Abstract
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) inhibits collagen production and cell proliferation in cultured rat cardiac fibroblasts, but its effect on differentiation of fibroblasts into myofibroblasts is not known. High amounts of transforming growth factor-β1 (TGF-β1) have been found in fibrotic cardiac tissue. TGF-β1 converts fibroblasts into myofibroblasts, which produce more extracellular matrix proteins than fibroblasts. We hypothesized that 1) Ac-SDKP inhibits TGF-β1-induced differentiation of fibroblasts into myofibroblasts; and 2) this effect is mediated in part by blocking phosphorylation of small-mothers-against-decapentaplegic (Smad) 2 and extracellular signal-regulated kinase (ERK) 1/2. For this study, we used human fetal cardiac fibroblasts (HCFs), which do not spontaneously become myofibroblasts when cultured at low passages. We investigated the effect of Ac-SDKP on TGF-β1-induced HCF transformation into myofibroblasts, Smad2 and ERK1/2 phosphorylation, Smad7 expression, cell proliferation, and collagen production. We also investigated TGF-β1 production by HCFs stimulated with endothelin-1 (ET-1). As expected, HCFs treated with TGF-β1 transformed into myofibroblasts as indicated by increased expression of α-smooth muscle actin and a higher proportion of the embryonic isoform of smooth muscle myosin compared with untreated cells. TGF-β1 also increased Smad2 and ERK1/2 phosphorylation but did not affect Smad7 expression. In addition, TGF-β1 stimulated HCF proliferation as indicated by an increase in mitochondrial dehydrogenase activity and collagen production (hydroxyproline assay). Ac-SDKP significantly inhibited all of the effects of TGF-β1. It also inhibited ET-1-stimulated TGF-β1 production. We concluded that Ac-SDKP markedly suppresses differentiation of human cardiac fibroblasts into myofibroblasts, probably by inhibiting the TGF-β/Smad/ERK1/2 signaling pathway, and thus mediating its anti-fibrotic effects.
Keywords: transforming growth factor-β1, human cardiac myofibroblasts, N-acetyl-seryl-aspartyl-lysyl-proline, collagen, signal transduction, fibroblast differentiation
fibrosis contributes to the cardiac remodeling caused by hypertension, myocardial infarction, and other cardiovascular diseases. Activated myofibroblasts are considered the major source of extracellular matrix components such as collagen types I and III (44). Cardiac fibroblasts transform into myofibroblasts in response to fibrogenic cytokines such as transforming growth factor-β1 (TGF-β1). Cardiac myofibroblasts are characterized by expression of the contractile protein smooth muscle α-actin (α-SMA) (11). We and others found that rat cardiac fibroblasts in culture spontaneously become myofibroblasts at very early passages (41), thus limiting their use in studying the transformation process. On the other hand, when human fetal cardiac fibroblasts (HCFs) are cultured at low passages (up to 6), the phenotype remains stable, with only 10–20% of cells transforming into myofibroblasts; thus, they offer a unique model to study differentiation of fibroblasts into myofibroblasts (9).
TGF-β1 is a key fibrogenic cytokine both in vitro and in vivo (2, 4, 5, 37). Increased concentrations of TGF-β1 have been observed at sites of cardiac fibrosis (17, 18, 39, 40). Studies showed blunted fibrosis in TGF-β-deficient mice (3) and rats treated with anti-TGF-β1 antibodies (42). TGF-β1 converts cardiac fibroblasts into myofibroblasts, which express α-SMA and produce more collagen than fibroblasts (37). Thus TGF-β1 plays a major role in cardiac remodeling under physiological and pathophysiological conditions. Myofibroblasts are increased in rats with cardiac fibrosis resulting from hypertension or myocardial infarction (6, 38). Hence conversion of fibroblasts into myofibroblasts by TGF-β1 is an important mechanism in the production of cardiac fibrosis.
