Abstract
Plasminogen activator inhibitor-1 (PAI-1) is an acute phase protein that has been shown to play a role in experimental fibrosis caused by bile duct ligation (BDL) in mice. However, its role in more severe models of hepatic fibrosis (e.g., carbon tetrachloride; CCl4) has not been determined and is important for extrapolation to human disease. Wild-type or PAI-1 knockout mice were administered CCl4 (1 ml/kg body wt ip) 2×/wk for 4 wk. Plasma (e.g., transaminase activity) and histological (e.g., Sirius red staining) indexes of liver damage and fibrosis were evaluated. Proliferation and apoptosis were assessed by PCNA and TdT-mediated dUTP nick-end labeling (TUNEL) staining, respectively, as well as by indexes of cell cycle (e.g., p53, cyclin D1). In contrast to previous studies with BDL, hepatic fibrosis was enhanced in PAI-1−/− mice after chronic CCl4 administration. Indeed, all indexes of liver damage were elevated in PAI-1−/− mice compared with wild-type mice. This enhanced liver damage correlated with impaired hepatocyte proliferation. A similar effect on proliferation was observed after one bolus dose of CCl4, without concomitant increases in liver damage. Under these conditions, a decrease in phospho-p38, coupled with elevated p53 protein, was observed; these results suggest impaired proliferation and a potential G1/S cell cycle arrest in PAI-1−/− mice. These data suggest that PAI-1 may play multiple roles in chronic liver diseases, both protective and damaging, the latter mediated by its influence on inflammation and fibrosis and the former via helping maintain hepatocyte division after an injury.
Keywords: experimental fibrosis, coagulation, regeneration, liver
liver fibrosis is a common pathological endpoint in a variety of liver diseases, such as alcoholic liver disease or viral infections (21). Eventually, fibrosis can progress into cirrhosis. Without liver transplantation, cirrhosis often leads to the death of the patient (17). It was recently shown in humans that liver fibrosis and subsequent cirrhosis can at least partially resolve when the underlying cause is effectively treated (e.g., hepatitis virus C infection) (27). However, the mechanisms leading to fibrosis and cirrhosis are still not completely understood, and there is no FDA-approved therapy yet to blunt the progression of fibrotic liver disease.
A basic limitation in fibrotic liver disease research is that no rodent model completely recapitulates the human disease. Indeed, rodents appear more resistant to hepatic fibrosis relative to humans. Thus surrogate models of hepatic fibrosis [e.g., bile duct ligation (BDL) and carbon tetrachloride (CCl4)] are predominantly used (15). Whereas these models have many similarities to the human disease, there are also differences. Furthermore, not only are there differences between the models and human fibrosis, but there are also differences between the models themselves (15). To account for these limitations, a weight-of-evidence approach using multiple models is most appropriate to identify new mechanisms or therapeutic targets (21).
Previous studies have identified a new potential role of plasminogen activator inhibitor-1 (PAI-1) in hepatic fibrosis. PAI-1 is an acute phase protein that can be induced during inflammation (19, 22, 28). PAI-1 is a major inhibitor of both tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) and, therefore, a key regulator of fibrinolysis by plasmin (18). In addition to regulating the accumulation of fibrinogen/fibrin in the extracellular space, plasmin can also directly degrade other ECM components such as laminin, proteoglycan, and type IV collagen (20, 23, 24). Plasmin can also indirectly degrade ECM via activation of matrix metalloproteinases (MMPs) (29). Thus, by impairing the plasminogen activating systems, PAI-1 can alter organ fibrogenesis. Indeed, a protective effect of pharmacological and/or genetic prevention of PAI-1 induction has been observed in models of renal, pulmonary, and vascular fibrosis (13, 14, 16). PAI-1 is known to be induced in models of hepatic fibrosis (6, 34), analogous to findings in other organs. It was shown recently that PAI-1-deficient mice are protected in fibrosis induced by BDL (2). The purpose of the present study was to determine the role of PAI-1 in another, more severe, model of hepatic fibrosis caused by CCl4.
MATERIALS AND METHODS
Animals and treatments.
Male (4–6 wk) C57BL/6J mice and PAI-1-deficient mice (B6.12952-Serpine1tm1Mg/J) were purchased from Jackson Laboratory (Bar Harbor, ME). This knockout strain has been backcrossed at least ten times onto C57BL/6J mice, avoiding concerns about genetic differences at nonspecific loci. Mice were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and procedures were approved by the University of Louisville's Institutional Animal Care and Use Committee. Food and tap water were provided ad libitum. Mice were administered CCl4 (1 ml/kg ip; diluted 1:4 in olive oil; Sigma-Aldrich, St. Louis, MO) 2×/wk for 4 wk. At 24 h after the last CCl4 administration, mice were anesthetized by injection of a ketamine HCl/xylazine solution (100/15 mg/kg im; Sigma-Aldrich). Other animals received the same dose of CCl4, but only once, and were euthanized 12–72 h after intoxication. Blood was collected from the vena cava just prior to euthanasia by exsanguination, and citrated plasma was stored at −80°C for further analysis. Portions of liver tissue were frozen immediately in liquid nitrogen, while others were fixed in 10% neutral buffered formalin or embedded in frozen specimen medium (Tissue-Tek OCT compound, Sakura Finetek, Torrance, CA) for subsequent sectioning and mounting on microscope slides.
Biochemical analyses and histology.
