Abstract
The injurious effect of nonsteroidal anti-inflammatory drugs (NSAIDs) in the small intestine was not appreciated until the widespread use of capsule endoscopy. Animal studies found that NSAID-induced small intestinal injury depends on the ability of these drugs to be secreted into the bile. Because the individual toxicity of amphiphilic bile acids and NSAIDs directly correlates with their interactions with phospholipid membranes, we propose that the presence of both NSAIDs and bile acids alters their individual physicochemical properties and enhances the disruptive effect on cell membranes and overall cytotoxicity. We utilized in vitro gastric AGS and intestinal IEC-6 cells and found that combinations of bile acid, deoxycholic acid (DC), taurodeoxycholic acid, glycodeoxycholic acid, and the NSAID indomethacin (Indo) significantly increased cell plasma membrane permeability and became more cytotoxic than these agents alone. We confirmed this finding by measuring liposome permeability and intramembrane packing in synthetic model membranes exposed to DC, Indo, or combinations of both agents. By measuring physicochemical parameters, such as fluorescence resonance energy transfer and membrane surface charge, we found that Indo associated with phosphatidylcholine and promoted the molecular aggregation of DC and potential formation of larger and isolated bile acid complexes within either biomembranes or bile acid-lipid mixed micelles, which leads to membrane disruption. In this study, we demonstrated increased cytotoxicity of combinations of bile acid and NSAID and provided a molecular mechanism for the observed toxicity. This mechanism potentially contributes to the NSAID-induced injury in the small bowel.
Keywords: bile acids, NSAIDs, phospholipid membrane
the ability of nonsteroidal anti-inflammatory drugs (NSAIDs) to cause small intestinal injury in patients has been highlighted by the use of capsule endoscopy in only recent years (13, 16, 20–22). It is estimated that 80% of regular NSAID users develop small intestinal injury (even more common than NSAID-induced injury in the gastroduodenal mucosa) (13, 16, 20, 21). Furthermore, chronic use of NSAIDs is one of the main contributors to small bowel ulceration, hemorrhage, and strictures (1, 22, 40). The ability of NSAIDs to inhibit activities of cyclooxygenase (COX) contributes to the drugs' cytotoxicity in the gastrointestinal (GI) tract (44, 45). However, many studies found that COX-independent mechanisms also contribute to NSAID cytotoxicity in the GI tract (9, 28, 37, 38, 46, 48). Animal studies demonstrated that bile acids, which enter the small bowel as a component of enterohepatic circulation, play an important role in the ability of NSAIDs to induce small intestinal injury, regardless whether the drugs are administered orally, intravenously, or by enema (12, 37, 38, 46, 49). The contribution of bile to the pathogenic process was highlighted by the finding of Yamada et al. (49) that the ability of indomethacin (Indo) to induce severe injury and perforation to the distal small bowel of rats was prevented by bile duct ligation, which halted secretion of bile acids into the small intestine. A striking correlation between the ability of an NSAID to be secreted into the bile and its efficacy to induce small intestinal injury in rodent model systems has been found (2, 37, 38, 46). Furthermore, if one focused on one type of NSAID (Indo) and compared the cytotoxicity of bile collected from a number of animal species after it was systemically administered, the most injurious bile was found in a species that secreted high concentrations of Indo into the bile via enterohepatic circulation (dog), and the least toxic bile was recorded in a species (rabbit) that had negligible levels of the NSAID (12). The ability of combinations of bile acids and NSAIDs to induce injury has also been demonstrated in cell cultures (10, 37, 38, 46). This leads to the question of whether the NSAID induces injury because it interacts with and changes the balance among the organic constituents of bile (e.g., bile acids, phospholipids, and cholesterol).
Bile acids are cytotoxic because of their detergent effects, which allow these molecules to associate with phospholipids and alter membrane integrity directly (3, 4, 50). Coincidentally, NSAIDs are also highly amphiphilic and associate strongly with phospholipids (28–30). In both laboratory animal and clinical studies, NSAIDs were found to attenuate the hydrophobic barrier properties of the upper GI tract, which is, in part, established by the surface-active phospholipids. Specifically, aspirin and other NSAIDs induced a rapid and dose-dependent decrease in the surface hydrophobicity of the mammalian gastric mucosa under both in vitro and in vivo conditions (17–19, 26, 28). The biomechanical technique of micropipette aspiration also demonstrated that salicylate associates with phosphatidylcholine (PC) and alters the integrity of the lipid bilayer (28, 30). Thus it is possible that NSAIDs secreted into the bile interact with amphipathic components in the bile, such as PC and bile acids. This close association potentially leads to alterations in the stability and structure of these components and consequentially may change the toxicity of bile acids in the small intestine.
In this study, we utilized both in vitro cell cultures and synthetic model membranes to examine the ability of a potent NSAID, Indo, to alter cytotoxicity of a hydrophobic and highly toxic bile acid, deoxycholic acid (DC) and its conjugated counterparts taurodeoxycholic acid (TDC) and glycodeoxycholic acid (GDC). A series of fluorescence techniques were used to deduce the molecular mechanism by which NSAIDs alter PC-bile acid association and influence bile acid toxicity.
MATERIALS AND METHODS
Materials.
Indo, DC, TDC, GDC, and powdered phosphate buffer solution (PBS) were purchased from Sigma-Aldrich (St. Louis, MO). Dilinoleoyl PC (di18:2 PC, or DLPC) and phosphatidylethanolamine (PE) rhodamine were purchased from Avanti Polar Lipids (Alabaster, AL). Purified soy PC, Phospholipon 90G, was purchased from Lipoid (Newark, NJ). Lipids were dissolved in chloroform and stored at −20°C under nitrogen. Lactate dehydrogenase (LDH) assay kit and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Nitrobenz-2-oxa-1,3-diazyl-4-yl covalently linked to 3α-hydroxy-5β-cholan-24-oic acid (lithocholic acid-NBD) was a gift kindly provided by Dr. Alan F. Hofmann. Hydrophilic probe calcein and the membrane potentiometric probe 4-[2-[6-(dioctylamino)-2-naphthalenyl]ethenyl]-1-(3-sulfopropyl)-pyridinium (di-8-ANEPPS) were purchased from Molecular Probes. AGS cells were provided by the Texas Medical Center Digestive Disease Center, and the IEC-6 cells were provided by the University of Texas Trauma Research Center.
Gastric AGS cell cultures: cell plasma membrane damage (LDH) and survival (MTT).
