Abstract
Endothelial function is impaired by oxidative stress in chronic heart failure (HF). Mechanisms that protect against increases in oxidative stress in HF are not clear. The goal of this study was to determine whether manganese superoxide dismutase (MnSOD) plays a key role in protecting against endothelial dysfunction in HF. Endothelial function and gene expression were examined in aorta from wild-type mice (MnSOD+/+) and mice deficient in MnSOD (MnSOD+/−) 12 wk after ligation of the left coronary artery (LCA). LCA ligation produced similar size myocardial infarctions in MnSOD+/+ and MnSOD+/− mice and reduced ejection fraction to ∼20% in both groups. Maximal relaxation in response to acetylcholine was 78 ± 3% (mean ± SE) and 66 ± 8% in sham-operated MnSOD+/+ and MnSOD+/− mice, respectively. Expression of antioxidant enzymes increased in MnSOD+/+ mice with HF, and maximal relaxation to acetylcholine was slightly impaired (68 ± 4%). Greater endothelial dysfunction was observed in MnSOD+/− mice with HF (46 ± 5%, P < 0.05), which was significantly improved by polyethylene glycol-catalase but not Tempol. Incubation with the nonspecific cyclooxygenase (COX) inhibitor indomethacin or the COX1 inhibitor valeryl salicylate, but not the COX-2 inhibitor NS-398, significantly improved relaxation to acetylcholine in HF mice (maximum relaxation = 74 ± 5, 91 ± 1, and 58 ± 5%). These data suggest that MnSOD plays a key role in protecting against endothelial dysfunction in HF. A novel mechanism was identified whereby chronic increases in oxidative stress, produced by mitochondrial SOD deficiency, impair vascular function via a hydrogen peroxide-dependent, COX1-dependent, endothelium-derived contracting factor.
Keywords: mitochondria, oxidative stress, vasomotor function, endothelium, endothelium-derived contracting factor, cyclooxygenase
endothelial dysfunction is associated with increases in oxidative stress and appears to play a significant role in the pathophysiology of several diseases (5). Humans and experimental animals develop endothelial dysfunction and increases in oxidative stress after the induction of chronic heart failure (HF; see Refs. 2, 17, 21, 24, 30, 32, 35, 42, 43). Additionally, several of the most effective treatments for HF (especially inhibitors of the renin-angiotensin system) are associated with improvements in endothelial function, reductions in NAD(P)H oxidase activity, and reductions in oxidative stress (19, 34, 38).
Superoxide dismutases (SOD) provide important protection against oxidative stress in blood vessels (14). Expression of copper-zinc SOD (CuZnSOD) and manganese SOD (MnSOD) appears to be relatively well preserved in a variety of tissue beds in humans with HF, but several groups have shown that expression of extracellular SOD (ecSOD) is significantly reduced (35). Although recent work from our laboratory has shown that overexpression of ecSOD significantly improved endothelial function in rats with HF (22), it is not known whether maintenance of expression of endogenous MnSOD or CuZnSOD plays an important role in protecting against endothelial dysfunction.
In the present investigation, we sought to determine whether increases in mitochondrial oxidative stress impair endothelial function in a mouse model of HF. We tested the hypothesis that deletion of one copy of the MnSOD gene would impair endothelial function in mice with HF primarily through reductions in nitric oxide bioavailability. Because previous work from Yang et al. (48, 49) and others (18, 44) has shown that increased reactive oxygen and nitrogen species can increase cyclooxygenase (COX) activity in blood vessels, we also tested the alternate hypothesis that increased mitochondrial oxidative stress impairs vascular function by increasing COX-derived contracting factors.
METHODS
Animals.
Studies were performed on 70 mice. All procedures and protocols were approved by the University of Iowa Animal Care and Use Committee. Wild-type (WT) and heterozygous MnSOD-deficient mice were derived from breeder pairs comprised of one WT and one MnSOD-deficient/heterozygous mouse (since complete ablation of MnSOD results in lethality at an early age). This strain has been backcrossed onto a C57BL/6J background for more than 10 generations. For this study, we used littermate-matched WT and MnSOD-deficient mice.
Coronary ligation.
Adult female mice (4–5 mo old) were anesthetized with ketamine (90 mg/kg ip) and xylazine (9 mg/kg ip). After tracheal intubation and establishment of mechanical ventilation, a small thoracotomy was made, and the proximal left coronary artery was ligated. Sham mice underwent a thoracotomy, and the coronary artery was exposed but not ligated.
Cardiac function.
