Abstract
Gastroparesis is a debilitating disease predominantly affecting young women. Recently, dysregulation of neuronal nitric oxide synthase (nNOS) in myenteric plexus neurons has been implicated for delayed solid gastric emptying/gastroparesis in diabetic patients. In this study, we have explored the role of tetrahydrobiopterin (BH4), a major cofactor for nNOS activity and NO synthesis in diabetic gastroparesis. Diabetes was induced with single injection of streptozotocin (55 mg/kg body wt, ip) in female rats, with experiments performed on week 3 or 9 following induction, with or without 3-wk BH4 supplementation. Gastric pyloric BH4 levels were significantly decreased in diabetic female rats compared with control (18.6 ± 1.45 vs. 31.0 ± 2.31 pmol/mg protein). In vitro studies showed that 2,4-diamino-6-hydroxypyrimidine (DAHP), an inhibitor of BH4 synthesis, significantly decreased gastric NO release and nitrergic relaxation. Three-week dietary supplementation of BH4 either from day 1 or week 6 significantly attenuated diabetes-induced delayed gastric emptying for solids (3 wk: BH4, 67 ± 6.7 vs. diabetic, 36.05 ± 7.09; 9 wk: BH4, 57 ± 8.45 vs. diabetic, 33 ± 9.91) and diabetes-induced reduction in pyloric nNOS-α protein expression in female rats. Supplementation of BH4 significantly restored gastric nNOS-α dimerization in 9-wk-old diabetic female rats. In addition, BH4 treatment reversed (17.23 ± 5.81 vs. 42.0 ± 2.70 mmHg × s) the diabetes-induced changes in intragastric pressures (IGP) and gastric pyloric nitrergic relaxation (−0.62 ± 0.01 vs. −0.22 ± 0.07). BH4 deficiency plays a critical role in diabetes-induced alterations including delayed solid gastric emptying, increased IGP, reduced pyloric nitrergic relaxation, and nNOS-α expression in female rats. Supplementation of BH4 accelerates gastric emptying by restoring nitrergic system in diabetic female rats. Therefore, BH4 supplementation is a potential therapeutic option for female patients of diabetic gastroparesis.
Keywords: nitrergic relaxation, diabetes, female rat, streptozotocin
gastroparesis is a clinical disorder characterized by delayed gastric emptying and symptoms such as nausea, vomiting, pain, and discomfort. A recent increase in prevalence of gastroparesis has been indicated, which may be an outcome of increased prevalence of diabetes (38). The most common known cause of this syndrome is diabetes, and it is estimated to affect 20–55% of patients with type 1 (insulin-dependent) and up to 30% of patients with type 2 (noninsulin-dependent) diabetes (31). There is a striking sex bias in gastroparetic patients, with women outnumbering men by a 4:1 ratio (18, 19, 34). Recent findings from our laboratory demonstrate that the reduction in gastric emptying is greater in female rats compared with males after diabetes induction (14). In addition, the study suggested that at least one biological reason for this sex bias may be differences in nitrergic regulation of gastric motility, with females being more vulnerable to changes in this system (14). Therefore, in the present study, we sought to examine the mechanisms underlying such changes.
Gastrointestinal motility is largely regulated by a synchronized interplay of excitatory (predominantly cholinergic) and inhibitory (predominantly nitrergic) neurons innervating smooth muscle bundles directly or through interstitial cells of Cajal (36, 40). Long-standing or uncontrolled diabetes may result in impairment of these regulatory mechanisms, particularly those involving inhibitory nitrergic neurons (32, 6, 7). In these neurons, nitric oxide (NO) is produced by neuronal NO synthase (nNOS), a process that requires an optimum supply of the substrate l-arginine and several cofactors such as flavin adenine dinucleotide (FAD), flavin adenine mononucleotide (FMN), calcium/calmodulin, and the small molecule, tetrahydrobiopterin (BH4). BH4 stimulates and stabilizes NOS dimerization (2). Deficiency of BH4 leads to uncoupling of NOS enzyme, functionally converting it into a peroxynitrite synthase, generating superoxide and NO, the precursors of peroxynitrite (ONOO−) (5, 10, 20, 29). BH4 supplementation or augmentation has been shown to restore nitrergic function in vascular endothelium in diabetes and other conditions (21, 30). However, the contribution of changes in the BH4 pathway to diabetic gastric nNOS dysfunction has not been studied. Therefore, this study was designed to test the hypothesis that BH4 deficiency plays an important role in diabetic gastric motility dysfunction through alterations in nitrergic regulation.
MATERIALS AND METHODS
Experimental rats and induction of diabetes.
Adult female Sprague-Dawley rats (9 wk old) were procured from Harlan (Houston, TX) and maintained in the institutional animal care facility under controlled temperature, humidity, and light-dark cycle (12-h:12-h), with free access to rodent chow and water. All experiments in this study were approved by the Institutional Animal Care and Use Committees at the University of Texas Medical Branch, Galveston, Texas and Meharry Medical College, Nashville, Tennessee, in accordance with the recommendations of National Institutes of Health, Guide for the Care and Use of Laboratory Animals.
Diabetes was induced in overnight fasted animals by a single intraperitoneal injection of streptozotocin (STZ, 55 mg/kg) (Sigma Chemical, St. Louis, MO) prepared in 9 mmol citrate buffer, pH 4.0. Nondiabetic control animals were injected with the vehicle (9 mmol citrate buffer, pH 4.0). Blood glucose levels were examined in overnight fasted animals, 48 h after STZ injection. Animals exhibiting blood glucose levels more than 250 mg/dl were considered diabetic and included in the study. Blood glucose levels in vehicle-treated overnight fasted rats ranged between 80–95 mg/dl. Both control and diabetic female rats were selected during the diestrus phase of the estrous cycle for further experiments. Body weights and blood glucose levels for female rats before or at 9 wk of diabetes induction are presented in Table 1.
