal | High Fat, kcal/100 kcal |
|---|---|---|
| Protein | 20 | 20 |
| Carbohydrate | 70 | 35 |
| Fat | 10 | 45 |
| Ingredients, g | ||
| Casein (80 mesh) | 200 | 200 |
| l-Cystine | 3 | 3 |
| Corn starch | 315 | 72.8 |
| Maltodextrin 10 | 35 | 100 |
| Sucrose | 350 | 172.8 |
| Cellulose, BW200 | 50 | 50 |
| Soybean oil | 25 | 25 |
| Lard | 20 | 177.5 |
| Mineral mix S10026 | 10 | 10 |
| Dicalcium phosphate | 13 | 13 |
| Calcium carbonate | 5.5 | 5.5 |
| Potassium citrate, 1H2O | 16.5 | 16.5 |
| Vitamin mix V10001 | 10 | 10 |
| Choline bitartrate | 2 | 2 |
Isolation and culture of primary adipocytes.
High-fat diet-fed mice were used to obtain primary adipose cells at the end of the experimental period. Briefly, the mice were anesthetized and killed via cervical dislocation. Epididymal fat pads were harvested, washed in PBS (pH 7.4) buffer at room temperature, and minced thoroughly (2–3 mm in diameter) in the collagenase solution (0.2 mg/ml collagenase A; 4 ml/g of adipose tissue). This mixture was incubated at 37°C with shaking at 120 rpm for 30 min. After digestion, the mixture was filtered through a 250-μm gauze mesh in a 50-ml conical polypropylene tube and allowed to stand for 2–3 min. The floating layer of adipocytes was washed three times and incubated at 37°C in Dulbecco's modified Eagle's medium containing 5% BSA. The cells and conditioned medium were taken at various times as indicated in the legends for Figs. 1–6.
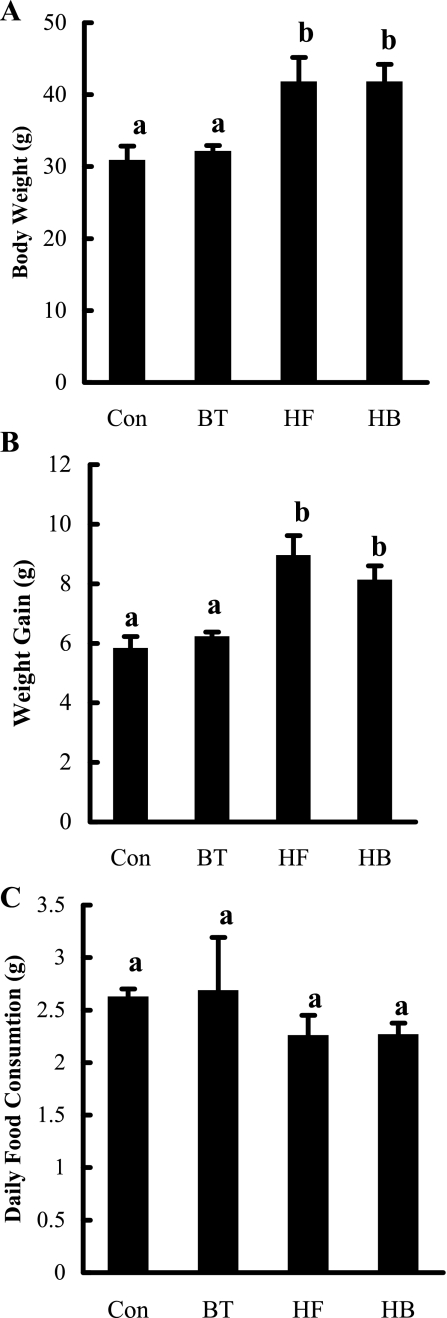
Fig. 1.
Betaine supplementation had no effect on body weight change and food consumption in C57BL/6 mice fed a high-fat diet. A: changes of absolute body weight. B: changes of body weight gain. C: daily food consumption. C57BL/6 mice were fed with control and high-fat diet with/without betaine supplementation (1%) in the drinking water (n = 8 mice/group) for 12 wk. Con, control diet; BT, control diet supplemented with betaine in the drinking water; HF, high-fat diet; HB, high-fat diet supplemented with betaine in the drinking water. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05).