Cardiac function was evaluated as described previously (13, 46). Briefly, mice were sedated with midazolam (0.15 mg sc), and the animal was cradled in the left lateral recumbent position while a 15-MHz linear-array probe was applied horizontally to the chest. The imaging probe was coupled to a Sonos 5500 imager (Philips Medical Systems, Bothell, WA), generating ≈180–200 two-dimensional frames per second in both short- and long-axis left ventricular planes. Left ventricular dimensions were measured in the long and short axes 12 wk after coronary ligation, and left ventricular volumes and ejection fraction were calculated as described previously.
Infarct area.
Infarct area was measured on immersion-fixed left ventricular sections. In brief, hearts were immersed in 4% paraformaldehyde for >24 h, after which point the ventricles were cut into 1-mm-thick sections. Images of each section were acquired using a stereoscope, and the percentage of the left ventricular free wall that was infarcted was measured using ImageJ software (National Institutes of Health).
Gene expression.
Tissue from the aortic arch and proximal descending thoracic aorta was used to measure gene expression 12 wk after coronary ligation. Quantitative real-time RT-PCR was used to measure expression of genes related to antioxidant defense mechanisms [MnSOD, CuZnSOD, ecSOD, catalase, glutathione peroxidase (GPx) 1, and GPx4], prooxidant mechanisms [NAD(P)H oxidase (Nox) 1, Nox2, and Nox4], nitric oxide synthase isoforms (endothelial NOS, inducible NOS, and neuronal NOS), and enzymes related to synthesis of endothelium-derived contracting factors (EDCF; COX1 and COX2) using previously described methods (8).
Oxidative stress.
We measured superoxide levels in mouse aortic rings using lucigenin-enhanced chemiluminescence (5 μM lucigenin). All relative light unit (RLU) counts were normalized to the surface area of the aortic ring. This method has been described previously in detail (23).
Evaluation of vascular function.
Vasomotor function of aorta was evaluated ex vivo by measurement of isometric tension, as we have described in many previous studies (1, 10, 33, 36). Briefly, 12 wk after the coronary ligation procedure, mice were anesthetized and killed with pentobarbital sodium (75–100 mg/kg ip). The aorta was excised, connective and adipose tissue were removed, and the aorta was placed in oxygenated Krebs buffer. Vessels were suspended between two triangular hooks in an organ bath, and isometric tension was measured. Responses to acetylcholine (endothelium dependent) and sodium nitroprusside (endothelium independent) were examined after preconstriction of the vessel to ∼50–60% of its maximal force.
The following compounds were used: the antioxidant Tempol (1 mM dissolved in saline; Sigma), the COX1 and -2 inhibitor indomethacin (10 μM dissolved in 0.1 M Na2CO3; Sigma), the COX1 specific inhibitor valeryl salicylate [3 mM dissolved in dimethyl sulfoxide (DMSO); Sigma], the COX2 specific inhibitor NS-398 (10 μM dissolved in saline; Sigma), pegylated catalase to degrade hydrogen peroxide (200 U/ml dissolved in saline; Sigma), and the nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME, 10 μM dissolved in saline; Sigma). Adjacent regions of descending thoracic aorta were used as time controls and incubated with identical concentrations of vehicle in the organ bath (DMSO, Na2CO3, or saline). Because we did not detect a significant time effect across any of the conditions, data are presented as the means of all time control vessels. The final concentration of vehicle in the organ bath was <1%.
Statistical analyses.
All data are expressed as means ± SE. Differences in contraction and relaxation across groups were detected using an ANOVA, with subsequent post hoc testing using Bonferroni-corrected t-tests.
RESULTS
Left ventricular function.
Left ventricular function was similar between WT and heterozygous sham-operated animals. Left ventricular function was significantly impaired in both WT and MnSOD-deficient mice with HF (ejection fraction was reduced by >70%; see Table 1) and was associated with significant increases in the heart wet weight-to-body weight ratio (Table 1). MnSOD deficiency did not alter infarct size or the magnitude of left ventricular dysfunction in mice with HF (see Table 1).
Table 1.
Indexes of LV dysfunction and severity of heart failure in wild-type and MnSOD-deficient animals
| SHAM |
HF |
|||
|---|---|---|---|---|
| WT | MnSOD+/− | WT | MnSOD+/− | |
| Body wt, g | 21.4 ± 1.2 | 21.0 ± 0.6 | 21.9 ± 0.6 | 21.3 ± 0.6 |
| LWW/BW | 8.4 ± 0.7 | 8.1 ± 0.6 | 7.9 ± 0.1 | 9.3 ± 0.6 |
| HWW/BW | 5.8 ± 0.6 | 5.3 ± 0.5 | 7.0 ± 0.2‡ | 7.3 ± 0.4‡ |
| Infarct size,* % | 51 ± 7 | 55 ± 4 | ||
| Cardiac output,† μl/min | 12.6 ± 0.3 | 10.1 ± 2.4 | 9.4 ± 2.0 | 12.2 ± 1.6 |
| EF,† % | 68 ± 9 | 66 ± 7 | 19 ± 4‡ | 20 ± 2‡ |
Values are means ± SE. HF, heart failure; WT, wild type; MnSOD+/−, manganese superoxide dismutase deficient; LWW/BW, lung wet weight-to-body weight ratio; HWW/BW, heart wet weight-to-body weight ratio; EF: ejection fraction.
