Abstract
Previous studies have demonstrated that apical Na-bile acid cotransport (ASBT) is inhibited during chronic ileitis by both a decrease in the affinity as well as a decrease in the number of cotransporters. Methylprednisolone (MP), a commonly used treatment for inflammatory bowel disease (IBD, e.g., Crohn's disease), has been shown to reverse the inhibition of several other Na-solute cotransporters during chronic enteritis. However, the effect of MP on ASBT in the chronically inflamed ileum is not known. MP stimulated ASBT in villus cells from the normal rabbit ileum by increasing the cotransporter expression without a change in the affinity of the cotransporter for bile acid. Western blot studies demonstrated an increase in cotransporter expression. MP reversed the inhibition of ASBT in villus cells from the chronically inflamed ileum. Kinetic studies demonstrated that the mechanism of MP-mediated reversal of ASBT inhibition was secondary to a restoration of both affinity as well as cotransporter numbers. Western blot analysis demonstrated restoration of cotransporter numbers after MP treatment of rabbits with chronic ileitis. Thus MP stimulates ASBT in the normal ileum by increasing cotransporter numbers. MP reverses the inhibition of ASBT during chronic ileitis. However, MP restores the diminished affinity as well as cotransporter expression levels during chronic ileitis. Thus MP differentially regulates ASBT in the normal and in the chronically inflamed ileum.
Keywords: inflammatory bowel disease, methylprednisolone, intestinal absorption
bile acids, which are synthesized from cholesterol, are essential for ileal digestion and absorption of dietary fats and fat-soluble vitamins such as vitamins A, K, D, and E. Fats are an important nutrient source for cell growth and metabolism. Fat-soluble vitamins are vital for many cellular functions (14, 37, 50).
Fat and fat-soluble vitamin absorption require bile acid. Bile acid absorption occurs in the terminal ileum by the apical sodium-bile acid cotransporter (ASBT). ASBT is an electrogenic secondary active transporter localized on the brush-border membrane (BBM) of absorptive ileal villus cell and requires the functioning of basolateral membrane Na-K-ATPase to provide the favorable Na gradient (52). ASBT has been cloned in the human (8) and multiple animal species including hamster (53), rat (38), and rabbit (20). In the normal ileum, regulation of ASBT has been studied. For example, Vitamin D (5), glucocorticoids (18), cholic acid (41), and postpartum state (25) have been shown to stimulate ASBT.
Inflammatory bowel disease (IBD, e.g., Crohn's disease) is characterized by chronic intestinal inflammation. The most common morbidities of IBD are malnutrition, weight loss, and diarrhea (13, 34, 51). These are the result of the inhibition of nutrient, electrolyte, and water absorption in the IBD intestine. Immune-inflammatory mediators are elevated in the chronically inflamed intestinal mucosa, which has been shown to affect electrolyte and nutrient transport pathways in patients as well as in animal models of IBD or Crohn's disease (3, 29, 35).
In a rabbit model of chronic ileal inflammation, it has been shown that Na-nutrient cotransport is inhibited by a several mechanisms. Na-glucose cotransport (SGLT1) is inhibited by a decrease in the number of cotransporters (48), whereas Na- amino acid cotransport (ASCT1) activity is decreased by a decrease in the affinity (47). However, ASBT activity is inhibited by both a decrease in the number of cotransporters as well as a decrease in the affinity (49). In an acute hamster model of ileitis, ASBT was also reported to be inhibited, but the mechanism of inhibition was not elucidated (40). Furthermore, studies have shown that, in patients with IBD, bile acid absorption is compromised (15, 21, 28, 31, 39, 42).
Methylprednisolone (MP), a glucocorticoid, is commonly used to treat IBD or Crohn's disease. MP inhibits many of the major immune pathways (13, 51). MP and other glucocorticoids have been shown to reverse the inhibition of nutrient and electrolyte malabsorption in animal models of IBD or Crohn's disease (7, 10, 16, 17, 30, 32, 33, 36, 43, 46). For example, we have demonstrated that SGLT1 (43), ASCT1(46), and the Na-K-ATPase inhibition (43, 46) are reversed by MP in a rabbit model of chronic ileal inflammation. However, how MP may affect ASBT in the chronically inflamed ileum is not known. Therefore, the first aim of this study was to determine whether MP alleviates the inhibition of ASBT during chronic ileal inflammation and to ascertain the cellular mechanisms of this reversal. The second aim was to determine whether MP regulates ASBT differentially in the normal vs. the chronically inflamed ileum and to determine cellular mechanisms of this regulation.
MATERIALS AND METHODS
Ethical approval.
The experiments involving the use of the animals in these studies were approved by the West Virginia University Animal Care Use Committee (WVU ACUC). The regulations overseen by the WVU ACUC are governed by the Federal Food and Drug Administration.
Induction of chronic ileal inflammation.
Chronic ileal inflammation was induced in pathogen-free New Zealand white rabbits (1.8–2.0 kg) by intragastric inoculation with Eimeria magna (∼10,000 oocytes per animal) as described previously (49). A total of 22 animals were confirmed by histology to have chronic ileal inflammation by 14 days after inoculation and were used for the studies. In addition 22 control animals were inoculated with saline.
MP treatment.
Both normal rabbits and rabbits with chronically inflamed ileum (13 and 14 days after inoculation) were administered soluble MP sodium succinate (40 mg per day per animal for 2 days) by intramuscular injection. Control animals were treated with physiological saline.
Cell isolation.