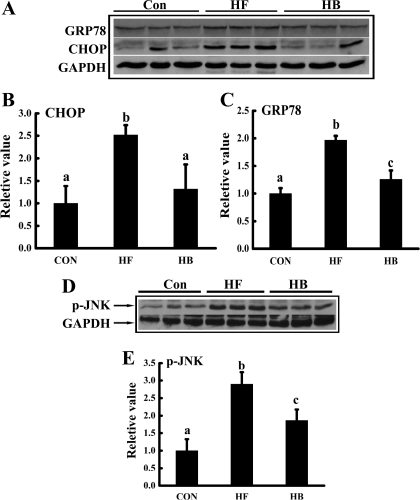
Fig. 6.
Betaine supplementation prevented the ER stress response in adipose tissue induced by high-fat feeding. Male C57BL/6 mice were fed with a high-fat diet with/without betaine supplementation (1%) in the drinking water for 12 wk. Total protein extracts from epididymal fat pads were prepared thereafter. Protein (40 μg) was subjected to Western blot analysis for glucose-regulated protein 78 (GRP78), C/EBP homologous protein (CHOP), and c-Jun NH2-terminal kinase (JNK) phosphorylation using specific antibodies. A: prolonged high-fat feeding caused a significant increase of both GRP78 and CHOP levels in adipose tissue, which was prevented by betaine supplementation. B and C: quantitation of Western blots of GRP78 and CHOP expression levels in adipose tissues from different groups, normalized by corresponding glyceraldehydes-3-phosphate dehydrogenase (GAPDH) expression levels (n = 8). D: betaine supplementation prevented JNK activation by high-fat feeding. E: quantitation of Western blots of JNK phosphorylation levels in adipose tissues from different groups, normalized by corresponding GAPDH expression levels (n = 8). Data are indicated as means ± SD. Bars with different letters differ significantly (P < 0.05).
Measurements of liver injury and hepatic fat content.
Liver injury was determined by measuring plasma alanine aminotransferase (ALT) activities using a commercially available kit (Infinity; Thermo Electron, Melbourne, Australia). Hepatic fat accumulation was determined by measuring total hepatic triglyceride content and by histopathological evaluation. For intrahepatic triglyceride measurement, liver tissues were homogenized, and hepatic total lipids were extracted according to Bligh and Dyer (5) and redissolved in 2% Triton X-100 in water. Hepatic triglyceride content was determined by enzymatic colorimetric methods using a commercially available kit (Infinity; Thermo Electron). For histopathological evaluation, fresh liver were flash-frozen, and cryostat sections were cut and prepared for staining with oil red O. Photomicrographs were taken on an a Nikon Eclipse E600 microscope (Fryer, Cincinnati, OH) equipped with a digital camera (SPOT; Diagnostic Instruments, Sterling Heights, MI).
Plasma biochemical assays.
The plasma biochemical assays were performed with commercially available kits: glucose, triglyceride, cholesterol (Infinity; Thermo Electron), free fatty acids (Waco Chemicals, Richmond, VA), adiponectin, leptin, resistin, and insulin (Linco Research, St. Charles, MO).
The homeostasis model assessment of basal insulin resistance (HOMA-IR) was used to calculate an index from the product of the fasting concentrations of plasma glucose (mmol/l) and plasma insulin (μU/ml) divided by 22.5 (23). Lower HOMA-IR values indicated greater insulin sensitivity, whereas higher HOMA-IR values indicated lower insulin sensitivity (insulin resistance).
Glucose tolerance test.
The glucose tolerance tests were conducted 1 wk before the end of feeding. After a 6-h fast, the mice were anesthetized, and, after the collection of an unchallenged sample (time 0), a solution of 50% glucose (2.0 g/kg body wt) was administered by oral gavage. During the test, blood was collected from the tail vein 30, 60, and 120 min after glucose administration. All blood glucose measurements were performed using a hand-held glucometer.
Western blotting detections.
Epididymal fat pads were lysed in Western lysis buffer consisting of the following: 20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 2% Nonidet P-40, 1 mM EDTA, pH 8.0, 20 mM sodium fluoride, 30 mM sodium pyrophosphate, 0.2% SDS, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1 mM sodium vanadate, 50 μM leupeptin, and 5 μM aprotinin. Samples were incubated on ice with frequent vortexing for 15 min and centrifuged for 20 min at 18,000 g. The protein content of each supernatant was quantified via a protein assay reagent from Bio-Rad Laboratories (Hercules, CA) in accordance with the manufacturer's instructions. Proteins were separated by SDS-PAGE and transferred to a 0.45-μm Immobilin-P polyvinylidene difluoride membrane (PerkinElmer Life Sciences). After transfer, membranes were blocked in 5% (wt/vol) nonfat dry milk in PBS-0.1% Tween 20 and probed with the antibodies specified. Horseradish peroxidase-conjugated secondary antibodies (Sigma) and enhanced chemiluminescence substrate kit (PerkinElmer Life Science) were used in the detection of specific proteins.
