Abstract
Disruption of the intestinal barrier is a causal factor in the development of alcoholic endotoxemia and hepatitis. This study was undertaken to determine whether zinc deficiency is related to the deleterious effects of alcohol on the intestinal barrier. Mice were pair fed an alcohol or isocaloric liquid diet for 4 wk, and hepatitis was detected in association with elevated blood endotoxin level. Alcohol exposure significantly increased the permeability of the ileum but did not affect the barrier function of the duodenum or jejunum. Reduction of tight-junction proteins at the ileal epithelium was detected in alcohol-fed mice although alcohol exposure did not cause apparent histopathological changes. Alcohol exposure significantly reduced the ileal zinc concentration in association with accumulation of reactive oxygen species. Caco-2 cell culture demonstrated that alcohol exposure increases the intracellular free zinc because of oxidative stress. Zinc deprivation caused epithelial barrier disruption in association with disassembling of tight junction proteins in the Caco-2 monolayer cells. Furthermore, minor zinc deprivation exaggerated the deleterious effect of alcohol on the epithelial barrier. In conclusion, epithelial barrier dysfunction in the distal small intestine plays an important role in alcohol-induced gut leakiness, and zinc deficiency attributable to oxidative stress may interfere with the intestinal barrier function by a direct action on tight junction proteins or by sensitizing to the effects of alcohol.
Keywords: tight junction, gut permeability, endotoxemia
endotoxemia plays an important role in the development of alcoholic liver disease through stimulating proinflammatory cytokine production (11). Disruption of the intestinal barrier has been suggested to be a leading cause of alcohol-induced endotoxemia (40). Alcoholic patients showed increased gut permeability to a variety of permeability markers, such as polyethyleneglycol, mannitol/lactulose, or 51CrEDTA (10, 22, 23, 38). In animal studies, gut permeability to macromolecules such as horseradish peroxidase (HRP) was also increased in association with alcohol-induced plasma endotoxemia and liver damage (13, 17, 24, 25, 32). Our previous work showed that orally administrated lipopolysaccharide can be detected in the plasma of alcohol-intoxicated mice but not in control mice (30), providing direct evidence that alcohol increases gut permeability to endotoxin. Animal studies also showed that prevention of gut leakiness results in suppression of alcohol exposure-induced endotoxemia and liver damage, suggesting that gut leakiness is a causal factor in the development of alcoholic endotoxemia and liver injury (17, 24, 30).
Zinc is known to play an important role in maintaining the physiological function of the gastrointestinal tract (42). Zinc deficiency has been documented in alcoholic liver disease (32). Zinc deficiency can be induced by both inadequate dietary zinc intake and zinc mobilization under stress conditions. Recent studies suggest that disruption of zinc homeostasis is a critical mediator in stress-mediated cell dysfunction and injury (26, 27). Reactive oxygen species (ROS), acetaldehyde, and lipid peroxidation products have been shown to release zinc from proteins, leading to cellular zinc dyshomeostasis. All these toxic molecules have been detected in the intestine after alcohol exposure (2, 15, 21), suggesting the possibility of intestinal zinc dyshomeostasis. Our previous studies demonstrated that zinc supplementation protects against alcohol-induced liver injury, and zinc is critical for maintenance of intestinal barrier function (20). However, the mechanism of how zinc modulates intestinal barrier function has not been determined. The present study was undertaken to determine 1) whether zinc deficiency is associated with alcohol-induced intestinal barrier dysfunction, 2) how alcohol induces zinc deficiency, and 3) how zinc deficiency affects intestinal barrier function.
MATERIALS AND METHODS
Alcohol feeding.
Male C57BL/J mice were obtained from Harlan (Indianapolis, IN). All the mice were treated according to the experimental procedures approved by the Institutional Animal Care and Use Committee. For chronic alcohol exposure, mice at 4 mo old were pair fed a Lieber-DeCarli alcohol or isocaloric maltose dextrin control liquid diet for 4 wk with a stepwise feeding procedure. The ethanol content in the diet (%, wt/vol) was 4.8 (34% of total calories) at study initiation and gradually increased up to 5.4 (38% of total calories). The food intake was measured daily, and the average daily alcohol intake was 18 g/kg body wt. At the end of the feeding experiment, the mice were fasted for 4 h and anesthetized with Avertin (300 mg/kg), and plasma, liver, and intestinal samples were harvested for assays.
Caco-2 monolayer cell culture.
Caco-2 cells from the American Type Culture Collection (Rockville, MD) were cultured in DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 10 mM HEPES, and 10% FBS, at 37°C in a 5% CO2 environment. Culture medium was changed every 2 days. Caco-2 cells were subcultured after partial digestion with 0.25% trypsin-EDTA, and passages 19–30 were used. Caco-2 cells grown on chamber slides (LabTek, Naperville, IL) were used for determination of tight junction proteins, whereas Caco-2 cells grown on six-well plates were used for immunoblotting analysis. For measurement of epithelial barrier function, Caco-2 cells were cultured on 24-well inserts (pore size 0.4 μm; BD Biosciences, San Jose, CA). For alcohol intoxication, ethanol was added to the culture medium at clinically relevant and noncytotoxic doses of 1, 2.5, 5, 7.5, and 10% (vol/vol) as described previously (31). N-acetyl-cysteine (NAC) was added at 2 mM 30 min before alcohol treatment to inhibit oxidative stress. To induce zinc deficiency, N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) was added to the culture medium at a final concentration of 2, 3, or 4 μM, and the culture time was 24 h. Zinc sulfate was added at 100 μM elemental zinc together with TPEN to confirm the specificity of the zinc-chelating action of TPEN. Alternatively, TPEN specificity was also tested by adding other divalent cations including 100 μM copper as cupric chloride or 100 μM iron as ferrous chloride. To determine the interaction of zinc deficiency with alcohol intoxication, Caco-2 cells were first treated with TPEN for 24 h, followed by incubation with 5% ethanol for 5 h.