AGS cells were cultured to 85–90% confluency in Ham's F-12K culture medium containing 10% FBS in a 24-well plate. IEC-6 cells were cultured in DMEM containing 10% FBS. Cells were washed with PBS buffer three times and incubated in Ham's medium (no FBS) (or DMEM in the case of IEC-6 cells) containing test agents, which include various concentrations of DC alone, DC with soy PC, Indo, or both DC and Indo. A 50 mM Indo stock was prepared in PBS and diluted in appropriate media for experiments. After 3 h of incubation, leakage of AGS/IEC-6 cell cytosolic enzyme LDH into the medium was tested by using LDH assay kit from Sigma-Aldrich (32).
Cell survival was tested by measuring levels of MTT incorporation into cells. After 3-h incubation, cells were washed with PBS and then exposed to fresh medium containing ∼0.5% MTT for 2 h. The MTT-containing medium was then discarded and cells were solubilized with MTT-solvent mix (0.2 mM SDS, 1 ml 1 N HCl, and 90 ml isopropanol in 100 ml solution). Absorbance of MTT was then measured at 570 nm (32).
Synthetic liposomes.
Approximately 20 mg PC lipids in chloroform were dried under nitrogen gas and then exposed to vacuum overnight to completely evaporate chloroform. The lipid film was rehydrated with ∼4 ml of PBS at pH 7.4 and incubated at room temperature under nitrogen gas for at least 30 min. Lipid solution was then extruded across a polycarbonate filter with 100 nm pores to form large unilamellar vesicles (LUVs) in a Mini-extruder setup (Avanti Polar Lipids). The average size of vesicles was verified to be ∼120 nm by dynamic light scattering (data not shown). In all experiments, liposomes were incubated for 30 min in PBS buffer containing test agents before measurements.
Dipole potential measurements.
The membrane dipole potential was obtained by incorporating a low level (∼0.2 mole%) of a voltage sensitive fluorescent probe, di-8 ANEPPS, which shifts its excitation spectrum when exposed to different dipole environments within the membrane (5–8, 42, 43). Approximately 50 μl of 1 mg/ml di-8 ANEPPS (in 100% ethanol) was added to the liposome solution (4 ml) to achieve a lipid-to-probe ratio of ∼400:1. The mixture was incubated overnight under nitrogen gas. The small amount of the probe was utilized to ensure that the overall membrane properties of liposomes are not affected by the presence of the probe. Moreover, a large lipid-to-probe ratio, long incubation time, and high affinity of the probe for hydrophobic environment within lipid membranes also guarantee that all probes partition into the membrane. Spectral measurements were performed via a SpectraMax Gemini X spectrophotometer (Molecular Devices, Sunnyvale, CA). The excitation spectra between 400 nm and 550 nm were recorded with the emission wavelength fixed at 680 nm (5, 8, 52). The ratio (F420/520) of fluorescence intensities at 420 and 520 nm of the excitation spectrum was then directly correlated with the dipole potential, Ψd (5, 8):
| 1 |
Liposome membrane permeability to calcein.
The calcein permeability technique has been well established to determine membrane permeability (47). Approximately 6 mg of DLPC in chloroform was dried under nitrogen gas and then vacuum overnight to completely evaporate chloroform. The lipid film was rehydrated with ∼2 ml of Tris buffer containing ∼4.5 mM calcein. The mixture was vortexed and sonicated for 10 min to form liposomes with high concentrations of calcein encapsulated inside. Liposome solution was then passed through a Sephadex G-50 column to separate liposomes (with encapsulated calcein) from free calcein in the bulk solution. After 30-min incubation with testing solutions, membrane leakage of calcein was indicated by an increase in the fluorescent intensity of the self-quenching calcein (excitation 472 nm; emission 516 nm). Total fluorescent intensity of calcein was obtained by adding ∼0.5% Triton X-100 to lyse all liposomes at the end of each experiment. The percent calcein leakage was calculated:
| 2 |
where Ft is the fluorescent intensity of a liposome solution exposed to testing solutions, Fo is the control fluorescent intensity of a liposome solution before being exposed to bile acid solutions, and Ftotal is the maximally available fluorescent intensity obtained after exposure to Triton X-100.
FRET.
The technique of fluorescence resonance energy transfer (FRET) was used to characterize molecular association between DC, PC, and Indo. In experiments involving PC-DC mixed micelles, various amount of DC was mixed with DLPC to achieve physiological molar ratios of either 3:1 or 6:1 (DC-PC) in 1:1 methanol-chloroform, then spiked with either lithocholic acid-NBD alone or both lithocholic acid-NBD and dioleoyl PE (DOPE)-rhodamine at 0.5 mole%. Mixtures were sonicated and methanol-chloroform solvents were evaporated under nitrogen. The bile acid-PC mix was then exposed to vacuum overnight to completely evaporate solvents. PBS buffer was added to achieve various bile acid concentrations that are physiologically significant. Fluorescence intensity of NBD in the presence or absence of the acceptor DOPE-rhodamine was measured at room temperature by using a QuantaMaster UV/VIS spectrofluorometer (Photon Technology International, Birmingham, New Jersey) at excitation wavelength of 472 nm and emission wavelength of 538 nm. The efficiency of energy transfer was calculated using the equation E = (ID − ID−A)/ID, where ID is the intensity of NBD in sample containing only donor probe and ID-A is the intensity of NBD in sample containing both donor and acceptor probes.
Membrane ζ-potential measurements.
The ζ-potential was obtained by measuring the electrophoretic mobility of LUVs. as described before (52). The electrophoretic mobility measurements were obtained by use of a Malvern Zetasizer Nano (Worcestershire, UK). The ζ-potential was calculated from the mobility measurements automatically by the instrument utilizing the Smoluchowski equation. Two to three batches of different vesicle sets were utilized to obtain the ζ-potential measurements.
Statistical analysis.
Student's t-test was performed as statistical analyses for all comparisons in this study.
EXPERIMENTAL RESULTS
Presence of Indo increased cytotoxicity of sodium DC and diminishes the ability of PC to protect AGS cells against DC.