Animals were euthanized with one dose of 100 mg/kg pentobarbital sodium. It was administered through the ear vein just before cell isolation on day 14 postinoculation. The ileum was then surgically removed and then washed thoroughly with phosphate-buffered saline. Villus cells were isolated from the ileum by a calcium chelation technique as previously described (49). Previously established villus and crypt cell separation criteria were used (e.g., alkaline phosphatase levels, Na:H exchange activity, cell size, and intracellular pH) to ensure good separation. Viability of the isolated villus cells was determined by trypan blue exclusion (45). Isolated cells were then used for intact cell uptake studies and for BBM vesicle (BBMV) preparation.
Na-K-ATPase assay.
Na-K-ATPase was measured from cell homogenate as previously described (12, 48). Specific activity was expressed as nanomoles Pi released/mg protein per minute.
BBMV preparation.
BBMV from rabbit ileal villus cells were prepared by MgCl2 precipitation and differential centrifugations as previously reported (43). BBMV were resuspended in medium appropriate for each experiment. BBMV purity was assured with marker enzyme enrichment (e.g., alkaline phosphatase).
Uptake studies in villus cells.
Cells maintained in short-term culture were washed with Na-free medium containing 4.5 mM KCl, 1.2 mM KH2PO4, 1 mM MgSO4, 1.25 mM CaCl2, 20 mM HEPES, and 130 mM choline chloride and were gassed with 100% 02 (pH 7.4 at 37°C). Following removal of Na, cells were washed and resuspended in reaction medium containing 0.1 mM taurocholate with 20 μM 3H-taurocholate, 4.5 mM KCl, 1.2 mM KH2PO4, 1.0 mM MgSO4, 1.25 mM CaCl2, 20 mM HEPES, and either 130 mM NaCl or choline chloride and were gassed with 100% 02 (pH 7.4 at 37°C). At 2- and 4-min time intervals, 100-μl aliquots were removed and uptake was arrested by mixing with 3 ml of ice-cold stop solution (Na-free medium). The mixture was filtered on 0.65-μm Millipore (HAWP) filters and washed with ice-cold stop solution. The filter was dissolved in 5 ml of scintillation fluid (Ecoscint; National Diagnostics), and radioactivity was determined in a Beckman 6500 Beta scintillation counter.
BBMV uptake.
BBMV uptake was performed by a rapid filtration technique as previously described (49). Five microliters of BBMV were resuspended in medium containing 100 mM choline chloride, 0.10 mM MgSO4, 50 mM HEPES-Tris (pH 7.5), 50 mM mannitol, and 50 mM KCl and then incubated in 95 μl reaction medium that contained 50 mM HEPES-Tris buffer (pH 7.5), 0.1 mM taurocholate with 20 μM 3H-taurocholate, 0.10 mM MgSO4, 50 mM KCl, 50 mM mannitol, and 100 mM of either NaCl or choline chloride. The vesicles were voltage clamped (10 μM valinomycin) and pH clamped [100 μM carbonyl cyanide 4-(triflouromethoxy)phenylhydrazone]. Time-course studies were performed at desired times. Uptake was arrested by mixing with ice-cold stop solution [50 mM HEPES-Tris buffer (pH 7.5), 0.10 mM MgSO4, 75 mM KCl, and 100 mM choline chloride]. The mixture was filtered on a 0.45-μm Millipore (HAWP) filter and washed with 3 ml of ice-cold stop solution. Filters were processed in the same manner as the intact cell uptakes. Kinetics studies were performed at 6 s because uptake of taurocholate was linear for at least 10 s. Iso-osmotic conditions were maintained by adjusting extravesicular mannitol concentrations.
Real-time quantitative PCR.
Real-time quantitative PCR (RTQ-PCR) was performed using total RNA using the manufacturer's protocols (Invitrogen). Total RNA was isolated from ileal villus cells using Trizol reagent (Invitrogen). First-strand cDNA was synthesized using oligo (dT) primer, random hexamers, and SuperScript III (Invitrogen). The cDNAs synthesized were used as templates for RTQ-PCR using TaqMan universal PCR master mix according to the manufacturer's protocol (Applied Biosystems). RTQ-PCR was performed in parallel for ASBT and β-actin, which was used as an endogenous control. Rabbit ASBT-specific sequence (on the basis of accession number 254357) was amplified using gene-specific primers (forward: 5′- TGGTCAGCCATGGTACAGG-3′, reverse: 5′- GCGGGAAGGTGAACACATAA-3′) and TaqMan probe (5′ FAM- CAGTTGCTTTGGAAACAGGGATGCAGAAC -TAMRA 3′). β-Actin-specific sequences were also amplified using gene-specific primers and TaqMan probe and were run along with the ASBT as an endogenous control. Gene-specific sequences of the rabbit β-actin primers (forward: - 5′GCTATTTGGCGCTGGACTT 3′, reverse: - 5′ GCGGCTCGTAGCTCTTCTC 3′) and TaqMan Probe (5′ FAM- AAGAGATGGCCACGGCCGCGAAC-TAMRA 3′) were used. The expression of β-actin was used to normalize the expression levels of ASBT between the individual samples. Final concentrations of primer and probe for both ASBT and β-actin were 500 nM and 100 nM, respectively. RTQ-PCR was performed at 95°C for 15 s and 58°C for 1 min and placed into an ABI 7300 RTQ-PCR system according to the manufacturer's protocol. Comparative threshold cycle method was used to compare the expression of ASBT in different conditions. Experiments using various different dilutions of the ASBT and β-actin cDNAs were also performed to ensure proper PCR efficiency. RTQ-PCR analyses were performed in triplicate from RNA isolated from three different rabbits.
Western blot.