The primary TGF-β signal transduction pathway is the highly conserved small-mothers-against-decapentaplegic (Smad) pathway (35). Smads are divided into the following three major groups: 1) receptor-regulated Smads (R-Smads: Smad2 and Smad3), 2) common mediator Smad (co-Smad: Smad4), and 3) inhibitory Smads (I-Smads: Smad6 and Smad7) (24). Activation of TGF-β receptors and R-Smad proteins through phosphorylation results in an R-Smad-co-Smad complex that translocates to the nucleus, interacts with other transcription factors, and regulates target gene expression (36). Overexpression of Smad7 has been shown to inhibit signal transduction induced by TGF-β (12, 25). In addition, one recent study showed that pharmacological inhibition of extracellular signal-regulated kinases (ERKs) completely blocked TGF-β-stimulated collagen gene expression in cardiac fibroblasts (22). Moreover, the ERK-mitogen-activated protein kinase pathway appears to enhance Smad-mediated collagen gene expression stimulated by TGF-β1 in human mesangial cells (14). More importantly, Smad2/3 activation has been shown to be a key mediator of TGF-β1-induced differentiation of human cardiac fibroblasts into myofibroblasts (9).
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is an endogenous tetrapeptide secreted by bone marrow cells (23) that is widely found in various tissues (30), including plasma and circulating mononuclear cells (31). It is a natural inhibitor of pluripotent hematopoietic stem cell proliferation and a natural substrate of angiotensin-converting enzyme inhibitor (ACEi) (1, 20). We previously found that Ac-SDKP inhibited endothelin-1 (ET-1)-induced collagen production and serum-induced proliferation of cultured rat cardiac fibroblasts (33). In in vivo studies, Ac-SDKP blunted cardiac fibrosis in rats with 2K-1C, ALDO-salt-, or ANG II-induced hypertension or myocardial infarction and also reduced cardiac TGF-β expression in rats with ANG II-induced hypertension (27, 32, 34, 45). However, it is not clear whether the anti-fibrotic effects of Ac-SDKP are partially the result of preventing fibroblasts from becoming transformed into myofibroblasts. Therefore, in the present study, we tested the hypotheses that: 1) in HCFs stimulated with TGF-β1, Ac-SDKP inhibits differentiation of fibroblasts into myofibroblasts and thus decreases collagen production; 2) Ac-SDKP blunts TGF-β1-mediated differentiation by antagonizing the Smad/ERK1/2 signaling pathway; and 3) Ac-SDKP blocks fibroblast proliferation and TGF-β1 secretion.
METHODS
Cell Cultures
Fibroblasts from normal human fetal hearts at passage 1 were purchased from Cell Applications (San Diego, CA) and grown in low-glucose fibroblast basal medium (FBM) containing 10% fibroblast growth supplement (FGS; Cell Applications). Cells from passages 3 to 5 were made quiescent by culturing them in FBM without FGS for 24 h before each treatment. In all protocols, an ACEi, captopril (1 μM; Sigma, St. Louis, MO), was added to the medium of all groups, including control, to prevent degradation of Ac-SDKP.
Protocols
Protocol 1: Effect of Ac-SDKP on TGF-β1-induced transformation of HCFs to myofibroblasts.