Table 1.
Blood glucose level and body weight in the control and diabetic female rats
| ND (n = 20) | DM (n = 30) | BH4DM (n = 20) | |
|---|---|---|---|
| Body Weight, g | |||
| Baseline | 193 ± 1.51 | ||
| 3 wk after injection | 210 ± 3* | 175 ± 4*† | 176 ± 9*† |
| 9 wk after injection | 228 ± 1.34* | 160 ± 1.36*† | 208 ± 0.80*†‡ |
| Blood Glucose, mg/dl | |||
| Baseline | 86 ± 0.57 | ||
| 3 wk after injection | 90 ± 1.9 | 492 ± 8*† | 500 ± 4*† |
| 9 wk after injection | 85 ± 1.45 | 592 ± 1.26*† | 600 ± 4*† |
Control group (nondiabetic, ND) was injected with vehicle (citrate buffer) only. Diabetes (DM) was induced with streptozotocin (55 mg/kg body wt ip).
P < 0.05 compared to baseline;
P < 0.01 compared to control (ND) group;
P < 0.001 compared to DM group. BH4DM, tetrahydrobiopterin (BH4) + DM.
Experimental design.
At the end of sixth week of diabetes induction, animals were divided into three groups, i.e., nondiabetic female rats (ND), diabetic female rats (DM), and BH4-supplemented diabetic female rats (BH4DM). BH4DM were provided with BH4 pellets (20 mg/kg body wt per day) for the next 3 wk. A group of nondiabetic female rats were also treated with BH4 for 3 wk, and solid gastric emptying, nitrergic relaxation, and nNOS protein expression were measured at the end of treatment period. On the last day of BH4 supplementation, animals were used either for various experiments or euthanized to collect gastric tissue for future analysis. Tissue samples collected from animals were snap frozen in liquid nitrogen and stored at −80°C until analyzed. Solid gastric emptying and nNOS-α expression in female gastric pylorus were also studied at the end of the third week of diabetes, following 3-wk BH4 supplementation. In both male and female animals, BH4 supplementation was initiated from day 1 of diabetes. BH4-pellets used in this study were prepared by compression of BH4 (Schircks Laboratories, Jona, Switzerland) with rodent chow and stored at −20°C until used. To avoid oxidation of BH4, heat and water was not employed in pellet preparation (TestDiet; Land O'Lakes Purina Feed, Richmond, IN).
Determination of biopterins in gastric pylorus.
Biopterin levels were determined in pyloric homogenates following acid (total biopterin, BH4, BH2, biopterin) and alkali oxidation (BH2 and biopterin), essentially as described by Cai et al. (5). HPLC separation of biopterin was performed on a Spherisorb ODS-1 column (Waters, Elstree, UK) using mobile phase consisting of 10% methanol in water. Fluorescence detection (350-nm excitation, 450-nm emission) was performed using an FP-2020 detector (Jasco UK, Essex, UK). BH4 concentration in tissue samples was calculated by subtracting BH2 + biopterin (alkali oxidation product) from total biopterins (acid oxidation product) and presented as picomoles per milligram of protein.
Tissue culture studies.
Animals from ND group were euthanized under anesthesia, the abdominal cavity opened, and the stomach dissected and transferred in chilled oxygenated Krebs bicarbonate solution of the following composition (in mmol): 118.0 NaCl, 4.7 KCl, 25.0 NaHCO3, 1.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 11.5 glucose (pH 7.4). Pyloric tissue was harvested and cut into mucosa-free strips. Pyloric strips were cultured for 24 h (37°C, 5% CO2) in phenol red-free DMEM supplemented with NB27 (2%) and antibiotics (1%) with or without 10 mmol 2,4-diamino-6-hydroxypyrimidine [DAHP; an inhibitor of GTP cyclohydrolase 1 (GTPCH1), a rate-limiting enzyme in BH4 de novo synthesis pathway; Ref. 41]. On completion of incubation, DMEM was collected and stored at −80°C for analysis of NO released in medium during incubation period. NO released in the medium was analyzed as total nitrite (metabolic byproduct of NO) following the protocol supplied with a commercially available kit (Calbiochem, Gibbstown, NJ).
Solid gastric emptying studies.
At the end of 3-wk BH4 supplementation, solid gastric emptying studies were performed as described previously (14). The rate of gastric emptying was calculated according to the following equation: gastric emptying (% in 4 h) = (1 − gastric content/food intake) × 100.
Ambulatory telemetric studies.
Gastric contractility was measured using an ambulatory telemetric apparatus as described in our previous study (14). Each animal was implanted with a pressure transducer in gastric lumen 1 wk before experiment. Area under curve (AUC) of contraction events (intragastric pressure, IGP: mmHg × s) was recorded in freely moving overnight-fasted rats for 3 h, between 9:00 AM to 12:00 AM, and used for further statistical analysis.
Organ bath studies.