Statistical analysis.
All data were expressed as means ± SD. Statistical analysis was performed using a one-way ANOVA and was analyzed further by Newman-Keuls test for statistical difference. Differences between treatments were considered to be statistically significant at P < 0.05.
RESULTS
Body weight changes and food consumptions.
Absolute body weight of each mouse from each group was measured at the end of the feeding period, and body weight gain was calculated and shown in Fig. 1. No difference in either absolute body weight or body weight gain between the Con and BT groups was observed at the end of the feeding period. Compared with the Con group, mice in the HF group had significantly larger body weight and greater body weight gain. Twelve-week high-fat diet feeding was accompanied by 53% (8.92 vs. 5.81 g) more body weight gain than those in the Con group. Supplementation of betaine in the HF group (HB) had no significant effects on either body weight or average weight gain when compared with the HF group. Food consumptions were monitored every week, and average daily food consumptions during the entire experimental period were calculated. As shown in Fig. 1C, there was no significant difference in daily food consumptions among groups.
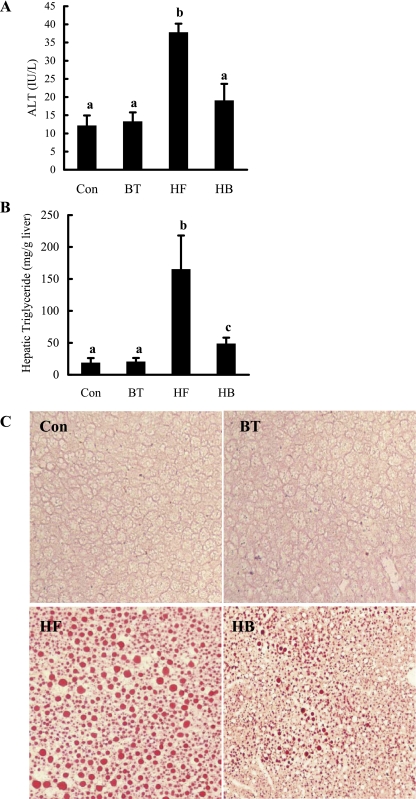
Betaine supplementation alleviated high-fat diet-induced fatty liver and liver injury.
The pathological alterations of livers from different groups were evaluated by measuring hepatic triglyceride contents and circulating liver enzyme levels. Consistent with previous studies (20, 33), long-term high-fat diet feeding induced liver injury (Fig. 2A) and fatty liver (Fig. 2B) in mice, which were manifested by increased plasma ALT levels and hepatic triglyceride contents, respectively. Moreover, massive fat accumulation in the liver of a mouse fed the high-fat diet was also observed via oil red O staining. When betaine was supplemented to the high-fat diet in the drinking water, both hepatic triglyceride accumulation and liver injury were significantly reduced. Because betaine supplementation to the control diet (BT group) had no effects on body weight changes, hepatic triglyceride content, and liver injury when compared with the Con group, we excluded this group in all of the following measurements to simplify our description.
Fig. 2.
Betaine supplementation attenuated liver injury and hepatic fat accumulation in mice fed a high-fat diet. A: plasma alanine aminotransferase (ALT) levels. B: hepatic triglyceride content. C57BL/6 mice were fed a high-fat diet with/without betaine supplementation (1%) in the drinking water for 12 wk. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05). C: fresh-frozen liver sections were stained with oil red O. Control group animals with/without betaine supplementation had no obvious fat accumulation in the liver. High-fat diet feeding resulted in profound hepatic steatosis, featured by a remarkable amount of fat droplet in the liver. Betaine supplementation significantly decreased hepatic fat accumulation induced by high-fat diet feeding.
