Abstract
Cyclic AMP (cAMP) induces translocation of multidrug resistant protein 2 (Mrp2) to the canalicular membrane and activates p38 MAPK in rat hepatocytes. In this study, we tested the hypothesis that cAMP-induced Mrp2 translocation may be mediated via p38 MAPK. Studies were conducted in rat hepatocytes and in a human hepatoma cell line, HuH-7. In rat hepatocytes, cAMP increased Mrp2 translocation and p38 MAPK activity. These effects of cAMP were inhibited by SB203580, an inhibitor of p38 MAPK. Wortmannin, a specific inhibitor of phosphoinositide-3-kinase (PI3K), did not inhibit cAMP induced activation of p38 MAPK, indicating PI3K-independent activation of p38 MAPK by cAMP. To further define the role of p38 MAPK, molecular approaches were used to up- or downregulate p38 MAPK activity in HuH-7 cells using constitutively active (CA) and dominant-negative (DN) MAPK kinase 3 and 6 (MKK3/6). MKK3/6 are upstream kinases responsible for the activation of p38 MAPK. Cells transfected with CAMKK6 showed increased p38 MAPK activity and MRP2 translocation compared with empty vector. cAMP-induced activation of p38 MAPK was inhibited in cells transfected with DNMKK3/6 and DNMKK3, but not with DNMKK6. DNMKK3/6 and DNMKK3 also inhibited cAMP-induced MRP2 translocation. cAMP selectively activated p38α MAPK in HuH-7 cells. Knockdown of p38α MAPK by short heterodimer RNA resulted in decreased level of p38 MAPK and failure of cAMP to stimulate MRP2 translocation. Taken together, these results suggest that cAMP-induced MRP2 translocation in hepatic cells is mediated via PI3K-independent and MKK3-mediated activation of p38α MAPK.
Keywords: p38 MAPK isoforms, rat hepatocytes, Huh-7 cells, MAPK kinase, phosphoinositide-3-kinase
multidrug resistance protein 2 (Mrp2; ABCC2) is an ATP-binding cassette (ABC) transporter located at the canalicular membrane of hepatocytes and is involved in biliary secretion of conjugated endogenous and exogenous organic anions (11, 26). Biliary excretion of Mrp2 substrates is decreased in cholestasis, and the underlying mechanism involves both transcriptional and posttranslational regulation. For example, transcription of Mrp2 is downregulated in cholestatic liver diseases (41), and Mrp2 is retrieved from the canalicular membrane during cholestasis induced by taurolithocholate (TLC) (2) or estradiol-17β-glucuronide (E217G) (25). The TLC-induced retrieval of Mrp2 may be mediated via a phosphoinositide-3-kinase (PI3K)/PKCϵ-dependent mechanism (3). Taurocholate (TC), tauroursodeoxycholate (TUDC), and cAMP, on the other hand, increase biliary excretion of Mrp2 substrates and induce Mrp2 translocation to the canalicular membrane (2, 9, 33), and cAMP can reverse E217G-induced retrieval of Mrp2 (25). However, little is known about the signaling pathways involved in cAMP-mediated Mrp2 translocation. We have recently reported that cAMP-induced Mrp2 translocation may involve PI3K-dependent PKCδ activation (37); however, another study reported that cAMP-induced translocation of bile salt export pump (Bsep), another ABC transporter located at the canalicular membrane of hepatocytes (38), and Mrp2 is independent of PI3K activity (24). Other studies raise the possibility that the effect of cAMP may also be mediated via PI3K-independent activation of the p38 MAPK signaling pathway (see below).
There are four known isoforms (α, β, γ and δ) of p38 MAPK (34) with only α and β isoforms expressed in human liver (18). Two upstream kinases, namely MAPK kinase 3 (MKK3) and MKK6, have been shown to activate p38 MAPK by dual phosphorylation (34) although MKK4 can also activate p38 MAPK (4, 7). The p38 MAPK signaling pathway has been implicated in the regulation of hepatocyte proliferation (1), hepatoprotection against hypoxic injury (6), gluconeogenesis (5), bile acid synthesis in hepatocytes (48), the antiapoptotic effect of TUDC (36), apoptosis in some cells (14, 20, 35, 47), and biliary excretion of bile acids (19). Cell swelling and TUDC-induced increases in bile acid excretion and Bsep translocation to the canalicular membrane require PI3K-independent activation of p38 MAPK (21). Whether p38 MAPK is also involved in Mrp2 translocation is not known. Because cAMP stimulates p38 MAPK in hepatocytes (44) and TUDC induced Bsep translocation requires p38 MAPK (21), we hypothesize that PI3K-independent activation of p38 MAPK may mediate cAMP-induced Mrp2 translocation in hepatocytes.
The aim of the present study was to determine whether cAMP-induced Mrp2 translocation is mediated via the p38 MAPK signaling pathway. Results of studies with activators and inhibitors of p38 MAPK in rat hepatocytes and HuH-7 cells suggest a role for p38α MAPK.