Plasma levels of alanine (ALT) and aspartate (AST) aminotransferases were determined with use of standard kits (Thermotrace, Melbourne, Australia). Paraffin sections of liver (5 μm) were stained with hematoxylin and eosin (H&E). Plasma levels of hepatocyte growth factor (HGF) were determined by using an ELISA kit purchased from B-Bridge International (Mountain View, CA), which was used according to manufacturer's instructions. Hepatic tissue inhibitor of metalloproteinases-1 (TIMP-1) levels were quantitated via an ELISA kit purchased from R&D Systems (Minneapolis, MN) and normalized to total protein; liver samples for the assay were homogenized in RIPA buffer [20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% (wt/vol) Triton X-100], containing protease and phosphatase inhibitor cocktails purchased from Sigma (St. Louis, MO). Neutrophil accumulation in the livers was assessed by staining tissue sections for chloroacetate esterase (CAE), a marker for neutrophils, using the naphthol AS-D CAE kit (Sigma) (12). ECM accumulation in liver sections was determined by staining with Sirius red/fast green and reticulin/neutral red staining. Sirius red staining was quantitated by image analysis as described previously (2). Liver cell apoptosis was assessed by TdT-mediated dUTP nick-end labeling (TUNEL) employing a commercially available kit (Chemicon, Temecula, CA). CAE- or TUNEL-positive cells were quantitated by counting (per 1,000 hepatocytes) in randomly selected fields (×400 final magnification), as described previously (33). Hepatocyte proliferation was assessed by proliferating-cell-nuclear-antigen (PCNA) staining employing a monoclonal mouse anti-PCNA Clone PC10 (Dako, Glostrup, Denmark) and a peroxidase-linked secondary antibody and diaminobenzidine (ARK peroxidase for mouse primary antibodies, DAKO, Carpinteria, CA). Cell cycle progression (per 1,000 hepatocytes) was estimated via PCNA staining as described by Greenwell et al. (11).
Zymographic determination of hepatic MMPs and plasminogen activators.
The activity of MMP-9 in liver lysates was determined by gelatin zymography, as described previously (2). The activity of tPA and uPA were determined in liver samples as described by Bezerra et al. (4). Briefly, total protein was extracted from frozen liver tissue samples using lysis buffer [1% (vol/vol) Nonidet P-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS in PBS (pH 7.4)]. Lysates were separated on 8% SDS-polyacrylamide gels containing 1.2 mg/ml casein and 75 mU/ml plasminogen (Sigma). Plasminogen-free gels run in parallel were used to confirm that the activity detected was plasminogen dependent. Gels were washed 4×20 min in 2.5% vol/vol Triton-X-100 solution and then 3×30 min in developing buffer (50 mM Tris, 0.1 M glycine, 0.1 M sodium chloride, pH 8.0), followed by a 6-h incubation in developing buffer at 37°C. The caseinolytic activity was detected by staining the gel overnight (0.1% amido black, 45% methanol, 10% acetic acid) and destaining (45% methanol, 10% acetic acid) for 30 min. Densitometric analysis was performed using Image Quant software (Amersham Biosciences, Piscataway, NJ).
RNA isolation and real-time RT-PCR.
Total RNA was extracted from liver tissue samples by a guanidium thiocyanate-based method (Tel-Test, Austin, TX). RNA concentrations were determined spectrophotometrically, and 1 μg total RNA was reverse transcribed using an avian myeloblastosis virus reverse transcriptase kit (Promega, Madison, WI) and random primers. PCR was carried out as described previously (3).
Immunoblots.
To prepare total hepatic protein, frozen liver samples were processed as described previously (3). Primary antibodies against actin, phosphorylated and total p38 and ERK were used, as well as antibodies against total cyclin D1 and p53 (all antibodies Cell Signaling, Danvers, MA, and Sigma-Aldrich). Horseradish peroxidase-coupled secondary antibodies and chemiluminescence detection reagents were from Pierce (Rockford, IL). The signals were detected employing Hyperfilm ECL (Amersham, Buckinghamshire, UK). Quantification was performed with Scion Image analysis software (Scion, Frederick, MD).
Statistical analyses.
Results are reported as means ± SE (n = 4–7). ANOVA with Bonferroni's post hoc test (for parametric data) or Mann-Whitney's rank sum test (for nonparametric data) was used for the determination of statistical significance among treatment groups, as appropriate.
RESULTS
PAI-1-deficient mice are more susceptible to liver damage and fibrosis caused by chronic CCl4.