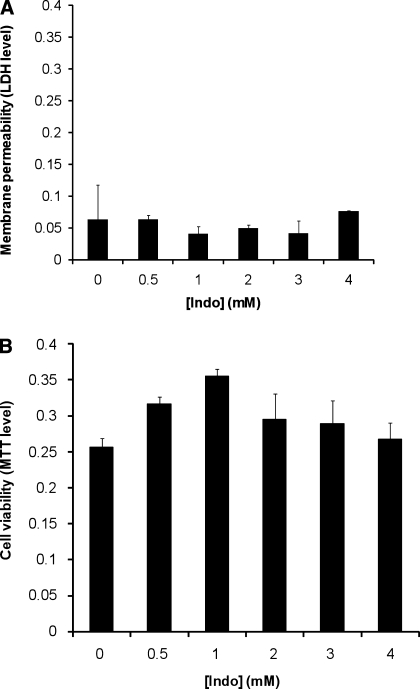
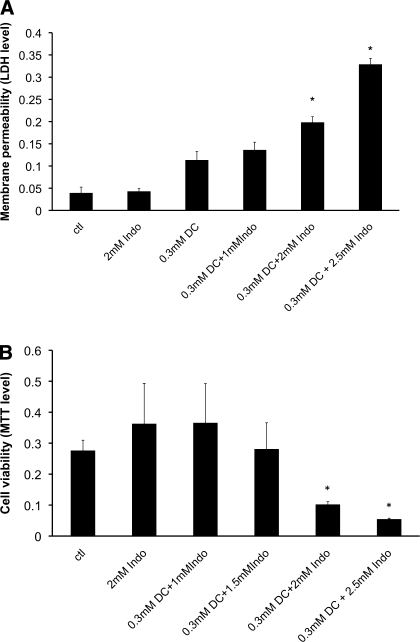
Gastric AGS cells cultured to 85–90% confluency were incubated in Ham's F12K medium containing various concentrations of Indo for 3 h. Cell plasma membrane permeability (indicated by leakage of cytosolic enzyme LDH into the medium) and cell survival (incorporation of MTT into the cell) were measured. We found that Indo at concentrations up to 4 mM did not alter the level of cytosolic enzyme LDH in the medium (Fig. 1A),suggesting that Indo at these concentrations had no detectable effect on AGS cell plasma membrane permeability. Our cell viability test also demonstrated that Indo at these concentrations had no detectable effect on the MTT levels incorporated into AGS cells (Fig. 1B), indicating that Indo at these concentrations did not affect cell survivability and is nontoxic. These nontoxic doses of Indo dose dependently increased the LDH level in the medium containing mildly cytotoxic 0.3 mM DC (Fig. 2A), indicating an increasing level of plasma membrane permeability. The MTT cell survival test also demonstrated that a combination of the noninjurious 2 mM Indo with 0.3 mM DC significantly decreased the cell number (Fig. 2B). These measurements strongly suggest that the injurious effects of DC and Indo were significantly higher than the additive effect of two individual agents.
Fig. 1.
A: effect of indomethacin (Indo) alone on the permeability of gastric AGS cell plasma membrane. Cells were incubated in Ham's medium containing various concentrations of Indo for 3 h. Level of cytoplasmic enzyme lactate dehydrogenase (LDH) in the medium, an indication of cell plasma membrane leakage, was measured. The LDH level is here quantified by the absorbance level at wavelength of 490 nm normalized by subtracting absorbance at 690 nm, which were plotted against Indo concentrations. B: effect of Indo on cell viability measured by the level of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) incorporated into cells. After Indo treatment, medium containing Indo was discarded and cells were incubated in fresh medium containing 0.5% MTT for 2 h. MTT in the cells was then dissolved in MTT-solvent mix and MTT levels were quantified as absorbance at 570 nm, which were plotted against Indo concentrations.
Fig. 2.
A: dose effect of Indo on the ability of deoxycholic acid (DC) to alter LDH level found in the medium, an indicator of membrane permeability. *Statistical significance vs. either 0.3 mM DC or 2 mM Indo alone. B: dose effect of Indo on the ability of DC to alter gastric AGS cell number. *Statistical significance vs. either 0.3 mM DC or 2 mM Indo alone. ctl, Control.
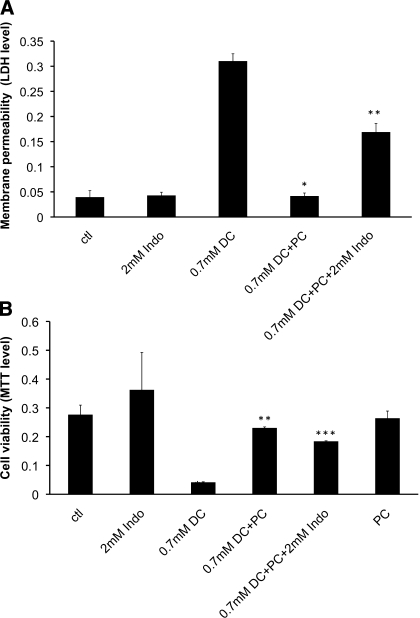
Furthermore, 0.7 mM DC was found to be highly damaging to the plasma membrane of AGS cells, indicated by high level of LDH detected in the medium (Fig. 3A) and low level of MTT (Fig. 3B). The presence of 2.8 mM purified soy PC (Phospholipon 90G) mixed with 0.7 mM DC was found to significantly decrease the level of LDH (Fig. 3A) and increase the MTT level (Fig. 3B), indicating that PC is able to effectively protect cells against toxic effect of the bile acid. Addition of 2 mM Indo partially reversed the protective effect of PC and caused a significant increase in membrane permeability of AGS cells (higher LDH level) (Fig. 3A) and observable decrease in cell number (Fig. 3B). These experiments suggest that Indo has detrimental effect on bile acid cytotoxicity and this effect is not related to its own toxicity since Indo alone at concentrations tested was nontoxic. It is then possible that a new type of macromolecule is formed when Indo aggregates with DC and PC.
Fig. 3.
A: detrimental effect of Indo on the protective effect of purified soy phosphatidylcholine (PC) on cell plasma membrane permeability as indicated by LDH levels found in the medium. *Statistical significance vs. 0.7 mM DC; **statistical significance vs. 0.7 mM DC + 2.8 mM PC. B: ability of Indo to diminish the protective effect of PC on cell viability as indicated by levels of MTT incorporated into cells. *Statistical significance over control; **statistical significance vs. 0.7 mM DC; ***statistical significance vs. 0.7 mM DC + 2.8 mM PC.
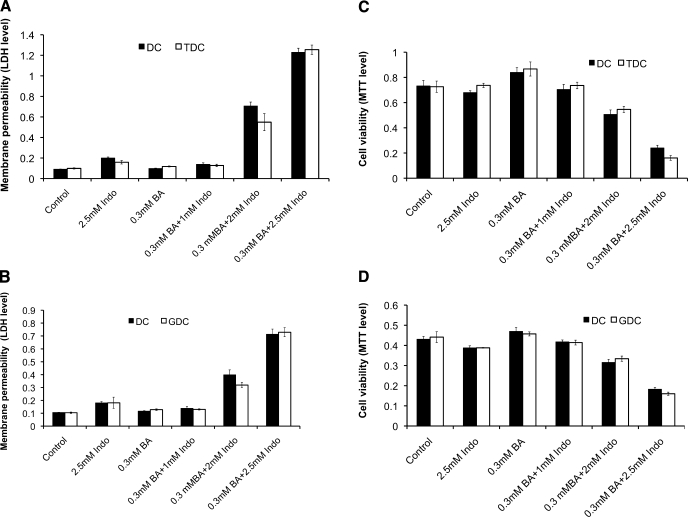
We also examined the effect of Indo and DC on intestinal epithelial (IEC-6) cells (Fig. 4).We found that Indo, DC, and the combinations of both have very similar effect on IEC-6 cells as AGS cells. Specifically, DC or Indo alone had limited effect on LDH and MTT levels of IEC-6 cells. Combinations of both agents markedly increased the LDH level, suggesting an increase in membrane permeability, and decreased the MTT level, indicating significantly lowered cell viability.