MATERIALS AND METHODS
Materials.
8-(4-Chlorophenylthio)-cAMP (CPT-cAMP), wortmannin and the antibody for human MRP2 were purchased from Sigma-Aldrich (St. Louis, MO). The antibody for rat Mrp2 was a generous gift from Dr. Keppler (Heidelberg, Germany). Sulfosuccinimidyl-6-(biotin-amido)hexanoate (sulfo-NHS-LC-biotin) was purchased from Pierce (Rockford, IL). Antibodies for phosphorylated p38 MAPK (Thr180/Tyr182) and PKB (Ser473) and total p38 MAPK and PKB were purchased from Cell Signaling Technology (Danvers, MA) and for p38α MAPK and p38β MAPK from Santa Cruz Biotechnology (Santa Cruz, California). Streptavidin beads were purchased from Novagen (Madison, WI). Plasmid constructs for FLAG-tagged constitutively active MKK6 (CAMKK6); dominant negative MKK6 (DNMKK6) and dominant negative MKK3 (DNMKK3) were kind gifts from Dr. R. Davis, University of Massachusetts Medical School, Worcester, MA (29), and wild-type p38α and p38β MAPKs were kindly provided by Dr. Sheila Collins, Duke University Medical Center, Durham, NC (32). p38α shRNA was obtained from SuperArray Bioscience (Frederick, MD). Four candidate human p38α MAPK shRNA plasmids were tested for their ability to inhibit p38 MAPK expression. One of these was found to inhibit p38 MAPK expression better than the others and was used in subsequent studies. Male Wistar rats (200–300 g) obtained from Charles River Laboratories served as liver donors, and the protocol for harvesting livers was approved by the Institutional Animal Care and Use Committee.
Hepatocyte preparation.
Hepatocytes were isolated from rat livers by collagenase perfusion as previously described (15, 43). All studies in rat hepatocytes were conducted in a HEPES assay buffer (pH 7.4) containing 20 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1.0 mM CaCl2, 0.8 mM KH2PO4, and 5 mM glucose. Details of these experiments are given in the legend of figures. All studies were repeated in at least three different cell preparations.
HuH-7 cell culture and transfections.
HuH-7 cells were cultured in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 100,000 U/l penicillin, and 100 mg/l streptomycin at 37°C in a 5% CO2-95% O2 air incubator. For transfection experiments involving CAMKK6, DNMKK6, DNMKK3, p38α MAPK, and p38β MAPK, the cells were cultured in six-well plates for 24 h and then transiently transfected with 1 μg of the desired plasmid using lipofectamine as previously described (44). Following 24 h of incubation in the transfection medium, the cells were cultured for an additional 24 h in culture medium. The expression of these plasmids was confirmed by immunoblot analysis using anti-FLAG, anti-MKK6, and anti-MKK3 antibodies. A similar method was used for transfections with p38α ShRNA except that the cells were cultured for additional 48 h after transfection. For all experiments, cells were then incubated in serum-free media for 3 h at 37°C before treatment with or without 100 μM CPT-cAMP for 15 min.
Protein kinase assay.
The p38 MAPK activity was determined from the ratio of phosphorylated (Thr180/Tyr182) p38 MAPK (active form) to total p38 MAPK (44), and the activity of PKB (PKB) was determined from the ratio of phosphorylated PKB (active form) to total PKB (23, 45). The determination of the activities of p38α and p38β MAPKs in cells transfected with FLAG-tagged p38α and p38β MAPKs involved immunoprecipitation of FLAG followed by immunoblot analysis of phosphorylated p38 and FLAG. The activity was expressed as the ratio of phosphorylated p38 MAPK to FLAG.
Mrp2 translocation.
A cell surface protein biotinylation method as previously described by us (37, 45, 46) was used to assess Mrp2 translocation in hepatocytes and HuH-7 cells. Briefly, following various treatments, cell surface proteins were biotinylated by exposing hepatocytes to sulfo-NHS-LC-biotin (0.5 mg/ml; Pierce) followed by preparation of cell lysate used to determine biotinylated and total Mrp2 mass using immunoblot analysis. Biotinylated proteins were isolated using streptavidin-agarose beads and then subjected to immunoblot analysis with rat Mrp2 or human MRP2 (HuH-7 cells) to determine plasma membrane Mrp2. The amount of Mrp2 present at the plasma membrane was expressed as a relative value compared with the level of total Mrp2.
Other methods.
The Lowry method (22) was used to determine cell protein. The blots were scanned using Adobe Photoshop (Adobe Systems, San Jose, CA), and the relative band densities were quantitated using Sigmal Gel (Jandel Scientific Software, San Rafael, CA). All values were expressed as means ± SE. Analysis of variance followed by paired t-test was used to determine statistically significant differences between two means with P < 0.05 considered significant.
RESULTS
SB203580 inhibits cAMP-induced increases in p38 MAPK activity and Mrp2 translocation in hepatocytes.