Western blot analysis of apical membranes was performed as described earlier (1, 19). Samples were dissolved in RIPA buffer (50 mM Tris·HCl pH 7.4, 1% Igepal, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, and 1 mM NaF) containing protease inhibitor cocktail (SAFC Biosciences), then mixed with denaturing buffer (100 mM Tris, 25% glycerol, 2% SDS, 0.01% bromphenol blue, 10% 2-ME, pH 6.8), and electrophoresed on a 4–20% gradient Ready Gel (Bio-Rad Laboratories). The separated proteins were transferred to nylon membrane (Schleicher & Schuell) and probed by the anti-ASBT antibody (1:1,000, rabbit anti-rat ASBT antibody produced by P.A. Dawson's laboratory and used previously, Ref. 6). Goat anti-rabbit second antibody coupled to horseradish peroxidase was used to detect the binding of the anti-ASBT antibody. The resulting chemiluminescence was measured using autoradiography. ASBT abundance was quantitated using a densitometric scanner (Molecular Dynamics).
Protein assay.
Proteins were assayed using the RC DC protein assay kit according to manufacturer's protocols (Bio-Rad).
Statistics.
Results presented represent means ± SE of experiments performed and calculated using GraphPad Instat program. All uptakes were done in triplicate. Students t-test was performed for statistical analysis and is significant if the P value is <0.05.
RESULTS
Na-bile acid cotransport in villus cells.
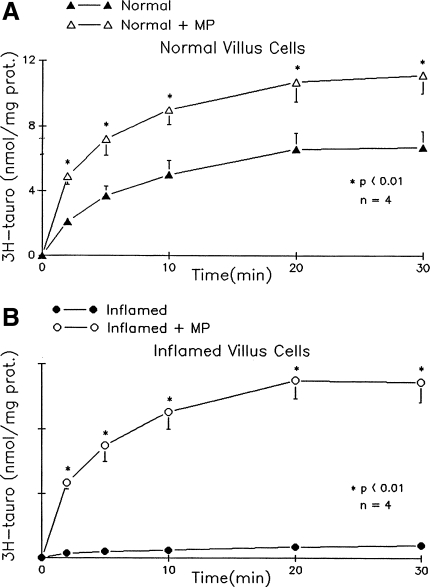
Na-dependent taurocholate uptake was present in villus cells from the normal ileum. MP treatment increased Na-dependent bile acid uptake in villus cells from the normal rabbit ileum. It was significantly reduced in villus cells isolated from chronically inflamed ileum (Fig. 1). MP treatment reversed the inhibition of ASBT in villus cells from the chronically inflamed ileum. These data indicate that MP treatment stimulates Na-bile acid cotransport in the normal rabbit ileum and reverses the inhibition of ASBT in the chronically inflamed ileum.
Fig. 1.
Effect of methylprednisolone (MP) treatment on (ASBT) in villus cells from the normal and chronically inflamed ileum. Na-dependent taurocholate (tauro) uptake is defined as taurocholate uptake in the presence of extracellular Na minus that in the absence of Na. Y-axis labeling is the same for A and B. Statistical comparisons are made of uptakes of different conditions for each time point. A: Na-dependent bile acid uptake as a function of time in villus cells from control rabbits. This uptake is significantly increased at all time points measured in villus cells from MP-treated normal rabbits. B: in villus cells from the chronically inflamed ileum, Na-dependent taurocholate uptake was significantly inhibited. Treatment of rabbits with chronic ileal inflammation with MP almost completely reversed the inhibition of Na-dependent taurocholate uptake. prot, protein.
Na-K-ATPase activity.
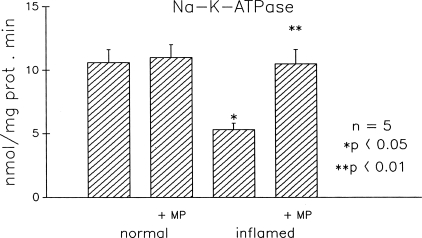
Because the Na-bile acid cotransport is dependent on the electrochemical gradient across the BBM that is maintained by the basolateral membrane Na-K-ATPase, we examined the effect of MP on Na-K-ATPase activity (Fig. 2). MP treatment did not alter Na-K-ATPase activity in villus cells from the normal ileum. MP treatment reversed the inhibition of Na-K-ATPase in villus cells from the chronically inflamed ileum (Pi released was 10.5 + 1.0 nmol mg protein per min in the normal, 11.1 + 1.1 in the normal + MP, 5.2 + 0.4* in the inflamed, and 10.6 + 0.8** in the inflamed + MP; n = 5, *P < 0.05, **P < 0.01). These results indicate that the reversal of Na-bile acid cotransport inhibition by MP treatment in the chronically inflamed ileal villus cells may, at least in part, be the result of the restoration of the Na-electrochemical gradient across the BBM. However, the stimulation of Na-bile acid cotransport by MP in the normal ileum is not secondary to an increase in Na-K-ATPase activity.
Fig. 2.
Effect of MP treatment on Na-K-ATPase activity in villus cells from the normal and chronically inflamed ileum. Villus cell Na-K-ATPase activity is expressed as nanomoles of Pi released per milligram protein per minute. MP has no effect on Na-K-ATPase activity in normal villus cells. However, Na-K-ATPase activity inhibition in the chronically inflamed ileum is reversed nearly back to normal levels by MP treatment.
Na-bile acid cotransport in BBMV.