Fig. 4.
Combined effect of Indo and conjugated bile acids (BA), taurodeoxycholic acid (TDC) and glycodeoxycholic acid (GDC), on intestinal epithelial IEC-6 cell plasma membrane permeability and cell viability. Similar effects were found as in AGS cells. IEC-6 cell plasma membrane permeability measured by LDH level in the cell medium was significantly increased by combining TDC and Indo (A) or GDC and Indo (B) compared with treatments with these agents alone. Cell viability measured by MTT incorporation into cells was markedly decreased by the combination of TDC and Indo (C) or GDC and Indo (D). A bile acid concentration of 0.3 mM was used in all bile acid-Indo combined treatments.
Because significant amount of bile acids in the small bowel is conjugated with either glycine or taurine, we tested possible effects of Indo on the toxicity of conjugated bile acids, GDC, or TDC, with or without Indo in IEC-6 cells. We found that the effect of Indo on TDC and GDC was found to be similar to the case of unconjugated deoxycholic acid (Fig. 4). Specifically, in the presence of Indo, both TDC and GDC were found to be significantly more toxic than either in the absence of Indo.
Effects of DC-Indo combination on permeability of model membranes.
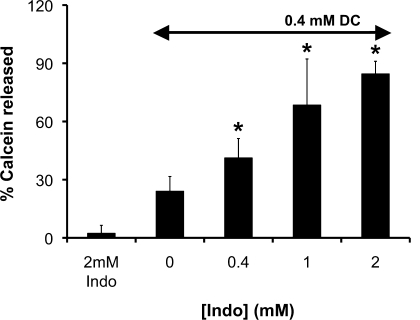
In an effort to better understand the ability of Indo to alter toxic effect of DC on phospholipid membrane, we utilized model liposomes to test the ability of Indo and DC to induce membrane permeability. This was achieved by monitoring membrane leakage of a hydrophilic fluorescence probe, calcein, incorporated into the liposomes composed of purified soy PC. Since calcein self-quenches at close distance or at high concentrations, these probes display minimal fluorescent intensity when entrapped inside of liposomes. When a membrane is damaged, calcein molecules permeate across the membrane and enter the bulk solution. The consequential significant dilution of the probes will cause the calcein fluorescent intensity to increase. Thus measuring the increase in calcein fluorescence intensity is an excellent way to monitor the level of membrane leakage and damage. We found that Indo (2 mM) alone caused little damage whereas DC (0.4 mM) alone led to a low level of permeation of purified PC liposomal membranes (Fig. 5). However, combining the two agents at the same concentrations led to significant and more-than-additive increase in the calcein intensity, indicating marked calcein leakage out of the liposomes, in agreement with our cell culture data. This set of experiments further indicates that a combination of NSAID with bile acids becomes highly damaging to cell membranes.
Fig. 5.
The amount of encapsulated calcein that permeated out of dilinoeoyl phosphatidylcholine (DLPC) liposomes was significantly increased in the presence of both DC and Indo, indicating the ability of a combination of DC and Indo to destabilize PL membranes. *Statistical significance vs. 0.4 mM DC + Indo.
Effect of DC and Indo combination on lateral packing of lipids in phospholipid membrane.
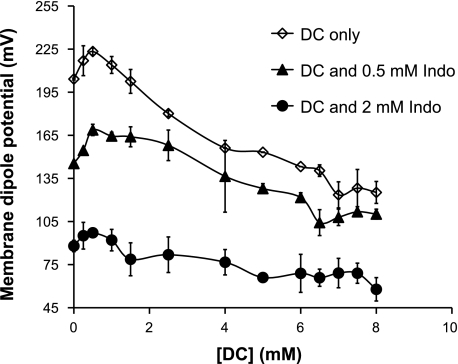
To deduce the molecular mechanism for this Indo-induced damage to phospholipid membranes, we measured the ability of DC alone or DC combined with Indo to alter intramembrane properties, such as the membrane dipole potential. Lipids in an undamaged bilayer membrane align with each other in an orderly fashion, where all molecular dipoles also align with each other. This ordered alignment gives a large dipole potential, which is the sum of molecular dipoles within the membrane. When the membrane is damaged, however, lipids change alignment and molecular dipoles within the membrane begin to cancel each other out. This leads to a decrease in the dipole potential. Hence, the change in the membrane dipole potential is an excellent indicator of disruption in the membrane (5–8, 42, 43). We first examined the ability of DC to alter the intramembrane dipole potential of liposomal membranes composed of DLPC (di18:2 PC), which is the main ingredient of the purified soy PC and many biological membranes. DC alone dose dependently decreased the dipole potential of DLPC liposomes (Fig. 5), indicating that DC disrupts the alignment of molecular dipoles of lipids and promotes the disorganization of membranes. Addition of Indo at 0.5 or 2 mM led to a significant downward shift of the dose curve (Fig. 6), suggesting that the presence of Indo and DC caused more disordering in the membrane than DC alone. It is also interesting that Indo alone in the absence of DC also decreased the membrane dipole potential (Fig. 6). These studies demonstrate that bile acids (DC) and NSAIDs (Indo) synergize to disorganize membrane packing and thereby integrity.
Fig. 6.
DC and Indo dose dependently decreased the membrane dipole potential, indicating that the combination of DC and Indo negatively affects the packing density of DLPC membrane and thereby integrity.
Use of FRET to deduce the molecular mechanism of DC/Indo toxicity.