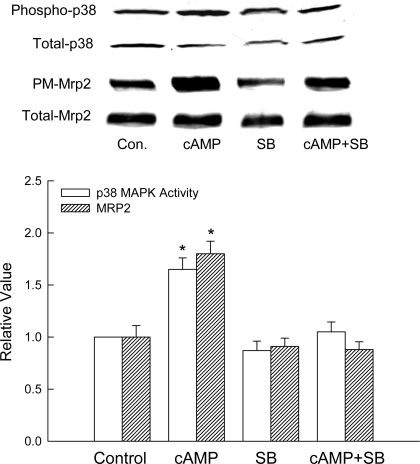
To determine whether p38 MAPK is involved in cAMP-induced Mrp2 translocation, studies were conducted in hepatocytes in the presence and absence of a p38 MAPK inhibitor, SB203580. We have previously reported that SB203580 inhibits cAMP-induced p38 MAPK activation at 1 and 10 μM without affecting cAMP-induced PKB activation (44). In the present study, 1 μM SB203580 completely inhibited cAMP-induced p38 MAPK activation without affecting the basal p38 MAPK activity (Fig. 1). cAMP stimulated Mrp2 translocation in hepatocytes by 65%, and this effect of cAMP was inhibited by 1 μM SB203580 (Fig. 1). These results raise the possibility that cAMP-induced Mrp2 translocation may be mediated by p38 MAPK.
Fig. 1.
SB203580 (SB) inhibits cAMP-induced p38 MAPK activity and multidrug-resistance protein 2 (Mrp2) translocation in rat hepatocytes. Hepatocytes were treated with or without 1 μM SB203580 for 30 min before addition of 100 μM 8-(4-chlorophenylthio)-cAMP (CPT-cAMP) or buffer. Cell lysates prepared 15 min after the addition of buffer (control, Con.) or CPT-cAMP (cAMP) were analyzed for phosphorylated (Phospho) and total p38 MAPK, and plasma membrane (PM) and total Mrp2 as described in materials and methods. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 4). *Significantly (P < 0.05) different from respective control values.
cAMP-induced activation of p38MAPK is PI3K independent.
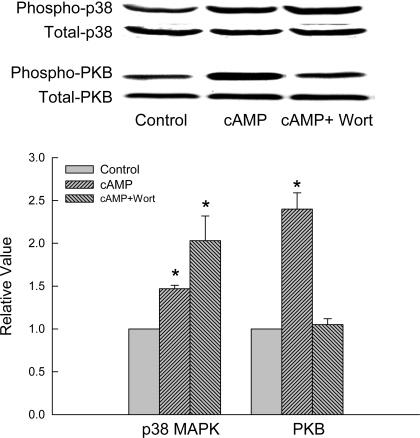
Whether cAMP-induced activation of p38 MAPK is PI3K dependent was evaluated by studying the effect of a PI3K inhibitor, wortmannin. As we have reported previously (45), wortmannin inhibited cAMP-induced activation of PKB (Fig. 2), indicating inhibition of PI3K. However, wortmannin failed to inhibit cAMP-induced activation of p38 MAPK in rat hepatocytes. Similar results were obtained in HuH-7 cell using another PI3K inhibitor, LY294002; relative values for cAMP-induced P38 MAPK activities were 1.51 ± 0.08 and 1.82 ± 0.12 in the presence and absence of LY294002, respectively (n = 4). Thus, cAMP-induced p38 MAPK activation is PI3K independent. Interestingly, cAMP-induced p38 MAPK activation appears to be enhanced in the presence of wortmannin (Fig. 2). However, this difference was not statistically significant.
Fig. 2.
Wortmannin does not inhibit cAMP-induced p38 MAPK activation in rat hepatocytes. Hepatocytes were treated with or without 50 nm wortmannin (Wort) for 15 min before addition of 100 μM CPT-cAMP or buffer. Cell lysates prepared 15 min after the addition of buffer (control) or CPT-cAMP (cAMP) were analyzed for phosphorylated (Phospho) and total p38 MAPK and PKB. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 3). *Significantly (P < 0.05) different from respective control values.
CAMKK6 induces p38 MAPK activity and MRP2 translocation.
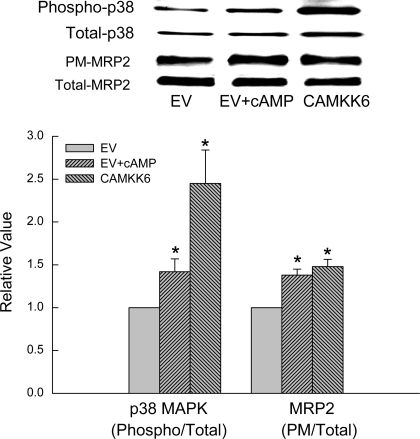
To further test whether the p38 MAPK pathway is involved in the translocation of Mrp2 to the plasma membrane, p38 MAPK activity and MRP2 translocation were assessed in HuH-7 cells. Our preliminary studies in HuH-7 cells showed that MRP2 is constitutively expressed and that cAMP stimulates MRP2 translocation and activates p38 MAPK (see Fig. 3), making this cell line a suitable model to study the role of p38 MAPK in cAMP-induced MRP2 translocation using molecular approaches. Two upstream MAPK kinases, namely MKK3 and MKK6 (also known as MEK3 and MEK6, respectively), can independently activate p38 MAPK, and this activation involves dual phosphorylation in the activation loop of p38 MAPK (34). Thus, if p38 MAPK is involved in cAMP-induced MRP2 translocation, CAMKK6 should mimic the effect of cAMP.
