Abstract
The majority of dietary amino acids are absorbed via the H+-di-/tripeptide transporter Pept1 of the small intestine. Proton influx via Pept1 requires maintenance of intracellular pH (pHi) to sustain the driving force for peptide absorption. The apical membrane Na+/H+ exchanger Nhe3 plays a major role in minimizing epithelial acidification during H+-di-/tripeptide absorption. However, the contributions of HCO3−-dependent transporters to this process have not been elucidated. In this study, we investigate the role of putative anion transporter-1 (Pat-1), an apical membrane anion exchanger, in epithelial pHi regulation during H+-peptide absorption. Using wild-type (WT) and Pat-1(−) mice, Ussing chambers were employed to measure the short-circuit current (Isc) associated with Pept1-mediated glycyl-sarcosine (Gly-Sar) absorption. Microfluorometry was used to measure pHi and Cl−/HCO3− exchange in the upper villous epithelium. In CO2/HCO3−-buffered Ringers, WT small intestine showed significant Gly-Sar-induced Isc and efficient pHi regulation during pharmacological inhibition of Nhe3 activity. In contrast, epithelial acidification and reduced Isc response to Gly-Sar exposure occurred during pharmacological inhibition of Cl−/HCO3− exchange and in the Pat-1(−) intestine. Pat-1 interacts with carbonic anhydrase II (CAII), and studies using CAII(−) intestine or the pharmacological inhibitor methazolamide on WT intestine resulted in increased epithelial acidification during Gly-Sar exposure. Increased epithelial acidification during Gly-Sar exposure also occurred in WT intestine during inhibition of luminal extracellular CA activity. Measurement of Cl−/HCO3− exchange in the presence of Gly-Sar revealed an increased rate of Cl−OUT/HCO3−IN exchange that was both Pat-1 dependent and CA dependent. In conclusion, Pat-1 Cl−/HCO3− exchange contributes to pHi regulation in the villous epithelium during H+-dipeptide absorption, possibly by providing a HCO3− import pathway.
Keywords: acid-base transport, anion exchange, Pept1, carbonic anhydrase II, mouse
the small intestinal enterocyte is the principal site for the absorption of dietary proteins that have been degraded enzymatically to free amino acids and small peptides. The majority of protein digestion products are absorbed in the form of di- and tripeptides by the intestinal oligopeptide transporter Pept1 (7). Pept1 expression is restricted to the apical membrane of the small intestine with greatest levels in the duodenum and jejunum, where it is not detectable in the crypts but increases from the villous base to its highest levels in the villous tips (10). The Pept1 protein has the ability to transport ∼400 different dipeptides and ∼8,000 different tripeptides derived from luminal protein digestion regardless of net charge, molecular size, or solubility (16). In addition, many orally delivered drugs, which have dipeptide and tripeptide structures, including β-lactam antibiotics and angiotensin-converting enzyme inhibitors, and bacterial products, such as the chemotactic tripeptide N-formyl-methionyl-leucyl-phenylalanine and muramyl dipeptide, cross the absorption barrier by means of Pept1 transport (3, 8, 20, 37).
Transport via Pept1 is electrogenic and couples peptide influx to an inwardly directed proton gradient that allows transport against a concentration gradient (18). H+/peptide influx exhibits a stoichiometry of 1:1, which occurs irrespective of the net charge associated with the peptide, although there is a clear preference for noncharged species (16). Studies of intestinal cell lines have shown that proton di-/tripeptide uptake results in intracellular acidification, and this decrease in intracellular pH (pHi) will diminish the driving force for Pept1-mediated transport unless the proton load is reduced by means of intracellular buffering or proton efflux (31, 32). Therefore, optimal peptide absorption must rely upon the coordination of a number of acid-base transport proteins present in intestinal epithelial cells. Studies performed in the absence of CO2/HCO3− have revealed that the intracellular acidification resulting from Pept1-mediated transport can be effectively minimized by means of apical membrane Na+/H+ exchange activity (i.e., Nhe2 and Nhe3) (14, 28, 33, 34). Furthermore, it has been shown in intestinal cell lines endogenously expressing Pept1 and Na+/H+ exchanger proteins that Pept1-mediated transport activates Nhe3, the major Na+/H+ exchanger in the mammalian intestine (14, 33, 34). As a result, H+ can recycle across the apical membrane to maintain pHi and the driving force for peptide absorption. In the presence of CO2/HCO3−, one previous study performed in murine small intestinal villous epithelia has demonstrated that intracellular carbonic anhydrase (CA) activity facilitates diffusive H+ movement, thereby maintaining a transmembrane ion gradient that allows for maximal absorption via Pept1 (29). However, the contribution of other acid-base transporters to the regulation of pHi during H+ peptide transport has not been fully investigated in the presence of physiological CO2/HCO3− buffering.
Three anion exchangers involved in Cl−/HCO3− exchange have been localized to the apical membrane in murine duodenum: downregulated in adenoma (Dra, Slc26a3), the putative anion transporter-1 (Pat-1, Slc26a6), and anion exchanger 4 (Ae4, Slc4a9) (12, 15, 39, 43). In a recent study on mice with gene-targeted deletions of these exchangers, Pat-1 was found to provide 70–80% of Cl−/HCO3− exchange (with 20–30% contributed by Dra) in epithelial cells of the upper half of duodenal villi (27), which is also the primary site for expression of Pept1 (8). Pat-1 is a multifunctional anion exchanger that has a physiological role in the intestinal transport of other anions, e.g., Cl−/oxalate and SO42−/HCO3− exchange (13). Pat-1, nonetheless, has a large capacity for Cl−/HCO3− exchange (mM/min) and contributes ∼20% to basal HCO3− secretion in the proximal small intestine (35, 38). Measurement of pHi under conditions that isolate apical membrane acid-base transport reveal a HCO3−-dependent acidic pHi in the Pat-1(−) compared with wild-type (WT) duodenum, suggesting a propensity for Pat-1 to act as a base importer (i.e., Cl−OUT/HCO3−IN) in native villous epithelium (27). The ability of Pat-1 to function as a base importer may be physiologically relevant as the upper villous epithelium of the duodenum is exposed to acid challenge, not only from gastric effluent, but also from H+ influx during nutrient absorption, e.g., Pept1-mediated absorption. Furthermore, Pat-1 can physically and functionally interact with cytosolic CAII, which has been shown to dissipate pHi gradients during H+-dipeptide absorption (1). Therefore, we investigated the relative contributions of Pat-1 and CAII to pHi regulation in the duodenal upper villous epithelium during absorption of the Pept1-dipeptide substrate glycyl-sarcosine (Gly-Sar) using mice with gene-targeted deletions of Pat-1 or CAII and their WT littermates.