Measurements made histologically, n = 4–5 mice/group,
Measurements derived from echocardiography, n = 4–5 mice/group.
P < 0.05 vs. sham-operated animals within the same genotype group.
Expression of antioxidant and prooxidant genes.
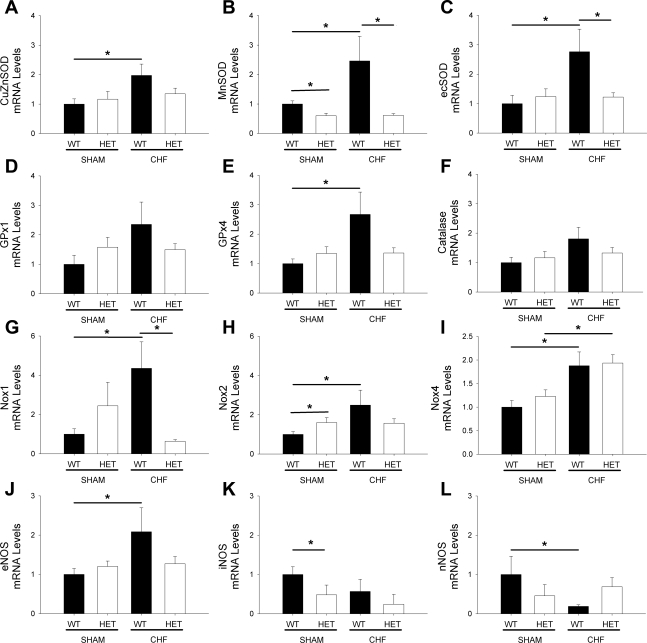
Expression of MnSOD was reduced by ∼40% in sham-operated MnSOD-deficient mice (Fig. 1B) and did not affect the expression of CuZnSOD and ecSOD (Fig. 1, A and C). After induction of HF in WT mice, expression of MnSOD, CuZnSOD, and ecSOD was significantly increased (Fig. 1, A–C). In contrast to WT mice, expression of MnSOD did not increase in MnSOD-deficient mice with HF (Fig. 1B). Furthermore, expression of CuZnSOD and ecSOD was markedly reduced compared with WT animals with HF (Fig. 1, A and C).
Fig. 1.
Expression of antioxidant genes (A–F), prooxidant genes (G–I), and nitric oxide synthase (NOS) isoforms (J–L) in wild-type (WT) and manganese superoxide dismutase (MnSOD)-deficient mice with or without heart failure (HF). Note that the compensatory increases in antioxidant enzymes (A–F) that occur in WT mice with HF are abolished in MnSOD-deficient mice with HF. Also, only endothelial nitric oxide synthase (eNOS) was significantly increased in WT mice with HF. Values are means ± SE; n = 7–15 mice/group. *P < 0.05. HET, MnSOD-deficient (+/−) mice; CuZnSOD, copper-zinc superoxide dismutase; ecSOD, extracellular SOD; GPx, glutathione peroxidase; Nox, NAD(P)H oxidase; iNOS, inducible NOS; nNOS, neuronal NOS; CHF, chronic HF.
Expression of GPx1, GPx4, and catalase was not significantly changed in sham-operated MnSOD-deficient mice compared with sham-operated WT mice (Fig. 1, D–F). After induction of HF, GPx1, GPx4, and catalase all tended to be elevated in WT mice (Fig. 1, D–F), although only GPx4 reached statistical significance (Fig. 1E).
Expression of Nox2 was significantly increased in sham-operated MnSOD-deficient mice (Fig. 1G). After induction of HF in WT mice, expression of Nox1, Nox2, and Nox4 was significantly increased (Fig. 1, G–I). In MnSOD-deficient mice with HF, levels of Nox1 and Nox2 did not increase significantly compared with sham-operated WT or MnSOD-deficient mice (Fig. 1, G and H). Nox4 expression, however, remained significantly elevated in MnSOD-deficient mice with chronic HF (Fig. 1I). Similar changes in other subunits associated with each NAD(P)H oxidase catalytic subunit were also observed (data not shown).