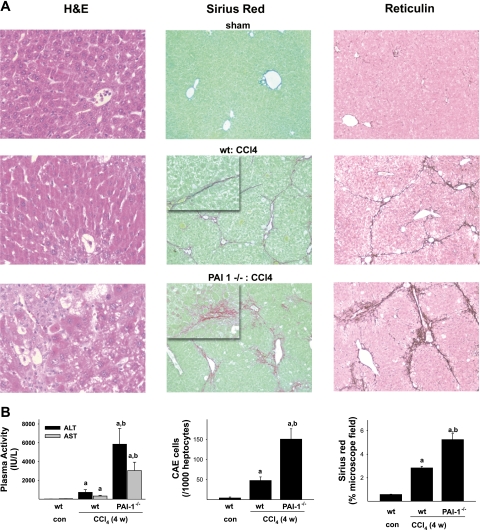
All animals survived 4-wk exposure to CCl4. In preliminary studies, no differences in parameters of liver damage were observed between wild-type and PAI-1−/− mice after vehicle (olive oil) injection; data with the former therefore are shown to represent both strains. Four weeks of CCl4 administration to wild-type mice increased plasma AST (∼7-fold) and ALT (∼40-fold) levels significantly compared with vehicle controls (Fig. 1B, left). In PAI-1-deficient mice, the administration of CCl4 for 4 wk caused an even more robust increase of ALT and AST activities, with values ∼10-fold greater than in wild-type mice receiving CCl4. This increase in plasma enzymes was also paralleled by hepatic histology, as assessed by H&E staining (Fig. 1A, left). CCl4 administration caused necroinflammatory liver damage in wild-type mice (Fig. 1A, left column, middle), with foci located predominantly in pericentral regions. Staining for CAE indicated that CCl4 increased neutrophil infiltration ∼20-fold (Fig. 1B, middle). CCl4 administration to PAI-1-deficient mice caused a dramatic exacerbation of liver damage compared with wild-type mice. Examination revealed widespread foci of necrotic cells, not only in the pericentral region, but also in midzonal and periportal (Fig. 1A, left column, bottom). PAI-1-deficient mice also showed an increase in inflammatory or necroinflammatory foci, which is in line with the threefold increase in CAE-positive cells compared with wild-type mice (Fig. 1B, middle).
Fig. 1.
Effect of plasminogen activator inhibitor-1 (PAI-1) deficiency on CCl4-induced liver damage. Male C57BL/6J or PAI-1 −/− mice were administered CCl4 (1 ml/kg; ip) 2×/wk for 4 wk. A: the effect of CCl4 on liver histology was assessed by hematoxylin and eosin (H&E, left, ×100), Sirius red (middle, ×100; insets are ×400), and reticulin (right, ×100) staining. Representative photomicrographs are shown. B: plasma transaminases (left) were determined as described inmaterials and methods. Neutrophil infiltration was assessed by chloroacetate esterase (CAE) staining and subsequent cell counting (middle) as described in materials and methods. Intensity of Sirius red staining (right) was assessed by image analysis. Quantitative data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wild type (wt or WT) exposed to CCl4 by ANOVA using Bonferroni's post hoc test. con, Control.
As expected, 4 wk of CCl4 administration caused hepatic fibrosis in mouse liver, as assessed by Sirius red (Fig. 1A, middle column, middle) and reticulin staining (Fig. 1A, right column, middle). In wild-type mice, the fibrotic response was pronounced with bridges between vessels; Sirius red staining in this group accounted for ∼3% of total hepatic area (Fig. 1B, right). As is known for CCl4 administration under these conditions, the macroscopic pattern of Sirius red staining in wild-type mice tended to be relatively tight, forming distinct septa (Fig. 1A, middle column, middle, inset). This pattern was also observable with reticulin staining (Fig. 1A, right column, middle). Whereas the lobular pattern of ECM accumulation (i.e., bridging) was not different between the mouse strains, there was a pronounced difference in the appearance of the ECM depositions in PAI-1−/− mice. Specifically, CCl4 exposure formed branched and irregular fibers in PAI-1−/− mice, instead of the distinct fibrils and septa found in wild-type mice (Fig. 1A, middle column, bottom, inset). This effect caused an almost twofold increase in the Sirius red-positive area of the liver (Fig. 1B, right). Similar patterns were also observed for reticulin staining (Fig. 1A, right column, bottom).
Effect of CCl4 on the expression of profibrotic genes.
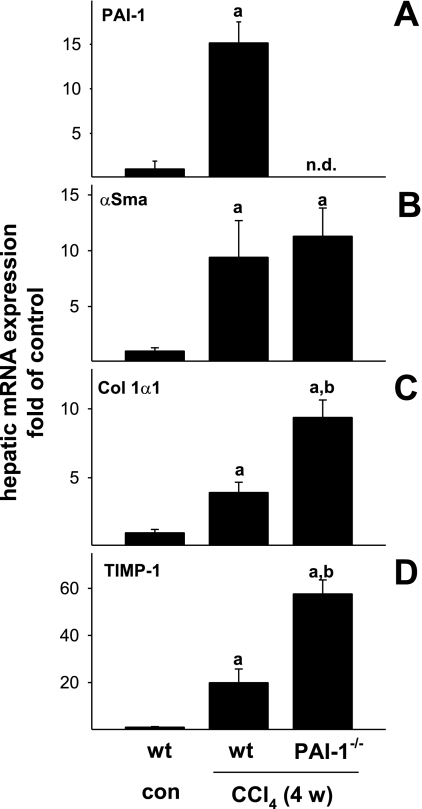
The expression of PAI-1 was previously shown to be induced in experimental hepatic fibrosis (2). Furthermore, increases in the expression of α-smooth muscle actin, collagen Iα1, and TIMP-1 are indicative of stellate cell activation, increased ECM synthesis and inhibited ECM degradation, respectively. The effect of CCl4 on hepatic expression of these genes was determined in samples from wild-type and PAI-1-deficient mice via real-time RT-PCR (Fig. 2). CCl4 administration robustly induced hepatic expression of all of these variables in livers from wild-type mice (Fig. 2). PAI-1 mRNA was not detectable in extracts of livers from PAI-1−/− mice, as expected (Fig. 2A). Whereas the increase in the expression of α-smooth muscle actin was not altered in PAI-1−/− mice, the expression of collagen Iα1 (Fig. 2C) and TIMP-1 (Fig. 2D) was induced to a greater extent in this strain, with values greater than twofold than those of wild-type mice exposed to CCl4 (Fig. 2, bottom).