MATERIALS AND METHODS
Animals.
The experiments in this study were performed on mice with gene-targeted and mutagen-induced disruption of the murine homologs of Pat-1 (38) and CAII (17), respectively. All comparisons were made with sex- and age-matched (+/+) siblings (WT). The mutant mice were identified using a PCR-based analysis of tail snip DNA, as previously described (4). All mice were maintained ad libitum on standard laboratory chow (Formulab 5008 Rodent Chow; Ralston Purina, St. Louis, MO) and tap water. The mice were housed singly in a temperature-controlled (22–26°C) and light-controlled (12-h:12-h light/dark cycle) room in the AAALAC accredited animal facility at the Dalton Cardiovascular Research Center, University of Missouri. Intestinal tissues for experiments were obtained from mice 2–4 mo of age. Mice were fasted overnight prior to experimentation but were provided with water ad libitum. All experiments involving animals were approved by the University of Missouri Animal Care and Use Committee.
Short-circuit current measurement.
Freshly excised duodenum or proximal jejunum was stripped of the underlying muscle layers and mounted on voltage-clamped Ussing chambers for the measurement of short-circuit current (Isc) as previously described (5). The apical surface of the intestine was bathed with Krebs bicarbonate Ringers (KBR) containing (in mM): 140.0 Na+, 5.2 K+, 2.8 PO42−, 119.8 Cl−, 25.0 HCO3−, 1.2 Ca2+, 1.2 Mg2+, 4.8 gluconate−, 10.0 mannitol and was gassed with 95% O2-5% CO2 at 37°C (pH 7.4). The basolateral perfusate was similar in composition to the apical solution except that 10 mM mannitol was replaced equimolar with glucose. All intestinal preparations contained 1 μM indomethacin (bilateral) and 0.1 μM tetrodotoxin (basolateral) to minimize the effects of endogenous prostaglandins and neural tone, respectively. In some experiments, 100 μM EIPA or 100 μM niflumic acid (NFA) from 100 mM stocks in DMSO were added to the apical perfusate. Following a 20-min equilibration period, 20 mM Gly-Sar, a poorly metabolized dipeptide, was added to the luminal bath, and the change in Isc was recorded at 5-min intervals.
Fluorescence measurement of pHi and image analysis.
The method used for imaging villous epithelial cells in intact murine intestine has been previously described (26). Briefly, freshly excised duodenum was stripped of the underlying muscle layers and mounted luminal side up in a horizontal Ussing-type perfusion chamber where luminal and serosal surfaces of the tissue were independently bathed. All intestinal preparations were treated with indomethacin (1 μM, bilateral) and tetrodotoxin (0.1 μM, serosal). The luminal side of the preparation was incubated 5 min with a Ringers solution containing 100 μM DL-dithiothreitol to remove mucus followed by a 10-min incubation with a Ringers solution containing 16 μM BCECF-acetoxymethyl ester. Using a ×40 water immersion objective (Olympus, Melville, NY), 10 epithelial cells from the mid to upper region of a single villus were selected for ratiometric analysis. Changes in pHi were measured by the dual excitation wavelength technique (440 and 495 nm) and imaged at 535-nm emission. Ratiometric images were obtained at 20-s intervals with a Sensi-Cam digital camera (Cooke, Auburn Heights, MI) and processed using Axon Imaging Workbench 2.2 (Axon Instruments, Union City, CA). The 495:440-nm ratios were converted to pHi using a standard curve generated by the K+/nigericin technique (2, 30). Intrinsic buffering capacity (βi) of duodenal villous cells was estimated by the ammonium prepulse technique, and the total buffering capacity (βtotal) was calculated from the equation βtotal = βi + βHCO3− = βi + 2.3 × [HCO3−]i, where βHCO3− is the buffering capacity of the CO2/HCO3− system and [HCO3−]i is the intracellular concentration of HCO3− (40). The rate of pHi change during the initial 90-s period of linear ΔpH/Δt change was converted to transmembrane flux (J) of HCO3− or H+ measured in mM/min using the equation J = ∇pH/∇ t × βtotal
Measurement of Gly-Sar-induced acidification.
For these studies, the luminal superfusate contained (in mM) 140.0 Na+, 110.0 Cl−, 25.0 HCO3−, 5.2 K+, 5.0 TES, 4.8 gluconate−, 2.8 PO42−, 1.2 Ca2+, 1.2 Mg2+, and 16.8 mannitol that was gassed with 95% O2-5% CO2 at 37°C (pH 7.4). The serosal perfusate was similar in composition to the luminal solution except that 10 mM mannitol was replaced equimolar with glucose. After attaining a stable pHi measurement (∼2 min), the tissue was exposed to 20 mM Gly-Sar on the luminal side. Following measurement of Gly-Sar-induced acidification, pHi recovery was induced by removal of Gly-Sar from the luminal superfusate. To examine the contribution of CA activity, the global CA inhibitor methazolamide (100 μM) was initially added to the luminal and serosal perfusates from a 10 mM stock in water. The contribution of extracellular CA activity was investigated using the sulphonamide compound 1-[4-aminosulphonyl] phenyl-2,4,6-trimethylpyridinium perchlorate (6a, 30 μM, from the laboratory of C. T. Supuran) added to the luminal perfusate from a 30 mM stock in DMSO (23). For transport inhibitor studies, 100 μM EIPA or 100 μM NFA was added to the luminal superfusate from a 100 mM stock in DMSO. In HCO3−-free studies, a TES-buffered ringer was used in which 25 mM HCO3− was replaced with 25 mM TES, and the luminal and serosal solutions were gassed with 100% O2. For Cl−-free studies, Cl− was replaced equimolar with isethionate−. Rates of Gly-Sar-induced acidification and alkalinization (ΔpHi/min) were calculated from a linear regression of the values from the first 90 s of the initial pHi changes during luminal Gly-Sar addition and removal, respectively. The flux of H+ equivalents into the cell (or, base− equivalents out of the cell) are denoted as negative, and the flux of H+ equivalents out of the cell are denoted as positive.
Measurement of apical membrane Cl−/HCO3− exchange.