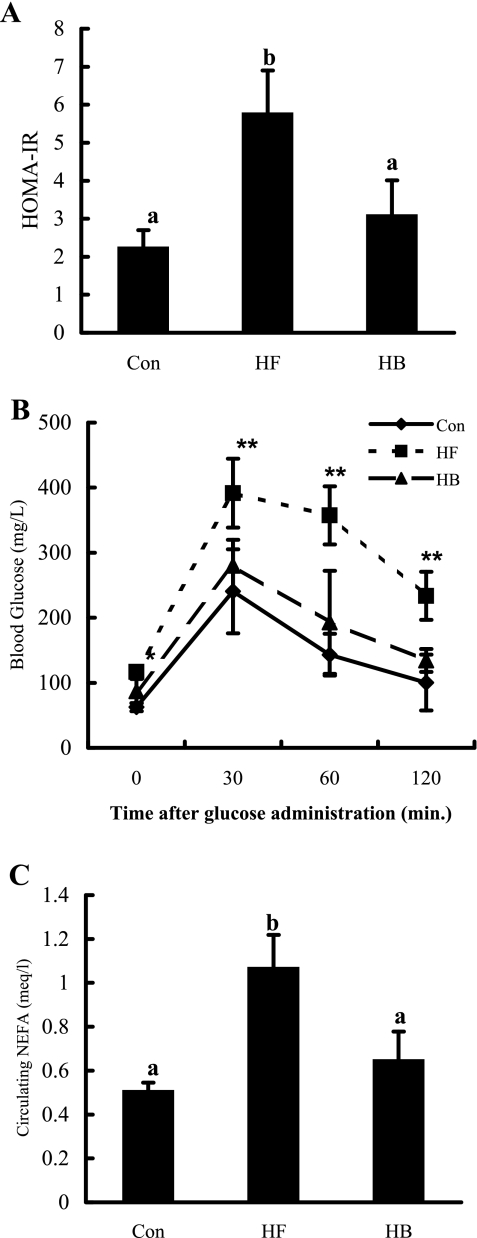
Betaine supplementation alleviated insulin resistance.
Insulin resistance plays a central role in the pathogenesis of NAFLD. To test whether the hepatoprotective effects of betaine supplementation are associated with the improved insulin resistance in the high-fat-fed mouse model, we evaluated insulin sensitivity by measuring three critical parameters: HOMA-IR, glucose tolerance test, and plasma NEFA levels. As shown in Fig. 3, high-fat diet feeding led to significantly higher HOMA-IR (Fig. 3A), severe hyperglycemia upon glucose administration, and impaired glucose tolerance (Fig. 3B), as well as significantly increased circulating NEFA levels (Fig. 3C), compared with control animals, which were consistent with previous studies. However, all of these alterations were alleviated through betaine supplementation.
Fig. 3.
Betaine supplementation alleviated insulin resistance in mice fed a high-fat diet. A: homeostasis model assessment of basal insulin resistance (HOMA-IR). B: glucose tolerance test. *P < 0.05 and **P < 0.01 compared with the control group or betaine supplementation group. C: circulating nonesterified free fatty acid (NEFA) levels. C57BL/6 mice were fed a high-fat diet with/without betaine supplementation (1%) in the drinking water for 12 wk. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05).
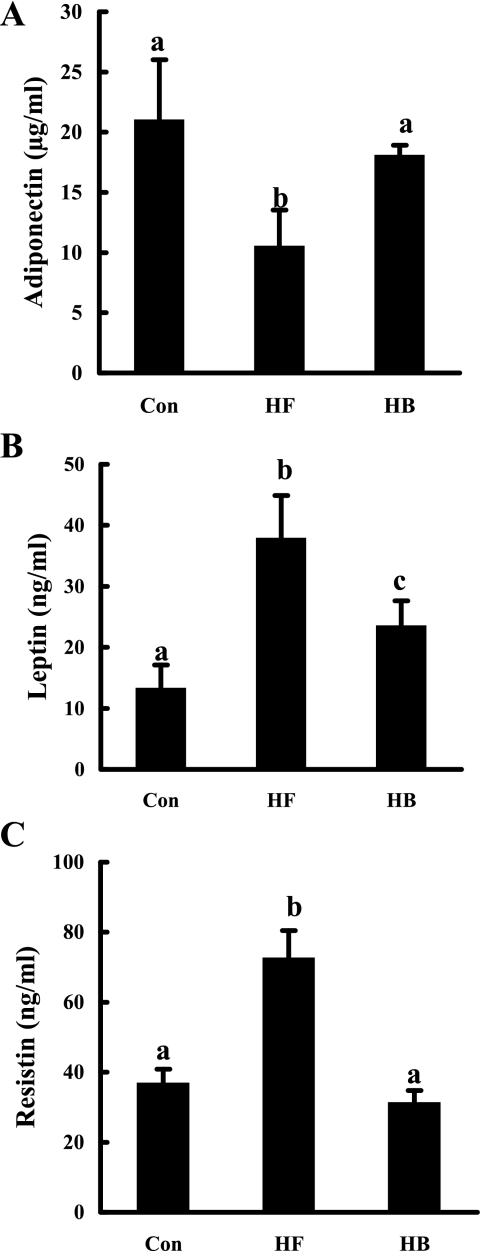
Betaine improved adipokine production.