Expression of endothelial NOS was significantly increased in WT mice with chronic HF but not in MnSOD-deficient mice with chronic HF (Fig. 1J). Expression of inducible NOS was significantly reduced in sham-operated MnSOD-deficient mice and tended to be reduced in both genotypes of mice with chronic HF (Fig. 1K). Expression of neuronal NOS was also significantly reduced in WT mice with chronic HF but was not significantly reduced in groups with MnSOD-deficient sham-operated mice or HF mice (Fig. 1L).
Superoxide levels in aorta.
In sham-operated mice, superoxide levels in aorta were relatively low (0.33 ± 0.21 RLU·min−1·mm−2). Superoxide levels were slightly elevated in sham-operated MnSOD-deficient mice (3.4 ± 1.4 RLU·min−1·mm−2) and WT mice with HF (3.9 ± 2.1 RLU·min−1·mm−2; P = not significant), and markedly elevated in MnSOD-deficient mice with HF (9.0 ± 1.5 RLU·min−1·mm−2; P < 0.05).
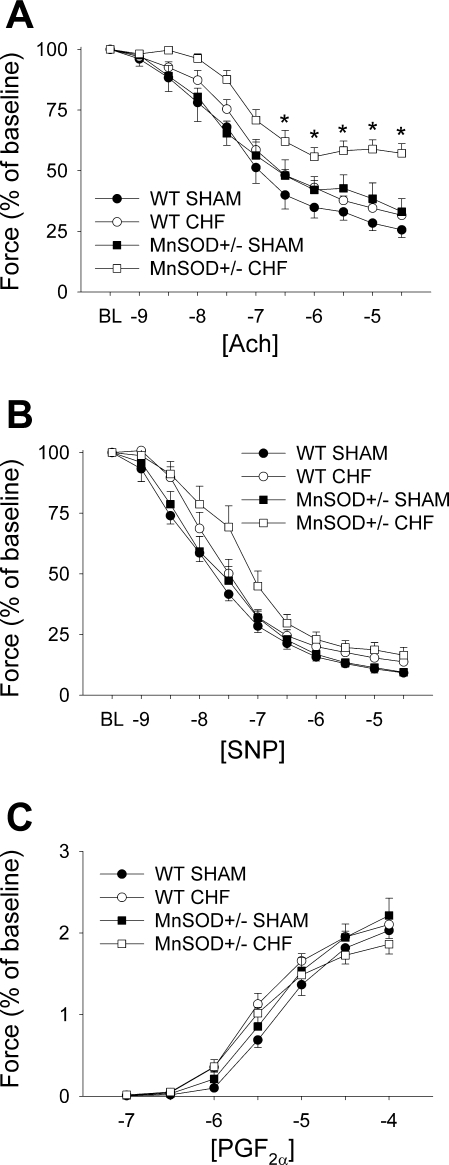
Vascular function in sham-operated mice.
Endothelial function in sham-operated MnSOD-deficient mice was modestly impaired compared with sham-operated WT mice (see Fig. 2A). Induction of HF did not significantly impair endothelial function in WT mice (Fig. 2A). In MnSOD-deficient mice, however, induction of HF resulted in marked impairment in vasorelaxation in response to acetylcholine. Relaxation to acetylcholine was virtually abolished by preincubation with l-NAME in all groups [see Online Supplement (Supplemental data for this article may be found on the American Journal of Physiology: Heart and Circulatory Metabolism website.)]. Responses to sodium nitroprusside or PGF2α were not altered in any of the groups (Fig. 2, B and C).
Fig. 2.
Vasomotor function in WT and MnSOD-deficient (MnSOD+/−) mice with or without HF. A: responses to acetylcholine (ACh). B: vascular responses to sodium nitroprusside (SNP). C: responses to prostaglandin F2α (PGF2α). Note that vasorelaxation in response to acetylcholine is relatively well preserved in WT mice with HF but profoundly impaired in MnSOD-deficient mice. BL, baseline. *P < 0.05 vs. sham-operated MnSOD-deficient mice; n = 7–15 mice/group.
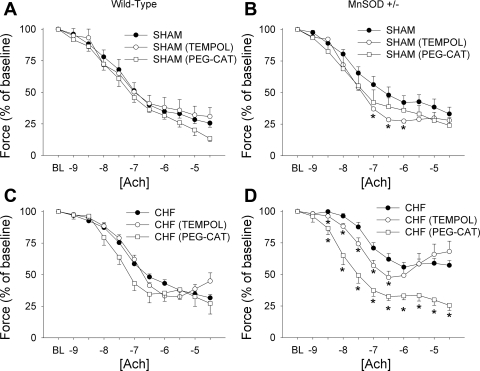
Redox mechanisms contributing to endothelial dysfunction.