For these studies, the luminal superfusate was a modified KBR solution (i.e., IBR) containing (in mM) 140.0 Na+, 55.0 Cl−, 55.0 isethionate−, 25.0 HCO3−, 5.2 K+, 5.0 TES, 4.8 gluconate−, 2.8 PO42−, 1.2 Ca2+, 1.2 Mg2+, 10.0 glucose, and 6.8 mannitol that was gassed with 95% O2-5% CO2 at 37°C (pH 7.4). The serosal superfusate was a Cl−-free IBR (Cl− replaced with isethionate) gassed with 95% O2-5% CO2 at 37°C (pH 7.4). Duodenal preparations were superfused with IBR on the luminal side, and pHi alkalinization was induced by replacement of Cl− with isethionate− on an equimolar basis. After attaining a stable pHi measurement (∼2 min), pHi recovery was initiated by readdition of Cl− to the luminal superfusate. For Gly-Sar studies, 20 mM Gly-Sar was included in the luminal solution throughout the experiment. For methazolamide studies, 100 μM methazolamide was initially added to luminal and serosal superfusates from a 10 mM stock in Cl−-free IBR. Rates of anion exchange during alkalinization and recovery (ΔpHi/min) were calculated from a linear regression of the values from the first 90 s of the initial pHi changes during Cl− removal and replacement, respectively. The flux of HCO3− (base−) equivalents into the cell (or H+ equivalents out of the cell) are denoted as positive, and the flux of HCO3− (base−) equivalents out of the cell are denoted as negative. Directionality of the Cl−/HCO3− exchange process is designated as Cl−IN/HCO3−OUT when referring to the movement of Cl− into the cell across the luminal membrane in exchange for HCO3− moving out of the cell, and Cl−OUT/HCO3−IN when referring to the movement of Cl− out of the cell across the luminal membrane in exchange for HCO3− moving into the cell.
Materials.
The fluorescent dye BCECF-acetoxymethyl ester was obtained from Invitrogen (Carlsbad, CA). Tetrodotoxin was obtained from Biomol International (Plymouth Meeting, PA). All other materials were obtained from either Sigma Aldrich (St. Louis, MO) or Fisher Scientific (Springfield, NJ).
Statistics.
All values are reported as means ± SE. Data between two treatment groups were compared using a two-tailed unpaired Student's t-test assuming equal variances between groups. Data from multiple treatment groups were compared using a one-way analysis of variance with a post hoc Tukey's t-test. A probability value of P < 0.05 was considered statistically significant.
RESULTS
Role of apical Na+/H+ exchangers in H+-dipeptide absorption under physiological conditions.
In the absence of CO2/HCO3− in the medium, the apical membrane Na+/H+ exchangers Nhe3 and Nhe2 can effectively minimize pHi acidification during proton di-/tripeptide transport (14, 28, 33, 34). However, relatively little is presently known regarding the role of apical membrane Na+/H+ exchangers in pHi maintenance during peptide absorption under physiological conditions (i.e., in the presence of CO2/HCO3−). To investigate the role of Na+/H+ exchange in sustaining H+-dipeptide transport in CO2/HCO3−-containing Ringers, Gly-Sar was added to the luminal superfusate of murine intestinal preparations in Ussing chambers containing physiological KBR. Recent Ussing-chamber studies have shown that the Gly-Sar-induced Isc is reduced by ∼80% in Pept1(-) intestinal preparations and therefore provides a good index of Pept1-mediated transport (11). As shown in Fig. 1A, Gly-Sar addition induced a robust increase in Isc that was sustained over the 15 min of study. Treatment of intestinal preparations with 100 μM EIPA, an effective inhibitor of apical Nhe2 and Nhe3 activity (9), reduced the Isc response, but significant Gly-Sar-induced Isc persisted. Because anion channel activity of the cystic fibrosis transmembrane conductance regulator (Cftr) is the major determinant of the Isc in the small intestine (4), a contribution of Cftr activity to the Gly-Sar-induced Isc was evaluated by pretreating WT intestinal preparations with a specific Cftr inhibitor GlyH-101 (20 μM, bilateral, Ref. 22) before Gly-Sar addition. GlyH-101 inhibits ∼30% of the maximum cAMP-stimulated Isc in murine small intestine (L. L. Clarke, unpublished data). However, GlyH-101 treatment did not affect the Gly-Sar-induced Isc (vehicle DMSO ΔIsc = 88.0 ± 27.4; GlyH-101 ΔIsc = 95.5 ± 23.9 μA/cm2, n = 5, ns).
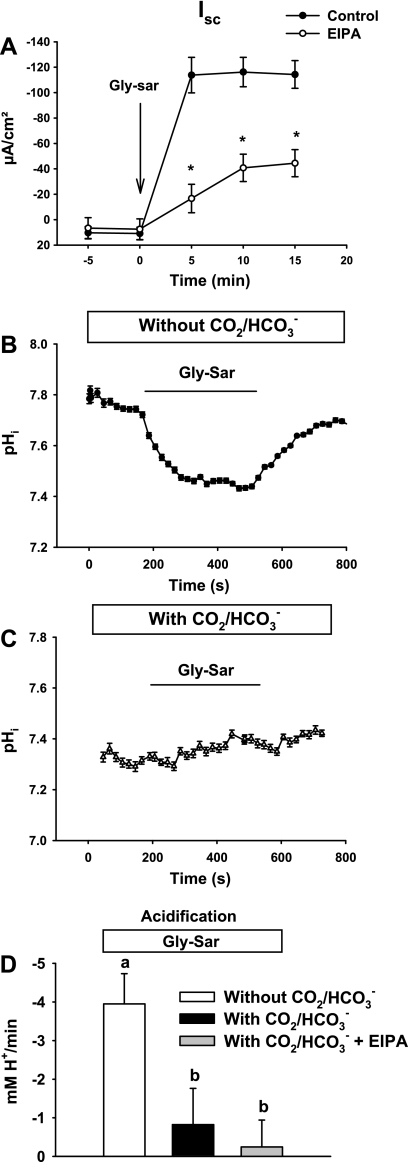
Fig. 1.