Assessment of liver injury.
Histopathological changes in the liver were examined by light microscopy with hematoxylin and eosin stain. Serum alanine aminotransferase (ALT) activity was colorimetrically measured using the Infinity ALT Reagent (Thermo Scientific, Waltham, MA).
Blood endotoxin assay.
Blood samples from control and treated mice were drawn from the dorsal vena cava. Plasma was obtained by centrifuging the blood at 300 g for 15 min at 4°C. Plasma samples were diluted 1:10 with sterile nanopure water, mixed by vortex, and placed in a 75°C water bath for 10 min. Samples were allowed to cool to room temperature for 10 min before colorimetric assay using the limulus ameobocyte lysate (LAL) kit (Lonza Walkersville, MD). Standards and samples were incubated with LAL for 10 min at 37°C followed by 6-min incubation with colorimetric substrate. The reaction was stopped with 25% acetic acid, and the absorbance at 405 nm was read.
Determination of gut permeability.
For ex vivo detection of intestinal permeability, the duodenum, jejunum, and ileum were freshly isolated and placed in modified Krebs-Henseleit bicarbonate buffer containing 8.4 mM HEPES, 119 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2, and 11 mM glucose (KHBB, pH 7.4). One end of the gut segment was first ligated with suture, and 100 μl FITC-dextran (molecular weight 4,000, FD-4, 40 mg/ml) was injected into the lumen using a gavage needle to avoid mucosal injury. Then the other end of the gut segment was ligated to form a 8-cm gut sac. After being rinsed in the KHBB buffer, the gut sac was placed in 2 ml of KHBB and incubated at 37°C for 20 min. The FD-4 that penetrated from the lumen into the incubation buffer was measured spectrofluorometrically with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The FD-4 permeability was expressed as micrograms per centimeter per minute.
Immunofluorescence microscopy of tight-junction proteins.
Cryostat sections of the ileum and Caco-2 cell chamber slides were fixed with cold methanol for 5 min at −20°C. The intestinal tissues or Caco-2 cells were then incubated with polyclonal rabbit anti-claudin-1, occludin or zonula occludens (ZO)-1 antibody (Zymed Laboratories, San Francisco, CA) overnight at 4°C, followed by incubation with a Cy3-conjugated antibody for tissue sections or FITC-conjugated antibody for Caco-2 cells for 30 min at room temperature.
Fluorescence microscopy of ROS.
ROS accumulation in the small intestine and Caco-2 cells was examined by dihydroethidium fluorescence microscopy. Nonfluorescent dihydroethidium is oxidized by ROS to yield the red fluorescent product, ethidium, that binds to nucleic acids, staining the nucleus a bright fluorescent red. Cryostat sections of small intestinal segments including duodenum, jejunum, and ileum or Caco-2 cell chamber slides were incubated with 5 μM dihydroethidium (Molecular Probes, Eugene, OR) for 30 min at 37°C in the dark. The ROS-catalyzed ethidium red fluorescence was examined under fluorescence microscopy. The relative fluorescence intensity was quantified by using SigmaScan Pro 5 software.
Intestinal zinc concentrations.
Zinc concentrations in the small intestinal segments including duodenum, jejunum, and ileum were determined by atomic absorbance spectrophotometry. The relative fluorescence intensity was quantified as described above.
Zinquin fluorescence microscopy.
Zinquin is a blue fluorescent zinc ion indicator. Caco-2 cells cultured on chamber slides were incubated with 25 μM Zinquin ethyl ester in PBS for 30 min at room temperature. The labeled zinc ion was detected by fluorescence microscopy, and the relative fluorescence intensity was quantified by using SigmaScan Pro 5 software.
Caco-2 monolayer barrier function analysis.
The Caco-2 monolayer barrier function was evaluated by measuring the electrical resistance and paracellular permeability. The transepithelial electrical resistance (TEER) of the filter-grown Caco-2 monolayers was measured with an epithelial volt ohmmeter (World Precision Instruments, Sarasota, FL). Electrical resistance was recorded with three consecutive measurements after subtracting the resistance value of the filters alone. For determination of paracellular permeability, FD-4 was added to the apical compartment of Caco-2 cells at the final concentration of 10 mg/ml in DMEM. After 90-min incubation, the basolateral media were collected and the FD-4 that penetrated to the basolateral media was measured using a microplate fluorescence reader with an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Immunoblotting analysis of tight junction proteins.