Fig. 3.
Constitutively active MAPK kinase 6 (CAMKK6) stimulates p38 MAPK activity and Mrp2 translocation. HuH-7 cells were transfected with either 1 μg empty vector (EV) or CAMKK6, and cells transfected with EV were treated with 100 μM CPT-cAMP (cAMP) for 15 min followed by determination of p38 activity and Mrp2 translocation. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 4). *Significantly (P < 0.05) different from respective EV controls.
Transient transfection with CAMKK6 increased p38 MAPK activity over twofold compared with cells transfected with the empty vector (Fig. 3); the expression of the transfected plasmid containing CAMKK6 was confirmed by immunoblotting (data not shown). cAMP also activated p38 MAPK in cell transfected with the empty vector. Concomitant with the increase in p38 MAPK activity, MRP2 translocation was also increased by cAMP treatment or CAMKK6 transfection compared with cells transfected with the empty vector (Fig. 3). These results would suggest that cAMP-induced MRP2 translocation may be mediated via p38 MAPK. Note that, although CAMKK6 increased p38 activity more than cAMP, the increases in MRP2 translocation were comparable. Because MKK6 preferentially activates p38β MAPK (17), it is possible that p38α MAPK instead of p38β MAPK may be involved in cAMP-induced MRP2 translocation (see discussion).
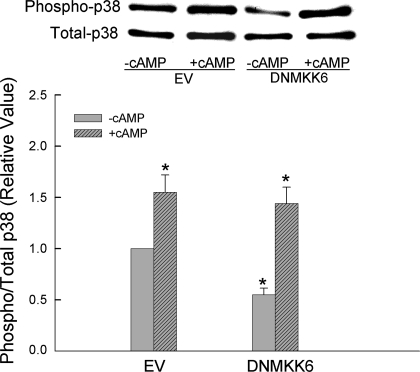
Effects of DNMKK3 and DNMKK6 on cAMP-induced p38 MAPK activity and MRP2 translocation.
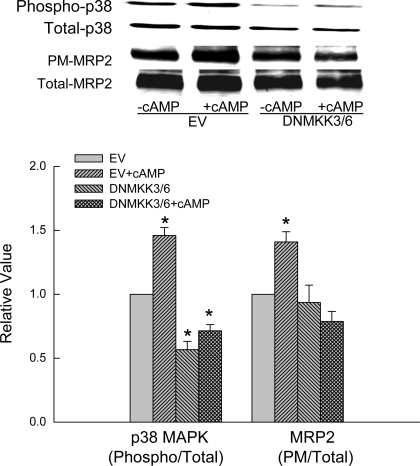
To further confirm the role of p38 MAPK, we determined whether inhibition of p38 MAPK affects cAMP-induced MRP2 translocation. Because both MKK3 and MKK6 are involved in the activation of p38 MAPK (34), we cotransfected HuH-7 cells with DNMKK3 and DNMKK6 (DNMKK3/6) to assure inhibition of p38 activity. As expected, transfection with DNMKK3/6 resulted in decreased basal p38 MAPK activity and inhibition of cAMP-induced p38 MAPK activation compared with cells transfected with the empty vector (Fig. 4). Transfection with DNMKK3/6 did not affect basal MRP2 level in the plasma membrane. However, the ability of cAMP to stimulate MRP2 translocation was inhibited in cells cotransfected with DNMKK3/6 (Fig. 4). These results suggest that activation of p38 MAPK is necessary for cAMP to stimulate MRP2 translocation.
Fig. 4.
Dominant negative MKK3 and MKK6 (DNMKK3/6) inhibits cAMP-induced p38 MAPK activity and Mrp2 translocation. HuH-7 cells transfected with EV or cotransfected with DNMKK3/6 were treated with or without 100 μM CPT-cAMP for 15 min. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 4). *Significantly (P < 0.05) different from respective EV controls.
The observed effect of DNMKK3/6 on p38 MAPK (Fig. 4) would also suggest that MKK6 and/or MKK3 are involved in cAMP-induced activation of p38 MAPK in hepatic cells. To determine whether the effect of cAMP on p38 MAPK is mediated via MKK6 and/or MKK3, HuH-7 cells were transfected with either DNMKK6 or DNMKK3 followed by determination of cAMP-induced activation of p38 MAPK. Interestingly, DNMKK3 (see Fig. 6) but not DNMKK6 (Fig. 5) inhibited cAMP-induced p38 MAPK activation. Moreover, DNMKK3 also inhibited cAMP-induced MRP2 translocation (Fig. 6). These results would suggest that cAMP-induced MRP2 translocation involves MKK3-mediated activation of p38 MAPK. Because MKK3 does not activate p38β MAPK (8) and p38γ and p38δ MAPKs are not present in human liver (18), it would follow that the effect of cAMP on MRP2 translocation is mediated via p38α MAPK. In that case, cAMP should activate p38α MAPK and not p38β MAPK. Thus we determined the effect of cAMP on p38α and p38β MAPKs.