Glycyl-sarcosine (Gly-Sar)-induced short-circuit current (Isc) response and intracellular pH (pHi) acidification in wild-type (WT) murine proximal intestine. A: Isc response to luminal addition of 20 mM Gly-Sar in the absence (control) or presence of EIPA (EIPA, 100 μM, luminal). *Significantly different from vehicle control (n = 5–8). B: representative trace of villous epithelial pHi during luminal addition of 20 mM Gly-Sar in the absence of CO2/HCO3− in the superfusate (TES-buffered). C: representative trace of villous epithelial pHi during luminal addition of 20 mM Gly-Sar in the presence of CO2/HCO3− buffering in the superfusate. D: rates of Gly-Sar-induced intracellular acidification in WT upper villous epithelium in the absence of CO2/HCO3− (without CO2/HCO3−), in the presence of CO2/HCO3− (with CO2/HCO3−), and in the presence of CO2/HCO3− during luminal treatment with 100 μM EIPA (with CO2/HCO3− + EIPA) (n = 3–6). The mean pHi before Gly-Sar addition for each condition was pHi (without CO2/HCO3−) = 7.78 ± 0.11, pHi (with CO2/HCO3−) = 7.68 ± 0.09, and pHi (with CO2/HCO3− + EIPA) = 7.59 ± 0.19. a,bMeans with the same letters are not significantly different.
To investigate the pH gradient for transmembrane H+-dipeptide uptake under physiological conditions, the intracellular pH of murine duodenal villous epithelial cells was measured in the absence and presence of CO2/HCO3− during Gly-Sar absorption. In the absence of CO2/HCO3−, addition of 20 mM Gly-Sar to the luminal bath resulted in an abrupt intracellular acidification, presumably attributable to the cotransport of H+ with Gly-Sar (Fig. 1B). Following removal of Gly-Sar from the luminal bath, pHi returned toward baseline in the villous epithelium. In the presence of CO2/HCO3−, the basal pHi was unchanged in villous epithelium during luminal Gly-Sar exposure (Fig. 1C). The mean rates of Gly-Sar-induced acidification in the absence and presence of CO2/HCO3− in the bathing medium are shown in Fig. 1D. To evaluate the role of Nhe2 and Nhe3 in the presence of CO2/HCO3−, apical membrane NHE activity was inhibited with 100 μM EIPA, but cell acidification was not acutely affected during Gly-Sar absorption (Fig. 1D). Taken together, these data indicate that, in the presence of CO2/HCO3−, villous epithelia sustain peptide absorption by effectively regulating pHi by processes other than H+ efflux via apical membrane Na+/H+ exchange.
Role of apical membrane Cl−/HCO3− exchange in the pHi regulation during H+-dipeptide absorption.
To evaluate the contribution of apical membrane Cl−/HCO3− exchange to H+ dipeptide absorption, the Gly-Sar-induced Isc in the murine proximal intestine was measured during inhibition of apical membrane Cl−/HCO3− exchange with luminal addition of 100 μM NFA. Previous studies have demonstrated that 100 μM NFA inhibits ∼60% of Cl−/HCO3− exchange in the upper villous epithelium (26). As shown in Fig. 2A, a significant reduction of Gly-Sar-induced Isc (∼70%) occurred in the presence of NFA compared with vehicle control. The large decrease of the Gly-Sar-induced Isc is likely an overestimation of the NFA effect on Cl−/HCO3− exchange because NFA also inhibits Ca2+-activated Cl− channels and may have had untoward effects on the metabolic status of the epithelium because of the long incubation time in these experiments (44) (L. L. Clarke, unpublished observations). To determine whether reduced Gly-Sar absorption during NFA was attributable to increased cellular acidification, the rate of Gly-Sar-induced acidification in the duodenal villous epithelium was measured in the presence of NFA and, for more complete inhibition, during Cl− removal from the luminal solution. As shown in Fig. 2B, NFA increased the rate of Gly-Sar-induced acidification compared with control. Likewise, an even greater rate of acidification occurred in absence of luminal Cl− compared with control (KBR solution).
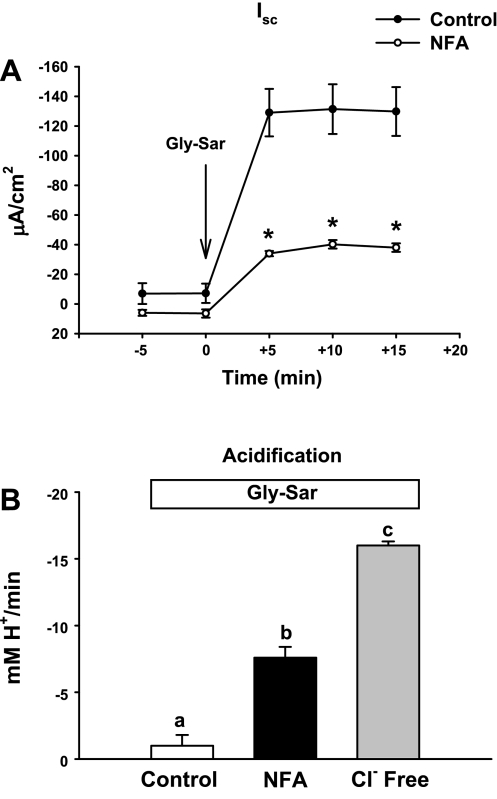
Fig. 2.
Gly-Sar-induced Isc response and pHi acidification in WT murine proximal intestine during inhibition of apical membrane Cl−/HCO3− exchange. A: Isc response to luminal treatment with 20 mM Gly-Sar for WT intestine in the absence (control) or presence of niflumic acid (NFA, 100 μM, luminal). *Significantly different from vehicle control (n = 4). B: summary of acidification rates for WT duodenal villous epithelial cells during luminal treatment with 20 mM Gly-Sar in the upper villous epithelium in Krebs bicarbonate Ringers (KBR) solution (control), in the presence of NFA, and in the absence of luminal Cl− (Cl− Free). The mean pHi before Gly-Sar addition for each condition was Control pHi = 7.68 ± 0.09, NFA pHi = 7.66 ± 0.08, and Cl−-free pHi = 7.75 ± 0.16. a,b,cMeans with the same letters are not significantly different.
Role of Pat-1 in pHi regulation during Gly-Sar exposure.
Significant levels of expression and functional activity of Pat-1 Cl−/HCO3− exchange are found in the upper villous epithelium, which parallels Pept1 activity (27). To determine the contribution of Pat-1 to H+-dipeptide absorption, Gly-Sar-induced ΔIsc was measured in Pat-1(−) and WT littermate small intestine bathed in CO2/HCO3−-containing Ringers. As shown in Fig. 3A, left, the Gly-Sar-induced ΔIsc of the Pat-1(−) intestine averaged less during a 15-min period than WT intestine, but the change was variable and did not attain statistical significance. However, when apical membrane NHE activity was inhibited by EIPA, the contribution of Pat-1 to the residual Gly-Sar absorption was less variable and significantly reduced in the Pat-1(−) intestine compared with WT (Fig. 3A, right). Because the contribution of Pat-1 to transepithelial Gly-Sar absorption is largely limited to the villous epithelium (27), the effect of Pat-1 ablation on Gly-Sar-induced acidification was examined in the upper villous epithelium of the duodenum. As shown in Fig. 3B, Gly-Sar-induced acidification of the villous epithelium in Pat-1(−) duodenum occurred at greater rates and, following removal of Gly-Sar, only partially recovered to baseline pHi compared with WT villous epithelium. Furthermore, as shown in Fig. 3C, steady-state pHi was significantly reduced in Pat-1(−) villous epithelium during Gly-Sar exposure, whereas pHi was unchanged in WT.