Tight junction proteins from Triton-soluble and -insoluble fractions were prepared. Briefly, ileum mucosa or cell monolayers were lysed on ice for 30 min in Triton-soluble buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 4 mM Na3VO4, 40 mM NaF, 1% Triton X-100, 1 mM PMSF, 1% protease inhibitor cocktail) and centrifuged at 14,000 g for 10 min. The supernatants were regarded as Triton-soluble fractions, and the pellets were resuspended in Triton-insoluble buffer (Triton-soluble buffer with 1% SDS) and sonicated. After being centrifuged at 14,000 g for 5 min, the supernatants were collected as Triton-insoluble fractions. Aliquots containing 30 μg protein were loaded on to a 8–15% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to polyvinylidene fluoride membrane. The membrane was probed with rabbit polyclonal antibody against claudin-1, occluding, ZO-1 (Zymed Laboratories), or GAPDH (Santa Cruz Biotechnologies, Santa Cruz, CA). The membrane was then processed with HRP-conjugated donkey anti-rabbit IgG (GE Healthcare, Piscataway, NJ). The protein bands were visualized by an enhanced chemiluminescence detection system (GE Healthcare) and quantified by densitometry analysis.
Statistics.
All data are expressed as means ± SD. The data were analyzed by ANOVA and Newman-Keuls multiple-comparison test. Differences between groups were considered significant at P < 0.05.
RESULTS
Alcohol exposure-induced liver injury.
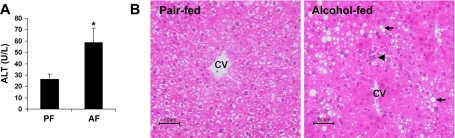
The body weights of the alcohol-fed mice (27.1 ± 1.0 g) at the end of 4-wk alcohol exposure were significantly lower compared with the pair-fed mice (29.0 ± 0.6 g). The liver/body weight ratios of the alcohol-fed mice (4.7 ± 0.2) were significantly higher than those of pair-fed mice(4.0 ± 0.3). Alcohol exposure significantly elevated the plasma ALT activities (Fig. 1A). Moreover, alcohol exposure caused liver pathological changes, including steatosis, necrosis, and neutrophil infiltration (Fig. 1B).
Fig. 1.
Liver injury in mice chronically fed alcohol (AF) for 4 wk. A: plasma alanine aminotransferase (ALT) activities. Results are means ± SD (n = 6–8). *Significantly different (P < 0.05, t-test) from the pair-fed (PF) mice. B: liver histopathology. Light microscopy shows lipid accumulation (arrows) and inflammation (arrowhead) in the liver of alcohol-fed mice. Hematoxylin and eosin stain. CV, central vein. Scale bar = 50 μM.
Alcohol exposure-induced disruption of intestinal barrier function.
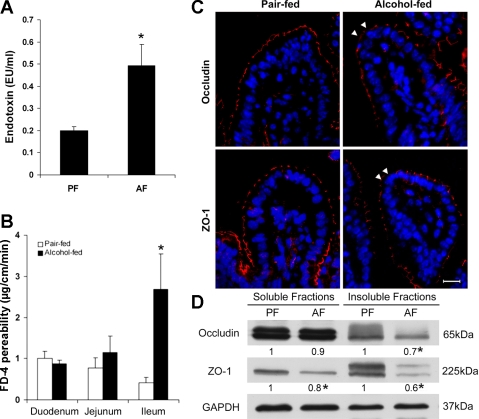
As an indication of intestinal barrier disruption, the plasma endotoxin concentrations were measured, and alcohol exposure significantly increased the plasma endotoxin level (Fig. 2A). The effect of alcohol on the intestinal barrier function was determined by ex vivo measuring the intestinal permeability to FD-4. As shown in Fig. 2B, alcohol exposure did not affect the FD-4 permeability of the duodenum and jejunum but significantly increased the ileal permeability. The distribution of the tight junction proteins, occludin, a transmembrane protein, and ZO-1, an intracellular plaque protein, at the ileal epithelium was then determined by immunofluorescence microscopy. Alcohol exposure caused a reduced distribution of both occludin and ZO-1 at the tight junctions between the adjacent epithelial cells (Fig. 2C). Immunoblotting analysis further confirmed that alcohol exposure reduced the protein levels of occludin and ZO-1 (Fig. 2D). However, light microscopy with hematoxylin and eosin stain did not reveal remarkable pathological changes of the intestine after alcohol exposure (data not shown).
Fig. 2.
Intestinal barrier dysfunction in mice chronically fed alcohol for 4 wk. A: plasma endotoxin. Endotoxin levels were measured by the limulus ameobocyte lystate method. B: intestinal permeability. The penetration of intralumen FITC-dextran (FD-4) to the incubation buffer was determined after incubation of the intestinal sac of duodenum, jejunum, and ileum for 20 min. C: immunofluorescence microscopy of the ileal tight-junction proteins. Arrowheads indicate the disappearance of occludin or zonula occludens (ZO)-1. Scale bar = 30 μM. D: immunoblotting of ileal occludin and ZO-1. The bands were quantified by densitometry analysis, and the ratio to GAPDH was calculated by setting the value of controls as 1. Results are means ± SD (n = 6–8 in A and B, n = 3 in D). *Significantly different (P < 0.05, t-test) from the pair-fed mice.