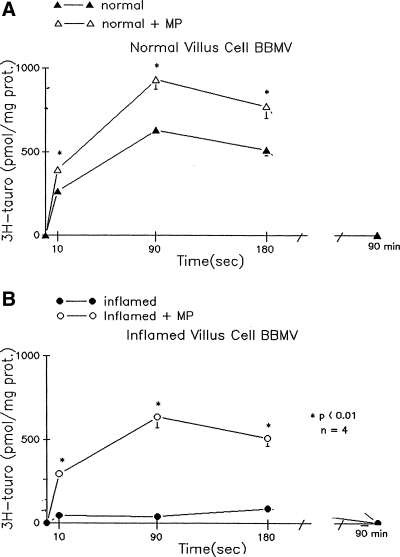
To determine whether MP has a direct effect at the level of the cotransporter on the BBM, we examined ASBT in BBMV prepared from isolated ileal villus cells. MP treatment stimulates ASBT in BBMV from the normal ileum (Fig. 3). ASBT is significantly reduced in BBMV from the chronically inflamed ileum. MP treatment of rabbits with chronic ileitis restores ASBT in BBMV. These data indicate that MP treatment stimulates Na-bile cotransport in BBMV from the normal ileum. It also reverses the inhibition of ASBT in BBMV from the chronically inflamed ileum. Thus MP has a direct effect at the level of the cotransporter in the BBM in both the normal ileum and in the chronically inflamed ileum.
Fig. 3.
Effect of MP treatment on ASBT in villus cell brush-border membrane vesicle (BBMV) isolated from the normal and chronically inflamed ileum. Na-dependent taurocholate uptake (pmol/mg of protein) is defined as taurocholate uptake in the presence of extravesicular Na minus that in the absence of Na. Y-axis labeling is the same for A and B. Statistical comparisons are made of uptakes of different conditions for each time point. All uptakes were done in triplicate, and each n represents BBMV preparations from different animals. Statistical comparisons are made of uptakes of different conditions for each time point. A: Na-dependent taurocholate uptake as a function of time in villus cell BBMV from control rabbits. This uptake was significantly increased at all time points measured in villus cell BBMV from MP-treated normal rabbits. B: in villus cell BBMV from the chronically inflamed ileum, Na-dependent taurocholate uptake was significantly reduced. Treatment of rabbits with chronic ileitis with MP almost completely reversed the inhibition of Na-dependent taurocholate uptake.
Kinetic studies.
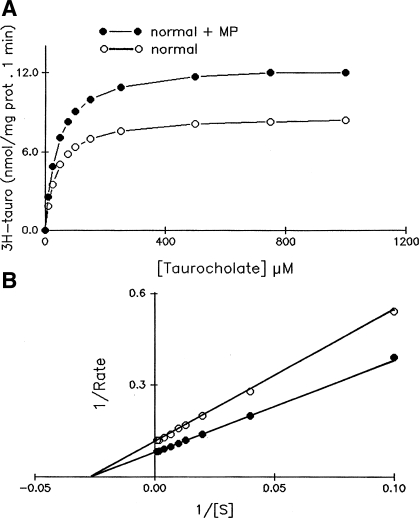
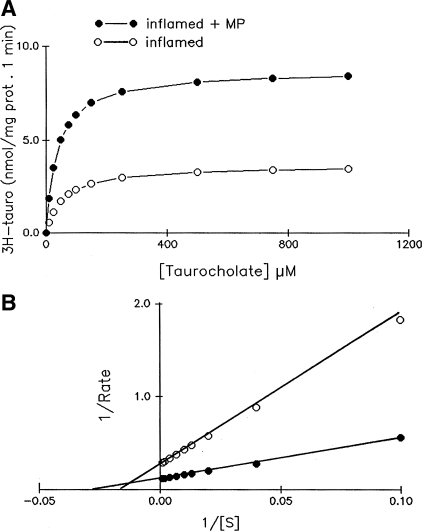
To determine the mechanism of ASBT stimulation by MP in the normal ileum and the mechanism of the MP-mediated reversal of inhibition of ASBT in the chronically inflamed ileum, kinetic studies were performed. Figure 4 shows that the uptake of Na-dependent bile acid as a function of increasing concentrations of extravesicular taurocholate in villus cell BBMV from the normal rabbits and normal rabbits treated with MP. As the concentration of extravesicular taurocholate was increased, the uptake of Na-dependent taurocholate was stimulated and subsequently became saturated in all conditions (Fig. 4A). Lineweaver Burk transformation yielded kinetic parameters (Fig. 4B.) The affinity (1/Km) for taurocholate uptake in the normal rabbit ileum was not altered by MP treatment (Km was 36.4 ± 4.2 μM in control and 39.4 ± 4.0 in control + MP, n = 3). However, the maximal rate of uptake (Vmax) of taurocholate was significantly increased in normal rabbits treated with MP compared with normal rabbits (Vmax for taurocholate uptake was 8.68 ± 0.8 nmol/mg protein at 6 s in normal rabbits and 12.69 ± 10 in normal + MP, n = 3, P < 0.01). These results indicate that the mechanism of ASBT stimulation by MP in the normal ileum is secondary to an increase in the number of cotransporters rather than altered affinity for taurocholate.
Fig. 4.
Kinetics of Na-dependent bile acid uptake in villus cell BBMV from control and MP-treated ileum. A: 3H-taurocholate uptake is shown as a function of varying concentrations of extravesicular taurocholate. Isosmolarity was maintained by adjusting the concentration of mannitol. Uptake for all concentrations was determined at 6 s. As the concentration of extravesicular taurocholate was increased, uptake of taurocholate was stimulated and subsequently became saturated in villus cell BBMV in all conditions. B: Lineweaver-Burk plot of these data with Enzfiter yielded kinetic parameters. Lineweaver Burk plot showed that the Km remains constant, whereas the Vmax changes significantly. The maximal rate of uptake of 3H-taurocholate (Vmax) was stimulated by MP. However, the affinity for 3H-taurocholate uptake in BBMV was unaffected in the MP-treated ileum. The data shown are an average of 3 experiments, and each uptake was done in triplicate.