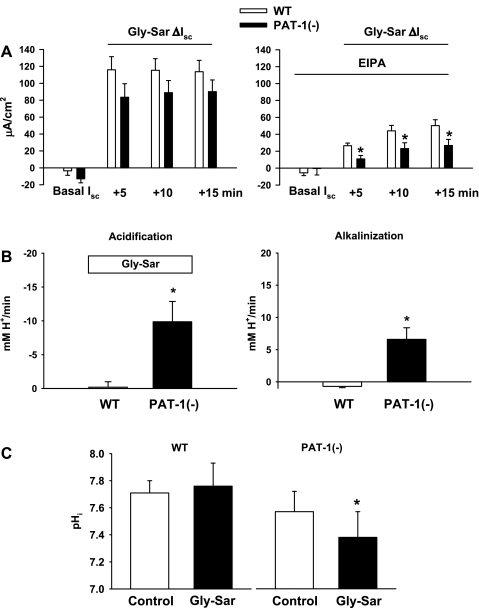
Fig. 3.
Gly-Sar-induced Isc response and pHi acidification in the proximal small intestine of WT and putative anion transporter-1 (Pat-1)(−) mice. A: Isc response to luminal addition of 20 mM Gly-Sar in WT and Pat-1(−) intestines in physiological KBR (left) and during luminal treatment with 100 μM EIPA (right). Changes in the Isc (ΔIsc = basal Isc − Gly-Sar Isc) at each time point after Gly-Sar addition are shown. *Significantly different from WT (n = 13–14). B: summary of acidification and alkalinization rates for upper villous epithelium of WT and Pat-1(−) duodena in physiological KBR solution during luminal addition and removal of 20 mM Gly-Sar, respectively. The mean pHi before Gly-Sar addition was WT pHi = 7.68 ± 0.07 and Pat-1(−) pHi = 7.58 ± 0.11 (n = 3). *Significantly different from WT. C: steady-state pHi under control conditions and during luminal exposure to 20 mM Gly-Sar in villous epithelium of WT and Pat-1(−) duodena superfused in physiological KBR solution (n = 4). *Significantly different from control.
Involvement of CA in pHi regulation during H+-dipeptide absorption.
A previous study has shown that CA activity in small intestinal enterocytes facilitates intracellular acid diffusion during H+/dipeptide transport (29). Studies of recombinant Pat-1 also demonstrate both a physical and functional coupling with CAII (1). Therefore, we investigated the role of CA activity in the regulation of pHi during luminal peptide absorption. Using membrane-permeant methazolamide to inhibit CA activity, pHi was measured in WT murine duodenal villous epithelial cells during Gly-Sar exposure in the presence of CO2/HCO3−-containing Ringers. As shown in Fig. 4A, the rate of cellular acidification during luminal Gly-Sar addition was increased by methazolamide treatment. To investigate the specific role of CAII in pHi regulation during peptide absorption, studies were then performed using duodenum from mice lacking cytosolic CAII, the major soluble isozyme of the villous epithelium (17). As shown in Fig. 4B, duodenal villous epithelia of CAII(−) mice demonstrated an impaired ability to maintain pHi, as evidenced by an increased rate of acidification upon luminal Gly-Sar addition. Extracellular CA activity on the luminal surface of the duodenum (21) may provide an important source of HCO3− for apical membrane Cl−/HCO3− exchange to act as a HCO3− importer during H+-dipeptide absorption, especially when luminal Pco2 is at high levels (>150 mmHg) following a meal (24, 41). To investigate the involvement of extracellular CA activity under basal conditions (Pco2 ≈ 38 mmHg), we monitored pHi in the villous epithelium during Gly-Sar exposure before and during treatment with the extracellular CA inhibitor, 6a. As shown in Fig. 4C, the inhibitor 6a significantly increased the rate of intracellular acidification within 2 min during Gly-Sar exposure.
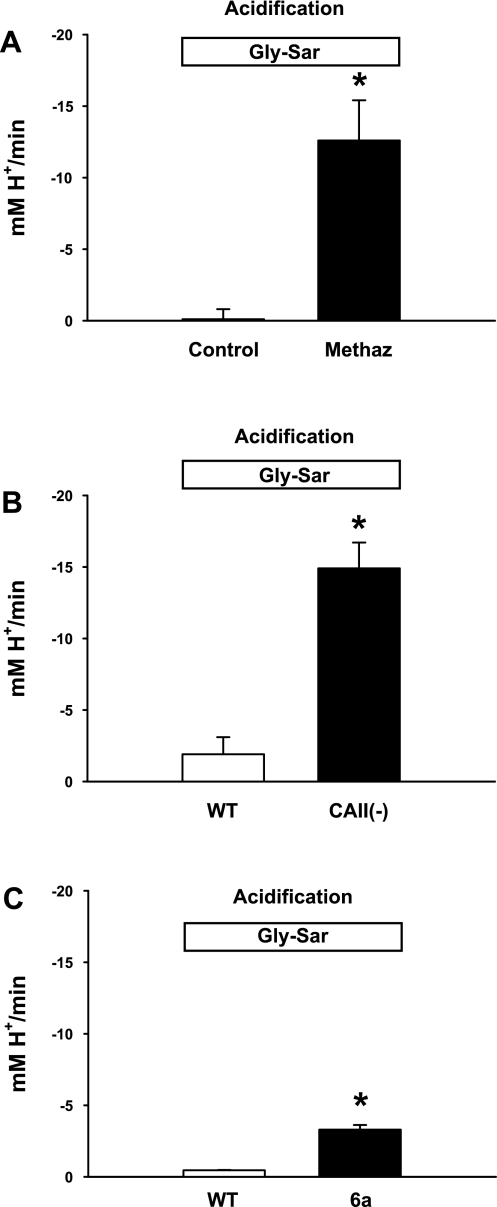
Fig. 4.