Electric-field stimulation (EFS)-induced nonadrenergic, noncholinergic (NANC) relaxation was studied in circular pyloric strips. Strips were tied with silk thread at both ends and were mounted in 5-ml water-jacketed organ baths containing Krebs buffer at 37°C and continuously bubbled with 95% O2-5% CO2 (Radnoti Glass Technology, Monrovia, CA). Tension for each muscle strip was monitored with an isometric force transducer and analyzed by a digital recording system (Biopac Systems, Santa Barbara, CA). A passive tension equal to 2 g was applied on each strip in the 1-h equilibration period through an incremental increase (0.5 g, 4 times, at 15-min intervals). Strips were exposed to atropine, phentolamine, and propranolol (10 μmol each) in bath solution for 1 h to block cholinergic and adrenergic responses. 5-Hydroxytryptamine (100 μmol)-precontracted pyloric strips were exposed to EFS (90 V, 2 Hz, 1-ms pulse for duration of 1 min) to elicit NANC relaxation. Relaxation response elicited by low-frequency (2 Hz) stimulus under NANC conditions, as used in this study, was demonstrated as predominantly nitrergic in origin (14).
To study the effect of pharmacologically created BH4 deficiency on EFS-induced NANC/nitrergic relaxation, pyloric strips from ND were incubated in tissue bath with DAHP (10 mmol) for 3 h, and EFS-induced NANC relaxation was elicited. At the end of each experiment, the muscle strip was blotted dry with filter paper and weighed. Comparisons between groups were performed by measuring the AUC of the EFS-induced relaxation (AUCR) for 1 min and the baseline for 1 min (AUCB) according to the formula (AUCR − AUCB)/weight of tissue (mg) = AUC/mg of tissue.
cNOS activity assay.
Total constitutive NOS (cNOS) activity was analyzed in pyloric homogenates by monitoring the formation of [3H]l-citrulline from [3H]l-arginine as described previously (4). In brief, the reaction was performed in 200-μl reaction volume, containing 2.5 mg protein, 20 μmol FAD, 4 μmol BH4, 50 μmol calmodulin, 1 μmol l-arginine, 10 nmol [3H]l-arginine with or without 1 mmol NADPH and with 1.25 mmol CaCl2 or with 1 mmol EGTA. All reactions were stopped by dilution with ice-cold HEPES buffer (80 mmol HEPES, 8 mmol EDTA, pH 5.2). [3H]l-citrulline, a product of reaction, was separated from [3H]l-arginine by DOWEX (AG 50W-8) cation exchange. For the blank reaction, tissue homogenate was replaced by equal volume of buffer. Count from blank reaction was subtracted from each tube to get corrected counts. Because NOS activity is NADPH dependent, the activity in the absence of NADPH was subtracted from total activity, and the results were expressed as NADPH-dependent activity. NOS activity was expressed as femtomole of l-citrulline per milligram protein per minute.
Western blot analysis.
Total nNOS and nNOS-α protein (40 μg protein was loaded in 6% gel/well) was quantified in gastric pyloric homogenates from all groups using standard Western blot analysis, as described in our previous study (14). Total nNOS and nNOS-α proteins were identified using primary antibodies directed to COOH-terminal (nNOS monoclonal antibody; Transduction Laboratory, San Jose, CA) and NH2-terminal (1–195 amino acids of nNOS-α, exon 2 region) nNOS-α-specific polyclonal antibody (Zymed Laboratories, San Francisco, CA), respectively. COOH-terminal antibody detects all (total) forms of nNOS (-α, -β, and -γ), whereas NH2-terminal antibody detects only the full-length nNOS-α protein and is the only catalytically active isoform (33). Quantification of proteins was achieved by enhanced chemiluminescence system (ECL; Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's protocol. Densitometry analysis was performed in the linear range using a Fluorchem Analysis System (Alpha InfoTech; San Leandro, CA) and the amount of nNOS-α (155 kDa) was normalized to the γ-tubulin (48 kDa) signal.
nNOS dimerization in rat gastric neuromuscular tissue.
Levels of nNOS monomer and dimer were quantified by Western blotting via low-temperature (LT)-PAGE in gastric pylorus homogenates as described previously (14). LT-SDS-PAGE was performed on ice. The LT process was used to identify nNOS dimers and monomers in the native state, as LT is known to prevent monomerization of nNOS dimmers. For the LT processing, 30 μg of protein in standard Laemmli buffer at 4°C was used for SDS-PAGE. The mixture was incubated at 0°C for 30 min before LT-SDS-PAGE using a 6% separating gel. All gels and buffers were preequilibrated to 4°C before electrophoresis and the buffer tank placed in an ice-bath during electrophoresis to maintain the gel temperature below 15°C. A polyclonal antibody specific to nNOS (Zymed Laboratories) and anti-rabbit IgG conjugated with horseradish peroxidase (Sigma Chemical) were used as the primary and secondary antibodies, respectively (14).
Statistical analysis.
Results are expressed as means ± SE obtained from four to eight animal data points. Data were analyzed for statistical differences with Student's t-test or two-way ANOVA followed by the Bonferroni t-test to verify the differences between individual groups. P < 0.05 was considered significant (n = 4–8).
RESULTS
Effect of supplementation of BH4 on blood glucose and body weight in female diabetic rats.
Initially, we examined whether supplementation of BH4 for 3 wk attenuated the elevated blood glucose and reduced body weights in female rats 3 or 9 wk after diabetes induction. Fasting blood glucose levels were significantly (P < 0.05) elevated in female rats both at 3 wk (492 ± 8 mg/dl, P < 0.05) and 9 wk (592 ± 1.26 mg/dl, P < 0.05) after diabetes induction (Table 1). Blood glucose levels were unchanged upon supplementation of BH4 in diabetic female rats. A significant (P < 0.05) weight loss was noted in the diabetic rats (Table 1). The mean body weight at 3 wk (175 ± 4 g) or 9 wk after STZ injections (160 ± 1.36 g) was significantly lower than either baseline (193 ± 1.51 g, P < 0.05) or age-matched ND group (P < 0.05). Supplementation of BH4 partially, but significantly, attenuated elevated body weights at 9 wk (208 ± 0.8 g, P < 0.05) but not at 3 wk (Table 1).