To examine the effects of betaine supplementation on adipokine production, circulating levels of three adipokines, adiponectin, leptin, and resistin, were measured. As shown in Fig. 4, compared with the control group, high-fat diet feeding caused a significant decrease of plasma adiponectin levels (Fig. 4A), whereas leptin and resistin levels were increased significantly (Fig. 4, B and C). With betaine supplementation in the drinking water, all of these abnormalities were attenuated substantially.
Fig. 4.
Betaine supplementation improved adipokine production in mice fed a high-fat diet. A: circulating adiponectin levels. B: circulating leptin levels. C: circulating resistin levels. C57BL/6 mice were fed a high-fat diet with/without betaine supplementation (1%) in drinking water for 12 wk. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05).
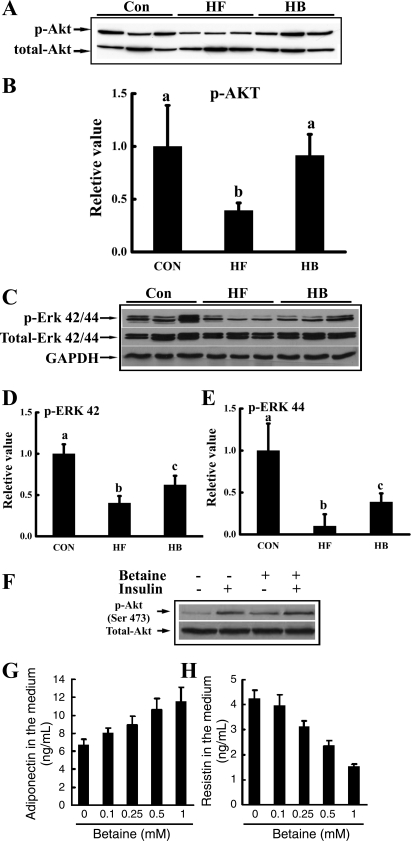
Betaine supplementation improved insulin sensitivity in adipose tissue.
We next examined the effects of betaine supplementation on insulin-signaling pathways in adipose tissue by Western blotting. As shown in Fig. 5, high-fat diet feeding impaired insulin signaling in adipose tissue, and this was illustrated by suppressed activations (phosphorylations) of both protein kinase B (Akt) (Fig. 5A) and extracellular signal-regulated kinases (ERK; Fig. 5B), two major downstream kinases in the insulin signaling pathway, in epididymal fat pad. With betaine supplementation, the suppressive effects of high-fat diet on both Akt and ERK activations were rescued. To further investigate the effect of betaine on insulin signaling in adipocytes, we then examined the effect of betaine on insulin-induced Akt phosphorylation status in primary adipocytes isolated from epididymal fat pads of mice fed the high-fat diet. Betaine pretreatment significantly improved insulin-stimulated Akt phosphorylation (Fig. 5C). Furthermore, compared with untreated adipocytes, betaine pretreatment increased adiponectin and decreased resistin productions from primary adipocytes, both in a dose-dependent manner (Fig. 5, D and E).
Fig. 5.
Betaine improved insulin signal transduction in adipose tissue both in vivo (A–E) and in vitro (F, G, and H). For in vivo studies, male C57BL/6 mice were fed a high-fat diet with/without betaine supplementation (1%) in the drinking water for 12 wk. Total protein extracts from epididymal fat pads were prepared thereafter. Protein (40 μg) was subjected to Western blot analysis for protein kinase B (Akt) and extracellular signal-regulated kinases (ERK) phosphorylation (p) using specific antibodies. A: betaine supplementation rescued adipose tissue Akt phosphorylation suppression induced by high-fat diet feeding. B: quantitation of Western blots of Akt phosphorylation levels in adipose tissues from different groups, normalized by corresponding total Akt expression levels (n = 8). C: betaine supplementation rescued ERK1/2 suppression induced by high-fat feeding. D and E: quantitation of Western blots of ERK42/44 phosphorylation levels in adipose tissues from different groups, normalized by corresponding total ERK42/44 expression levels (n = 8). Data are indicated as means ± SD. Bars with different letters differ significantly (P < 0.05). For in vitro investigation of insulin signaling, primary adipocytes from mouse fed a high-fat diet were isolated and cultured in a 6-well plate (1 × 106 cells/ml). Adipocytes were pretreated with 0.5 mM betaine for 2 h followed by stimulation with 10 nM insulin for 10 min. F: Akt phosphorylation status. For in vitro investigation of adipokine productions, primary adipocytes from mouse fed a high-fat diet were treated with different doses of betaine (0–1 mM) for 20 h. G: dose-response of adiponectin production. H: dose-response of resistin production. A total of three mice were used for the in vitro studies. Data are indicated as means ± SD from three independent batches of primary cultured high-fat diet adipocytes.