Fig. 2.
Effect of PAI-1 deficiency on CCl4-induced expression of profibrotic genes. Expression of PAI-1 (A), α-smooth muscle actin (α-SMA; B), collagen Iα1 (Col 1α1; C), and tissue inhibitor of metalloproteinase-1 (TIMP-1; D) mRNA was determined by real-time RT-PCR (see materials and methods). n.d., Not detectable. Data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wt exposed to CCl4 by ANOVA using Bonferroni's post hoc test.
Effect of PAI-1 deficiency and CCl4 on indexes of ECM metabolism.
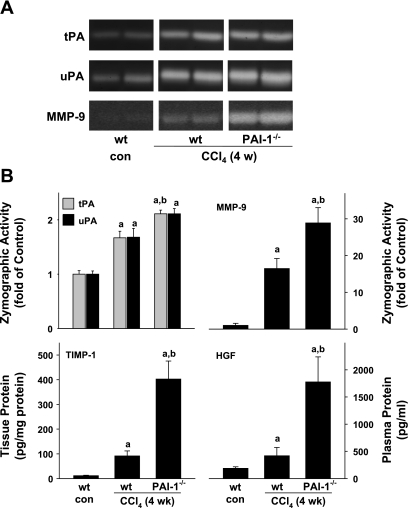
In addition to regulation at the level of production (see Fig. 2), ECM deposition is also controlled at the level of degradation (e.g., by MMPs). It was shown that the protection in PAI-1-deficient mice against BDL-induced liver fibrosis correlated with increased plasminogen activator (i.e., tPA and uPA) and MMP activity (2). The activities of uPA, tPA, and MMP-9 were therefore determined zymographically (Fig. 3, A and B). Protein levels of the major MMP-9 inhibitor in liver (TIMP-1) were also determined by ELISA (Fig. 3B). HGF, a major mitogenic cytokine that is proteolytically activated by the plasminogen system, was also determined by ELISA (Fig. 3B). Four weeks of CCl4 exposure significantly increased the activity of both tPA and uPA almost twofold in wild-type mice (Fig. 3, A and B); the effect of CCl4 on tPA activity was significantly enhanced in PAI-1−/− mice. A similar trend for uPA was also observed but did not reach significance (P = 0.052). After 4 wk of CCl4 administration, MMP-9 activity was induced ∼16-fold compared with sham-treated animals; this increase in MMP activity caused by CCl4 was significantly enhanced approximately twofold in PAI-1−/− mice compared with wild-type mice (Fig. 3, A and B). Analogous to mRNA expression (Fig. 2D), protein levels of TIMP-1 were also dramatically increased by CCl4 in wild-type mice ∼20-fold; this effect of CCl4 was more robust in PAI-1−/− mice, the relative TIMP-1-to-MMP-9 ratio was therefore ∼2.5-fold higher in PAI-1−/− mice compared with wild-type mice. Chronic exposure to CCl4 significantly increased plasma levels of HGF in wild-type mice (Fig. 3B); this effect of CCl4 was significantly greater in PAI-1−/− mice compared with wild types by a factor of ∼3.5.
Fig. 3.
Effect of PAI-1 deficiency on CCl4-stimulated matrix metalloproteinase 9 (MMP-9) activity and hepatic TIMP-1 expression. Urokinase-type plasminogen activator (uPA), tissue-type plasminogen activator (tPA), and MMP-9 activity were assessed by zymography gel electrophoresis. TIMP-1 and hepatocyte growth factor (HGF) levels were determined by ELISA. A: representative zymography bands from the same gel. B: summary results of image analysis of protease activity (top) and ELISA analyses (bottom). Quantitative data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wt exposed to CCl4 by Mann-Whitney's rank sum test (tPA, uPA, and MMP-9) or by ANOVA using Bonferroni's post hoc test (TIMP-1 and HGF).
Effect of CCl4 on proliferation and apoptosis in wild-type and PAI-1-deficient mice.