Effect of diabetes on BH4 concentration in gastric pylorus.
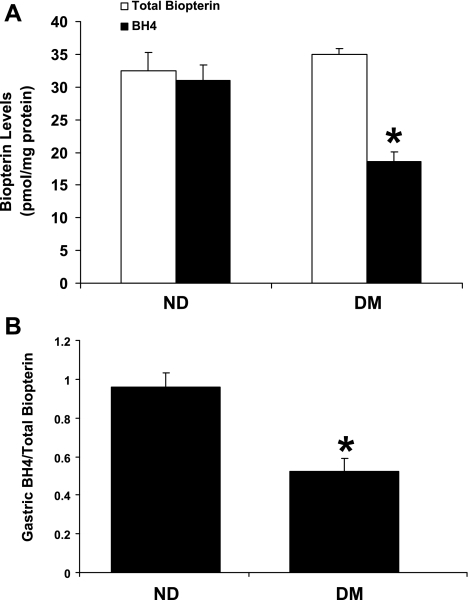
In the present study, we analyzed BH4 and total biopterin concentrations (pmol/mg protein) in gastric pylorus using HPLC with fluorescence detection. Our results show that total biopterin levels in gastric pylorus were unchanged in the DM group (35 ± 0.53 pmol/mg protein) compared with the ND group (32.5 ± 2.80 pmol/mg protein) (Fig. 1A). However, pyloric BH4 content was significantly reduced in the DM groups compared with the ND group (18.6 ± 1.45 vs. 31.0 ± 2.31 pmol/mg protein; P < 0.05), resulting in a significant decrease in the relative concentration of BH4 to total biopterin in diabetic specimens (Fig. 1B).
Fig. 1.
Effect of diabetes on gastric pyloric tetrahydrobiopterin (BH4) content in female rats. A: changes in total biopterin and BH4 content in gastric pylorus from nondiabetic (ND) and diabetic (DM) female rats. B: ratio of total biopterin and BH4 in ND and DM female gastric pylorus. The values are means ± SE for 4–6 animals in each group. *P < 0.05 compared with ND group.
Effect of GTPCH1 inhibition on nitrergic regulation of gastric pylorus in vitro.
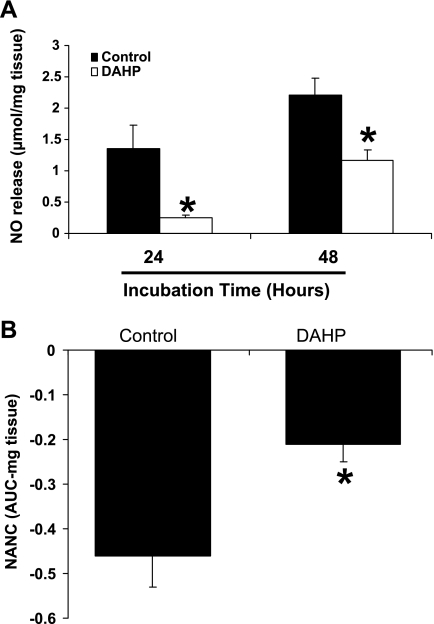
We next examined the effects of BH4 deficiency on NO release from pyloric strips in vitro. Gastric muscle strips were incubated with 10 mmol of DAHP, a specific inhibitor of GTPCH1, for 24 and 48 h. Compared with controls, DAHP exposure resulted in a significant reduction in NO released from pyloric strips at both 24 h (0.26 ± 0.03 vs. 1.35 ± 0.38 μmol/mg tissue; P < 0.05) and 48 h (1.16 ± 0.18 vs. 2.21 ± 0.27 μmol/mg tissue; P < 0.05) (Fig. 2A). We also examined the effects of GTPCH1 inhibition by DAHP in organ bath studies carried out on isolated pyloric strips. As shown in Fig. 2B, a significant reduction in EFS-induced NANC relaxation (AUC, mg tissue) of pyloric strips was observed following 3-h incubation with DAHP in bath solution.
Fig. 2.
Effect of 2, 4-diamino-6-hydroxypyrimidine (DAHP), a selective inhibitor of GTP cyclohydrolase-1, the rate-limiting enzyme for de novo BH4 synthesis, on nitric oxide (NO) production (A) and nitrergic relaxation (B) in rat gastric pyloric neuromuscular tissue. A: gastric neuromuscular pyloric tissues were incubated in the presence or absence of 10 mmol DAHP either for 24 or 48 h. B: Nonadrenergic, noncholinergic (NANC) relaxation in response to transmural nerve stimulation was measured in control and DAHP (10 mM, 3 h preincubation)-treated circular neuromuscular pyloric strips (electric-field stimulation, 90 V, 2 Hz, 1-ms pulse for duration of 1 min). The values are means ± SE for 6–8 strips. *P < 0.05 compared with control group; AUC, area under the curve.
Effect of BH4 supplementation on solid gastric emptying in vivo.