Fibroblasts undergo a phenotypic change to myofibroblasts, leading to expression of contractile protein α-SMA and an increase in the proportion of the embryonic isoform of smooth muscle myosin (SMemb) in relation to nonmuscle myosin heavy chain B (10). We used immunocytochemical staining to detect α-SMA and SMemb, which are both markers of myofibroblast transformation. In our preliminary experiments, we found that TGF-β1 at 5 and 10 ng/ml caused a significant change in the fibroblast phenotype without causing cell death. Hence, a dose of 5 ng/ml was selected in the present study based on published studies (15). Quiescent cells grown on four-well slides (Thermo Fisher Scientific, Rochester, NY) were first either maintained untreated or treated with Ac-SDKP (0.1–10 nM; Bachem, Torrance, CA) for 30 min and then exposed to TGF-β1 (R&D Systems) for 24 h, using cells grown in FBM alone as a control. Cells were first fixed with 2% paraformaldehyde/PBS for 15 min and then permeabilized with 1% Triton X-100/PBS for 30 min. α-SMA and SMemb were detected with avidin biotinylated enzyme. Endogenous peroxide was quenched with 3% H2O2, and cells were first blocked in 2% BSA/PBS and then incubated with a mouse monoclonal (clone 1A4) antibody against α-SMA (Sigma) or a mouse monoclonal [3H2] antibody against SMemb (Abcam, Cambridge, MA) for 60 min, followed by incubation for 20 min with a biotinylated secondary antibody (goat anti-mouse IgG; Sigma) and another 20 min with ExtraAvidin-conjugated peroxidase (Sigma). Cells were rinsed in PBS three times for 5 min each after antibody incubation. Staining was visualized using substrate 3-amino-9-ethylcarbazole (Zymed Laboratories, South San Francisco, CA). Cells were counterstained with hematoxylin to visualize the nuclei and counted (>500 cells/slide). α-SMA- and SMemb-positive cells containing rose-red to brownish-red filaments in the cytoplasm were expressed as a percentage of total cell number.
To confirm the immunocytochemical data, only α-SMA expression was measured by Western blot, since the mouse monoclonal [3H2] antibody against SMemb is not recommended for use in Western blot by Abcam. Quiescent cells on six-well plates were pretreated with vehicle or Ac-SDKP (0.1–10 nM) for 30 min before adding TGF-β1 to the medium and then incubated for 24 h. Control cells were cultured in FBM. Whole cell lysates were prepared in cell lysis buffer (20 mM Tris·HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1% Triton X-100; 2.5 mM sodium pyrophosphate; 1 mM β-glycerol phosphate; 1 mM Na3VO4; protease inhibitor mixture) and sonicated on ice. Protein was measured with Coomassie reagent (Thermo Scientific). Equal amounts of protein were subjected to 10% SDS-PAGE under reducing conditions and electrotransferred to a nitrocellulose membrane. Membranes were treated with blocking buffer (TBS/0.1% Tween 20 containing 5% nonfat dry milk) and incubated with the primary antibody overnight at 4°C. Bound antibodies were visualized using secondary antibodies combined with conjugated horseradish peroxidase (Cell Signaling Technology, Danvers, MA) and ECL reagent (Amersham Biosciences, Piscataway, NJ). After α-SMA was located, the blots were treated with a stripping buffer (Pierce, Rockford, IL) and reblotted with a rabbit polyclonal antibody against actin (1:2,000; Cell Signaling Technology). Intensity of the bands was quantified by densitometry. α-SMA was normalized to actin and the results expressed as the degree of increase compared with control.
Protocol 2: Effect of Ac-SDKP on TGF-β1-induced phosphorylation of Smad2 and ERK1/2 and Smad7 expression.
Quiescent cells on six-well plates were pretreated with vehicle or Ac-SDKP (0.1–10 nM) for 30 min. TGF-β1 was added to the medium, and cells were incubated for 30 min for detection of phosphorylated Smad (pSmad) 2 and Smad7 and 15 min for phosphorylated ERK1/2 (pERK1/2). Nontreated cells served as a control. Whole cell lysates were prepared in cell lysis buffer, and proteins were analyzed on SDS-PAGE as described above for α-SMA expression. As primary antibodies, we employed a rabbit polyclonal antibody against pSmad2, pERK1/2 (1:1,000; Cell Signaling Technology), or Smad7 (1:500; Santa Cruz Technology, Santa Cruz, CA). Once pSmad2 or pERK1/2 was detected, the blots were treated with a stripping buffer (Pierce) and reblotted with a mouse monoclonal antibody against total Smad2 (tSmad2), a rabbit polyclonal antibody against ERK1/2 (tERK1/2) (1:1,000; Cell Signaling Technology), or a rabbit monoclonal antibody against glyceraldehydes-3-phosphate dehydrogenase (GAPDH, 1:5,000; Cell Signaling Technology). Intensity of the bands was quantified by densitometry. pSmad2 and pERK1/2 were normalized to tSmad2 and tERK1/2, respectively; Smad7 was normalized to GAPDH. Results were expressed as fold increase compared with control.