Fig. 6.
DNMKK3 inhibits cAMP-induced p38 MAPK activity and Mrp2 translocation. HuH-7 cells transfected with EV or DNMKK3 were treated with or without 100 μM CPT-cAMP for 15 min. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 4). *Significantly (P < 0.05) different from respective EV controls.
Fig. 5.
DNMKK6 does not inhibit cAMP-induced p38 MAPK activity. HuH-7 cells transfected with EV or DNMKK6 were treated with or without 100 μM CPT-cAMP for 15 min followed by determination of p38 MAPK phosphorylation. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 5). *Significantly (P < 0.05) different from EV controls.
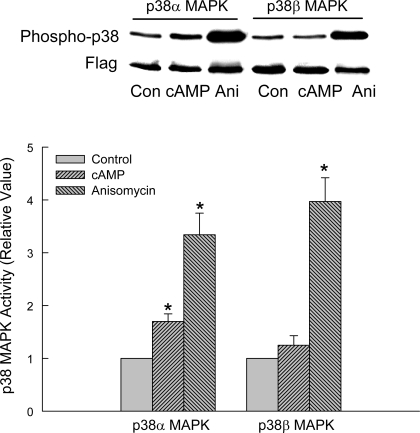
cAMP activates p38α MAPK in HuH-7 cells.
In this study, cells transfected with FLAG-tagged p38α MAPK or p38β MAPK were treated with CPT-cAMP or anisomycin followed by immunoprecipitation of FLAG and then immunoblot analysis for phosphorylated p38 and FLAG. Anisomycin, a known activator of p38 MAPK (10), was used as a positive control. We chose FLAG-tagged p38 MAPK-transfected cells to assure immunoprecipitation of specific isoforms of p38 MAPK. As expected, anisomycin activated p38α MAPK as well as p38β MAPK (Fig. 7). In contrast, cAMP only activated p38α MAPK significantly. In some experiments there was an increase in p38β MAPK activity by cAMP, but the increase was not statistically significant. Thus cAMP selectively activates p38α MAPK in HuH-7 cells, as it has been shown in HIB-1B brown preadipocytes (32). Next we determined whether the effect of cAMP on MRP2 translocation is mediated via p38α MAPK.
Fig. 7.
cAMP activates p38α and not p38β MAPK in HuH-7 cells. Cells transfected with FLAG-tagged wild-type P38α MAPK or p38β MAPK were treated with 100 μM CPT-cAMP or 20 μM anisomycin (Ani) for 15 min. Cell lysates were subjected to immunoprecipitation of FLAG followed by immunoblotting for phosphorylated p38 MAPK and FLAG. The activity of p38 MAPK isoform was expressed as the ratio of phosphorylated p38 MAPK to FLAG. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 6). *Significantly (P < 0.05) different from respective untreated control cells.
p38α MAPK shRNA inhibits cAMP-induced p38 activation and MRP2 translocation.
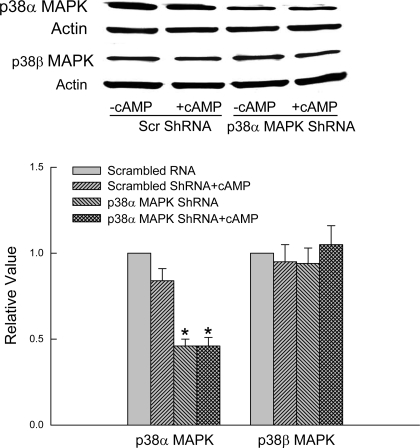
The role of p38α MAPK was evaluated using p38α MAPK shRNA to knockdown p38α MAPK. Preliminary studies showed that one of the four constructs (supplied by the vendor) significantly decreased expression of p38α MAPK in HuH-7 cells 48 h after transfection. Thus studies were conducted with this construct. We first determined the specificity of p38α MAPK shRNA by studying its effect on expression of p38α MAPK and p38β MAPK. Cells transfected with p38α MAPK shRNA showed more than 50% lower expression of p38α MAPK compared with cells transfected with the scrambled shRNA, and this decreased expression was not affected by cAMP treatment (Fig. 8). In contrast, the expression of p38β MAPK was unaffected, indicating specific effect on p38α MAPK expression.
Fig. 8.
p38α MAPK shRNA decreases expression of p38α but not p38β MAPK. HuH-7 cells transfected with scrambled (Scr) shRNA or p38α MAPK shRNA were treated with or without 100 μM CPT-cAMP for 15 min followed by immunoblotting of p38α or p38β MAPK with actin as loading control. Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE; n = 3). *Significantly (P < 0.05) different from respective scrambled shRNA controls in the absence of cAMP.