We next investigated whether dietary BH4 supplementation can restore delayed gastric emptying that occurs in diabetic male and female animals. Female DM and ND groups were treated with BH4 (20 mg/kg per day) for 3 wk, either from day 1 or the start of the sixth week of diabetes induction. Male DM and ND groups were treated with BH4 (20 mg/kg per day) for 3 wk from day 1 of diabetes induction.
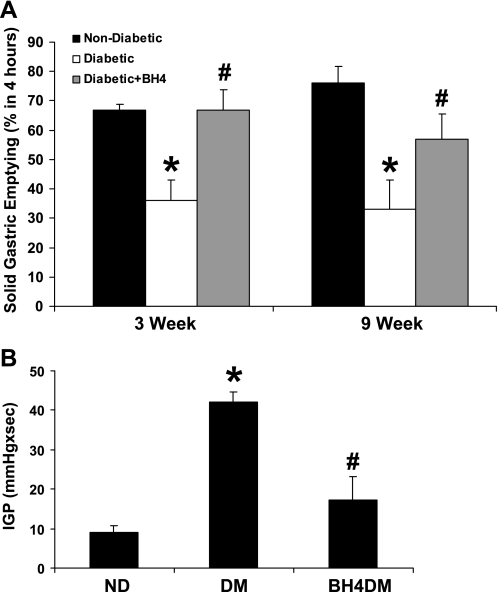
BH4 supplementation did not affect solid gastric emptying (percentage in 4 h) in female ND compared with female BH4ND (78 ± 3 vs. 70 ± 3.5). A significant reduction of solid gastric emptying (Fig. 3A) was observed, both at 3 and 9 wk after diabetes induction in female rats. A 3-wk course of BH4 supplementation to diabetic female animals, either from day 1 or week 6 of diabetes induction, resulted in reversal of this decrease in solid gastric emptying to levels comparable with nondiabetic rats (Fig. 3A). However, BH4 supplementation begun on day 1 of induction of diabetes was ineffective in restoring delayed gastric emptying in male rats after 3 wk [ND, 86 ± 1.78 (n = 6); DM, 58 ± 6.5 (n = 4) vs. BH4DM, 68 ± 4.16 (n = 5 animals per group)].
Fig. 3.
A: effect of BH4 on solid gastric emptying in ND and DM female rats. Groups (n = 4–6) of diabetic rats were given BH4 (BH4DM) tablets daily for 3 wk either from day 1 (5 mg/kg body wt per day) or 6 wk (20 mg/kg body wt per day) after diabetic induction with single injection of streptozotocin (STZ: 55 mg/kg body wt ip). The values are means ± SE for 6–8 animals. *P < 0.05 compared with ND group. #P < compared with DM group. B: effect of BH4 on intragastric pressure (IGP; mmHg × s) in DM female rats. IGPs were measured using ambulatory telemetric devices (see materials and methods and Ref. 14) in groups of freely moving ND and DM female rats. A group of DM female rats received BH4 tablets (BH4DM) for 3 wk after 6 wk of diabetic induction by single injection of STZ (55 mg/kg body wt ip). The values are means ± SE for 4–6 animals. *P < 0.05 compared with ND group. #P < 0.05 compared with DM group.
We also examined IGP (mmHg × s) in freely moving female rats using telemetric recording technique in ND, DM, and BH4DM groups. We have previously shown that increases in IGP are associated with delays in gastric emptying, nNOS dimerization, and nitrergic relaxation (14). As shown in Fig. 3B, a significant rise (>4.5-fold) in IGP was seen in DM compared with ND, an effect that was reversed by supplementation of BH4 in diet of BH4DM rats.
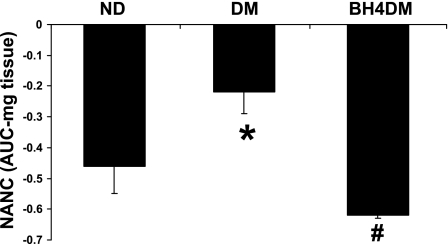
Effect of BH4 supplementation on nitrergic relaxation.
Pyloric strips from ND animals exhibited substantial relaxation following EFS (2 Hz). Compared with ND, EFS-induced NANC relaxation (AUC, mg tissue) was significantly less in pyloric strips from 9-wk diabetic animals. Supplementation of BH4 did not alter nitrergic relaxation compared with ND group (data not shown). As depicted in Fig. 4, supplementation of BH4 from the sixth to ninth week resulted in complete reversal of diabetes-induced alteration of nitrergic relaxation of pyloric strips.
Fig. 4.
Effect of BH4 on nitrergic relaxation in diabetic rat gastric muscular tissues. A group of DM female rats received BH4 tablets (BH4DM) for 3 wk after 6 wk of diabetic induction by single injection of STZ (55 mg/kg body wt ip). The values are means ± SE for 4–6 animals. *P < 0.05 compared with ND group. #P < 0.05 compared with DM group.
Effect of BH4 supplementation on cNOS activity in gastric pylorus.