Protocol 3: Effect of Ac-SDKP on TGF-β1-induced collagen production.
Collagen content of fibroblast cultures was measured by hydroxyproline assay as described previously (8, 27). Briefly, quiescent cells on six-well plates were pretreated with vehicle or Ac-SDKP (0.1–10 nM) for 30 min and then exposed to TGF-β1 (5 ng/ml) for 48 h, using cells grown in FBM as a control. Protein content of cell lysates was measured using Coomassie reagent (Thermo Scientific). Data were expressed as micrograms per milligram protein, assuming that collagen contains an average of 13.5% hydroxyproline (7).
Protocol 4: Effect of Ac-SDKP on TGF-β1-induced cell proliferation.
Fibroblasts were seeded onto 96-well plates at a density of 5 × 103 cells/well and grown to 50–60% confluence. Quiescent cells were treated with either 5% FGS or TGF-β1 for 48 h in the presence or absence of Ac-SDKP (0.1–10 nM); control cells were grown in FBM. Cell proliferation was quantified using an assay from BioVision (Mountain View, CA) based on cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. The formazan dye produced by viable cells was quantified by multiwell spectrophotometry (microtiter plate reader) by measuring absorbance of the dye solution at 450 nm.
Protocol 5: ET-1-induced TGF-β1 secretion.
Total TGF-β1 in the culture medium was detected with a quantitative sandwich enzyme immunoassay. Quiescent cells on six-well plates were cultured in FBM supplemented with vehicle (control) or ET-1 (10−7 M; Bachem) for 36-48 h in the presence or absence of Ac-SDKP (0.1–100 nM). Aliquots were stored at −72°C until assay. TGF-β1 concentration in the medium was detected using a Quantikine immunoassay kit (R&D Systems). Whole cell lysates were prepared in cell lysis buffer and sonicated on ice. Protein in cell lysates was measured using Coomassie reagent (Thermo Scientific, Rockford, IL). TGF-β1 was expressed as picograms per microgram protein.
Statistical Analysis
All variables were analyzed by a set of paired t-tests. Comparison of TGF-β1 to each dose of Ac-SDKP was considered as a set of experiments, and Hochberg's method was used to determine significance. Control vs. TGF-β1 was considered a single comparison where significance was defined as P < 0.05. Values are expressed as means ± SE.