Alcohol-induced oxidative stress and zinc deficiency in the intestine.
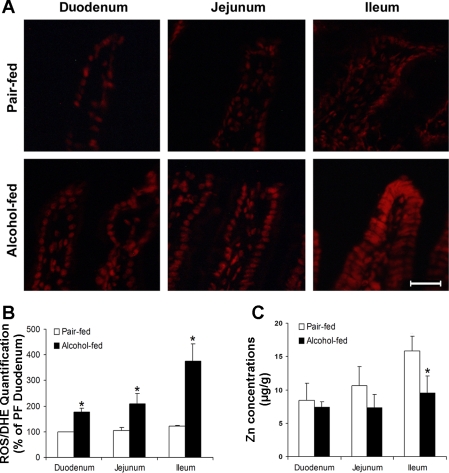
Oxidative stress in the small intestine was assessed by measuring ROS accumulation with ethidium fluorescence microscopy (Fig. 3A) and image quantification (Fig. 3B). Whereas only trace amounts of ROS were detected in the duodenum, jejunum, and ileum in the pair-fed mice, chronic alcohol exposure caused ROS accumulation in the small intestine as indicated by increased red fluorescence intensity. The ROS levels gradually increased along the small intestine from the caudal to the distal segment, and the ileum showed the strongest labeling. To determine whether zinc dyshomeostasis in the intestine is associated with oxidative stress, intestinal zinc concentrations were analyzed by atomic absorbance spectrophotometry. Alcohol exposure did not affect the zinc status in the duodenum and jejunum but significantly decreased the zinc concentrations in the ileum (Fig. 3C).
Fig. 3.
Intestinal reactive oxygen species (ROS) accumulation and zinc concentrations in mice chronically fed alcohol for 4 wk. A: fluorescence microscopy of ROS. Cryostat intestinal sections were incubated with dihydroethidium (DHE) at 5 μM, and red fluorescence was formed in the nuclei upon oxidation by ROS. Scale bar = 40 μM. B: quantitative analysis of the ethidium fluorescence intensity. C: intestinal zinc concentrations. Total zinc was measured by atom absorption spectroscopy. Results are means ± SD (n = 6–8). *Significantly different (P < 0.05, t-test) from the pair-fed mice.
Association of zinc mobilization with alcohol-induced oxidative stress in Caco-2 cells.
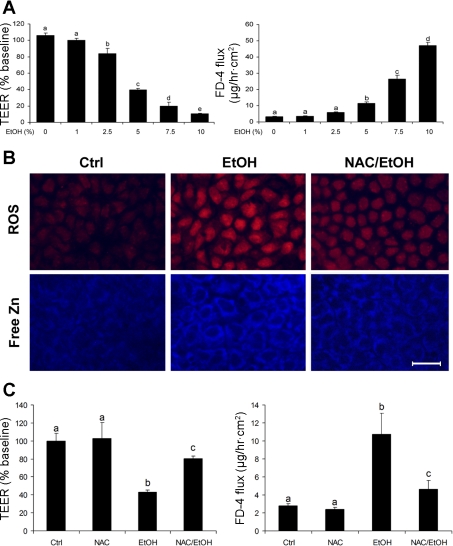
Caco-2 cell culture was performed to determine the link between zinc dyshomeostasis and oxidative stress in alcohol-induced epithelial barrier disruption. The effect of alcohol treatment on the epithelial barrier function was assessed by measuring TEER and FD-4. As shown in Fig. 4A, alcohol treatment for 5 h caused a significant decrease in the epithelial TEER in a dose-dependent manner. Consistent with these results, the paracellular permeability to FD-4 was significantly increased by alcohol in a dose-dependent manner. ROS accumulation was detected by dihydroethidium fluorescent microscopy, and alcohol treatment generated ROS as indicated by increased red fluorescence in the nuclei (Fig. 4B). To determine whether alcohol induces zinc mobilization, the intracellular free zinc was detected by Zinquin, which binds free zinc to generate blue fluorescence. As shown in Fig. 4B, alcohol treatment markedly increased the intensity of cytoplasmic Zinquin labeling. To determine the link of zinc mobilization with ROS accumulation, inhibition of ROS was achieved by pretreatment with NAC. Inhibition of ROS with NAC was accompanied by reduction of zinc release. In accordance, NAC pretreatment attenuated alcohol-induced epithelial barrier dysfunction (Fig. 4C).
Fig. 4.
Association of zinc mobilization with ROS generation in alcohol-induced epithelial barrier disruption in Caco-2 cells. A: epithelial barrier function. The Caco-2 cells on the inserts were treated with ethanol at a concentration of 1, 2.5, 5, 7.5, or 10% (vol/vol) for 5 h, and alteration in epithelial barrier function was assessed by measuring transepithelial electrical resistance (TEER) and FD-4 permeability. B: fluorescence microscopy of ROS and free zinc. Caco-2 cells were cultured on chamber slides and treated with 5% ethanol (vol/vol) for 5 h in the presence or absence of 2 mM N-acetyl-cysteine (NAC). ROS and free zinc were detected by fluorescent microscopy after incubation with dihydroethidium (5 μM) or Zinquin (25 μM), respectively. Scale bar = 25 μM. C: effect of NAC on alcohol-induced epithelial barrier disruption. Caco-2 cells were treated with 5% ethanol with or without 2 mM NAC pretreatment. Results are means ± SD (n = 8). Significant differences (P < 0.05, ANOVA) are identified by different letters, a-e. Ctrl, control; E or EtOH, ethanol.