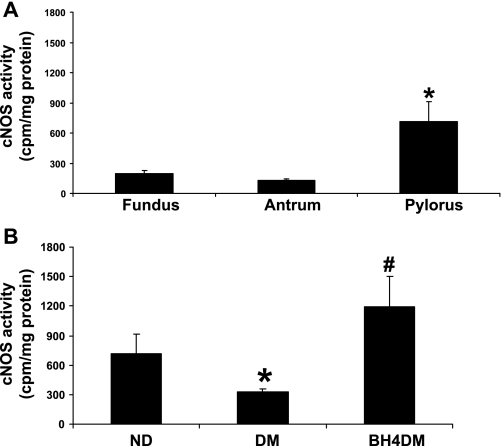
Constitutively expressed NOS includes both nNOS and endothelial NOS (eNOS) isoforms (3). Because specific inhibitors for these individual isoforms are not available, we have measured total cNOS activity (cpm/mg protein) in pyloric homogenates following the method described by Bush et al. (3). Figure 5A depicts that pyloric region of stomach contains maximum cNOS activity compared with fundus and antrum regions in ND group. As shown in Fig. 5B, diabetes results in significant reduction of pyloric cNOS activity. BH4 treatment resulted in complete reversal of cNOS activity in BH4DM as shown in Fig. 5B.
Fig. 5.
Effect of BH4 on constitutive nitric oxide synthase (cNOS) activity in diabetic rat gastric tissues. A: cNOS activity in gastric fundus, antrum, and pyloric tissues in healthy (control) female rats (n = 4 in each group). B: cNOS activity in BH4-treated diabetic female rat gastric pyloric tissues. A group of DM female rats received BH4 tablets (BH4DM) for 3 wk after 6 wk of diabetic induction. The ND group received citrate buffer (vehicle). The values are means ± SE for 4–6 animals. *P < 0.05 compared with ND group. #P < 0.05 compared with DM group.
Effect of BH4 supplementation on total nNOS, nNOS-α expression, and nNOS-α dimerization in gastric pylorus.
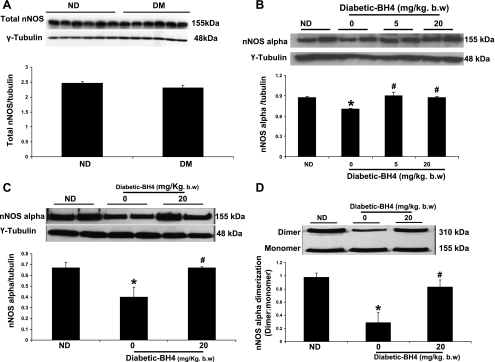
As shown in Fig. 6A, total nNOS (combined α, β, γ isoforms) protein expression (nNOS/tubulin) was unchanged in the pylorus of DM compared with ND female rats. However, we observed that protein expression of nNOS-α, the only functional isoform of nNOS in gastric tissue, was significantly decreased following 3 and 9 wk of diabetes (Fig. 6, B and C). In addition, BH4 supplementation significantly (P < 0.05) restored gastric nNOS-α dimerization in diabetic female rats (Fig. 6D). This finding was in agreement with our organ bath findings shown in Fig. 4. Supplementation of BH4 to diabetic female but not ND group (data not shown) results in significant restoration of nNOS-α expression at both 3 and 9 wk of diabetes (Fig. 6, B and C).
Fig. 6.
Effect of BH4 in the expression and dimerization of neuronal NO synthase (nNOS) in diabetic rat gastric tissues. A: representative immunoblot and densitometric analysis data for nNOS protein expression in ND and 9-wk DM female rat gastric pylorus. The values are means ± SE for 4 samples in each group. B: representative immunoblot and densitometric analysis data for nNOS-α protein expression in ND and DM female rat gastric pylorus. A group of DM female rats received BH4 tablets (BH4DM) for 3 wk after day 1 (5 mg/kg body wt per day) or 6 wk (20 mg/kg body wt per day) of diabetic induction by single injection of STZ (55 mg/kg body wt ip). The values are means ± SE for 4 samples in each group. *P < 0.05 compared with ND group. #P < compared with untreated (0) diabetic group. C: representative immunoblot and densitometric analysis data for nNOS-α protein expression in ND and 9-wk DM female rat gastric pylorus. D: representative immunoblot and densitometric analysis data for nNOS-α dimerization in ND and 9-wk DM female rat gastric pylorus. A group of DM female rats received BH4 tablets (BH4DM) for 3 wk after 6 wk (20 mg/kg body wt per day) of diabetic induction. The values are means ± SE for 3–4 samples in each group. *P < 0.05 compared with ND group. #P < 0.05 compared with untreated (0) diabetic group.
DISCUSSION
The major findings of this study are the following: 1) STZ-induced diabetes results in gastric pyloric BH4 deficiency in female rats. 2) This is associated with reductions in both expression and dimerization of nNOS-α, impaired nitrergic regulation of pyloric motility, and reduced gastric emptying. 3) BH4 supplementation reverses all these changes. Collectively, our studies support our original hypothesis that BH4 deficiency plays a significant role in gastric dysmotility in DM and that BH4 supplementation may represent a reasonable and rational approach to the treatment of gastroparesis in female diabetics.
BH4 is an endogenously synthesized molecule and serves as a cofactor for aromatic amino acid hydroxylases (phenylalanine-4-hydroxylase, tyrosine-3-hydroxylase, and tryptophan-5-hydroxylase) and NOS enzymes (nNOS, eNOS, and inducible NOS) (27). Tissue BH4 concentration is determined by its production through de novo and salvage pathway and degradation by catalytic processes. In de novo pathway, GTP is converted to BH4 through the sequential action of three enzymes, i.e., GTPCH1, 6-pyruvoyltetrahydroprerin synthase, and sepiapterin reductase (SR) (37). Alternatively, BH4 can be synthesized from the exogenous pterin precursor sepiapterin, which is metabolized to dihydrobiopterin (BH2) by SR first, and further to BH4 by dihydrofolate reductase (DHFR) (37). When BH4 is endogenously oxidized to BH2 (23), it can also be reduced back to BH4 by DHFR (27).