RESULTS
Protocol 1: Effect of Ac-SDKP on TGF-β1-Induced Transformation of HCFs to Myofibroblasts
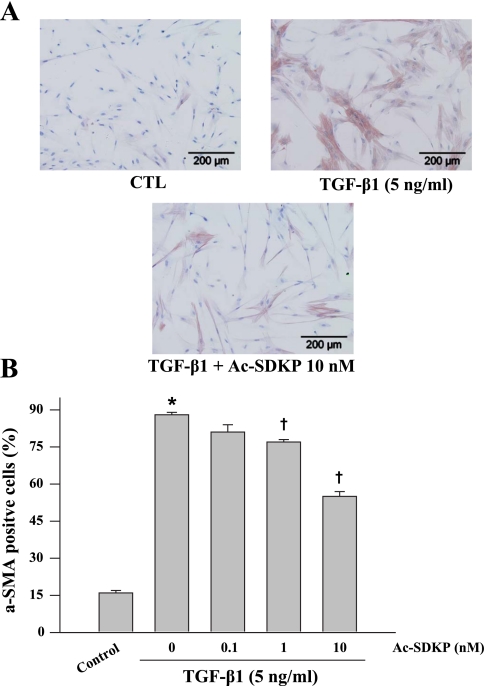
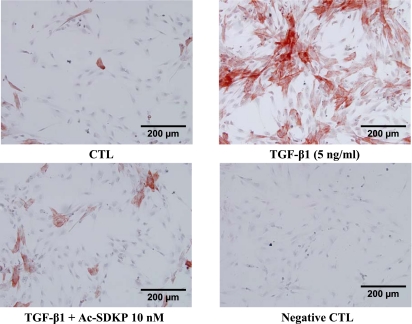
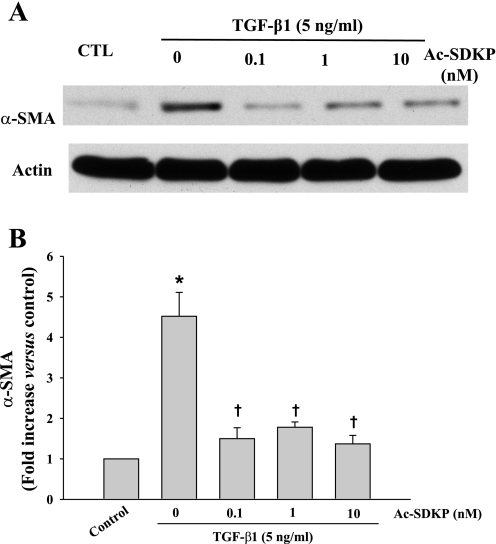
Immunochemical staining of the control HCFs showed relatively few α-SMA-positive cells (16 ± 1%), characterized by disorganized intracellular α-SMA. In contrast, >80% of the TGF-β1-treated HCFs became α-SMA-positive and contained well-organized α-SMA filaments (Fig. 1A), indicating nearly complete differentiation of fibroblasts to myofibroblasts. In TGF-β1-treated HCFs, Ac-SDKP reduced the number of α-SMA-positive cells in a dose-dependent manner but did not normalize cell number (Fig. 1B). Cells stained with SMemb exhibited a similar pattern as α-SMA (Fig. 2). Likewise control cultures contained only a few SMemb-positive cells (14 ± 1%); Ac-SDKP at 1 and 10 nM markedly decreased SMemb-positive cells treated with TGF-β1 from 81 ± 4% to 60 ± 4% (P = 0.053) and 42 ± 1% (P = 0.0013), respectively. Western blot revealed a fourfold increase in α-SMA expression in cells treated with TGF-β1 compared with control, which was significantly reduced by Ac-SDKP (Fig. 3).
Fig. 1.
Representative micrographs (A) and quantitative data (B) representing smooth muscle α-actin (α-SMA)-stained cultured fibroblasts treated with transforming growth factor-β1 (TGF-β1, 5 ng/ml for 24 h) in the presence or absence of N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP). Cells were counterstained with hematoxylin to show the nuclei. P = 0.001, control vs. TGF-β1 (*) and Ac-SDKP + TGF-β1 vs. TGF-β1 alone (†) (n = 5 experiments/group).
Fig. 2.
Representative micrographs showing the effect of Ac-SDKP on the embryonic isoform of smooth muscle myosin expression in human fetal cardiac fibroblasts treated with TGF-β1 for 24 h. Conditions are the same as in Fig. 1.
Fig. 3.
Representative Western blot (A) and quantitative data (B) representing α-SMA expression in fibroblasts treated with TGF-β1 (5 ng/ml) for 24 h in the presence or absence of Ac-SDKP. α-SMA was normalized to actin. *P = 0.004, control vs. TGF-β1; †P < 0.007, Ac-SDKP + TGF-β1 vs. TGF-β1 alone (n = 5/group).