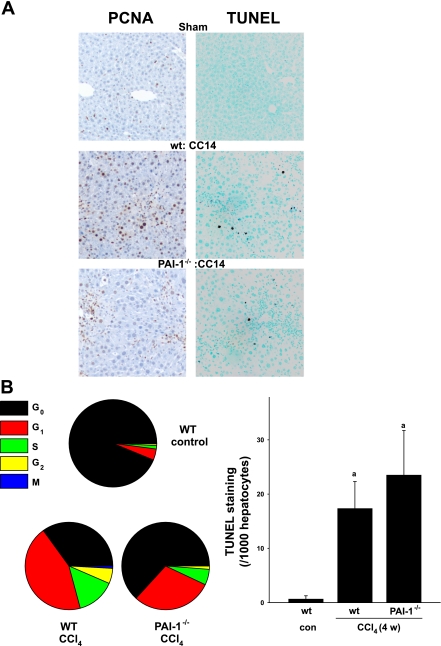
It is proposed that impaired regeneration in response to cell death is one mechanism by which liver diseases progress to cirrhosis (10). Therefore, the effect of CCl4 administration on proliferation (PCNA; Fig. 4, A and B, left) and apoptosis (TUNEL; Fig. 4, A and B, right) in wild-type and PAI-1−/− mice was assessed. In control mice, relatively few (∼6%) of the cells were stained for active cycling (Fig. 4, A and B) and the majority of the cells (937 ± 76 per 1,000 hepatocytes) were found in G0. Chronic exposure to CCl4 dramatically increased the number of PCNA-positive cells in livers from wild-type mice to ∼65%, with 350 ± 7 per 1,000 hepatocytes in G0 (Fig. 4A, left column, middle; Fig. 4B, left). In contrast, the number of cells still in G0 in PAI-1−/− exposed to CCl4 was significantly higher (632 ± 26), with only ∼37% of the total population actively cycling (i.e., not in G0; Fig. 4A, left, and B, left). Furthermore, the effect of knocking out PAI-1 on hepatocyte proliferation was not evenly distributed among the cell cycle phases (Fig. 4B, left); specifically, whereas the number of cells in G1 was ∼30% lower in PAI-1 knockout mice compared with wild-type mice (297 ± 22 vs. 442 ± 23, P < 0.05), the number of cells was ∼60% lower (57 ± 11 vs. 143 ± 26, P < 0.05) in S phase, ∼80% lower (12 ± 2 vs. 56 ± 7, P < 0.05) in G2, and ∼95% lower (1 ± 1 vs. 9 ± 1, P < 0.05) in M phase. These differences in cell cycle affected the relative G1-to-S (∼3 vs. 5) ratios for the wild-type and PAI-1−/− mice, respectively. Chronic CCl4 administration also significantly increased positive TUNEL staining in mouse liver (Fig. 4A, right), but to a similar extent (∼20-fold) in both strains (Fig. 4B, right).
Fig. 4.
PAI-1-deficient mice show reduced proliferation. Markers of proliferation and apoptosis were assessed by PCNA and TdT-mediated dUTP nick-end labeling (TUNEL) staining, respectively. A: representative photomicrographs (×200). B: number of staining-positive hepatocytes per 1,000 hepatocytes for PCNA (bottom left) and TUNEL (bottom right) staining. Quantitative data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wt exposed to CCl4 by ANOVA using Bonferroni's post hoc test.
Hepatocyte proliferation is impaired in PAI-1-deficient mice after acute CCl4.
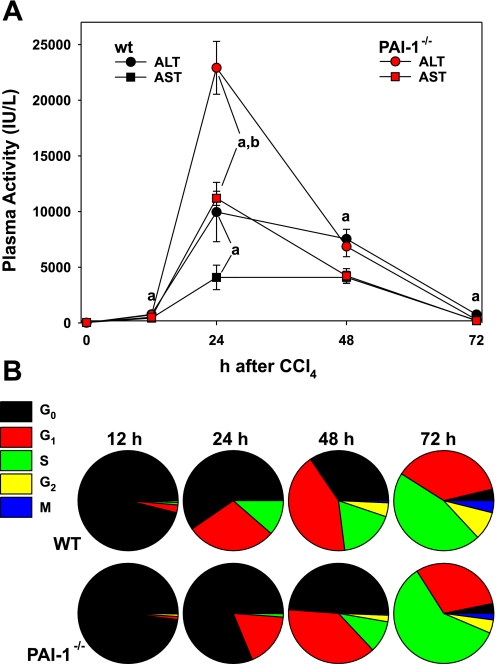
Fibrosis caused by chronic CCl4 exposure is a sum of numerous insults and responses, which makes it difficult to delineate mechanisms. An acute model of CCl4 exposure was therefore chosen to further investigate the effect of knocking out PAI-1 on proliferation and repair after injury. Accordingly, wild-type and PAI-1-deficient mice were administered one dose of CCl4 and were euthanized 12–72 h later. Bolus CCl4 exposure significantly increased plasma transaminase values (Fig. 5A), which peaked 24 h after exposure. At the 24-h time point, plasma AST and ALT were significantly (>2-fold) higher in knockout mice compared with control. There were no differences in transaminases between wild-type and PAI-1−/− mice after acute CCl4 exposure at any other time point (Fig. 5A).
Fig. 5.
Blunted proliferation after acute CCl4 exposure in PAI-1 knockout mice. Liver enzyme activity (A) was determined as described in materials and methods. Hepatocyte proliferation was assessed by PCNA staining and reported as the number of staining-positive hepatocytes per 1,000 hepatocytes (B). ALT, alanine aminotransferase; AST, aspartate aminotransferase. Transaminase data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wt exposed to CCl4 by ANOVA using Bonferroni's post hoc test.
The cell cycle profile for both strains was similar to untreated controls (Fig. 4B) 12 h after acute CCl4 exposure (Fig. 5B). However, 24 h after exposure, there were significant differences between the strains. For example, ∼40% of the cells were actively cycling 24 h after CCl4 exposure in the wild-type strain, with 596 ± 46 per 1,000 hepatocytes in G0. In contrast, only ∼20% of total cells were actively cycling at this time point in the PAI-1−/− mice, with significantly more (813 ± 63 per 1,000 ; P < 0.05) hepatocytes in G0 at this time point. Analogous to findings with chronic CCl4 exposure (Fig. 4B), this effect in the PAI-1 knockout mice was unevenly distributed in the cell cycle phases at the 24-h time point. Specifically, whereas the number of cells in G1 was ∼40% lower in PAI-1 knockout mice compared with wild-type mice (176 ± 58 vs. 290 ± 13, P < 0.05), the number of cells was ∼90% lower (11 ± 5 vs. 114 ± 33, P < 0.05) in S phase. The relative G1-to-S ratio for wild-type and PAI-1−/− mice at this time point was ∼2.5 vs. 16, respectively. At 48 h after acute CCl4 exposure, there were still significantly fewer cells actively cycling in the knockout strain compared with wild types, as well as significantly fewer cells in S and G2. At 72 h after CCl4 exposure, the total number of cells actively cycling was similar between the two strains; however, there were significantly more (596 ± 22 vs. 459 ± 39, P < 0.05) in S phase in the PAI-1−/− strain at this time point.