Recently, BH4 deficiency has been recognized associated with many diabetic complications (such as hypertension, cardiopathy, nephropathy), sickle cell disease, and phenylketonuria (8, 25, 28, 27, 42). In this study, we show that diabetes also results in significant reduction in BH4 levels in the gastric pylorus (Fig. 1). Hyperglycemia has been reported to cause decreased synthesis of BH4 by the de novo pathway through proteasome-dependent degradation of GTPCH1 (39, 42). Increased generation of superoxide, a common finding in diabetes can enhance oxidation of BH4 and may constitute another possible explanation of reduced BH4 availability (8, 12, 13, 16, 26). Low BH4 levels not only impair the production of NO but also, because of nNOS uncoupling, lead to increased superoxide radical production. Superoxide radical thus produced reacts with NO to produce peroxynitrite, which further reduces biological availability of NO (29).
These findings provide a rational basis for the use of supplemental BH4 in diabetic conditions. Supplementation of BH4, either pharmacologically (BH4 or its precursor sepiapterin) or genetically (increased GTPCH1 expression and/or enzyme activity), has shown promising effects on cardiovascular tissue and function both in in vitro (cultured endothelial cells, isolated organs/tissue) and in vivo experimental studies (8, 21, 27). We now show that supplementation of BH4 can normalize the delayed gastric emptying associated with diabetes in female rats (Fig. 3A). These results are further supported by our data on IGP, showing normalization of diabetes-induced altered IGP in BH4DM (Fig. 3B). Our results in male rats demonstrated that BH4 was ineffective in restoring delayed gastric emptying in 3-wk diabetic rats. These results together with our recent studies suggest that the beneficial effects of BH4 are limited to female but not male perhaps because nitrergic-mediated motility is greater in these animals, possibly attributable to elevated levels of BH4 biosynthesis and/or enzyme activity required for improved BH4 content in targeted tissue (14). However, further studies are warranted to investigate whether gastric BH4 biosynthesis is higher in female compared with male rats. In addition, studies are also required to investigate dose- and time-dependent effects of BH4 on gastric emptying in male diabetic rats before we can confidently draw this conclusion.
Gastric tissues express both constitutive isoforms of NOS, i.e., smooth muscle eNOS and nNOS, which makes it difficult to study nNOS activity in pyloric homogenate (15). The data presented in Fig. 5, A and B, therefore represent both nNOS and eNOS activity levels in female control and diabetic rats. It is likely that the change in cNOS represents a change in eNOS; however, given the lack of specific inhibitors, it is impossible to distinguish the contributions from individual isoforms of the enzyme. Thus we presented NOS activity in gastric pyloric tissue as cNOS activity. Our results demonstrate that BH4 administration to diabetic animals significantly restores cNOS activity in the pylorus, a portion of the stomach with the highest cNOS activity (Fig. 5, A and B).
The literature is somewhat conflicting on the effect of diabetes on nNOS expression in gastric tissue, with studies suggesting both increase (1) and decrease in nNOS expression (6, 17). In our previous study, we reported an increased expression of total nNOS (α, β, γ) in the gastric antrum of diabetic male but not female rat (14). Similar to our previous findings, in our present study, gastric pyloric total nNOS expression does not change in diabetic female animals (Fig. 6A). However, total nNOS expression may be misleading. Among the three isoforms of nNOS present in rat gastric tissue, nNOS-α is the only catalytically active isoform (33). Our study demonstrates a significant decrease in the expression of this isoform along with dimerization in the pylorus in diabetic animals (Fig. 6, B, C, and D). Supplementation of BH4 to diabetic animals resulted in significant restoration of both nNOS-α expression and dimerization level in gastric pylorus (Fig. 6, B and C).
Studies in human volunteers demonstrated that supplementation of NO donors slows, rather than accelerates, gastric emptying and raises the issue of the utility of improving nNOS function in diabetes (35). Apart from difficulty in extrapolation from animal studies to humans, it is also worth pointing out that pharmacological studies in mixed-sex groups of healthy humans may not predict the response in diabetic patients, particularly if the results are sex specific. Furthermore, it is possible that supplementation of BH4 may work only by maintaining endogenous NO homeostasis at a physiological level rather than pharmacological level, as we might observe with supplementation of NO donors.
Although our experiments were focused on the pylorus, it is likely that similar changes will be seen in the rest of the stomach. Recently, pyloric injection of botulinum toxin (BTX) has been tried in patients of gastroparesis, with both positive and negative outcomes. Even though BTX has been shown to reverse the gastric emptying in gastroparesis patients (24), it may not improve the symptoms (3, 22). This may be because 1) nausea and vomiting may not correlate with emptying and 2) the site of action of BTX is localized to the pylorus where it is injected. On the other hand, dietary BH4 supplementation may effect other regions of the stomach as well as influence other pathophysiological abnormalities in addition to gastric emptying. However, more studies are needed to understand the beneficial role of BH4 in a clinical setting.
Finally, it should be emphasized that the effects of BH4 are probably more complex than those of an NO donor (35). Supplementation of BH4 stabilizes the functionally active, dimeric form of nNOS in vitro. BH4 also inhibits monomerization of nNOS, as well as inactivation of the enzyme (11, 20). Our in vivo data, for the first time, have shown that dietary BH4 supplementation restores both nNOS dimerization and enzyme activity in diabetic rat stomach. This has two major consequences. The first, as indicated previously, is the production of free radicals attributable to nNOS uncoupling (29). Secondly, the monomeric form of this enzyme is highly susceptible to proteolytic degradation (11, 20). Both of these effects may be reversed with BH4 supplementation without necessarily resulting in superphysiological levels of NO production. Many other factors may also contribute to the beneficial effect of BH4. BH4 also modulates the metabolism of norepinephrine, dopamine, and serotonin, all of which may affect gastric emptying and function (9, 23, 27).