Protocol 2: Effect of Ac-SDKP on TGF-β1-Induced Phosphorylation of Smad2 and ERK1/2 and Smad7 Expression
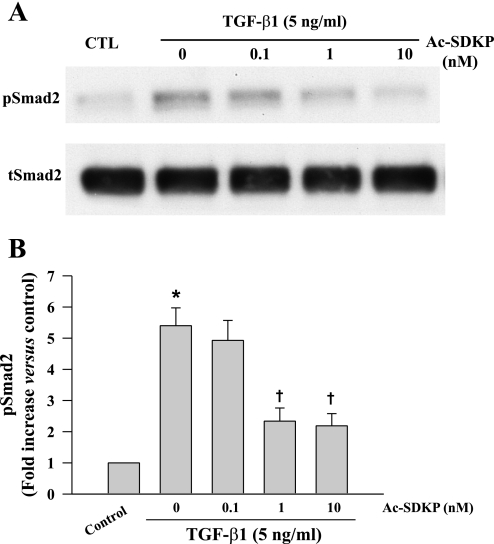
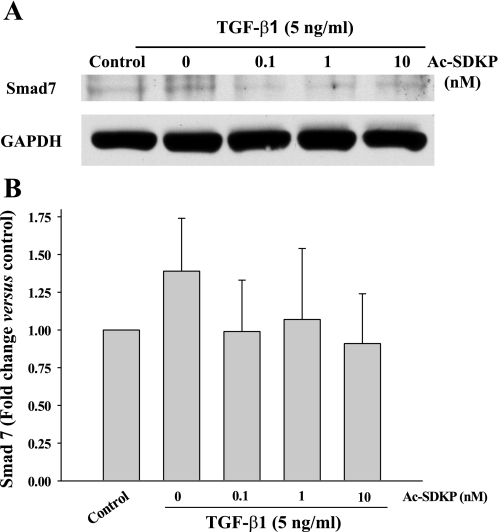
Western blot showed that at 30 min TGF-β1-induced Smad2 phosphorylation (pSmad2) was significantly increased but there was no difference in Smad7 expression between controls and TGF-β1-treated cells (Figs. 4 and 5). Ac-SDKP significantly blocked TGF-β1-induced pSmad2 but had no effect on Smad7 expression (Figs. 4 and 5).
Fig. 4.
Representative Western blot (A) and quantitative data (B) representing phosphorylated (p) small-mothers-against-decapentaplegic (Smad) 2 levels in fibroblasts treated with TGF-β1 for 30 min in the presence or absence of Ac-SDKP. pSmad2 was normalized to total (t) Smad2. *P = 0.005, control vs. TGF-β1; †P < 0.05, Ac-SDKP + TGF-β1 vs. TGF-β1 alone (n = 4/group).
Fig. 5.
Representative Western blot (A) and quantitative data (B) representing Smad7 levels in fibroblasts treated with TGF-β1 for 30 min in the presence or absence of Ac-SDKP. Smad7 was normalized to glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (n = 4/group).
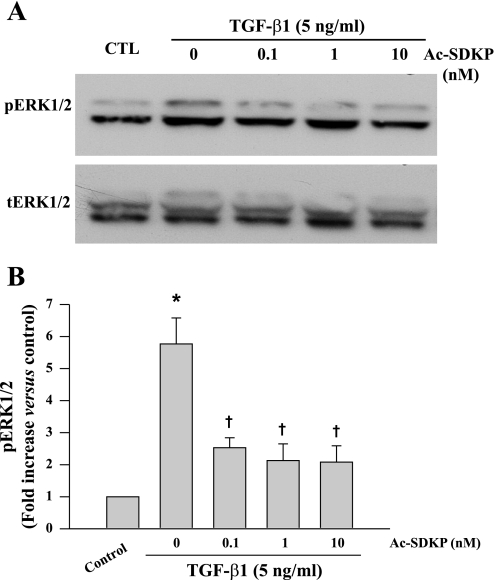
To test whether ERK1/2 activation is stimulated by TGF-β1 stimulation in fibroblasts and whether Ac-SDKP has any effect on this process, cells were preincubated with Ac-SDKP for 30 min and then incubated with TGF-β1 for 15 min. Western blot was performed to detect pERK1/2 and tERK1/2. We found that phosphorylation of ERK1/2 increased in TGF-β1-stimulated fibroblasts (P = 0.001) but was significantly blunted by pretreatment with Ac-SDKP (Fig. 6).
Fig. 6.