Figure 5 shows the uptake of Na-dependent bile acid as a function of increasing concentrations of extravesicular taurocholate in villus cell BBMV from chronic ileitis and ileitis rabbits treated with MP. Treatment of rabbits with chronically inflamed ileum with MP reversed the inhibition of Na-bile acid cotransport back to near normal levels. The mechanism of reversal of Na-bile acid cotransport inhibition was the result of both the restoration of maximal rate of uptake of taurocholate (Vmax for taurocholate uptake in BBMV was 8.68 ± 0.8 nmol/mg protein at 6 s in normal rabbits, 3.66 ± 0.4 nmol/mg protein at 6 s in inflamed, and 8.70 ± 0.7 in inflamed + MP; n = 3, P < 0.01) as well as restoration of the affinity for taurocholate (Km was 36.4 ± 4.2 μM in normal, 56.8 ± 6.0 in inflamed, and 37.0 ± 3.4 in inflamed + MP; n = 3, P < 0.01). These data indicate that the mechanism of reversal of the inhibition of ASBT by MP during chronic ileitis was secondary to a restoration in both the affinity for bile acid as well as in cotransporter numbers.
Fig. 5.
Kinetics of Na-dependent bile acid uptake in villus cell BBMV from chronically inflamed and MP treated ileum. A) 3H-taurocholate uptake is shown as a function of varying concentrations of extravesicular taurocholate. Isosmolarity was maintained by adjusting the concentration of mannitol. Uptake for all concentrations was determined at 6 s. As the concentration of extravesicular taurocholate was increased, uptake of taurocholate was stimulated and subsequently became saturated in villus cell BBMV in all conditions. B: Lineweaver-Burk plot of these data with Enzfiter yielded kinetic parameters. The diminished maximal rate of uptake of 3H-taurocholate (Vmax) in the chronically inflamed ileum was reversed by MP treatment. Affinity (1/Michaelis constant) for taurocholate uptake, which was significantly diminished during chronic ileitis, was also reversed by MP in chronically inflamed ileum. The data shown are an average of 3 experiments, and each uptake was done in triplicate.
Real-time quantitative PCR.
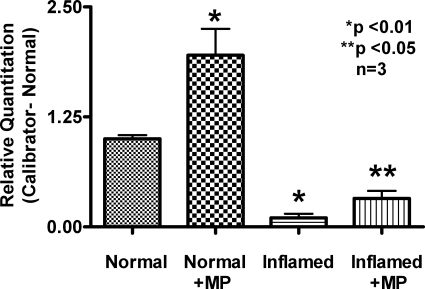
To determine the molecular mechanisms of stimulation of ASBT by MP in the normal ileum and the reversal of ASBT inhibition by MP during chronic ileitis, ASBT mRNA levels were determined by RTQ-PCR. MP treatment increased ASBT mRNA abundance in villus cells from the normal ileum. MP treatment restored ASBT mRNA levels in villus cells from the chronically inflamed ileum (Fig. 6). Thus these data indicate that MP treatment increases cotransporter numbers in normal ileum. Furthermore, in the chronically inflamed ileum, MP restores the numbers of Na-bile acid cotransporters.
Fig. 6.
Real-time PCR analysis of the ASBT in the normal, inflamed, and MP-treated rabbit ileum. Four experiments each with different animals are shown. RTQ-PCR demonstrated that the message for ASBT is increased significantly in the normal when treated with MP. Treatment of rabbits with chronic ileitis with MP partially restored the message levels of ASBT.
Western blot.
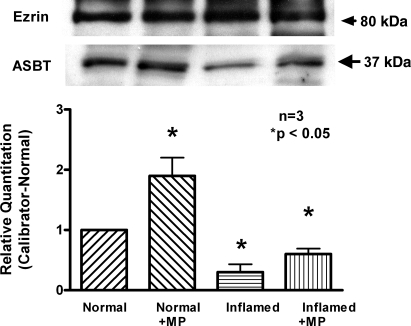
Because mRNA levels may not necessarily correlate with functional protein on the BBM, Western blot analysis was performed. MP treatment increased immunoreactive ASBT levels in villus cell BBM from the normal ileum. Furthermore, MP treatment restored immunoreactive ASBT levels in villus cell BBM from the chronically inflamed ileum as shown in Fig. 7. These data indicate that MP treatment reverses the reduction in the number of cotransporters back to normal levels in the chronically inflamed ileum, whereas it increases the number of cotransporters in the normal ileum.
Fig. 7.
Immunoreactive levels of ASBT in the normal, inflamed, and MP-treated rabbit ileum. Representative data of 4 experiments each with different animals are shown. Top: Western blot analysis demonstrated that the amounts of immunoreactive ASBT protein were increased in normal ileum treated with MP. Western blot analysis also demonstrated that the MP treatment reversed the amounts of immunoreactive ASBT protein nearly restored back to normal levels. Bottom: densitometry demonstrated the relative quantitation of ASBT, which confirmed both Western blot findings.