Effect of CCl4 and PAI-1 deficiency on MAPK activities and cell cycle regulators.
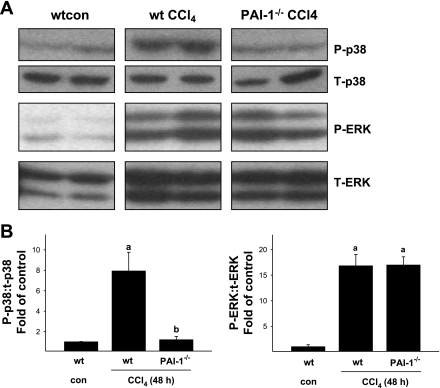
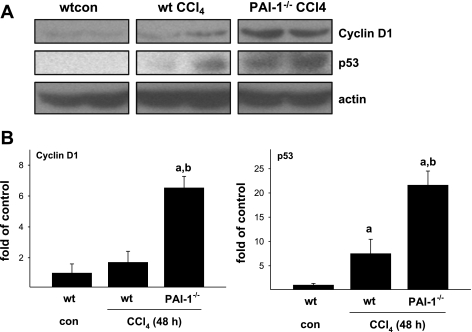
Hepatic regeneration after injury is mediated by a myriad of factors. A critical component in maintaining regeneration, once initiated, is HGF (see Ref. 8 for review). The mitogenic effects of HGF are mediated, at least in part, by MAPK [e.g., p38 and ERK (26)]. The phosphorylation status of these proteins was therefore assessed by immunoblot (Fig. 6). The phosphorylation of both p38 and ERK was significantly increased by acute CCl4 exposure and peaked at the 48-h time point (Fig. 6). Whereas the increase in ERK1/2 phosphorylation caused by CCl4 was not significantly different between the mouse strains, the increase in p38 phosphorylation caused by CCl4 was almost completely blunted in PAI-1-deficient mice at the 48-h time point (Fig. 6). Liver regeneration also involves the progression of the hepatocytes through the G1/S checkpoint (9). The increased ratio of G1/S cells in PAI-1 knockout mice after chronic and acute CCl4 exposure (Figs. 4 and 5) suggests a potential G1/S transition block. Therefore, expression of key regulators of transition through this checkpoint (cyclin D1 and p53) were determined (Fig. 7). Whereas cyclin D1 abundance was not influenced by acute CCl4 in wild-type mice, CCl4 administration significantly increased cyclin D1 protein in PAI-1-deficient mice approximately sixfold (Fig. 7). The levels of p53 were increased in the livers of both strains after CCl4 exposure, but to a far greater extent in PAI-1−/− mice (Fig. 7).
Fig. 6.
Blunted p38 phosphorylation in PAI-1-deficient mice after acute CCl4 administration. Phosphorylation of p38 and ERK was assessed by immunoblot analysis as described in materials and methods. A: representative bands from the same immunoblot. B: results of image analysis of the blots. Quantitative data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wt exposed to CCl4 by Mann-Whitney's rank sum test. P, phospho; T, total.
Fig. 7.
PAI-1-deficient mice show an increase in cyclin D1 and p53 protein after acute CCl4 administration. Cyclin D1 and p53 total protein were assessed by immunoblot analysis as described in materials and methods. A: representative bands from the same immunoblot. B: results of image analysis of the blots. Quantitative data are reported as means ± SE, n = 4–7. aP < 0.05 compared with absence of CCl4; bP < 0.05 compared with wt exposed to CCl4 by Mann-Whitney's rank sum test.
DISCUSSION
As mentioned in the introduction, there still is no optimal rodent paradigm of human fibrosis. Consequently, several models (e.g., BDL or toxin-induced liver injury) are used to represent various aspects of human fibrogenesis. Previous studies have indicated that PAI-1-deficient mice are protected against liver damage and fibrosis after BDL (2), a paradigm in which liver damage is less severe compared with other models of hepatic fibrogenesis. Here, the effect of knocking out PAI-1 on liver damage and fibrosis in the more severe CCl4 model was investigated. In contrast to findings in the BDL model (2), liver damage and fibrosis was dramatically enhanced in PAI-1 knockout mice after chronic CCl4 exposure.
The enhanced fibrosis after chronic CCl4 in PAI-1−/− mice may be explained, in part, by increases in fibrogenesis and decreases in ECM degradation in this strain. Specifically, the expression of collagen Iα1 was significantly elevated in PAI-1−/− mice (Fig. 2), which indicates increased fibrogenesis. Furthermore, although MMP-9 activity was significantly elevated in the knockout mice compared with wild-type mice (Fig. 3), the mRNA (Fig. 2D) and protein (Fig. 3B) expression of the major inhibitor of MMPs in liver (TIMP-1) was enhanced to even a greater extent, indicating impaired collagen degradation in this strain compared with wild-type mice. These changes indicate a shift in PAI-1−/− mice to more collagen deposition in the liver after chronic CCl4 exposure. However, there are other effects in the knockout strain that are less likely to be explained by the above-mentioned changes. For example, the robustly elevated liver damage (Fig. 1) and impaired proliferation (Fig. 4) are unlikely to be explained by alterations in collagen metabolism.