Addition of Tempol or polyethylene glycol (PEG)-catalase did not alter vasomotor function in sham-operated WT mice (Fig. 3A). Addition of Tempol, however, completely normalized endothelial function in MnSOD-deficient sham mice (Fig. 3B), although PEG-catalase did not significantly affect vascular relaxation in this group (Fig. 3, A and B). Addition of Tempol or PEG-catalase did not significantly affect vasomotor function in vessels from WT mice with HF (Fig. 3C).
Fig. 3.
Effects of Tempol (an antioxidant) or polyethylene glycol (PEG)-catalase (CAT) on vascular function in WT and MnSOD-deficient mice with or without HF. A and C: effects of Tempol or PEG-catalase on responses to acetylcholine in sham-operated and HF WT mice. B and D: effects of Tempol or PEG-catalase on vascular responses to acetylcholine in sham-operated and HF MnSOD-deficient mice. Tempol did not improve vascular function in vessels from WT mice and only improved relaxation in response to acetylcholine in sham-operated, MnSOD-deficient mice. PEG-catalase significantly improved vascular function only in MnSOD-deficient mice with HF. *P < 0.05 vs. vessels without antioxidants; n = 7–12 mice/group.
In MnSOD-deficient mice with HF, addition of Tempol improved relaxation in response to low doses of acetylcholine [log(ACh) from −8.5 to −6.5; see Fig. 3D]. Relaxation in response to acetylcholine in the presence of Tempol, however, tended to be more impaired at the higher doses when compared with untreated vessels, and even unmasked contraction at the highest doses of acetylcholine (log[ACh] above −6; Fig. 3D). In contrast, vasomotor responses to acetylcholine were completely normalized by PEG-catalase in MnSOD-deficient mice with HF (see Fig. 3D). Both of these observations are consistent with the notion that EDCF production in MnSOD-deficient mice is mediated by hydrogen peroxide.
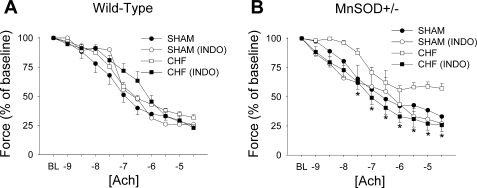
Effects of COX inhibition on vascular function.
Addition of indomethacin (an inhibitor of both COX1 and COX2) did not significantly alter vasomotor responses to acetylcholine in sham mice of either genotype (Fig. 4, A and B), nor did it affect endothelium-dependent relaxation in WT mice with HF (see Fig. 4A). In MnSOD-deficient mice with HF, however, indomethacin completely normalized vasorelaxation in response to acetylcholine (Fig. 4B).
Fig. 4.
Effects of indomethacin (INDO; an inhibitor of cyclooxygenase 1 and 2) on responses to acetylcholine in WT (A) and MnSOD-deficient (B) mice with or without HF. Note that preincubation with indomethacin significantly improved responses to acetylcholine only in MnSOD-deficient mice with HF. *P < 0.05 vs. MnSOD-deficient HF group; n = 6–10/group.
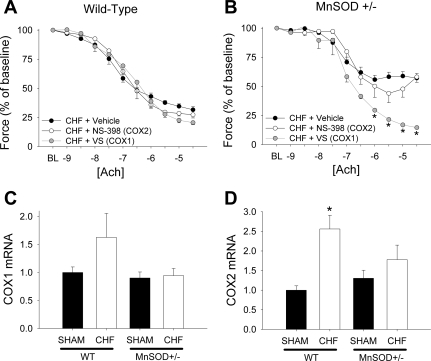
Consistent with the absence of effects of indomethacin in WT mice with HF, incubation of vessels with valeryl salicylate (a specific inhibitor of COX1) or NS-398 (a specific COX2 inhibitor) did not affect endothelial function in WT mice with HF (Fig. 5A). Inhibition of COX1, however, completely normalized vasomotor responses to acetylcholine in MnSOD-deficient mice with HF (Fig. 5B). In contrast, inhibition of COX2 did not significantly affect vascular function in HF mice of either genotype (Fig. 5, A and B).
Fig. 5.
Effects of valeryl salicylate [VS, a cyclooxygenase (COX) 1 inhibitor; A] or NS-398 (a COX2 inhibitor; B) on responses to acetylcholine in WT or MnSOD-deficient mice with HF. C: expression of cyclooxygenase-1 in WT and MnSOD-deficient mice with and without HF. D: expression of cyclooxygenase-2 in WT and MnSOD-deficient mice with and without HF. Only valeryl salicylate, which blocks cyclooxygenase-1, improved responses to acetylcholine in MnSOD-deficient mice with HF. *P < 0.05 vs. HF + vehicle within each panel; n = 5–7/group.