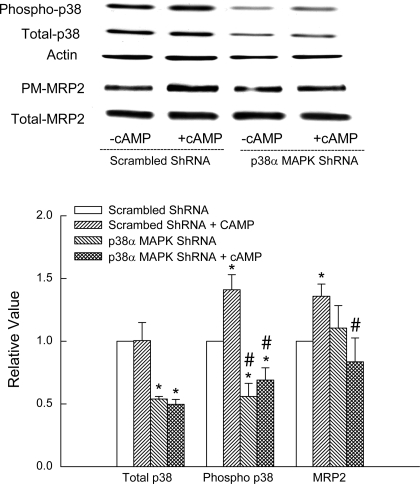
We then determined the effect of p38α MAPK shRNA on cAMP-induced p38 MAPK phosphorylation and MRP2 translocation. Cells transfected with p38α MAPK shRNA showed decreased expression of total and phosphorylated p38 MAPK (Fig. 9). cAMP-induced p38 phosphorylation was inhibited in cells transfected with p38α MAPK shRNA. The expression of p38α MAPK shRNA did not affect basal plasma membrane expression of Mrp2. However, the ability of cAMP to induce MRP2 translocation was significantly inhibited in cells transfected with p38α MAPK shRNA (Fig. 9). These results suggest that cAMP-induced MRP2 translocation involves p38α MAPK.
Fig. 9.
p38α MAPK shRNA inhibits cAMP-induced p38 MAPK activity and Mrp2 translocation. HuH-7 cells transfected with scrambled shRNA or p38α MAPK shRNA were treated with or without 100 μM CPT-cAMP for 15 min followed by determination of phosphorylated and total p38 MAPK (n = 4) and Mrp2 translocation (n = 6). Top: representative immunoblots. Bottom: results of densitometric analysis (means ± SE). *Significantly (P < 0.05) different from respective scrambled ShRNA controls and #significantly (P < 0.05) different from respective cAMP value.
DISCUSSION
The aim of the present study was to test the hypothesis that cAMP-induced Mrp2 translocation is mediated via the p38 MAPK signaling pathway. Chemical inhibitors and molecular approaches were used to study the role of p38 MAPK. Studies in rat hepatocytes showed that chemical inhibition of cAMP-induced activation of p38 MAPK with SB203580 was associated with an inhibition of cAMP-induced Mrp2 translocation. In addition, activation of p38 MAPK by cAMP was not dependent on PI3K. Studies in HuH-7 cells showed that activation of p38 MAPK by CAMKK6 stimulated MRP2 translocation. In addition, inhibition of cAMP-induced activation of p38 MAPK by DNMKK3 resulted in attenuation of MRP2 translocation by cAMP. In HuH-7 cells, cAMP activated p38α but not p38β MAPK. In addition, knockdown of p38α MAPK by shRNA inhibited cAMP-induced MRP2 translocation. These results suggest that PI3K-independent activation of p38α MAPK is involved in cAMP-stimulated Mrp2/MRP2 translocation in hepatic cells.
The present study showed for the first time that p38 MAPK is involved in Mrp2 translocation in hepatic cells. Studies with chemical inhibitors have shown that TUDC-mediated Bsep translocation from Golgi to plasma membranes is mediated via p38 MAPK (19). Our previous studies suggested that p38 MAPK is not involved in cAMP-mediated translocation of Na-TC-cotransporting polypeptide to the sinusoidal membrane in hepatocytes (44). It would thus appear that p38 MAPK may stimulate translocation of hepatic solute transporters located in the canalicular membrane. Although p38 MAPK is involved in insulin-stimulated glucose transport, this does not involve glucose transporter 4 translocation in 3T3-L1 adipocytes and L6 myotubules (18, 39). Thus p38 MAPK appears to stimulate translocation of specific transporters.
The present study provided further insight into the isoform-specific effect of p38 MAPK on cAMP-induced MRP2 translocation. Of the four p38 MAPK isoforms, only α and β isoforms are present in human liver (18). Our study showed that the effect of cAMP on MRP2 translocation is mediated via p38α MAPK. This conclusion is supported by our observations that cAMP-induced p38 MAPK activation and MRP2 translocation is inhibited by DNMKK3 and p38α MAPK shRNA. Note that MKK3 does not activate p38β isoform (8, 18). In addition, cAMP selectively activates p38α MAPK in HUH-7 cells (Fig. 7). A recent study showed that cAMP activates p38α, but not p38β, in HIB-1B brown preadipocytes, and this activation relies solely on the presence of MKK3 (32). Thus the results of present study would suggest that cAMP-induced MRP2 translocation involves MKK3-mediated activation of p38α MAPK in hepatic cells. These results can also explain why CAMKK6 activates p38 MAPK more than cAMP and still stimulates MRP2 translocation to the same extent as cAMP (Fig. 3). Because p38α MAPK mediates cAMP-induced MRP2 translocation and MKK6 activates both p38α and p38β MAPK, CAMKK6-mediated p38 MAPK activation (sum of both α and β isoforms) would be expected to be higher that CAMKK6-mediated MRP2 translocation.