As mentioned in results, it is often difficult to distinguish between effects and proximate causes in chronic models of liver damage. To address this concern, the effects of acute CCl4 exposure on liver damage and proliferation was determined (Figs. 5–7). Under these conditions, liver damage was higher in PAI-1−/− mice 24 h after exposure but was similar to that in wild-type mice at other time points (Fig. 5, top). Analogous to findings in the chronic model (Fig. 4), the compensatory increase in hepatocyte proliferation caused by CCl4-induced liver damage was also significantly blunted in the PAI-1 knockout strain in the acute model (Fig. 5, bottom). These data suggest that the increased liver damage observed after chronic exposure to CCl4 (Fig. 1) is likely secondary to an impaired proliferative response. A recent study has also observed a similar apparent proproliferative role of PAI-1 in mouse liver after experimental acetaminophen toxicity (1), which is in line with these findings.
In response to chemical toxicity, cells in target tissues undergo numerous changes, including altering the expression of genes that regulate proliferation and differentiation and/or apoptosis (8). Many of these processes are controlled by cues received from circulating growth factors, such as HGF. Most studies to date have assumed that PAI-1 plays an antiproliferative role in vivo. This assumption is due to the fact that PAI-1 inhibits the maturation of HGF by plasminogen activators. In support of this hypothesis, mice deficient in plasminogen activators have impaired liver regeneration after partial hepatectomy (31), and this effect correlates with low levels of HGF. Indeed, HGF levels in PAI-1−/− mice after chronic CCl4 exposure were significantly higher than in wild-type mice (Fig. 3B).
Despite this increase in plasma levels of HGF in PAI-1−/− mice, the proliferative response of this strain was significantly impaired in the chronic (Fig. 4) and acute (Fig. 5) CCl4 models. HGF-induced hepatocyte proliferation requires signaling via MAPK. For example, Müller et al. (26) showed that HGF-induced proliferation in cultured liver cells is attenuated by inhibiting either p38 or ERK MAPKs. Here, the activation of p38 caused by CCl4 was completely attenuated in PAI-1−/− mice (Fig. 6). The mechanisms by which PAI-1 deficiency may mediate this effect are unclear. A direct effect of PAI-1 on MAPK signaling pathways is not clearly elucidated in the literature. However, some studies indicate that PAI-1 may enhance or prolong MAPK signaling via the uPA/PAI-1 complex (see Ref. 5 for review). Such a potential effect of PAI-1 on MAPK signaling is in line with the results observed here.
Another possibility to explain the disconnect between plasma HGF levels and proliferation is that PAI-1 deficiency impairs HGF maturation. Whereas the plasma compartment of HGF is assumed to represent HGF cleaved from the ECM, the HGF ELISA does not necessarily differentiate between active and latent forms of this protein. Transgenic mice that overexpress TIMP-1 have impaired liver regeneration after partial hepatectomy, in part via impaired proteolytic activation of HGF (25). Given the fact that TIMP-1 levels were much more elevated in PAI-1−/− mice vs. wild-types after CCl4 (Figs. 2 and 3), a similar mechanism by which TIMP-1 levels are inhibiting HGF maturation is distinctly possible here. Taken together, these data suggest that despite increased HGF levels in plasma (Fig. 3B), livers from PAI-1-deficient mice have an impaired regenerative response to injury in part via mechanisms involving impaired HGF maturation (via TIMP-1 superinduction; Figs. 2 and 3) or via impaired HGF signaling via p38 (Fig. 5) phosphorylation. These mechanisms are of course not mutually exclusive.
The cell cycle is not only regulated at the level of initiation, but also at the level of progression, with the G1/S transition being a key checkpoint (8). Here, the ratio of G1/S cells after acute and chronic CCl4 was higher in livers from PAI-1−/− mice compared with wild-type (see results text), which is indicative of G1/S transition block. This transition is regulated by cell cycle mediators (e.g., cyclin D1) and inhibitors (e.g., p53). The protein level p53 was robustly induced in the PAI-1−/− mice (Fig. 7), supporting the G1/S ratio data. The induction of p53 in PAI-1-deficient mice may be related to the mechanisms described above (i.e., impaired HGF maturation and signaling) or via independent mechanisms. For example, plasminogen activators induce protein expression of p53 in cultured lung epithelial cells (30). Whereas protein levels of cyclin D1 are generally directly associated with proliferation, this protein is rapidly degraded when the cell enters S phase (7); an elevated level of this protein (Fig. 7) may therefore also represent impaired entry into S phase.
Taken together, these results indicate that PAI-1 appears to play a protective role in CCl4-induced liver damage and fibrosis. Results with acute exposure to CCl4 suggest that this effect is mediated, at least in part, by a significant impairment in hepatocyte proliferation in the knockout strain. It is hypothesized that with chronic exposure to CCl4, this impaired ability of the knockout liver to restitute itself enhances the amount of liver damage and accelerates the fibrotic process. Given that previous studies have shown a profibrotic effect of PAI-1 in the BDL model (2), the results observed here are somewhat surprising. Furthermore, as mentioned above, the apparent proproliferative role of PAI-1 under these conditions is also surprising. It is known that high PAI-1 levels correlate with poor prognosis for several cancers and that PAI-1 levels correlate with cell growth and tumor aggressiveness in vitro and in animal models (5). Therefore, further elucidation of the mechanisms by which PAI-1 mediates the effects observed here may shed light on the “PAI-1 paradox” in cancer research.