Gly-Sar-induced pHi acidification in the proximal small intestine during inhibition of carbonic anhydrases (CAs) and in the CAII(−) knockout mouse. A: summary of acidification rates for WT villous epithelium during luminal addition of 20 mM Gly-Sar in the absence (control) or presence of methazolamide (Methaz, 100 μM, bilateral addition). The mean pHi before Gly-Sar addition was control pHi = 7.67 ± 0.27 and Methaz pHi = 7.63 ± 0.06. *Significantly different from vehicle control (n = 3). B: summary of acidification rates for upper villous epithelial cells during luminal addition of 20 mM Gly-Sar in WT and CAII(−) duodena. The mean pHi before Gly-Sar addition was WT pHi = 7.74 ± 0.10 and CAII(−) pHi = 7.61 ± 0.08. *Significantly different from WT (n = 4). C: summary of acidification rates for WT villous epithelium during luminal exposure to 20 mM Gly-Sar (control) followed by treatment with the extracellular CA inhibitor, sulphonamide compound 1-[4-aminosulphonyl] phenyl-2,4,6-trimethylpyridinium perchlorate (6a) (30 μM, luminal addition for 5 min). The mean pHi before Gly-Sar addition was 7.35 ± 0.06. *Significantly different from control (n = 3).
Gly-Sar exposure increases HCO3− influx via a Pat-1- and CA-dependent mechanism in duodenal villous epithelium.
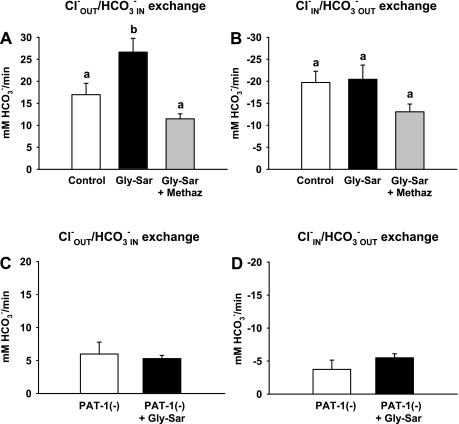
The rate of Cl−/HCO3− exchange was measured in the presence of luminal Gly-Sar to evaluate the role of Pat-1 in regulating pHi during Gly-Sar absorption. As shown in Fig. 5A, the rate of Cl−OUT/HCO3−IN exchange (i.e., HCO3− influx) during luminal Cl− removal is increased in the presence of Gly-Sar in WT compared with control villous epithelium. In contrast, the rate of Cl−IN/HCO3−OUT exchange (i.e., HCO3− efflux) during luminal Cl− replacement is unchanged by Gly-Sar exposure (Fig. 5B). The discovery that Cl−OUT/HCO3−IN exchange increased in the presence of Gly-Sar was further investigated by treating WT villous epithelium with the CA inhibitor methazolamide. As shown in Fig. 5A, methazolamide eliminated the increased rate of Cl−OUT/HCO3−IN exchange in WT epithelium during Gly-Sar exposure. To evaluate the role of Pat-1, rates of Cl−/HCO3− exchange during Gly-Sar exposure in villous epithelium were compared between Pat-1(−) and WT intestine. As shown in Fig. 5, C and D, Gly-Sar exposure did not affect the rate of residual Cl−/HCO3− exchange in the Pat-1(−) upper villous epithelium.
Fig. 5.
Comparison of Cl−/HCO3− exchange activity in upper villous epithelium of WT and Pat-1(−) duodena during luminal treatment with 20 mM Gly-Sar. A and B: rates of Cl−OUT/HCO3−IN and Cl−IN/HCO3−OUT exchange of WT upper villous epithelium induced by luminal Cl− removal and replacement, respectively, during vehicle treatment (control), in the presence of Gly-Sar (20 mM, luminal), and in the presence of Gly-Sar during methazolamide (100 μM, bilateral addition) treatment. Duodenal preparations were exposed to 20 mM Gly-Sar for 20 min either in the presence or absence of methazolamide before measurement of Cl−/HCO3− exchange rates. The mean pHi before measurement of Cl−/HCO3− exchange rates for each condition were control pHi = 7.62 ± 0.18, Gly-Sar pHi = 7.67 ± 0.27, and Gly-Sar + Methaz pHi = 7.63 ± 0.06. a,bMeans with the same letters are not significantly different (n = 5–6). C and D: rates of Cl−OUT/HCO3−IN and Cl−IN/HCO3−OUT exchange of upper villous epithelium induced by luminal Cl− removal and replacement, respectively, in Pat-1(−) duodenum under control conditions in KBR solution (open bar) and after 20-min exposure to 20 mM Gly-Sar (solid bar). The mean pHi before measurement of Cl−/HCO3− exchange rates were Pat-1(−) pHi 7.57 ± 0.06 and Pat-1(−) + Gly-Sar pHi = 7.44 ± 0.12 (n = 3–4).
DISCUSSION
Efficient H+-dipeptide absorption via Pept1 requires a sustained inward H+ electrochemical gradient across the apical membrane. Although this and previous studies have established a major role for apical membrane Na+/H+ exchange in maintaining a favorable pH gradient for H+-peptide absorption (14, 28, 33, 34), a contribution of apical membrane Cl−/HCO3− exchange was revealed when intestinal studies of Gly-Sar absorption were performed in media containing physiological CO2/HCO3− buffering. Under this condition, both transepithelial Gly-Sar transport and epithelial pHi regulation were sustained during Gly-Sar exposure despite pharmacological inhibition of apical membrane Na+/H+ exchange activity. Further investigation indicated a contribution of apical membrane Cl−/HCO3− exchange. Given the requirement to buffer H+ influx during peptide absorption, it was hypothesized that Cl−/HCO3− exchange would function as a base (HCO3−) importer, i.e., Cl−OUT/HCO3−IN exchange.