Betaine supplementation attenuated endoplasmic reticulum stress in adipose tissue.
Endoplasmic reticulum (ER) stress plays a critical role in adipose tissue dysfunction and insulin resistance in a variety of experimental models, including high-fat diet-fed mice (13, 42). To test if the observed beneficial effects of betaine supplementation in our study are related to its alleviative effects on ER stress response in adipose tissue, we measured three representative proteins induced/activated during ER stress, glucose-regulated protein 78 (GRP78), C/EBP homologous protein (CHOP), and c-Jun NH2-terminal kinase (JNK), in epididymal fat pad. As shown in Fig. 6, long-term high-fat diet feeding induced both GRP78 and CHOP protein productions and JNK activation, indicative of the ER stress response. With betaine supplementation, the abnormal changes in all three proteins were rescued, suggesting that alleviating ER stress may play an important role in betaine's beneficial effects on adipose tissue function.
DISCUSSION
We reported previously that betaine supplementation alleviated hepatic steatosis in mice fed a high-sucrose diet. Mechanistic studies revealed that activation of the hepatic AMPK system and subsequent inhibition of de novo lipogenesis contributed, at least partially, to betaine's hepatoprotective effects (37). In addition to AMPK activation in the liver, we also found, in that study, that betaine supplementation increased circulating adiponectin levels, whereas insulin and glucose levels were decreased, implying that betaine had the potential to improve adipose tissue function. In the present study, a long-term high-fat diet feeding mouse model of NAFLD was used to investigate the potential beneficial effects of betaine on adipose tissue function. Our results showed that betaine, when supplemented in the drinking water, significantly attenuated hepatic steatosis and injury, and these changes were associated with improved insulin sensitivity and adipokine production. Mechanistic investigations revealed that betaine supplementation alleviated the ER stress response in adipose tissue of mice fed a high-fat diet. Taken together, our results suggest that multiple mechanisms are involved in the hepatoprotective effects of betaine in NAFLD. Apart from acting on hepatocytes to activate the AMPK system, betaine also improved adipose tissue function.
Insulin resistance has been well-recognized to play a central role in the development of steatosis and the transitional process to steatohepatitis in NAFLD (16, 34, 40). Because of the strong relationship between insulin resistance and NAFLD, pharmacological treatment using insulin sensitizers, such as TDZs and metformin, has been shown to be beneficial in the prevention or improvement in NAFLD (22, 26–28, 33), although the varying degrees of side effects, specifically increases in body weight and body fat content (29, 35), have also been reported. There is currently no FDA-approved treatment for NAFLD or nonalcoholic steatohepatitis; therefore, searching for the safe compound with high preventive and/or therapeutic efficacies is urgently needed. In the present study, we demonstrated that long-term high-fat diet feeding resulted in significantly increased fat accumulation in the liver and plasma ALT levels, an indicator of liver injury. These changes were associated with increased body weight gain and systemic insulin resistance, which was manifested by significantly elevated HOMA-IR and circulating NEFA levels, as well as impaired blood glucose clearance compared with these in mice fed control diet. Betaine supplementation alleviated hepatic steatosis and lowered plasma ALT levels compared with the high-fat group, which were concomitant with improved HOMA-IR, circulating NEFA levels, and glucose tolerance test without affecting body weight changes and daily food consumptions, suggesting that betaine may act as an insulin sensitizer.
Although there had been some debates with regard to the primary site of insulin resistance in NAFLD (peripheral insulin resistance vs. hepatic insulin resistance), recent data indicate the periphery, primarily adipose tissue insulin resistance, to be the initial site, followed by hepatic steatosis, which in turn exacerbates the degree of insulin resistance (39). In normal circumstances, insulin inhibits NEFA release (lipolysis) from adipose tissues via suppressing hormone-sensitive lipase. As insulin resistance develops, insulin-mediated suppression of lipolysis is overwhelmed, and increased release of NEFA in the portal circulation ensues. Enhanced exposure and uptake of NEFA by the liver lead to not only excessive fat accumulation in the hepatocyte but also hepatic insulin resistance (6). Although beneficial effects of betaine in NAFLD were reported in both clinical and experimental studies (1, 17, 21, 24, 37), the underlying mechanisms remain to be clearly defined. Clinically, betaine supplementation improved aminotransferases and hepatic steatosis in nonalcoholic steatosis patients (1, 24). Experimentally, a couple of very recent studies showed that betaine alleviated fatty liver and liver injury by correcting abnormal hepatic methionine metabolism and improving hepatic insulin sensitivity in mice fed a high-fat diet (17, 21). Considering the critical role of adipose tissue in the initiation and progression of NAFLD, in present study, we examined the effects of betaine supplementation on insulin sensitivity in epididymal fat by detecting activations of Akt kinase and ERK, two major downstream kinases activated by insulin binding to its receptors. High-fat diet feeding decreased phosphorylated (p)-Akt and p-ERK42/44 protein abundance in epididymal fat pad, indicative of dampened insulin signal transduction in adipose tissue. Importantly, our study showed that suppression of both kinases was rescued by betaine supplementation in the drinking water, suggesting that betaine may act directly on adipose tissue to prevent insulin resistance induced by high-fat diet feeding.