Effect of zinc deprivation on Caco-2 epithelial barrier function.
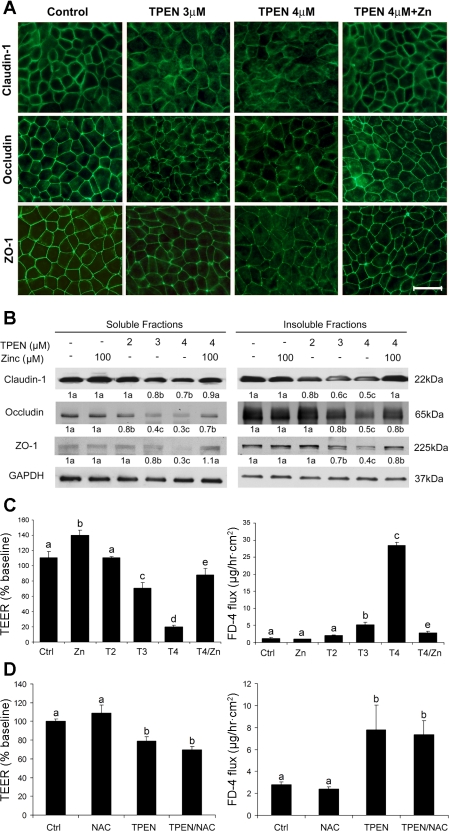
To define the role of zinc in epithelial barrier function, zinc deprivation was induced by TPEN. The effects of zinc deprivation on epithelial barrier integrity were determined by immunofluorescence labeling of tight junction proteins. As shown in Fig. 5A, TPEN at 3 and 4 μM caused significant reduction of tight-junction proteins, including claudin-1, occludin, and ZO-1. TPEN at 2 μM did not appear to affect the distribution of the tight-junction proteins (Fig. 6A). Immunoblotting analysis showed that TPEN at 3 and 4 μM significantly decreased the protein levels of all the three tight-junction proteins in either soluble or insoluble fractions, and TPEN at 2 μM only reduced the protein levels of insoluble fractional claudin-1 and soluble fractional occludin (Fig. 5B). Barrier function analysis showed that TPEN treatment caused a decrease in the epithelial TEER and an increase in FD-4 permeability in a dose-dependent manner (Fig. 5C). NAC pretreatment did not affect TPEN-induced epithelial barrier dysfunction (Fig. 5D). Addition of zinc attenuated TPEN-impaired epithelial barrier function in association with preservation of the tight junction proteins (Fig. 5), whereas the effects of TPEN on epithelial barrier were not reversed by addition of either copper or iron (data not shown).
Fig. 5.
Effect of zinc deprivation on the epithelial barrier of Caco-2 cells. Caco-2 cells were cultured on inserts and treated with N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) at 2, 3, and 4 μM or 4 μM TPEN plus 100 μM zinc for 24 h. A: distribution of tight-junction proteins were examined by detected by immunofluorescence microscopy. Scale bar = 25 μM. B: protein levels of tight-junction proteins were determined by immunoblotting analysis. Immunoblot bands were quantified by densitometry analysis, and the ratio to GAPDH was calculated by setting the value of controls as 1. C: epithelial barrier function was assessed by measuring TEER and FD-4 permeability. D: effect of NAC on TPEN-induced epithelial barrier disruption. Caco-2 cells were treated with 3 μM TPEN with or without 2 mM NAC pretreatment. Results are means ± SD (n = 4 in B, n = 8 in C and D). Significant differences (P < 0.05, ANOVA) are identified by different letters, a-e. T, TPEN.
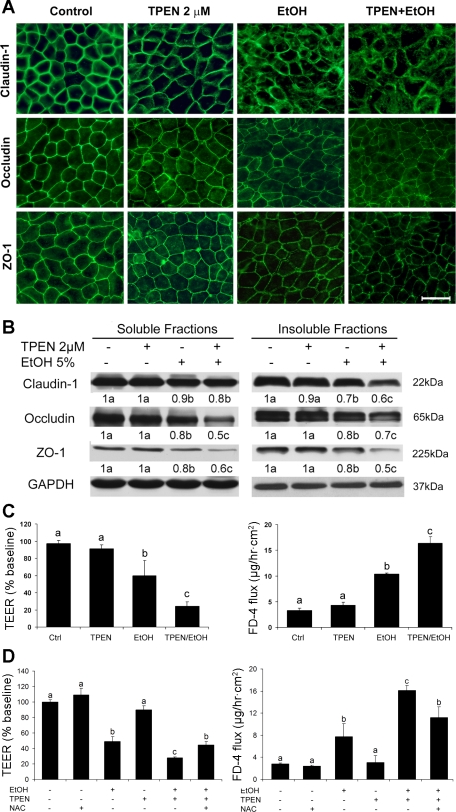
Fig. 6.