DISCUSSION
This study demonstrates that glucocorticoids (MP) stimulate ASBT in the normal ileum. Furthermore, it demonstrates that MP reverses the inhibition of ASBT during chronic ileitis. However, the MP-mediated mechanisms of regulation of ASBT in the normal and chronically inflamed ileum are novel and different. MP stimulates ASBT in the normal ileum by increasing the synthesis of cotransporter numbers in the villus cell BBM. In contrast, it reverses the inhibition of ASBT by restoring both the affinity for bile acid as well as BBM cotransporter numbers in the chronically inflamed ileum.
Bile acids are important for the assimilation of fat and fat-soluble vitamins. Bile acids are primarily absorbed in the distal ileum by ASBT (14, 37, 50). Stimulation of ASBT has been reported in the normal ileum. For example, glucocorticoids (18), vitamin D (5), and cholic acid (41) have been shown to increase ASBT. However, the cellular and molecular mechanisms of regulation were not completely delineated in these studies.
This study demonstrates that MP stimulates ASBT in rabbit ileal villus cells. At the cellular level, the mechanism of stimulation of ASBT is not secondary to altered Na-extruding capacity of the cell. Rather, MP appears to exert a direct effect at the level of the cotransporter on the BBM of villus cells. Because MP increases the mRNA levels of ASBT as well as the immunoreactive protein levels of ASBT in the BBM of villus cells, the likely mechanism of stimulation of ASBT in villus cells in the normal ileum is secondary to increased number of cotransporters. Indeed, other studies have also concluded that, in the normal intestine, MP may regulate Na-solute cotransport and electrolyte transport pathways directly (16, 17, 27, 32, 36). However, glucocorticoids do not indiscriminately stimulate all Na-solute cotransport processes. For example, we have demonstrated that ASCT1 is enhanced by MP treatment in normal villus cells, whereas SGLT1 is unaffected (46). Alternatively, glucocorticoids may upregulate cotransporters such as ASBT by facilitating the normal development of the intestinal mucosa (2).
Stimulation of ASBT via an increase in the number of cotransporters by glucocorticoids may occur by several mechanisms in the normal ileum. This upregulation of cotransporter numbers may be mediated by activation of the ASBT promoter by glucocorticoid-responsive elements (18). Glucocorticoids may increase ASBT levels by exerting effects on the farnesoid X receptor-short heterodimer partner-fetoprotein transcription factor (FXR-SHP-FTF) cascade, which is a bile acid feedback mechanism. Glucocorticoids share regulatory pathways associated with FXR-SHP-FTF cascade to exert effects on Na-taurocholate cotransporting peptide in hepatocytes. Although there is no direct evidence, it is possible that the bile acid feedback mechanisms of ASBT may also share regulatory pathways that may be affected by glucocorticoids in the ileum (11, 22). Glucocorticoids might also bind to the nuclear glucocorticoid receptor in the ASBT promotor, which has been shown to upregulate Na-bile acid cotransporters in the normal rat ileum (11, 18). Thus, although there is some understanding of the mechanism of regulation of ASBT by MP in the normal ileum, how MP may regulate ASBT in pathological states of the ileum is not well understood.
Human IBD is characterized by chronic inflammation in the gastrointestinal tract, most commonly in the terminal ileum. Although malabsorption of electrolytes and water has been well documented in IBD, until recently the alterations that occur in Na-nutrient cotransport processes had not been investigated at the cellular level. During chronic intestinal inflammation these cotransport processes are inhibited because of a decrease in the Na-K-ATPase activity on the BLM of villus cells because this enzyme maintains the favorable electrochemical gradient necessary for Na-nutrient cotransport. Several Na-solute cotransporters are inhibited at the level of the cotransporter in the BBM as well. However, each of these cotransporters is uniquely altered during chronic ileitis at the transporter level (47–49). SGLT1 was inhibited by a decrease in the number of cotransporters without a change in the affinity for glucose in the chronically inflamed ileum (48). In contrast, ASCT1 was inhibited by a reduction in the affinity for the amino acid without a change in the number of cotransporters during chronic ileitis (47). Unlike these two Na-solute cotransport processes, ASBT was inhibited by both a decrease in the affinity for the bile acid as well as in cotransporter numbers in the chronically inflamed ileum (49). Because a variety of immune-inflammatory mediators are increased in the chronically inflamed ileum, it is hypothesized that different immune-inflammatory mediators may differentially regulate these three cotransport pathways in the chronically inflamed ileum (44). Previous studies have shown that there are increases in many of the cytokines and inflammatory mediators produced in the rabbit model of chronic ileal inflammation, which is similar to what is found in patients with IBD (44, 45). In addition, MP does not significantly change the architecture of the chronically inflamed intestine in these animals, which indicates an effect of MP on the inflammatory mediators, which may affect the cotransporters (43).
Glucocorticoids may act as a broad-spectrum immune modulator and decrease the levels of immune inflammatory mediators. If steroids inhibit the immune inflammatory mediator responsible for ASBT inhibition, then it should alleviate the inhibition in ASBT by the same mechanism that produced the inhibition during chronic ileitis. We have shown that glucocorticoids can reverse the inhibition of SGLT1 and ASCT1 in the chronically inflamed intestine. Indeed MP reverses the inhibition of SGLT1 and ASCT1 by reversing the same mechanisms (43, 46) that caused the inhibition. In the case of SGLT1, MP reversed the inhibition by restoring the diminished cotransporter numbers. In the case of ASCT1, MP reversed the inhibition by reversing the altered affinity. These observations support the hypothesis that, rather than having a direct effect on the cotransporter, MP simply blocks the yet to be determined immune mediator in the chronically inflamed intestine that caused the unique cotransporter alterations.