Mechanism(s) involved in the induction of insulin resistance in adipocytes are multifactorial, with ER stress, specifically JNK activation, being well-established (13, 38, 41). ER stress is initiated by protein overload or misfolding in the ER. Cells cope with ER stress by an adaptive protective response termed unfolded protein response (UPR), which includes enhancing protein folding and degradation in the ER and downregulating overall protein synthesis. When the UPR adaption to ER stress is insufficient, the ER stress response unleashes pathological consequences. The link between ER stress and adipose tissue insulin resistance has been shown in a variety of experimental models, including high-fat diet-fed mice and ob/ob mice (12, 30, 30). Furthermore, treatment with orally active chemical chaperones reversed ER stress and insulin resistance in high-fat diet-fed mice (30), suggesting that the ER stress response plays a mechanistic role in adipose tissue insulin resistance. In the present study, we characterized the ER stress response in adipose tissue via Western blot detection of three representative proteins induced/activated by the ER stress response, GRP78, CHOP, and JNK. Among these proteins, JNK activation represents a critical link between the ER stress response and insulin resistance in that JNK can directly phosphorylate insulin receptor substrate (IRS)-1 at several sites, including serine-307 (2), and this modification leads to reduced insulin-stimulated IRS-1 tyrosine phosphorylation. In obese animals, deletion of the JNK gene reversed the obesity-induced increase in JNK activity and IRS-1 serine phosphorylation and protected against insulin resistance (13). In the present study, we showed that high-fat diet feeding increased protein productions of both GRP78 and CHOP and activation of JNK, which were in line with previous studies that high-fat diet feeding induced the ER stress response in adipose tissue (30). With betaine supplementation, the high-fat diet-induced ER stress response in adipose tissue was significantly alleviated. Our data clearly showed that betaine supplementation not only reduced GRP78 and CHOP abundance but also prevented JNK activation in the epididymal fat pad. Therefore, alleviation of the ER stress response in adipose tissue represents an important mechanism underlying the beneficial effects of betaine on adipose tissue function.
Apart from insulin resistance, another critical mechanism implicated in the pathogenesis of NAFLD is dysregulated adipokine production. Adipokines are implicated in the pathogenesis of NAFLD, through their metabolic and pro-/anti-inflammatory activity (9, 25). Accumulated evidence supports that decreased adiponectin levels are related to hepatic steatosis and inflammation in NAFLD (25). Leptin promotes insulin resistance and hepatocyte injury/fibrogenesis in cell cultures and animal models through activation of the transforming growth factor-β axis and stellate cells (10). In animal models, circulating resistin levels link adiposity and insulin resistance (31). In the present study, we measured circulating levels of three adipokines in high-fat diet-feeding mice with/without betaine supplementation. We found that long-term high-fat diet feeding significantly decreased circulating adiponectin levels, whereas leptin and resistin levels were significantly elevated compared with control animals. Importantly, we found that all changes were recovered by betaine supplementation, implying that betaine can prevent not only the early stage steatosis but also its transition to nonalcoholic steatohepatitis and fibrosis.
Betaine is a metabolite in the choline metabolism pathway and an important participant in methionine metabolism. Via a reaction catalyzed by betaine homocysteine methyltransferases, betaine can convert homocysteine to methionine, thereby preventing homocysteine accumulation. Although the beneficial effects of betaine supplementation on fatty liver diseases have been reported, exact mechanisms remain elusive. Correction of abnormal methionine metabolism seems to represent a major mechanism proposed by these studies. For instance, using a mouse model of alcoholic liver disease, Ji et al. (15) demonstrated that betaine prevented fatty liver and liver injury induced by chronic alcohol consumption through attenuating hyperhomocysteinemia and subsequent ER stress in the liver. Moreover, a very recent study demonstrated that betaine alleviated high-fat diet-induced NAFLD in rats by correcting abnormal hepatic methionine metabolism (21). Although we also showed that improved liver abnormalities by betaine supplementation were associated with improved hepatic methionine/homocysteine metabolism in a high-sucrose diet feeding model of NAFLD (37), our results revealed that betaine could directly activate the AMPK system in hepatocytes, implying that the mechanisms underlying the beneficial effects of betaine on NAFLD may be multifactorial.