Sensitizing effect of zinc deprivation on alcohol-induced epithelial barrier dysfunction. Caco-2 cells were cultured on inserts and treated with TPEN at 2 μM for 24 h, followed by treatment with 5% (vol/vol) ethanol for 5 h. A: distribution of tight-junction proteins detected by immunofluorescence microscopy. Scale bar = 25 μM. B: protein levels of tight-junction proteins were determined by immunoblotting analysis. Immunoblot bands were quantified by densitometry analysis, and the ratio to GAPDH was calculated by setting the value of controls as 1. C: epithelial barrier function assessed by measuring TEER and FD-4 permeability. D: effects of NAC on TPEN- or/and ethanol-induced epithelial barrier disruption. Caco-2 cells were treated with 5% ethanol (vol/vol) or/and 2 μM TPEN with or without 2 mM NAC pretreatment. Results are means ± SD (n = 4 in B, n = 8 in C and D). Significant differences (P < 0.05, ANOVA) are identified by different letters, a-c.
Interaction of zinc deficiency with alcohol in induction of Caco-2 epithelial barrier disruption.
To determine whether zinc deficiency might interact with alcohol to induce epithelial barrier disruption, Caco-2 cells were first cultured under minor zinc deprivation, followed by treatment with alcohol. As shown in Fig. 6A, alcohol treatment reduced the distribution of claudin-1, occludin, and ZO-1 at the tight junctions, whereas TPEN at 2 μM did not affect the tight junction proteins. The Caco-2 cells treated with alcohol after zinc deprivation showed a dramatic reduction of tight junction proteins. Immunoblotting analysis showed that alcohol treatment following zinc deprivation significantly reduced the protein levels of all three tight-junction proteins in both soluble and insoluble fractions compared with alcohol alone (Fig. 6B). TPEN at 2 μM did not significantly affect Caco-2 epithelial barrier function as indicated by measurements of TEER and FD-4 permeability (Fig. 6C). Alcohol treatment caused a significant decrease in TEER and a significant increase in FD-4 permeability, indicating an impaired epithelial barrier function. However, Caco-2 cells treated with alcohol after zinc deprivation showed significantly lower TEER and higher FD-4 permeability than alcohol alone. NAC pretreatment attenuated TPEN and alcohol-induced epithelial barrier dysfunction (Fig. 6D).
DISCUSSION
The barrier function of intestinal epithelium is provided by the epithelial cells and the paracellular apical junction complex, including tight junctions and adherence junctions (14, 35). Tight junctions are the most apical organelle of the apical junction complex and are primarily involved in the regulation of paracellular permeability. Either loss of the epithelial cells or disruption of the tight junctions will lead to an increase in intestinal permeability. Clinical studies demonstrated that acute alcohol administration can cause apparent histopathological changes in the proximal part of the small intestine, whereas chronic alcohol consumption only induces minor histopathological changes (9, 12, 34). Our previous study (30) also showed that intestinal histopathological alterations, such as loss of epithelial cells at the top of the intestinal villi, were associated with acute alcohol intoxication-induced intestinal barrier disruption in mice (30). The present study demonstrated that chronic alcohol exposure causes gut leakiness without obviously affecting intestinal histology. Most importantly, we found that the gut leakiness after chronic alcohol exposure occurs in the ileum but not in the duodenum or jejunum. These data provide evidence for the first time that the lower part of the small intestine plays an important role in the development of alcoholic endotoxemia.
Tight junctions are composed of several transmembrane proteins such as occludin and claudins and intracellular molecules such as ZO-1 (18). The interactions of the cell surface molecules ensure intercellular adhesion and regulate paracellular permeability. Disassembling of proteins at the tight junctions will result in disruption of the intestinal barrier to allow the diffusion of macromolecules such as endotoxin and pathogens from the intestinal lumen into the blood. This appears to be a common mechanism involved in the pathogenesis of a number of gastrointestinal diseases such as inflammatory bowel disease and celiac disease (28, 38, 36). A recent study demonstrated that the ZO-1 protein levels in colon biopsies from alcoholic patients are significantly lower than in normal subjects (46). The present study detected a reduced distribution of transmembrane protein, occludin, and intracellular plaque protein, ZO-1, at the tight junctions of the ileal mucosal epithelium in the mice chronically fed alcohol. Thus disassembly of tight-junction proteins is likely a causal factor in the pathogenesis of chronic alcohol exposure-induced intestinal barrier dysfunction.