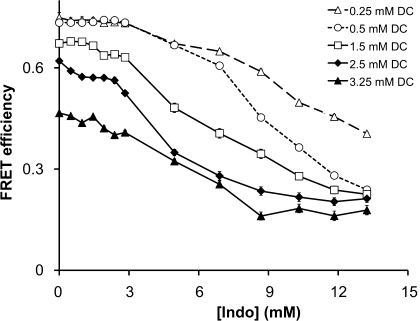
To examine the molecular mechanism by which NSAIDs interact with bile acids and phospholipids, we utilized FRET, which characterizes the effect of NSAID-bile acid-phospholipid association. This technique utilizes two fluorescent probes that form an energy transfer pair, which is an excellent “molecular ruler” for measuring distance between two labeled particles (27). Approximately 2.5 mg of DLPC liposomes was labeled with ∼0.5 mole% of PE rhodamine, which was then mixed with various concentrations of DC (from 0.25 to 3.25 mM) spiked with equal amount of lithocholic acid covalently linked to NBD (less than 0.5 mole% of total bile acid level). Since probes NBD and rhodamine have been established to be highly effective FRET pair, we were able to examine PC-bile acid association by monitoring energy transfer between the two probes. FRET efficiency was then monitored as a function of increasing Indo concentration.
We found that increasing concentrations of DC alone led to a decrease in FRET efficiency (see the y-intercept in Fig. 7). This trend was found to be continuous from DC monomeric concentrations below its critical micellar concentration [CMC; 3 mM in a solution with an ionic strength of 150 mM (39)] to micelles at concentrations greater than 3.25 mM. The observed decrease in interaction between PC and the increasing concentrations of the bile acid supports the formation of isolated bile acid complexes within the bilayer (see discussion). This finding is in good agreement with previous findings in the literature proposing that bile acid monomers form isolated aggregates, called reversed micelles, within lipid bilayers (31, 33, 41) and that bile acid/PC mixed micelles also contain isolated bile acid complexes segregated from the surrounding lipids (33, 34). At all DC concentrations tested, Indo was able to further decrease FRET efficiency between PC and DC in dose-dependent manner, suggesting that Indo weakens association between DC and PC (Fig. 7). As discussed in more detail in the next section, our data suggest that Indo promotes the formation of isolated bile acid aggregates forming in the PC membrane or mixed PC-bile acid micelles.
Fig. 7.
Indomethacin (Indo) was added to a mixture of DC and dilinoleoyl PC (DLPC) liposomes. Energy transfer between bile acid and PC was weakened in the presence of Indo. FRET, fluorescence resonance energy transfer.
As suggested by our dipole potential and membrane permeability experiment, Indo partitions into the membrane and loosens the lipid packing. This general disruption of the membrane should not affect the observed changes in FRET efficiency in our study. This is because, in another study, we demonstrated that Indo had no effect on the energy transfer between NBD-PE and rhodamine-PE incorporated in PC liposomes (51). This means that the Indo-induced increase in distance between the probes does not change FRET efficiency. Thus the observed changes in FRET efficiency between the PC and the bile acid were likely caused by the possible formation of isolated aggregates.
Changes in liposome surface charges indicate that both Indo and DC bind with PC liposomes.
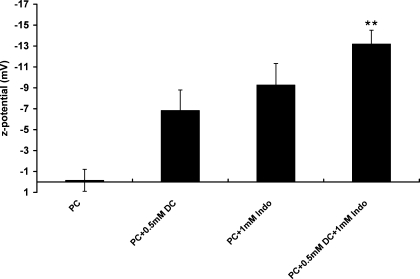
Weakened energy transfer between bile acid and PC can also be caused by dissociation of bile acid molecules from PC liposomes. To examine this possibility and further understand the binding of Indo molecules to PC membranes, we measured changes in surface charges of PC liposomes. Because both DC and Indo are weak acids and are negatively charged at neutral pH, changes in PC liposome surface charges indicate binding of these charged amphiphiles. Membrane ζ-potential is a measure of apparent surface electrical properties of phospholipid bilayers (35). We found that the ζ-potential of zwitterionic purified soy PC alone was nearly zero (Fig. 8), indicating that the PC bilayer was neutral in PBS buffer at pH 7.4. The presence of 0.5 mM DC alone caused the ζ-potential to decrease to −7 mV (Fig. 8), indicating significant association of DC with PC lipids. Addition of 1 mM Indo alone decreased the ζ-potential to −9 mV. When the PC liposomes were exposed to both 0.5 mM DC and 1 mM Indo, the ζ-potential was significantly decreased to −13 mV (Fig. 8), approximately the sum of the ζ-potential values of PC liposomes exposed to DC alone and Indo alone. This strongly suggests that DC is still associated with PC liposomes and Indo molecules also bind with the bilayer.
Fig. 8.
Zeta (ζ) potential of purified soy PC liposomes was measured in the presence of either DC alone, Indo alone, or the combination of both. Both DC and Indo alone caused the PC membrane to become negatively charged since both amphiphiles are weak acids and possess negative charge at neutral pH. The ζ-potential of PC bilayer exposed to both amphiphiles was found to be approximately the sum of the ζ-potential of PC membrane exposed to either amphiphile alone, indicating the association of both types of molecules with PC membrane. **Statistical significance vs. PC + 0.5 mM DC.
DISCUSSION
In this study, we utilized in vitro cell cultures and model liposomes to demonstrate that combinations of the bile acids, such as DC, TDC, or GDC, and NSAIDs such as Indo are significantly more injurious than one of these agents alone. Although a significant portion of Indo molecules in the GI tract is glucuronidated, only the unconjugated Indo was tested in this study because a large portion of the Indo molecules is secreted into the small intestine as a component of enterohepatic circulation, where bacteria deconjugate Indo molecules (25, 36). Thus examining the unconjugated Indo is still important physiologically. Evidence has been obtained by our group and those of other investigators that the cytotoxicity of both bile acids and NSAIDs is attributable, in part, to their interactions with phospholipid membranes because of their amphiphilic structures (3, 11, 14, 15, 30, 37, 38, 46). In exposure to both bile acid (DC, TDC, or GDC) and Indo, a simple additive effect would suggest that both membrane-damaging agents are present at the same time, but not necessarily interacting with each other. Here, however, we found that with exposure to both bile acid (DC, TDC, or GDC) and Indo the damaging effect on gastric AGS cells, intestinal IEC-6 cells, and liposomes was significantly more than an additive effect, suggesting that physicochemical properties, such as molecular aggregation and association, of these agents may be altered when present together. This change in physicochemical properties potentially leads to alteration in toxicity of these agents, observed in our cell culture experiments, which were also confirmed in liposome experiments. Liposome permeability experiments demonstrated that combinations of DC and Indo led to significant disruption of the model membrane, the level of which was higher than added effects of single agent on liposomes. Similarly, the intramembrane dipole potential, an indicator of membrane integrity, was also significantly decreased in the presence of both DC and Indo. This series of in vitro cell culture and model membrane experiments supports our argument that combination of DC and Indo is more injurious to phospholipid membranes than individual components.
Indo alters molecular association of bile acid and PC.