At the cellular level, MP treatment reversed the inhibition of ASBT activity attributable to a reversal of diminished Na-K-ATPase activity as well as reversal of cotransporter inhibition in the BBM. However, at the cotransporter level, MP reversed the inhibition by reversing the precise mechanism that caused the specific inhibition of ASBT in the chronically inflamed intestine. ASBT is diminished in the chronically inflamed ileum by both a decrease in the affinity of cotransporter for its substrate as well as a decrease in the number of cotransporters on the BBM. However, the mechanism for the complete reversal of transporter numbers may not only be secondary to an increase in transcription but also an increase in trafficking to the membrane. Trafficking may be a part of the restoration because mRNA levels are not completely restored with MP treatment even though the numbers of ASBT proteins are restored. MP treatment reversed ASBT inhibition by restoring the affinity and the cotransporter numbers nearly back to normal levels. Thus MP likely reversed the inhibition of ASBT by inhibiting the inflammatory mediator(s) that affected ASBT in the chronically inflamed ileum.
As previously noted, although the ASBT promoter and the steroid-dependent transcription factors may regulate ASBT in the normal ileum, how ASBT is regulated in IBD is presently unknown because, other than this study, ASBT has not been studied during chronic ileal inflammation. Certain cis binding sites such as activator protein (AP)-1 or AP-2 motifs are located in the promotor region of some transport proteins that may bind to proinflammatory transcription factors during inflammation (4, 9, 23, 24, 54). By this mechanism the upregulated inflammatory mediators during inflammation may downregulate Na-solute cotransporters such as ASBT (26). This may occur to ASBT in this rabbit model of chronic ileitis.
In summary, Na-bile acid cotransport is differentially regulated by glucocorticoids. In the normal rabbit ileum, glucocorticoids stimulate ASBT by increasing the numbers of cotransporters on the BBM. The increased numbers of transporters are likely the result of increased rates of transcription and protein synthesis. Glucocorticoids reverse the inhibition of ASBT during chronic ileal inflammation. ASBT inhibition is alleviated to normal levels by restoring the numbers of cotransporters as well as restoring the affinity of the cotransporter. It is likely that this reversal may be secondary to a reduction in the levels of inflammatory mediators released during chronic ileal inflammation, which produced the ASBT inhibition. Thus these studies demonstrate that glucocorticoids differently regulate ASBT in the normal and in the chronically inflamed ileum.
GRANTS
This work was supported by the National Institutes of Health Research Grants DK45062 and DK58034 to U. Sundaram.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. P. A. Dawson for generously providing antibodies for the Western blot studies.
REFERENCES
- 1.Ausubel FM, Kingston RE, Moore DD, Seidman JG, Smith JA, Stuhl K. Current Protocols in Molecular Biology New York: John Wiley & Sons, 1995 [Google Scholar]
- 2.Barnard JA, Ghishan FK. Methylprednisolone accelerates the ontogeny of sodium-taurocholate cotransport in rat ileal brush border membranes. J Lab Clin Med 108: 549–555, 1986 [PubMed] [Google Scholar]
- 3.Castro G. Immunological Regulation of Electrolyte Transport New York: Raven, 1990, p. 31–46 [Google Scholar]
- 4.Chen F, Ma L, Al-Ansari N, Shneider B. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J Biol Chem 276: 38703–38714, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Chen F, Liu S, Glaeser H, Dawson PA, Hofmann AF, Kim RB, Shneider BL, Pang KS. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1alpha,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol Pharmacol 69: 1913–1923, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol Gastrointest Liver Physiol 271: G377–G385, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Coon S, Sundaram U. Mechanism of glucocorticoid-mediated reversal of inhibition of Cl−/HCO−(3) exchange during chronic ileitis. Am J Physiol Gastrointest Liver Physiol 278: G570–G577, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol Gastrointest Liver Physiol 274: G157–G169, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Cuff MA, Shirazi-Beechey SP. The human monocarboxylate transporter MCT1: gene structure and regulation. Am J Physiol Gastrointest Liver Physiol 289: G977–G979, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Domenech E. Inflammatory bowel disease: current therapeutic options. Digestion 73, Suppl 1: 67–76, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol 20: 65–79, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Forbush B., 3rd Assay of Na-K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal Biochem 128: 159–163, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 12, Suppl 1: S3–S9, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hofmann AF. Intestinal Absorption of Bile Acids In Biliary Constituents New York: Raven, 1994, p. 1845–1866 [Google Scholar]
- 15.Holmquist L, Andersson H, Rudic N, Ahren C, Fallstrom SP. Bile acid malabsorption in children and adolescents with chronic colitis. Scand J Gastroenterol 21: 87–92, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Iannoli P, Miller JH, Ryan CK, Sax HC. Glucocorticoids upregulate intestinal nutrient transport in a time-dependent and substrate-specific fashion. J Gastrointest Surg 2: 449–457, 1998 [DOI] [PubMed] [Google Scholar]
- 17.James PS, Smith MW, Tivey DR, Wilson TJ. Dexamethasone selectively increases sodium-dependent alanine transport across neonatal piglet intestine. J Physiol 393: 569–582, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut 53: 78–84, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knickelbein R, Aronson PS, Schron CM, Seifter J, Dobbins JW. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol Gastrointest Liver Physiol 249: G236–G245, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res 40: 1604–1617, 1999 [PubMed] [Google Scholar]