Oxidative stress and alcohol reactive metabolites have been suggested to critically mediate alcohol-induced intestinal barrier dysfunction. Previous studies have shown that alcohol exposure induces oxidative stress in the intestine, and prevention of oxidative stress leads to inhibition of the gut leakiness (2, 17, 21, 24, 25). Acetaldehyde, the major alcohol-reactive metabolite, has also been detected in the intestine after alcohol exposure (8, 15). Caco-2 enterocyte culture studies demonstrated that alcohol treatment induced a progressive disruption of ZO-1 from the tight junction and led to the formation of gaps between the adjacent cells (31). ROS and nitric oxide production in Caco-2 cells were detected after alcohol treatment, and oxidation and nitration of tubulin were found in association with epithelial barrier disruption (3). Treatment with antioxidants, such as l-cysteine, superoxide dismutase, and rebamipide, iNOS inhibitors, and growth factors, such as epididymal growth factor or TGF-β, inhibited alcohol-induced ROS accumulation, tubulin nitration, and epithelial barrier disruption (4–7). These findings suggest that ROS critically mediate alcohol-induced epithelial barrier disruption. The present study showed that chronic alcohol exposure causes significant accumulation of ROS in the ileum but not in the duodenum and jejunum, which correlates well with the measurements of gut permeability. Interestingly, the zinc levels in the ileum, but not in the duodenum and jejunum, were also decreased by chronic alcohol exposure. To define the link between alcohol-induced oxidative stress and zinc release, Caco-2 cell culture model was introduced. However, high ethanol concentration (5–10%, vol/vol) was required for significant reduction of TEER (>60%). Western blotting assay of alcohol metabolic enzymes showed that Caco-2 cells express abundant aldehyde dehydrogenase but only a minimum of alcohol dehydrogenase (data not shown), indicating a lower alcohol metabolic capacity. Caco-2 cell culture demonstrated that attenuation by NAC of ROS led to attenuation of alcohol-induced zinc mobilization and epithelial barrier dysfunction in Caco-2 cells. However, NAC did not affect TPEN-induced epithelial barrier dysfunction. These data suggest that zinc deprivation under oxidative stress condition critically mediates alcohol-induced intestinal epithelial barrier dysfunction.
Zinc is an important trace element that is involved in all the major aspects of cell function, including metabolism, detoxification, antioxidant defense, signal transduction, and gene regulation (44). Although it is known that zinc is necessary for proper liver function, increasing evidence suggests that zinc plays an important role in maintaining epithelial integrity of the gastrointestinal tract (20, 42, 43). Oral zinc supplementation has been shown to prevent gut leakiness under the a variety of disease conditions such as Crohn's disease, experimental colitis, malnutrition, enteral pathogen challenge, and methotrexate treatment (1, 41, 45, 47, 48). Electron microscopic studies showed that percentages of the disrupted (opened) tight junctions in experimental colitis were reduced by 50% with zinc supplementation (45). Mechanistic studies showed that zinc preservation of the intestinal barrier function was associated with induction of metallothionein (MT) and inhibition of oxidative stress (37, 48). A recent study showed that zinc deficiency attributable to reduction of zinc transporters accounts for alcohol-induced alveolar epithelial barrier dysfunction (19). Zinc deficiency in Caco-2 cells not only induced membrane barrier damage but also increased neutrophil transmigration (16). Our previous study (29) showed that zinc protects against acute alcohol intoxication-induced intestinal histopathological changes independent of MT, suggesting the possibility that other zinc-binding proteins may mediate the effects of zinc. The present study demonstrated that zinc deprivation disassembles the tight-junction proteins, suggesting a novel mechanism of how zinc modulates the epithelial barrier. We also found that minor zinc deficiency has a sensitizing effect to alcohol-induced epithelial barrier disruption. These data suggest that zinc deficiency may not only mediate but also sensitize to alcohol effects on the epithelial barrier. Because ROS, aldehyde, and lipid peroxidation products have been shown to release zinc from zinc proteins, the significance of dysfunction of zinc proteins in the pathogenesis of alcohol-induced gut leakiness should be further defined in future investigations.
In conclusion, the present study demonstrated that chronic alcohol exposure induces ileal oxidative stress, which leads to zinc deficiency by mobilizing intracellular zinc. Zinc deficiency may directly disassemble tight-junction proteins or indirectly sensitize the epithelial cells to the action of alcohol, leading to epithelial barrier dysfunction with subsequent increase in gut permeability. These results thus indicate that zinc deficiency critically modulates alcohol-induced intestinal barrier dysfunction and the resulting development of endotoxemia and liver injury in alcoholic liver disease.
GRANTS
This research was supported in part by the National Institutes of Health (Z. Zhou, C.J. McClain, Y.J. Kang), Office of Dietary Supplements (Z. Zhou) grants, University of Louisville Intramural Grant Program (M. Cave), and the Veterans Administration (C. McClain).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this article with any of the authors or any of the authors' academic institutions or employers.
ACKNOWLEDGMENTS
We thank Xinguo Sun for technical assistance and Marion McClain for review of this article. Wei Zhong (D.V.M.) is supported by the State Scholarship Fund of China Scholarship Council. Y.J. Kang and C.J. McClain are Distinguished University Scholars of the University of Louisville.