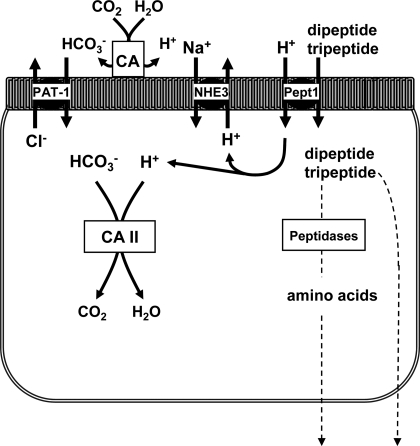
Both expression and functional studies colocalize Pat-1 Cl−/HCO3− exchange activity with Pept1 in the apical membrane of the upper villous epithelium (10). Loss of Pat-1 activity in knockout mice paradoxically results in cell acidification (27), and, unlike WT villous epithelium, Pat-1(−) epithelium rapidly acidified during H+-dipeptide transport in the presence of CO2/HCO3−-buffered medium. Previous studies have also implicated CA activity in efficient H+-peptide absorption and demonstrated that CAII binds to and interacts with Pat-1 to form a “HCO3− transport metabolon” in heterologous cell expression systems (1). Both pharmacological inhibition of CA activity in WT mice and genetic ablation of CAII resulted in increased rates of Gly-Sar-induced acidification in the upper villous epithelium. Extracellular CAs (CAs IV, IX, and possibly XII and XIV; Ref. 21) can provide a source of HCO3−, especially during luminal acidity, for Pat-1 acting as HCO3− importer, and pharmacological inhibition of extracellular CAs significantly increased Gly-Sar-induced acidification. Taken together, these data are consistent with a novel derivation of the HCO3− metabolon concept, whereby Pat-1 and CA activity mediate HCO3− uptake during H+/peptide transport to minimize intracellular acidification (see model in Fig. 6). However, it should be kept in mind that CAs may interact with other HCO3− transport proteins, e.g., basolateral membrane NaHCO3 cotransporters (1), so the role of these enzymes may be significantly more complex and only parallel to Pat-1 activity.
Fig. 6.
Cell model depicting the contributions of Nhe3, Pat-1, and CAs to pHi regulation during H+-dipeptide absorption via Pept1.
Consistent with the model shown in Fig. 6, measurement of Cl−/HCO3− exchange in WT villous epithelium during H+-dipeptide transport revealed an apparent enhancement of the Cl−OUT/HCO3−IN exchange during removal of Cl− from the luminal bath and no change in the rate of Cl−IN/HCO3−OUT exchange when Cl− was returned to the luminal bath. Increased Cl−OUT/HCO3−IN exchange during Gly-Sar was eliminated by methazolamide and did not occur in the Pat-1(−) intestine, indicating a dependence on these changes for Pat-1 and CA activity. If the subapical membrane pHi during Gly-Sar absorption is less than the measured cytosolic pHi (19), then an inward [HCO3−] gradient would favor Cl−OUT/HCO3−IN exchange and disadvantage Cl−IN/HCO3−OUT exchange by Pat-1. However, recent evidence by others suggests that Pat-1 is electrogenic (1Cl−:2HCO3− stoichiometry) (25, 42). In this case, depolarization of the apical membrane potential (Va) by the combined effects of luminal Cl− removal and H+-peptide absorption would also favor Cl−OUT/HCO3−IN exchange. When Cl− is returned to the luminal bath to initiate Cl−IN/HCO3−OUT exchange, the resulting hyperpolarization of Va may offset the inward [HCO3−] gradient so Cl−OUT/HCO3−IN exchange rate would be unchanged from control. If Pat-1 is electroneutral (1Cl−:1HCO3− stoichiometry), an alternative explanation is that signaling pathways activated by Gly-Sar absorption may stimulate Pat-1 Cl−/HCO3− exchange activity in both directions. Previous studies have shown that Na+-coupled glucose transport via sodium-glucose cotransporter-1 initiates signaling that increases Na+/H+ exchanger Nhe3 activity by a p38 mitogen-activated protein kinase pathway (36). Further investigation will be necessary to determine the specific cause of increased Cl−/HCO3− activity during H+-dipeptide absorption.
To predict whether Pat-1 Cl−OUT/HCO3−IN exchange occurs in balanced CO2/HCO3−-buffered KBR during Gly-Sar absorption, the relative driving force for HCO3− movement across the apical membrane was estimated using the villous epithelial pHi in the absence of Pat-1, i.e., the Pat-1(−) intestine. Calculating [HCO3−]i from the Henderson-Hasselbalch equation, the apical membrane HCO3− driving force for an electroneutral Pat-1 is Δ ũ a HCO3− =(RT/F).ln ([HCO 3−] i/[HCO3−]o); whereas, for an electrogenic Pat-1, Δ ũ a HCO3− =(RT/F).ln ([HCO 3−] i/[HCO3−]o)V a, where R is the gas constant, T is temperature (°K), F is the Faraday constant and [HCO3−]o is 25 mM (6). In the absence of Gly-Sar and assuming Va = −45 mV (L. L. Clarke, unpublished observations), Cl−IN/HCO3−OUT exchange is favorable for both electroneutral and electrogenic Pat-1 (ΔũaHCO3− = +12.4 mV and +57.5 mV, respectively). However, in the presence of Gly-Sar, Cl−OUT/HCO3−IN exchange is favorable for electroneutral Pat-1 (ΔũaHCO3− = −4.1 mV) but not for electrogenic Pat-1 (ΔũaHCO3− = +20.9 mV, assuming a depolarized Va = −25 mV). Although these calculations suggest that electrogenic Pat-1 is disadvantaged for HCO3− influx during Gly-Sar absorption, the pHi at the subapical membrane during H+-dipeptide uptake is likely less than the cytosolic pHi used to calculate ΔũaHCO3−. To achieve Cl−OUT/HCO3−IN exchange via an electrogenic Pat-1, the subapical membrane [HCO3−]i would need to be 9.8 mM, or a pHi = 7.04, which is easily within the range of subapical membrane pHi measured during weak acid absorption in polarized enterocytes (19).
Pat-1 Cl−/HCO3− exchange may be important for intraepithelial pH regulation under varying luminal conditions in the upper small intestine, but our studies do not indicate that this function is critical for H+-dipeptide absorption. On the basis of the Gly-Sar-induced Isc, the effect of Pat-1 was variable and only apparent when apical membrane Na+/H+ exchange was pharmacologically inhibited, indicating only a ∼15–20% contribution in sustaining Gly-Sar absorption. Thus it is not surprising that Pat-1(−) mice demonstrate normal growth on a standard diet (38). The major factor in sustaining H+-dipeptide absorption appears to be the contribution of Nhe2/Nhe3 activity. Nonetheless, Pat-1 is localized to the villous epithelium in the proximal small intestine, which is exposed to an acidic gastric effluent containing a Pco2 >150 mmHg (24, 41). Operation in Cl−OUT/HCO3−IN exchange may provide a larger contribution to pHi regulation during H+-peptide or -amino acid absorption in the presence of high CO2 tension. Under this condition, the role of extracellular CA activity may take on an even greater role than measured with basal Pco2 (38 mmHg) in providing a source of luminal HCO3− for Pat-1 operation as a HCO3− importer (see model in Fig. 6). Additional considerations that would affect Pat-1 activity as a HCO3− importer are changes in luminal Cl− concentration and the availability of other Pat-1 substrates (SO42−, oxalate) during and after a meal.