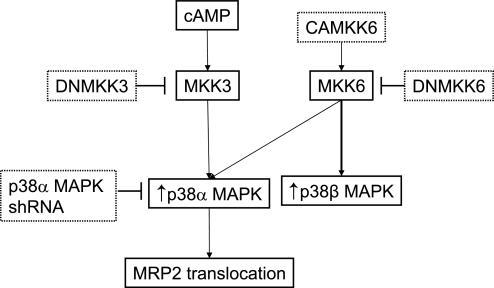
The known effects of p38 MAPK in the liver include regulation of proliferation (1), protection against hypoxic injury (6), gluconeogenesis (5), bile acid synthesis (48), antiapoptotic effect of TUDC (36), and biliary excretion of bile acids (19). Choleretic (TUDC, TC, cAMP) as well as cholestatic (glycodeoxycholate, TLC) agents have been shown to activate p38 MAPK (12–14, 21, 28, 44). Thus p38 MAPK appears to mediate both toxic and beneficial effects in the liver, and the underlying mechanism is unclear. Whether these effects are p38 MAPK isoform specific is not known. One possibility could be that the opposing effects are mediated via different isoforms. Indeed, hypertrophy and apoptosis in myocytes appear to be mediated via p38β and p38α isoforms, respectively (42). It is therefore possible that p38α MAPK and p38β MAPK mediate some of the effects of choleretic and cholestatic agents, respectively. However, further studies will be necessary to test the proposed isoform-specific effects of p38 MAPK (Fig. 10).
Fig. 10.
Proposed p38 MAPK signaling pathway involved in cAMP-induced MRP2 translocation in hepatic cells. cAMP acting via MKK3 activates p38α MAPK, which in turn induces Mrp2 translocation. Probes used to activate (arrows) and inhibit (error bars) kinases are also shown. Note that MKK6 preferentially activates p38β MAPK.
Acute cholestasis induced by TLC or E217G is associated with the retrieval of Mrp2 from the canalicular membrane, and TUDC and cAMP can reverse Mrp2 retrieval by TLC and E217G, respectively (2, 25). The present study shows that cAMP induces MRP2 translocation via p38α MAPK, raising the possibility that cAMP-induced reversal of E217G-mediated retrieval of Mrp2 may be mediated via p38α MAPK. Similarly, because TUDC activates p38 MAPK (21), it is likely that TUDC-induced reversal of TLC-induced Mrp2 retrieval is also mediated via p38α MAPK. Thus p38α MAPK may play an important role in reversing acute cholestasis. In this context, it would be interesting to find out whether p38α MAPK activity is decreased in acute cholestasis.
The present study also showed that cAMP-induced p38 MAPK activation is PI3K independent in hepatocytes. A previous study in HepG2 cells showed that TUDC-induced activation of p38 MAPK is also independent of PI3K (21). PI3K-independent activation of p38 MAPK is also suggested by results in LNCaP cells showing that PMA activates p38 MAPK but inhibits PKB (40), which requires PI3K for activation. However, PI3K-dependent activation of p38 MAPK has been reported in Hl-60 granulocytes (30) and neutrophils (31) when formyl-methionyl-leucyl-phenylalanine was used to stimulate these cells. Thus the PI3K dependency of p38 MAPK activation appears to be cell type and stimulus specific.
PKCs have been reported to be an upstream regulator of p38 MAPK. PMA, a known activator of conventional and novel PKCs, activates p38 MAPK in LNCaP prostrate cancer cells (40), and PKCδ has been shown to activate p38 MAPK (16) via MKK3 (27) in cardiac myocytes. We have recently reported that cAMP activates PKCδ in hepatocytes (37). These results raise the possibility that cAMP-induced p38 MAPK activation may be mediated via cAMP-induced activation of PKCδ. However, cAMP-induced PKCδ activation in hepatocytes is PI3K dependent (37), and our present study showed that cAMP-induced p38 MAPK activation is PI3K independent. Thus it is unlikely that PKCδ is involved in the activation of p38 MAPK by cAMP in hepatic cells.
In summary, the present study represents the first evidence that cAMP-induced Mrp2/MRP2 translocation in hepatic cells is mediated via p38α MAPK. It remains to be established whether p38 MAPK isoform-specific effects may explain the observed beneficial as well as toxic effects of this kinase in the liver.
GRANTS
This study was supported in part by National Institutes of Health Grants DK-33436 (M. Anwer) and DK-65975 (C. Webster).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this article with any of the authors or any of the authors' academic institutions or employers.
ACKNOWLEDGMENTS
We thank Holly Jameson and Ariel Hobson for excellent technical assistance.