As already mentioned, it is sometimes difficult to extrapolate from these models to human disease, especially under conditions where the results in different models are contradictory, as has been observed for PAI-1 in the BDL and CCl4 models. However, the relative differences in the two models may yield some information. Specifically, liver damage in the BDL model is relatively moderate (i.e., transaminases <1,000 IU/l). In contrast, CCl4 causes massive hepatocyte damage (Figs. 1 and 5). It is therefore likely that the damaging role of PAI-1 observed experimentally (e.g., Ref. 2) better represents the role of PAI-1 in chronic liver diseases in humans with less severe hepatocyte death (e.g., alcoholic liver disease). However, if liver injury is severe [e.g., after acetaminophen (1) and liver failure and in liver transplant], the beneficial role of PAI-1 may be unmasked.
GRANTS
This work was supported, in part, by a grant from the National Institute of Alcohol Abuse and Alcoholism (AA003624). J. P. Kaiser was supported by a predoctoral (F31) fellowship from the National Institute of Alcohol Abuse and Alcoholism (AA017346) and J. I. Beier was supported by a postdoctoral (T32) fellowship from National Institute of Environmental Health Science (ES011564).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
C. von Montfort is currently a postdoctoral fellow at the Department of Cell Death and Proliferation, Instituto Investigaciones Biomédicas de Barcelona, Consejo Superior de Investigaciones Científicas, Barcelona, Spain.
REFERENCES
- 1.Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol Sci 104: 419–427, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther 316: 592–600, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology 130: 2099–2112, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezerra JA, Currier AR, Melin-Aldana H, Sabla G, Bugge TH, Kombrinck KW, Degen JL. Plasminogen activators direct reorganization of the liver lobule after acute injury. Am J Pathol 158: 921–929, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder BR, Mihaly J. The plasminogen activator inhibitor “paradox” in cancer. Immunol Lett 118: 116–124, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bueno MR, Daneri A, Armendariz-Borunda J. Cholestasis-induced fibrosis is reduced by interferon alpha-2a and is associated with elevated liver metalloprotease activity. J Hepatol 33: 915–925, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Caldon CE, Daly RJ, Sutherland RL, Musgrove EA. Cell cycle control in breast cancer cells. J Cell Biochem 97: 261–274, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43: S45–S53, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 9: 1527–1536, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwell A, Foley JF, Maronpot RR. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett 59: 251–256, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Richardson KS, Tucker LM, Doll MA, Hein DW, Arteel GE. Role of the renin-angiotensin system in hepatic ischemia reperfusion injury in rats. Hepatology 40: 583–589, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 106: 1341–1350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest 112: 379–388, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117: 539–548, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation 104: 839–844, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Kim WR, Brown RS, Jr, Terrault NA, El Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology 36: 227–242, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kruithof EK. Plasminogen activator inhibitors—a review. Enzyme 40: 113–121, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Lagoa CE, Vodovotz Y, Stolz DB, Lhuillier F, McCloskey C, Gallo D, Yang R, Ustinova E, Fink MP, Billiar TR, Mars WM. The role of hepatic type 1 plasminogen activator inhibitor (PAI-1) during murine hemorrhagic shock. Hepatology 42: 390–399, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res 41: 4629–4636, 1981 [PubMed] [Google Scholar]
- 21.Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 45: 605–628, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Luyendyk JP, Maddox JF, Green CD, Ganey PE, Roth RA. Role of hepatic fibrin in idiosyncrasy-like liver injury from lipopolysaccharide-ranitidine coexposure in rats. Hepatology 40: 1342–1351, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mackay AR, Corbitt RH, Hartzler JL, Thorgeirsson UP. Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res 50: 5997–6001, 1990 [PubMed] [Google Scholar]
- 24.Mochan E, Keler T. Plasmin degradation of cartilage proteoglycan. Biochim Biophys Acta 800: 312–315, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, Khokha R. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology 41: 857–867, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Muller M, Morotti A, Ponzetto C. Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol Cell Biol 22: 1060–1072, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 122: 1303–1313, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Quax PH, van den Hoogen CM, Verheijen JH, Padro T, Zeheb R, Gelehrter TD, van Berkel TJ, Kuiper J, Emeis JJ. Endotoxin induction of plasminogen activator and plasminogen activator inhibitor type 1 mRNA in rat tissues in vivo. J Biol Chem 265: 15560–15563, 1990 [PubMed] [Google Scholar]
- 29.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem 274: 13066–13076, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Shetty S, Gyetko MR, Mazar AP. Induction of p53 by urokinase in lung epithelial cells. J Biol Chem 280: 28133–28141, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Shimizu M, Hara A, Okuno M, Matsuno H, Okada K, Ueshima S, Matsuo O, Niwa M, Akita K, Yamada Y, Yoshimi N, Uematsu T, Kojima S, Friedman SL, Moriwaki H, Mori H. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology 33: 569–576, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Von Montfort C, Beier JI, Guo L, Kaiser JP, Arteel GE. Contribution of the sympathetic hormone epinephrine to the sensitizing effect of ethanol on LPS-induced liver damage in mice. Am J Physiol Gastrointest Liver Physiol 294: G1227–G1234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang LP, Takahara T, Yata Y, Furui K, Jin B, Kawada N, Watanabe A. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. J Hepatol 31: 703–711, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.