Expression of COX isoforms.
We detected expression of both COX1 (Fig. 5C) and COX2 (Fig. 5D) in aorta. Expression of both COX isoforms was significantly increased by HF in WT mice (Fig. 5, C and D). Expression of both COX1 and COX2 was not significantly elevated in MnSOD-deficient mice with HF (Fig. 5D).
DISCUSSION
The main findings of this study are: 1) WT mice with HF are remarkably resistant to endothelial dysfunction, 2) expression of multiple antioxidant defenses is impaired in MnSOD-deficient mice, 3) MnSOD-deficient mice with HF have markedly impaired endothelial function, and 4) MnSOD-deficient mice have increased COX1-dependent EDCF production in HF.
Cardiac function in WT and MnSOD-deficient mice.
In sham-operated mice, MnSOD deficiency did not affect ejection fraction, cardiac output, and heart or lung weight-to-body weight ratios. After ligation of the left coronary artery, ejection fraction was greatly reduced, and the heart weight-to-body weight ratio was increased, but the lung weight-to-body weight ratio was not increased. Myocardial infarction size and reductions in ejection fraction following coronary ligation were similar between WT and MnSOD-deficient mice. Although reducing NAD(P)H oxidase activity by deletion of the p47phox subunit attenuates impairment of left ventricular function following coronary ligation in mice (11), our data suggest that the severity of the ischemic insult (and consequent HF) was similar between WT and MnSOD-deficient mice following coronary ligation.
Endothelial function in WT mice with HF.
In WT mice, endothelial dysfunction was relatively modest following the induction of HF. This was a rather surprising finding, since previous studies have reported that humans, rats, and mice with HF have substantial endothelial dysfunction (2, 3, 22, 23, 34). Interestingly, we did observe a significant increase in endothelial NOS in WT mice with HF. One potential explanation for the more modest endothelial dysfunction in the present study could be that we only studied female mice, and estrogen can protect against endothelial dysfunction through the upregulation of NOS (39).
A second mechanism that may contribute to the small magnitude of endothelial dysfunction in WT mice with HF is that expression of all three SOD isoforms and enzymes related to hydrogen peroxide production were increased in aorta from WT mice following the induction of HF. Although our group has demonstrated that antioxidants or viral overexpression of SOD improve vascular function in HF (22, 23) and other disease states (6, 16, 37), these studies did not examine the role of endogenous antioxidant defense mechanisms in protecting against endothelial dysfunction. Thus coordinated increases in antioxidant enzymes in WT mice may play an important role in protecting against endothelial dysfunction in HF.
Pro- and antioxidant defense responses are altered in MnSOD-deficient mice with HF.
In sham-operated mice, there was no significant effect of MnSOD deficiency on expression of other SOD isoforms or hydrogen peroxide-degrading enzymes. This finding is consistent with previous reports in which the genetic ablation of either CuZnSOD or ecSOD levels does not affect expression of other SOD isoforms (20, 27, 40). Additionally, we found that MnSOD deficiency significantly increased Nox2 expression in aorta.
After induction of HF in MnSOD-deficient mice, expression of CuZnSOD, ecSOD, GPx1, and GPx4 was markedly reduced compared with WT mice. Previous work has demonstrated that the promoter regions of several of these enzymes have numerous redox-sensitive regions, thus making this finding rather paradoxical (50). Nevertheless, the current findings suggest that MnSOD may play an important role in coordinating specific antioxidant defenses in HF (25). Reductions in antioxidant defense mechanisms appear to have a functional consequence because vasomotor function can be normalized by acute treatment with PEG-catalase in MnSOD-deficient mice with HF.
Interestingly, increases in expression of Nox1 and Nox2 in WT mice with HF were markedly attenuated in MnSOD-deficient mice with HF. This change is qualitatively and quantitatively different from what was observed in sham-operated MnSOD-deficient mice. We speculate that this may be an adaptive response to reductions in mitochondrial antioxidant defenses and excessive increases in intracellular oxidant stress, since previous work has shown that Nox2 plays a key role in inducing cardiac, vascular, and mitochondrial dysfunction following ANG II administration (4, 12, 26). Nox4 expression, however, was elevated in both WT and MnSOD-deficient mice with HF. Thus Nox4 may be a major contributor to increases in reactive species production and vascular dysfunction in these animals.
Endothelial dysfunction in MnSOD-deficient mice with HF is the result of an EDCF.