- 21.Kruis W, Kalek HD, Stellaard F, Paumgartner G. Altered fecal bile acid pattern in patients with inflammatory bowel disease. Digestion 35: 189–198, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Chen F, Shang Q, Pan L, Shneider BL, Chiang JY, Forman BM, Ananthanarayanan M, Tint GS, Salen G, Xu G. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am J Physiol Gastrointest Liver Physiol 288: G60–G66, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Malakooti J, Memark VC, Dudeja PK, Ramaswamy K. Molecular cloning and functional analysis of the human Na+/H+ exchanger NHE3 promoter. Am J Physiol Gastrointest Liver Physiol 282: G491–G500, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Miller RT, Counillon L, Pages G, Lifton RP, Sardet C, Pouyssegur J. Structure of the 5′-flanking regulatory region and gene for the human growth factor-activatable Na/H exchanger NHE-1. J Biol Chem 266: 10813–10819, 1991 [PubMed] [Google Scholar]
- 25.Mottino AD, Hoffman T, Dawson PA, Luquita MG, Monti JA, Sanchez Pozzi EJ, Catania VA, Cao J, Vore M. Increased expression of ileal apical sodium-dependent bile acid transporter in postpartum rats. Am J Physiol Gastrointest Liver Physiol 282: G41–G50, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Neimark E, Chen F, Li X, Magid MS, Alasio TM, Frankenberg T, Sinha J, Dawson PA, Shneider BL. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology 131: 554–567, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Nowicki MJ, Shneider BL, Paul JM, Heubi JE. Glucocorticoids upregulate taurocholate transport by ileal brush-border membrane. Am J Physiol Gastrointest Liver Physiol 273: G197–G203, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut 35: 90–93, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell DW. Immunophysiology of intestinal electrolyte transport. In: Handbook of Physiology. The Gastrointestinal System. Intestinal Absorption and Secretion Bethesda, MD: Am. Physiol. Soc., 1991, sect. 6, vol. IV, chapt. 25, p. 591–641 [Google Scholar]
- 30.Rhoads JM, Macleod RJ, Hamilton JR. Effect of glucocorticoid on piglet jejunal mucosa during acute viral enteritis. Pediatr Res 23: 279–282, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutgeerts P, Ghoos Y, Vantrappen G. Kinetics of primary bile acids in patients with non-operated Crohn's disease. Eur J Clin Invest 12: 135–143, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Sandle GI, Binder HJ. Corticosteroids and intestinal ion transport. Gastroenterology 93: 188–196, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Sandle GI, Hayslett JP, Binder HJ. Effect of glucocorticoids on rectal transport in normal subjects and patients with ulcerative colitis. Gut 27: 309–316, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol 42: 16–25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartor DW. Mechanisms of Diarrhea in Inflammation and Hypersensitivity: Immune System Modulation of Intestinal Transport New York: Elsevier Science, 1991 [Google Scholar]
- 36.Sellin JH, Field M. Physiologic and pharmacologic effects of glucocorticoids on ion transport across rabbit ileal mucosa in vitro. J Clin Invest 67: 770–778, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr 32: 407–417, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest 95: 745–754, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MJ, Cherian P, Raju GS, Dawson BF, Mahon S, Bardhan KD. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond 34: 448–451, 2000 [PMC free article] [PubMed] [Google Scholar]
- 40.Stelzner M, Somasundaram S, Khakberdiev T. Systemic effects of acute terminal ileitis on uninflamed gut aggravate bile acid malabsorption. J Surg Res 99: 359–364, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Stravitz RT, Sanyal AJ, Pandak WM, Vlahcevic ZR, Beets JW, Dawson PA. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology 113: 1599–1608, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Suchy FS, Balistreri WF. Ileal dysfunction in Crohn's disease assessed by the postprandial serum bile acid response. Gut 22: 948–952, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundaram U, Coon S, Wisel S, West AB. Corticosteroids reverse the inhibition of Na-glucose cotransport in the chronically inflamed rabbit ileum. Am J Physiol Gastrointest Liver Physiol 276: G211–G218, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Sundaram U, Hassanain H, Suntres Z, Yu JG, Cooke HJ, Guzman J, Christofi FL. Rabbit chronic ileitis leads to up-regulation of adenosine A1/A3 gene products, oxidative stress, and immune modulation. Biochem Pharmacol 65: 1529–1538, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Sundaram U, West AB. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am J Physiol Gastrointest Liver Physiol 272: G732–G741, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Sundaram U, Wisel S, Coon S. Neutral Na-amino acid cotransport is differentially regulated by glucocorticoids in the normal and chronically inflamed rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 292: G467–G474, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Sundaram U, Wisel S, Fromkes JJ. Unique mechanism of inhibition of Na+-amino acid cotransport during chronic ileal inflammation. Am J Physiol Gastrointest Liver Physiol 275: G483–G489, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Sundaram U, Wisel S, Rajendren VM, West AB. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am J Physiol Gastrointest Liver Physiol 273: G913–G919, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Sundaram U, Wisel S, Stengelin S, Kramer W, Rajendran V. Mechanism of inhibition of Na+-bile acid cotransport during chronic ileal inflammation in rabbits. Am J Physiol Gastrointest Liver Physiol 275: G1259–G1265, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83: 633–671, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Van Assche G, Vermeire S, Rutgeerts P. Focus on mechanisms of inflammation in inflammatory bowel disease sites of inhibition: current and future therapies. Gastroenterol Clin North Am 35: 743–756, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Weinman SA, Carruth MW, Dawson PA. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J Biol Chem 273: 34691–34695, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem 269: 1340–1347, 1994 [PubMed] [Google Scholar]
- 54.Wood IS, Scott D, Beechey RB, Shirazi-Beechey SP. Cloning and sequencing of the ovine intestinal Na+/glucose transporter (SGLT1). Biochem Soc Trans 22: 266S, 1994. [DOI] [PubMed] [Google Scholar]