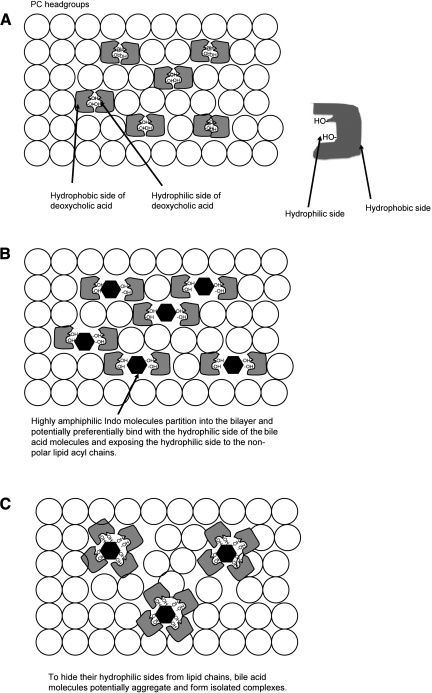
The significantly increased toxicity of combinations of Indo and DC may arise from altered physicochemical interactions among involved factors. Since the toxicity of either DC or Indo is correlated with their ability to associate with phospholipids, such as PC, which is a major constituent of cell membranes, we tested whether Indo affects association of PC and DC in model membranes. The use of model membranes, instead of biological cell membranes, was intended for better control of the test system and for clearer data interpretation. We found that FRET efficiency between the bile acid and PC was decreased as DC concentration was increased, suggesting weaker interaction between the particles. This seems counterintuitive at first: more DC should enhance the binding between the bile acid and PC since DC is hydrophobic and prefers associating with PC. However, it has been established that bile acids and PC do not form a homogeneous mixture either within the membrane when bile acid concentration is below its CMC, or in mixed micelles when bile acid concentration is above its CMC (3, 33, 34). Bile acids as a class possess “two faces,” a hydrophobic side and a hydrophilic side with respect to the long axis of the molecule (Fig. 9A) (24). Bile acid molecules typically partition into a phospholipid bilayer vertically with their long axes aligned parallel with the acyl chains of lipids (3, 33, 34). Exposure of the hydrophilic side of the bile acid molecule to the surrounding lipid environment is unfavorable because the bilayer core is highly hydrophobic. Two bile acid molecules will have a tendency to aggregate to form a dimer, termed reverse micelle, within the lipid bilayer (Fig. 9A). In these dimers, hydrophilic portions of the bile acid molecules are hidden inside of the complex, away from the highly nonpolar environment in the phospholipid bilayer, while exposing the hydrophobic side to the lipid bilayer core (31, 33, 41). The higher the concentration of DC, the more bile acid molecules incorporated into the bilayer will induce the formation of more isolated bile acid aggregates and the population of PC lipids in direct contact with DC molecules will decrease (3, 33, 34), thus yielding less FRET efficiency.
Fig. 9.
A: schematic demonstrating bile acid monomers partitioning into a phospholipid bilayer. A typical bile acid molecule possesses 2 faces: a hydrophilic face with hydroxyl groups attached and a hydrophobic face with the cholesterol backbone (see right). A top view of a lipid bilayer (lipids depicted by open circles) containing bile acids illustrates that 2 bile acid monomers form a dimer with the hydrophilic faces of the bile acid molecules hidden inside the dimer complex while exposing the hydrophobic face to the bilayer core. This reverse micelle structure ensures that the highly polar hydrophilic face of the bile acid would not interact unfavorably with the nonpolar bilayer core. A similar packing pattern is also found in PC-bile acid mixed micelles. B: amphiphilic Indo molecules (solid hexagons) potentially interact extensively with the hydrophilic face of the bile acid molecules and further expand the area that is polar. The original dimer structure becomes inadequate in protecting the polar portion of the bile acid/Indo molecules. C: unfavorable interactions between now-exposed hydrophilic face of the bile acid molecules and nonpolar bilayer core allows association of several bile acid and Indo molecules to form a larger complex. This change potentially further isolates bile acid molecules from PC lipids. Formation of the “polar pocket” composed of bile acids and Indo molecules induces increase in membrane permeability and disruption of the entire membrane. This is thought to occur when bile acid concentration is either below or above its critical micellar concentration.
A similar phenomenon, i.e., a decrease in FRET efficiency between DC and PC, was also found at bile acid concentration above its CMC, where bile acids and PC lipids form mixed micelles (Fig. 7). There are two competing models attempting to explain bile acid-PC interactions in mixed micelles: the stacked-disk model and the radial-shell model (3, 23, 33, 34). Because a FRET experiment measures energy transfer between two probes covalently attached to biological molecules, this experiment has been thought to act as a molecular ruler and demonstrates how two types of biological molecules interact. Our FRET study is novel in that it directly monitors the molecular interactions between bile acid and PC. To be consistent with the radial-shell model, which predicts that bile acid molecules are in constant contact with PC lipids (23), increasing bile acid concentration would enhance the energy transfer between bile acid and PC and show a dose-dependent increase in FRET efficiency. However, our experimental data demonstrated a consistent and dose-dependent decrease in FRET efficiency, indicating that the distance between the bile acid molecules and PC increases as bile acid concentration increases. Other experiments in our study, such as the ζ-potential, the dipole potential, and the membrane permeability, show that the bile acid molecules are definitely binding to PC. The interesting “contradiction” between these studies and the FRET data can be explained by formation of segregated areas enriched with bile acid molecules and those with PC lipids in the complexes. And this view is most consistent with the models proposed by the stacked-disk model (3, 33, 34), in which a disc-shaped bile acid/PC micelle contains a bilayer of PC lipids located primarily in the center and bile acids around the perimeter (3, 33, 34). This will lead to less interaction between the bile acid and PC and less energy transfer. Furthermore, bile acid dimers isolated from PC lipids, similar to reverse micelles, have also been found within the PC domains in the center of the mixed micelles. The formation of bile acid aggregates explains the observed decrease in FRET efficiency between DC and PC as the concentration of the bile acid increased.