Endothelial vasomotor function was markedly impaired in MnSOD-deficient mice with HF. This exaggeration of endothelial dysfunction was not associated with increased severity of ventricular dysfunction. Previous work has shown that genetic reductions in MnSOD increase susceptibility to endothelial dysfunction in cerebral vessels (15) and in conduit vessels of aged animals (7). Unlike previous studies, however, we were not able to restore endothelial function by acutely incubating vessels with SOD mimetics (15).
The major finding of this study was that endothelial dysfunction in MnSOD-deficient mice with HF was ameliorated by compounds that inhibit COX activity. Previous work has suggested that coronary vascular function can be improved by indomethacin in humans and dogs with long-term severe chronic HF, which suggests a role for an EDCF in the endothelial dysfunction observed in HF (28, 29, 31, 41).
We initially expected that increased EDCF production would be the result of increased COX-2 expression and activity. Expression of COX-2 is inducible by humoral factors (including tumor necrosis factor-α) that are increased in HF (47), and COX-2 has been shown to be increased in activated endothelial cells from patients with HF (9). Administration of the COX-2 inhibitor NS-398, however, did not improve endothelial function in mice with HF.
In contrast, inhibition of COX-1 with valeryl salicylate significantly improved endothelial function in MnSOD-deficient mice with HF. Tang and colleagues (45) have provided pharmacological and genetic evidence that COX-1 can contribute importantly to EDCF production in mice. The EDCF produced by COX-1 is likely to be an endoperoxide, which subsequently binds to thromboxane/prostanoid receptors on vascular smooth muscle (45, 48, 49). Relevant to the present report, increases in oxidative stress produce an increase in activity of COX-1 (48, 49), and reducing peroxidative stress by pretreatment of the vessels with PEG-catalase abrogates COX-1-dependent EDCF production in MnSOD-deficient mice with HF. Thus we speculate that, in vivo, mitochondria-derived reactive oxygen species are an important determinant of COX-1 activity, and this effect does not require an increase in COX-1 expression.
Limitations.
The definition of HF in humans is complex and evolving and is based not only on impaired cardiac function but also on other pathophysiological changes. In the present study, we used echocardiography to evaluate cardiac function and to define the presence or absence of HF, with an a priori definition of HF being an ejection fraction <35%. In mice that underwent coronary ligation, ejection fractions were well below 35%. The mice, however, did not have significant increases in lung wet weight at the time of death, and other measures of HF were not obtained. Thus this mouse model may be more representative of severe, chronic left ventricular dysfunction than that of chronic HF. Nevertheless, it is likely that mitochondrial oxidative stress is elevated even more in stages of decompensated HF, providing a milieu that is conducive to the production of EDCF by COX1.
We did not measure protein or enzyme activity levels for several of our outcome variables (e.g., COX and NOS isoforms), which is an important limitation to the current study. The amount of tissue available for evaluating protein levels from each mouse was small because tissue was used for studies of vascular function and gene expression. Thus meaningful measurements of protein levels or enzyme activity were not obtained.
Finally, we studied only female mice in the present investigation. In our experience, females have a much better survival rate compared with male mice following coronary ligation (with >80% mortality in male MnSOD-deficient mice undergoing coronary ligation). Consequently, we used female mice for all of our studies. We did not control for variations in the estrous cycle or other sex-related variations in hormone levels. It is likely that careful littermate matching and group caging minimized the influence of these factors on the findings in the present investigation.
In conclusion, these studies indicate that reductions in mitochondrial antioxidant mechanisms markedly exacerbate endothelial dysfunction in mice with chronic HF. A key mechanism of this augmented endothelial dysfunction is generation of EDCF by COX1. We speculate that identification of potential contributors to mitochondrial dysfunction may ultimately lead to novel therapies for treatment of vascular dysfunction in chronic HF.
GRANTS
This work was supported by National Institutes of Health Grants HL-086160, HL-092235, HL-62984, NS-24621, and RR-017369, funds from the Department of Veterans Affairs, and funds from a Carver Research Trust Program of Excellence at the University of Iowa.
DISCLOSURES
None.
Supplementary Material
ACKNOWLEDGMENTS
We thank Samantha Ryan, Lauren Castaneda, and Kristine Serrano for assistance with data analysis and production of figures, Kathy Walters for help with immunostaining, and Geoff Kraemer for assistance with genotyping the mice.