In summary, the present investigation found that Pat-1 Cl−/HCO3− function in the villous epithelium contributes to pHi regulation during H+-peptide absorption. Evidence suggested a model in which Pat-1 operates in concert with CA activity to import HCO3− (Cl−OUT/HCO3−IN exchange) and offset cellular acidification. In balanced CO2/HCO3−-buffered solutions, the contribution of Pat-1 activity is small and likely serves an auxiliary function to the significantly greater pHi regulation provided by apical membrane Na+/H+ exchange. However, reversible Cl−/HCO3− function of Pat-1 provides another means of pHi regulation for villous epithelium that is subjected to physiological extremes in pH, CO2, and ionic composition from gastric effluent.
GRANTS
This study was supported by grants from the National Institutes of Health DK 48816 (to L. Clarke), T32-RR 07004 (to J. Simpson), DK 62809 (to M. Soleimani), the Cystic Fibrosis Foundation CLARKE05P0 (to L. Clarke), and by an European Union framework programme 7 grant, METOXIA (to C. Supuran).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this article with any of the authors, or any of the authors' academic institutions or employers.
Supplementary Material
REFERENCES
- 1.Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J 24: 2499–2511, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells I. Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844–C856, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Buyse M, Tsocas A, Walker F, Merlin D, Bado A. PEPT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol 283: C1795–C1800, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol Gastrointest Liver Physiol 270: G259–G267, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Clarke LL, Paradiso AM, Mason SJ, Boucher RC. Effects of bradykinin on Na+ and Cl− transport in human nasal epithelium. Am J Physiol Cell Physiol 262: C644–C655, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66: 361–384, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ganapathy V, Ganapathy ME, Leibach FH. Intestinal transport of peptides and amino acids. In: Gastrointestinal Transport Molecular Physiology, edited by Barrett KE, Donowitz M. San Diego, CA: Academic, 2001, p. 379–412 [Google Scholar]
- 9.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Groneberg DA, Doring F, Eynott PR, Fischer A, Daniel H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am J Physiol Gastrointest Liver Physiol 281: G697–G704, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Smith EE, Ma K, Jappar D, Thomas W, Hillgren KM. Targeted disruption of peptide transport Pept1 gene in mice significantly reduces dipeptide absorption in the intestine. Mol Pharm 5: 1122–1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflügers Arch 445: 139–146, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Ko SBH, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/HCO3− exchanger in the basolateral membrane of the renal CCD and SMG duct. Am J Physiol Cell Physiol 283: C1206–C1218, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Kottra G, Stamfort A, Daniel H. PEPT1 as a paradigm for membrane carriers that mediate electrogenic bidirectional transport of anionic, cationic and neutral substrates. J Biol Chem 277: 32683–32691, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Lewis SE, Erickson RP, Barnett LB, Venta PJ, Tashian RE. N-ethyl-N-nitrosurea-induced null mutation at the mouse Car-2 locus: an animal model for human carbonic anhydrase II deficiency syndrome. Proc Natl Acad Sci USA 85: 1962–1966, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie B, Loo DD, Fei Y, Liu WJ, Ganapathy V, Leibach FH, Wright EM. Mechanisms of the human intestinal H+-coupled oligopeptide transporter hPEPT1. J Biol Chem 271: 5430–5437, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Maouyo D, Chu S, Montrose MH. pH heterogeneity at intracellular and extracellular plasma membrane sites in HT29-C1 cell monolayers. Am J Physiol Cell Physiol 278: C973–C981, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Merlin D, Steel A, Gewirtz AT, Si-Tahar M, Hediger MA, Madara JL. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest 102: 2011–2018, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573.3: 827–842, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJV, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124: 125–137, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastorekova S, Casini A, Scozzafava A, Vullo D, Pastorek J, Supuran CT. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett 14: 869–873, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Rune SJ, Henriksen FW. Carbon dioxide tensions in the proximal part of the canine gastrointestinal tract. Gastroenterology 56: 758–762, 1969 [PubMed] [Google Scholar]
- 25.Shcheynikov N, Wang Y, Park M, Ko SBH, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol 127: 511–524, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Simpson JE, Walker NM, Clarke LL. Recovery from proton di-tripeptide transport-mediated intracellular acidification in the duodenal villous epithelium of Na+/H+ exchanger isoform 3 (NHE3) knockout mice. FASEB J 19(4): A146, 2005. [Google Scholar]
- 29.Stewart AK, Boyd CAR, Vaughan-Jones RD. A novel role for carbonic anhydrase: cytoplasmic pH gradient dissipation in mouse small intestinal enterocytes. J Physiol 516: 209–217, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979 [DOI] [PubMed] [Google Scholar]
- 31.Thwaites DT, Brown DA, Hirst BH, Simmons NL. H+-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers displays distinctive characteristics. Biochim Biophys Acta 1151: 237–245, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Thwaites DT, Brown DA, Hirst BH, Simmons NL. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H+-coupled carriers at both apical and basal membranes. J Biol Chem 268: 7640–7642, 1993 [PubMed] [Google Scholar]
- 33.Thwaites DT, Ford D, Glanville M, Simmons NL. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest 104: 629–635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thwaites DT, Kennedy DJ, Raldua D, Anderson CMH, Mendoza ME, Bladen CL, Simmons NL. H+/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na+/H+ exchanger. Gastroenterology 122: 1322–1333, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger Slc26a6 in PGE2- but not forskolin-stimulated murine duodenal HCO3 secretion. Gastroenterology 130: 349–358, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na+-glucose cotransport in intestinal epithelia. Am J Physiol Cell Physiol 281: C1533–C1541, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-KB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127: 1401–1409, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestine transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Wang ZH, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Weintraub WH, Machen TE. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol Gastrointest Liver Physiol 257: G317–G327, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Winship DH, Robinson JE. Acid loss in the human duodenum. Volume change, osmolal loss, and CO2 production in response to acid loads. Gastroenterology 66: 188, 1974 [PubMed] [Google Scholar]
- 42.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Barone S, Petrovic S, Wang Z, Seidler U, Riederer B, Ramaswamy K, Dudeja PK, Shull GE, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in gastric surface mucous and duodenal villus cells. Am J Physiol Gastrointest Liver Physiol 285: G1225–G1234, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1215, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.