Interestingly, Indo induced a dose-dependent decrease in the efficiency of energy transfer between PC and DC below and above its CMC of 3 mM, indicating an increase in the distance between the two types of molecules. Further aggregation of bile acid molecules into isolated bile acid complexes isolated from PC lipids in the bilayer would lead to separation of bile acid molecules from PC molecules. Thus our data suggest that Indo enhanced further segregation of DC molecules in the PC bilayer (see Fig. 9C). As the ability of Indo to alter interaction between DC and PC follows the same pattern both below and above CMC of the bile acid, we believe that the Indo-induced bile acid aggregation occurs in both PC bilayers containing reversed micelles and PC-DC mixed micelles. Of course, it is also possible that Indo competitively binds with PC and induces dissociation of bile acid from PC lipids in either bilayer membranes or mixed micelles, which cannot be differentiated from the first possibility by using the FRET data since both involve weakened interaction between the bile acid and PC. However, our experiments with ζ-potential, an indicator of membrane surface charge and binding of negatively charged bile acids and/or NSAIDs, illustrated that the surface charge of PC liposomes exposed to both DC and Indo was approximately the sum of those exposed to individual amphiphiles. Although we only measured the changes in ζ-potential of PC-bile acid-Indo complexes at bile acid concentrations below CMC, we believe that the same type of binding would occur for bile acid-PC mixed micelles. This is because the binding of bile acids, PC, and NSAIDs originates from the unfavorable interactions with water. Since the aqueous environment stays the same, the hydrophobic association among three components should stay the same. This is, indeed, demonstrated by our FRET experiments, which show that the effect of Indo on DC-PC association maintains qualitatively the trend. The data therefore strongly support the possibility that both DC and Indo associate with PC lipids. Therefore, the weakened association of the bile acid and PC observed in our FRET experiments can best be explained by binding of Indo to the PC bilayer and the consequential formation of more isolated bile acid aggregates in the PC bilayer either below or above CMC of the bile acid.
Taking the data from our biophysical experiments together, we propose a molecular model for Indo-bile acid-PC interactons. It is possible that close association of amphiphilic Indo molecules to DC molecules modifies the hydrophobicity of the “faces” of the bile acid molecule. Indo (shown as solid hexagons in Fig. 9B) is highly amphiphilic and most likely interacts with the hydrophilic face of the bile acid molecules, in contrast to the highly hydrophobic lipid acyl chains or the hydrophobic face of the bile acid molecule. This association would increase the polar portion of the bile acid molecule (Fig. 9B), which cannot be adequately protected by only two bile acid molecules in a dimer. Thus Indo binding to the bile acid molecules provides the driving force for more bile acid molecules binding together to form a larger complex (Fig. 9C). Combining small bile acid dimers into larger complexes decreases the total surface area of bile acid molecules in the membrane and minimizes the interaction between bile acid and PC. This explains the Indo-induced weakening of interaction between PC and DC observed in our FRET experiments. Formation of these bile acid complexes, which act as “detergent pockets,” as well as incorporation of additional amphiphilic Indo molecules significantly increases the detergent effect of the complex and the consequent breakdown in the membrane integrity. Our study provides an explanation for the NSAID-induced increase in cytotoxicity of the bile, which contains high level of PC-bile acids mixed micelles. Our findings are in good agreement with previous animal studies in showing that bile acids are crucial to the ability of NSAIDs to cause injury in the small bowel.
Conclusions.
NSAID-induced small intestinal injury in human subjects has only been recently appreciated by the use of capsule endoscopy. Animal studies found no correlation between NSAID-induced lesions and bleeding with the COX-inhibitory activity of these drugs. It has, however, been found that bile contents contribute to the increased toxicity of NSAIDs in the small bowel. Here, we found that combinations of Indo and DC led to a significant increase in membrane damage, consistent with previous findings that bile acids induce NSAID toxicity in the small intestine. The molecular mechanism for this event potentially comes from the ability of the NSAID to incorporate into the PC bilayers in either biomembranes or mixed micelles and enhance segregation of bile acid molecules from PC lipids, possibly by forming larger complexes in the bilayer or in the mixed micelles. The presence of both the NSAID and the bile acid, which are effective detergents, enhances the toxicity of the complex and contribute to the NSAID-induced injury in the small bowel.
GRANTS
Funding was in part provided by NIH F-32 DK07664, Texas Medical Center Digestive Disease Center PHS P-30DK56338, and NIH Challenge grant IRC DK086304.
DISCLOSURES
Dr. Lichtenberger is founder and office holder and has equity in PLx Pharma, a university-based start-up that is developing phospholipid-conjugated NSAIDs.
ACKNOWLEDGMENTS
We appreciate Nanospectra for equipment access.
REFERENCES
- 1.Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104: 1832–1847, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Brune K, Nurnberg B, Szelenyi I. The Enterohepatic Circulation of Some Anti-Inflammatory Drugs May Cause Intestinal Ulcerations Boston, MA: MTP Press, 1985 [Google Scholar]
- 3.Carey MC, Montet JC, Phillips MC, Armstrong MJ, Mazer NA. Thermodynamic and molecular basis for dissimilar cholesterol-solubilizing capacities by micellar solutions of bile salts: cases of sodium chenodeoxycholate and sodium ursodeoxycholate and their glycine and taurine conjugates. Biochemistry 20: 3637–3648, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Carey MC, Small DM. Micellar properties of dihydroxy and trihydroxy bile salts: effects of counterion and temperature. J Colloid Interface Sci 31: 382–396, 1969 [DOI] [PubMed] [Google Scholar]
- 5.Clarke RJ. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim Biophys Acta 1327: 269–278, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Clarke RJ. The dipole potential of phospholipid membranes and methods for its detection. Adv Colloid Interface Sci 89–90: 263–281, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Clarke RJ, Kane DJ. Optical detection of membrane dipole potential: avoidance of fluidity and dye-induced effects. Biochim Biophys Acta 1323: 223–239, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Clarke RJ, Lupfert C. Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: a basis for the Hofmeister effect. Biophys J 76: 2614–2624, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology 127: 94–104, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dial EJ, Darling RL, Lichtenberger LM. Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J Gastroenterol Hepatol 23: e384–e389, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Dial EJ, Rooijakkers SH, Darling RL, Romero JJ, Lichtenberger LM. Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol 23: 430–436, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Duggan DE, Hooke KF, Noll RM, Kwan KC. Enterohepatic circulation of indomethacin and its role in intestinal irritation. Biochem Pharmacol 24: 1749–1754, 1975 [DOI] [PubMed] [Google Scholar]