REFERENCES
- 1.Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol 287: H1141–H1148, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 100: 292–298, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep 60: 119–126, 2008 [PubMed] [Google Scholar]
- 4.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100: 1016–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 290: H2600–H2605, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol 22: 611–616, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation 111: 58–62, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res 88: 1203–1209, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 100: 894–903, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol 100: 2089–2093, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Fennell JP, Brosnan MJ, Frater AJ, Hamilton CA, Alexander MY, Nicklin SA, Heistad DD, Baker AH, Dominiczak AF. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther 9: 110–117, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 26: 65–69, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto Y, Uno E, Sakuma S. Effects of reactive oxygen and nitrogen species on cyclooxygenase-1 and -2 activities. Prostagland Leukot Essent Fatty Acids 71: 335–340, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Giannattasio C, Achilli F, Grappiolo A, Failla M, Meles E, Gentile G, Calchera I, Capra A, Baglivo J, Vincenzi A, Sala L, Mancia G. Radial artery flow-mediated dilatation in heart failure patients: effects of pharmacological and nonpharmacological treatment. Hypertension 38: 1451–1455, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Van Remmen H, Yang H, Chen X, Mele J, Vijg J, Epstein CJ, Ho YS, Richardson A. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol 21: 1131–1138, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V, Meinertz T. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol 25: 1174–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Iida S, Chu Y, Francis J, Weiss RM, Gunnett CA, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase improves endothelial function in rats with heart failure. Am J Physiol Heart Circ Physiol 289: H525–H532, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Iida S, Chu Y, Weiss RM, Kang YM, Faraci FM, Heistad DD. Vascular effects of a common gene variant of extracellular superoxide dismutase in heart failure. Am J Physiol Heart Circ Physiol 291: H914–H920, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Indik JH, Goldman S, Gaballa MA. Oxidative stress contributes to vascular endothelial dysfunction in heart failure. Am J Physiol Heart Circ Physiol 281: H1767–H1770, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Jackson RM, Helton ES, Viera L, Ohman T. Survival, lung injury, and lung protein nitration in heterozygous MnSOD knockout mice in hyperoxia. Exp Lung Res 25: 631–646, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93: 622–629, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Kaiser L, Spickard RC, Olivier NB. Heart failure depresses endothelium-dependent responses in canine femoral artery. Am J Physiol Heart Circ Physiol 256: H962–H967, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 19: 918–925, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 111: 310–314, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Katz SD, Schwarz M, Yuen J, LeJemtel TH. Impaired acetylcholine-mediated vasodilation in patients with congestive heart failure. Role of endothelium-derived vasodilating and vasoconstricting factors. Circulation 88: 55–61, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Knecht M, Burkhoff D, Yi GH, Popilskis S, Homma S, Packer M, Wang J. Coronary endothelial dysfunction precedes heart failure and reduction of coronary reserve in awake dogs. J Mol Cell Cardiol 29: 217–227, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Lake-Bruse KD, Faraci FM, Shesely EG, Maeda N, Sigmund CD, Heistad DD. Gene transfer of endothelial nitric oxide synthase (eNOS) in eNOS-deficient mice. Am J Physiol Heart Circ Physiol 277: H770–H776, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation 110: 1933–1939, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106: 3073–3078, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice. Am J Physiol Heart Circ Physiol 279: H970–H975, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Lund DD, Gunnett CA, Chu Y, Brooks RM, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase improves relaxation of aorta after treatment with endotoxin. Am J Physiol Heart Circ Physiol 287: H805–H811, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Luscher TF. Vascular protection: current possibilities and future perspectives. Int J Clin Pract Suppl 117: 3–6, 2001 [PubMed] [Google Scholar]
- 39.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest 112: 1871–1879, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura M, Funakoshi T, Arakawa N, Yoshida H, Makita S, Hiramori K. Effect of angiotensin-converting enzyme inhibitors on endothelium-dependent peripheral vasodilation in patients with chronic heart failure. J Am Coll Cardiol 24: 1321–1327, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Nakamura R, Egashira K, Arimura K, Machida Y, Ide T, Tsutsui H, Shimokawa H, Takeshita A. Increased inactivation of nitric oxide is involved in impaired coronary flow reserve in heart failure. Am J Physiol Heart Circ Physiol 281: H2619–H2625, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Prasad A, Higano ST, Al Suwaidi J, Holmes DR, Jr, Mathew V, Pumper G, Lennon RJ, Lerman A. Abnormal coronary microvascular endothelial function in humans with asymptomatic left ventricular dysfunction. Am Heart J 146: 549–554, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Smith WL, Song I. The enzymology of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandin Other Lipid Mediat 68–69: 115–128, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2-/- knockout but not COX1-/- knockout mice. J Cardiovasc Pharmacol 46: 761–765, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation 114: 2065–2069, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Wu KK. Control of cyclooxygenase-2 transcriptional activation by pro-inflammatory mediators. Prostaglandin Leukot Essent Fatty Acids 72: 89–93, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol 136: 104–110, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension 41: 143–148, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33: 337–349, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.