REFERENCES
- 1.Awad MM, Enslen H, Boylan JM, Davis RJ, Gruppuso PA. Growth regulation via p38 mitogen-activated protein kinase in developing liver. J Biol Chem 275: 38716–38721, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology 33: 1206–1216, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, Boyer JL. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol-3 kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem 278: 17810–17818, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev 17: 1969–1978, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG, Jr, Xiong Y, Daniel KW, Floering L, Collins S. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem 280: 42731–42737, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Carini R, Alchera E, Baldanzi G, Piranda D, Splendore R, Grazia De CM, Caraceni P, Graziani A, Albano E. Role of p38 map kinase in glycine-induced hepatocyte resistance to hypoxic injury. J Hepatol 46: 692–699, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267: 682–685, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem 273: 1741–1748, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Gatmaitan ZC, Nies AT, Arias IM. Regulation and translocation of ATP-dependent apical membrane proteins in rat liver. Am J Physiol Gastrointest Liver Physiol 272: G1041–G1049, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Geiger PC, Wright DC, Han DH, Holloszy JO. Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 288: E782–E788, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther 302: 407–415, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Haussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology 36: 829–839, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Graf D, Reinehr R, Kurz AK, Fischer R, Haussinger D. Inhibition of taurolithocholate 3-sulfate-induced apoptosis by cyclic AMP in rat hepatocytes involves protein kinase A-dependent and -independent mechanisms. Arch Biochem Biophys 415: 34–42, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Grambihler A, Higuchi H, Bronk SF, Gores GJ. cFLIP-L inhibits p38 MAPK activation: an additional anti-apoptotic mechanism in bile acid-mediated apoptosis. J Biol Chem 278: 26831–26837, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Grüne S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinases in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J Biol Chem 268: 17734–17741, 1993 [PubMed] [Google Scholar]
- 16.Heidkamp MC, Bayer AL, Martin JL, Samarel AM. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ Res 89: 882–890, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta). J Biol Chem 271: 17920–17926, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di PF, Ulevitch RJ, Han J. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem 272: 30122–30128, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kubitz R, Sutfels G, Kuhlkamp T, Kolling R, Haussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology 126: 541–553, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem 272: 20490–20494, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Kurz AK, Graf D, Schmitt M, Dahl SV, Haussinger D. Tauroursodeoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology 121: 407–419, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lowry DH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 23.McConkey M, Gillin H, Webster CR, Anwer MS. Cross-talk between protein kinases Czeta and B in cyclic AMP-mediated sodium taurocholate co-transporting polypeptide translocation in hepatocytes. J Biol Chem 279: 20882–20888, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol 285: G316–G324, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology 35: 1409–1419, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2). Pflügers Arch 453: 643–659, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, Baker KM. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol 202: 536–553, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Qiao L, Han SI, Fang Y, Park JS, Gupta S, Gilfor D, Amorino G, Valerie K, Sealy L, Engelhardt JF, Grant S, Hylemon PB, Dent P. Bile acid regulation of C/EBPbeta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol 23: 3052–3066, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rane MJ, Carrithers SL, Arthur JM, Klein JB, McLeish KR. Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways: role in activation of reduced nicotinamide adenine dinucleotide oxidase. J Immunol 159: 5070–5078, 1997 [PubMed] [Google Scholar]
- 31.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem 276: 3517–3523, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Robidoux J, Cao W, Quan H, Daniel KW, Moukdar F, Bai X, Floering LM, Collins S. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38alpha MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol Cell Biol 25: 5466–5479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci 111: 1137–1145, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saldeen J, Lee JC, Welsh N. Role of p38 mitogen-activated protein kinase (p38 MAPK) in cytokine-induced rat islet cell apoptosis. Biochem Pharmacol 61: 1561–1569, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Schoemaker MH, Conde de la RL, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 39: 1563–1573, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology 47: 1309–1316, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflügers Arch 453: 611–620, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin- stimulated glucose transport but not glucose transporter translocation in 3T3–L1 adipocytes and L6 myotubes. J Biol Chem 274: 10071–10078, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem 278: 33753–33762, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology 113: 255–264, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem 273: 2161–2168, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Webster CR, Anwer MS. Phosphoinositide 3-kinase, but not mitogen-activated protein kinase, pathway is involved in hepatocyte growth factor-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology 33: 608–615, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem 277: 28578–28583, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Webster CRL, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol Gastrointest Liver Physiol 277: G1165–G1172, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Webster CRL, Blanch CJ, Philips J, Anwer MS. Cell swelling-induced translocation of rat liver Na+/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J Biol Chem 275: 29754–29760, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Xu Z, Tavares-Sanchez OL, Li Q, Fernando J, Rodriguez CM, Studer EJ, Pandak WM, Hylemon PB, Gil G. Activation of bile acid biosynthesis by the p38 mitogen-activated protein kinase (MAPK): hepatocyte nuclear factor-4alpha phosphorylation by the p38 MAPK is required for cholesterol 7alpha-hydroxylase expression. J Biol Chem 282: 24607–24614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]