- 13.Endo H, Hosono K, Inamori M, Kato S, Nozaki Y, Yoneda K, Akiyama T, Fujita K, Takahashi H, Yoneda M, Abe Y, Kirikoshi H, Kobayashi N, Kubota K, Saito S, Matsuhashi N, Nakajima A. Incidence of small bowel injury induced by low-dose aspirin: a crossover study using capsule endoscopy in healthy volunteers. Digestion 79: 44–51, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Evans EA, Rawicz W, Hofmann A. Lipid bilayer expansion and mechanical degradation in solutions of water-soluble bile-acids. In: XIII Bile Acids in Gastroenterology: Basic and Clinical Advances. Falk Symposium 80: Proceedings of the 80th Falk Symposium (XIII International Bile Acid Meeting), held in San Diego, California, USA, September 30–October 2, 1994, edited by Hofmann A, Paumgartner G, Stiehl A.Boston MA: Kluwer Academic, p. 59–68, 1995 [Google Scholar]
- 15.Fahey DA, Carey MC, Donovan JM. Bile acid/phosphatidylcholine interactions in mixed monomolecular layers: differences in condensation effects but not interfacial orientation between hydrophobic and hydrophilic bile acid species. Biochemistry 34: 10886–10897, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Fortun PJ, Hawkey CJ. Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol 23: 134–141, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Giraud MN, Motta C, Romero JJ, Bommelaer G, Lichtenberger LM. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: a possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs). Biochem Pharmacol 57: 247–254, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Goddard PJ, Hills BA, Lichtenberger LM. Does aspirin damage canine gastric mucosa by reducing its surface hydrophobicity? Am J Physiol Gastrointest Liver Physiol 252: G421–G430, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Goddard PJ, Lichtenberger LM. In vitro recovery of canine gastric mucosal surface hydrophobicity and potential difference after aspirin damage. Dig Dis Sci 40: 1357–1359, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 3: 133–141, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol 3: 55–59, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hara AK, Leighton JA, Sharma VK, Heigh RI, Fleischer DE. Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiographics 25: 697–711; discussion 711–698, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Hjelm R, Thiyagarajan P. Organization of phosphatidylcholine and bile salt in rodlike mixed micelles. J Phys Chem 96: 8653–8661, 1992 [Google Scholar]
- 24.Hofmann AF, Hagey LR. Bile acids: chemistry pathochemistry, biology, pathobiology, therapeutics. Cell Mol Life Sci 65: 2461–2483, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hucker HB, Zacchei AG, Cox SV, Brodie DA, Cantwell NH. Studies on the absorption, distribution and excretion of indomethacin in various species. J Pharmacol Exp Ther 153: 237–249, 1966 [Google Scholar]
- 26.Kao YC, Goddard PJ, Lichtenberger LM. Morphological effects of aspirin and prostaglandin on the canine gastric mucosal surface. Analysis with a phospholipid-selective cytochemical stain. Gastroenterology 98: 592–606, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Leidy C, Wolkers WF, Jorgensen K, Mouritsen OG, Crowe JH. Lateral organization and domain formation in a two-component lipid membrane system. Biophys J 80: 1819–1828, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol 57: 565–583, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Lichtenberger LM, Wang ZM, Romero JJ, Ulloa C, Perez JC, Giraud MN, Barreto JC. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med 1: 154–158, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Lichtenberger LM, Zhou Y, Dial EJ, Raphael RM. NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J Pharm Pharmacol 58: 1421–1428, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lindblom G, Wennerstrom H, Arvidson G, Lindman B. Lecithin translational diffusion studied by pulsed nuclear magnetic resonance. Biophys J 16: 1287–1295, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods 96: 147–152, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Mazer NA, Benedek GB, Carey MC. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry 19: 601–615, 1980 [DOI] [PubMed] [Google Scholar]
- 34.Mazer NA, Carey MC, Kwasnick RF, Benedek GB. Quasielastic light scattering studies of aqueous biliary lipid systems. Size, shape, and thermodynamics of bile salt micelles. Biochemistry 18: 3064–3075, 1979 [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem 18: 113–136, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Moolenaar F, Crancrinus S, Visser J, De Zeeuw D, Meijer DK. Clearance of indomethacin occurs predominantly by renal glucuronidation. Pharm Weekbl Sci 14: 191–195, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Petruzzelli M, Moschetta A, Renooij W, de Smet MB, Palasciano G, Portincasa P, van Erpecum KJ. Indomethacin enhances bile salt detergent activity: relevance for NSAIDs-induced gastrointestinal mucosal injury. Dig Dis Sci 51: 766–774, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Petruzzelli M, Vacca M, Moschetta A, Cinzia Sasso R, Palasciano G, van Erpecum KJ, Portincasa P. Intestinal mucosal damage caused by non-steroidal anti-inflammatory drugs: role of bile salts. Clin Biochem 40: 503–510, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Roda A, Hofmann AF, Mysels KJ. The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem 258: 6362–6370, 1983 [PubMed] [Google Scholar]
- 40.Scholz FJ, Heiss FW, Roberts PL, Thomas C. Diaphragmlike strictures of the small bowel associated with use of nonsteroidal antiinflammatory drugs. AJR Am J Roentgenol 162: 49–50, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Small DM, Bourges MC, Dervichian DG. The biophysics of lipidic associations. I. The ternary systems: lecithin-bile salt-water. Biochim Biophys Acta 125: 563–580, 1966 [PubMed] [Google Scholar]
- 42.Starke-Peterkovic T, Turner N, Else PL, Clarke RJ. Electric field strength of membrane lipids from vertebrate species: membrane lipid composition and Na+-K+-ATPase molecular activity. Am J Physiol Regul Integr Comp Physiol 288: R663–R670, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Starke-Peterkovic T, Turner N, Vitha MF, Waller MP, Hibbs DE, Clarke RJ. Cholesterol effect on the dipole potential of lipid membranes. Biophys J 90: 4060–4070, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231: 232–235, 1971 [DOI] [PubMed] [Google Scholar]
- 45.Vane JR. The mode of action of aspirin-like drugs. Agents Actions 8: 430–431, 1978 [DOI] [PubMed] [Google Scholar]
- 46.Venneman NG, Petruzzelli M, van Dijk JE, Verheem A, Akkermans LM, Kroese AB, van Erpecum KJ. Indomethacin disrupts the protective effect of phosphatidylcholine against bile salt-induced ileal mucosa injury. Eur J Clin Invest 36: 105–112, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Weinstein JN, Yoshikami S, Henkart P, Blumenthal R, Hagins WA. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science 195: 489–492, 1977 [DOI] [PubMed] [Google Scholar]
- 48.Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology 80: 94–98, 1981 [PubMed] [Google Scholar]
- 49.Yamada T, Deitch E, Specian RD, Perry MA, Sartor RB, Grisham MB. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation 17: 641–662, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y, Doyen R, Lichtenberger LM. The role of membrane cholesterol in determining bile acid cytotoxicity and cytoprotection of ursodeoxycholic acid. Biochim Biophys Acta 1788: 507–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Hancock JF, Lichtenberger LM. The nonsteroidal anti-inflammatory drug indomethacin induces heterogeneity in lipid membranes: potential implication for its diverse biological action. PLoS One 5: e8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Raphael RM. Solution pH alters mechanical and electrical properties of phosphatidylcholine membranes: relation between interfacial electrostatics, intramembrane potential and bending elasticity. Biophys J 92: 2451–2462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]