Although the detailed mechanism(s) involved in the association between betaine supplementation and improvement of adipose tissue dysfunction remain to be fully understood, our data clearly demonstrate that long-term feeding of a high-fat diet to mice induced fatty liver and liver injury, which were associated with obesity, insulin resistance, and dysregulated adipokine production, and betaine supplementation in the drinking water alleviated hepatic steatosis and liver injury, which were associated with improved adipose tissue function, including insulin resistance and abnormal adipokine production. Our further investigations revealed that alleviation of the ER stress response represented a critical mechanism underlying the beneficial effects of betaine on adipose tissue function. Betaine is a nature component of many foods and safe in doses ranging from 3 to 30 g/day; therefore, unlike other insulin sensitizers with potential side effects, betaine may represent an ideal therapeutic agent for NAFLD. Our present study provides strong evidence for further evaluation of the potential therapeutic role of betaine.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants K01 AA-015344-01A1 (Z. Song), R01 AA-017442A (Z. Song), and AA-014623-01A2 (Z. Zhou).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol 96: 2711–2717, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17: S186–S190, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Barak AJ, Beckenhauer HC, Badakhsh S, Tuma DJ. The effect of betaine in reversing alcoholic steatosis. Alcohol Clin Exp Res 21: 1100–1102, 1997 [PubMed] [Google Scholar]
- 5.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 6.Carmen GY, Víctor SM. Signaling mechanisms regulating lipolysis. Cell Signal 18: 401–408, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis 8: 575–894, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Craig SA. Betaine in human nutrition. Am J Clin Nutr 80: 539–549, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gambino R, Cassader M, Pagano G, Durazzo M, Musso G. Polymorphism in microsomal triglyceride transfer protein: a link between liver disease and atherogenic postprandial lipid profile in NASH? Hepatology 45: 1097–1107, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Haussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology 36: 829–839, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gregor MG, Hotamisligil GS. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48: 1905–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 14.James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol 29: 495–501, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124: 1488–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 28: 370–399, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kathirvel E, Morgan K, French SW, Morgan TR. Betaine reduced insulin resistance and hepatic steatosis, and augments hepatic insulin signaling, in an animal model of NAFLD (Abstract). Hepatology 48: 486A, 2008 [Google Scholar]
- 18.Kharbanda KK, Rogers DD, 2nd, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol 70: 1883–1890, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 74: 4734–4740, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kosone T, Takagi H, Horiguchi N, Ariyama Y, Otsuka T, Sohara N, Kakizaki S, Sato K, Mori M. HGF ameliorates a high-fat diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G204–G210, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kwon do Y, Jung YS, Kim SJ, Park HK, Park JH, Kim YC. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139: 63–68, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet 358: 893–894, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Miglio F, Rovati LC, Santoro A, Setnikar I. Efficacy and safety of oral betaine glucuronate in non-alcoholic steatohepatitis. A double-blind, randomized, parallel-group, placebo-controlled prospective clinical study. Arzneimittelforschung 50: 722–727, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Rabbione L, Premoli A, Cassader M, Pagano G. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 42: 1175–1183, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nair S, Diehl AM, Perrillo RP. Metformin in non-alcoholic steatohepatitis (NASH): efficacy and safety–a preliminary report (Abstract). Gastroenterology Suppl 122: 4, 2002 [Google Scholar]
- 27.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther 20: 23–28, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38: 1008–1017, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palanivel R, Maida A, Liu Y, Sweeney G. Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia 49: 183–190, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105: 9793–9798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 39: 188–196, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 13: 3540–3553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Pioglitazone for type 2 diabetes mellitus, Cochrane Database Syst Rev 18: CD006060, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto A, Nishimura Y, Ono H, Sakura N. Betaine and homocysteine concentrations in foods. Pediatr Int 44: 409–413, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, McClain CJ. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 293: G894–G902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniguchi CM, Aleman JO, Ueki K, Luo J, Asano T, Kaneto H, Stephanopoulos G, Cantley LC, Kahn CR. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol Cell Biol 27: 2830–2840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 131: 934–1945, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA 103: 10741–10746, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133: 1302–1307, 2003 [DOI] [PubMed] [Google Scholar]