In summary, the present study highlights the role of BH4 in maintaining nitrergic expression and function and gastric motility and suggests a novel strategy to restore diabetes-induced delayed gastric emptying through supplementation of BH4.
GRANTS
The original research was supported by the NIH-NIDDK R21DKO76704 awarded to Pandu R. Gangula. This study was also made possible in part by Grant G12 RR003032 from the National Center for Research Resources (NCRR), a component of NIH. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
DISCLOSURES
There are no conflict of interest to disclose for the authors except that Pandu R.R. Gangula and Pankaj J Pasricha (the University of Texas Medical Branch, Galveston, TX) have filed a patent application in their names.
ACKNOWLEDGMENTS
We thank Praveen Gupta for helping in manuscript preparation. We also thank Dr. Veera Rajaratnam, Director of Scientific Publications and Grant Support at the Center for Women's Health Research, Meharry Medical College, for the excellent editing of the revised manuscript.
REFERENCES
- 1.Adeghate E, Al-Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K, Howarth FC, El-Sharkawy T. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci 60: 1172–1179, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res 43: 521–531, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Xu MJ, Yang X, Xu C, Gao J, Zou DW, Li ZS. A systemic review on intrapyloric botulinum toxin injection for gastroparesis. Digestion 81: 27–34, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Bush PA, Gonzalez NE, Ignarro LJ. Biosynthesis of nitric oxide and citrulline from L- arginine by constitutive nitric oxide synthase present in rabbit corpus cavernosum. Biochem Biophys Res Commun 186: 308–314, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, Heales S, Channon KM. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res 55: 838–849, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes 52: 2353–2362, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil 19: 951–960, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14: 323–327, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cooper SM, McRitchie B. Role of dopamine and alpha-adrenoreceptors in the control of gastric emptying in the rat: possible involvement in the mechanism of action of metoclopramide. J Auton Pharmacol 5: 325–331, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem 284: 1136–1144, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dunbar AY, Kamada Y, Jenkins GJ, Lowe ER, Billecke SS, Osawa Y. Ubiquitination and degradation of neuronal nitric-oxide synthase in vitro: dimer stabilization protects the enzyme from proteolysis. Mol Pharmacol 66: 964–969, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord 9: 301–314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 292: G725–G733, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grider JR, Murthy KS. Autoinhibition of endothelial nitric oxide synthase (eNOS) in gut smooth muscle by nitric oxide. Regul Pept 151: 75–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–E22, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol 41: 1076–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care 24: 1264–1269, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Jung HK, Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 136: 1225–1233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamada Y, Jenkins GJ, Lau M, Dunbar AY, Lowe ER, Osawa Y. Tetrahydrobiopterin depletion and ubiquitylation of neuronal nitric oxide synthase. Brain Res Mol Brain Res 142: 19–27, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 30: 48–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoo J, Rayner CK, Jones KL, Horowitz M. Pathophysiology and management of gastroparesis. Expert Rev Gastroenterol Hepatol 3: 167–181, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Hasegawa H, Kaneko E, Ichiyama A. Gastrointestinal serotonin: depletion due to tetrahydrobiopterin deficiency induced by 2,4-diamino-6-hydroxypyrimidine administration. J Pharmacol Exp Ther 256: 773–779, 1991 [PubMed] [Google Scholar]
- 24.Lacy BE, Crowell MD, Schettler-Duncan A, Mathis C, Pasricha PJ. The treatment of diabetic gastroparesis with botulinim toxin injection of the pylorus. Diabetes Care 27: 2341–2347, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J 349: 353–356, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263: 681–684, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol 50: 238–246, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Okumura M, Masada M, Yoshida Y, Shintaku H, Hosoi M, Okada N, Konishi Y, Morikawa T, Miura K, Imanishi M. Decrease in tetrahydrobiopterin as a possible cause of nephropathy in type II diabetic rats. Kidney Int 70: 471–476, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO- cycle. Med Hypotheses 69: 821–825, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol 140: 701–706, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 127: 1592–1622, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis (Abstract). BMC Gastroenterol 8: 21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao YM, Chaudhury A, Goyal RK. Active and inactive pools of nNOS in the nerve terminals in mouse gut: implications for nitrergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 294: G627–G634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci 43: 2398–2404, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Sun WM, Doran S, Jones KL, Ooi E, Boeckxstaens G, Hebbard GS, Lingenfelser T, Morley JE, Dent J, Horowitz M. Effects of nitroglycerin on liquid gastric emptying and antropyloroduodenal motility. Am J Physiol Gastrointest Liver Physiol 275: G1173–G1178, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol 38: 421–430, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347: 1–16, 2000 [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol 103: 313–322, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase 1. Diabetes 58: 1893–1901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut 45, Suppl 2: II6–II16, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie L, Smith JA, Gross SS. GTP cyclohydrolase I inhibition by the prototypic inhibitor 2, 4-diamino-6-hydroxypyrimidine. Mechanisms and unanticipated role of G cyclohydrolase I feedback regulatory protein. J Biol Chem 273: 21091–21098, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 116: 944–953, 2007. [DOI] [PubMed] [Google Scholar]