REFERENCES
- 1.Altaf W, Perveen S, Rehman KU, Teichberg S, Vancurova I, Harper RG, Wapnir RA. Zinc supplementation in oral rehydration solutions: experimental assessment, and mechanisms of action. J Am Coll Nutr 21: 26–32, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bagchi D, Carryl OR, Tran MX, Krohn RL, Bagchi DJ, Garg A, Bagchi M, Mitra S, Stohs SJ. Stress, diet, and alcohol-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. J Appl Toxicol 18: 3–13, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction, and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther 291: 1075–1085, 1999 [PubMed] [Google Scholar]
- 4.Banan A, Farhadi A, Fields JZ, Mutlu E, Zhang L, Keshavarzian A. Evidence that nuclear factor-kappa B activation is critical in oxidant-induced disruption of the microtubule cytoskeleton, and barrier integrity and that its inactivation is essential in epidermal growth factor-mediated protection of the monolayers of intestinal epithelia. J Pharmacol Exp Ther 306: 13–28, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Banan A, Zhang LJ, Farhadi A, Fields JZ, Shaikh M, Keshavarzian A. PKCβ1 isoform activation is required for EGF-induced NF-κB inactivation, and IκB α stabilization and protection of F-actin assembly and barrier function in enterocyte monolayers. Am J Physiol Cell Physiol 286: C723–C738, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Inhibition of oxidant-induced nuclear factor-kappaB activation, and inhibitory-kappaB alpha degradation and instability of F-actin cytoskeletal dynamics and barrier function by epidermal growth factor: key role of phospholipase-gamma isoform. J Pharmacol Exp Ther 309: 356–368, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Novel effect of NF-κB activation: carbonylation, and nitration injury to cytoskeleton and disruption of monolayer barrier in intestinal epithelium. Am J Physiol Cell Physiol 287: C1139–C1151, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions, and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol 289: G367–G375, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bhonchal S, Nain CK, Prasad KK, Nada R, Sharma AK, Sinha SK, Singh K. Functional, and morphological alterations in small intestine mucosa of chronic alcoholics. J Gastroenterol Hepatol 23: e43––e48., 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1: 179–182, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res 29: 166S–171S, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bode JC, Knüppel H, Schwerk W, Lorenz-Meyer H, Dürr HK. Quantitative histomorphometric study of the jejunal mucosa in chronic alcoholics. Digestion 23: 265–270, 1982 [DOI] [PubMed] [Google Scholar]
- 13.Enomoto N, Takei Y, Hirose M, Ikejima K, Miwa H, Kitamura T, Sato N. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization, and TNF-alpha production. Gastroenterology 123: 291–300, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health, and disease. J Gastroenterol Hepatol 18: 479–497, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora, and mast cell activation in rodents. Am J Pathol 168: 1148–1154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr 138: 1664–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43: 163–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Förster C. Tight junctions, and the modulation of barrier function in disease. Histochem Cell Biol 130: 55–70, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol 41: 207–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang YJ, Zhou Z. Zinc prevention, and treatment of alcoholic liver disease. Mol Aspects Med 26: 391–404, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kaur M, Kaur J, Ojha S, Mahmood A. Ethanol effects on lipid peroxidation, and glutathione-mediated defense in rat small intestine: role of dietary fats. Alcohol 15: 65–69, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute, and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol 89: 2205–2211, 1994 [PubMed] [Google Scholar]
- 23.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther 299: 442–448, 2001 [PubMed] [Google Scholar]
- 25.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50: 538–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kröncke KD. Cellular stress, and intracellular zinc dyshomeostasis. Arch Biochem Biophys 463: 183–187, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kroencke KD, Klotz LO. Zinc fingers as biological redox switches? Antioxid Redox Signal 11: 1015–1027, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 159: 2001–2009, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Preservation of intestinal structural integrity by zinc is independent of metallothionein in alcohol-intoxicated mice. Am J Pathol 164: 1959–1966, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther 305: 880–886, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 276: G965–G974, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology 32: 1008–1017, 2000 [DOI] [PubMed] [Google Scholar]
- 33.McClain CJ, Antonow DR, Cohen DA, Shedlofsky S. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res 10: 582–589, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Millan MS, Morris GP, Beck IT, Henson JT. Villous damage induced by suction biopsy, and by acute ethanol intake in normal human small intestine. Dig Dis Sci 25: 513–525, 1980 [DOI] [PubMed] [Google Scholar]
- 35.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology, and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 279: G250–G254, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Montalto M, Cuoco L, Ricci R, Maggiano N, Vecchio FM, Gasbarrini G. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated, and treated celiac disease. Digestion 65: 227–233, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Pathak A, Mahmood A, Pathak R, Dhawan D. Role of zinc on lipid peroxidation, and antioxidative enzymes in intestines of ethanol-fed rats. Biol Trace Elem Res 100: 247–257, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules, and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742–747, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 140: 12–19, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease. I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 286: G881–G884, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J Nutr 133: 4077–4082, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Semrad CE. Zinc, and intestinal function. Curr Gastroenterol Rep 1: 398–403, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Stamoulis I, Kouraklis G, Theocharis S. Zinc, and the liver: an active interaction. Dig Dis Sci 52: 1595–1612, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol 80: 1–9, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D'inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med 139: 311–315, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells, and its potential role in alcoholic liver disease. Alcohol Clin Exp Res 32: 355–364, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Inca R. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm Bowel Dis 7: 94–98, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Tran CD, Howarth GS, Coyle P, Philcox JC, Rofe AM, Butler RN. Dietary supplementation with zinc, and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine. Am J Clin Nutr 77: 1296–1303, 2003 [DOI] [PubMed] [Google Scholar]