Representative Western blot (A) and quantitative data (B) representing phosphorylated extracellular signal-regulated kinase (ERK) 1/2 levels in fibroblasts treated with TGF-β1 for 15 min in the presence or absence of Ac-SDKP. pERK1/2 was normalized to tERK1/2. *P = 0.001, control vs. TGF-β1; †P < 0.02, Ac-SDKP + TGF-β1 vs. TGF-β1 alone (n = 6/group).
Protocol 3: Effect of Ac-SDKP on TGF-β1-Induced Collagen Production
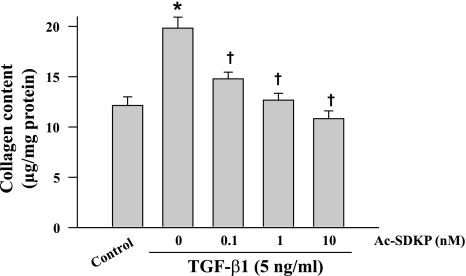
Treatment with TGF-β1 for 48 h increased collagen production from control levels of 12.1 ± 0.8 μg/mg protein to 19.8 ± 1.1 (P = 0.002). Ac-SDKP inhibited TGF-β1-stimulated collagen production in a dose-dependent manner (Fig. 7).
Fig. 7.
Effects of Ac-SDKP on TGF-β1-stimulated collagen production in cultured fibroblasts (5 ng/ml TGF-β1 for 48 h). *P = 0.002, control vs. TGF-β1; †P = 0.001, Ac-SDKP + TGF-β1 vs. TGF-β1 alone (n = 6/group).
Protocol 4: Effect of Ac-SDKP on TGF-β1-Induced Cell Proliferation
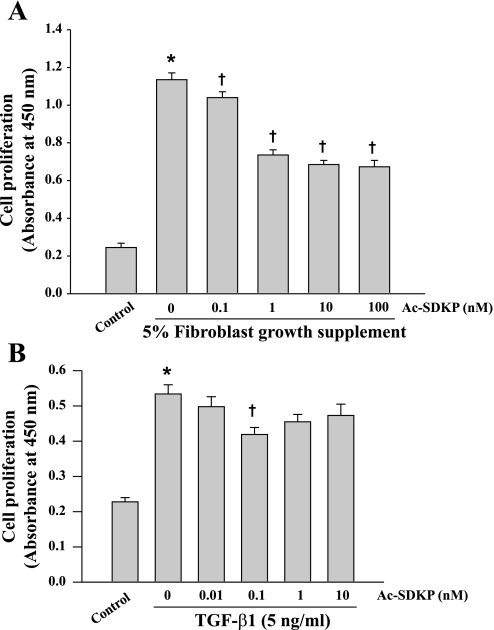
Absorbance in untreated fibroblasts (controls) was 0.21 ± 0.02 arbitrary units. It increased to 1.13 ± 0.04 after 48 h of incubation with 5% FGS and 0.53 ± 0.03 with TGF-β1. Ac-SDKP inhibited cell proliferation stimulated with both FGS and TGF-β1, with more pronounced inhibitory effect against FGS's effect (Fig. 8).
Fig. 8.
Effects of Ac-SDKP on proliferation of fibroblasts stimulated with either fibroblast growth supplement (FGS) (A) or TGF-β1 (B). Cell proliferation was measured by measuring mitochondrial dehydrogenase activity (A: n = 28–29/group; B: n = 12/group). Absorbance was recorded using a microtiter plate reader at 450 nm and expressed as arbitrary units (AU). P = 0.001, control vs. TGF-β1 (*) and Ac-SDKP + TGF-β1 vs. TGF-β1 alone (†).
Protocol 5: ET-1-Induced TGF-β1 Secretion
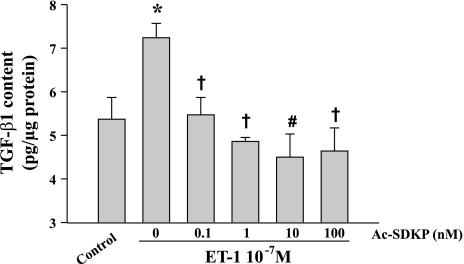
ET-1 (10−7 M) significantly increased TGF-β1 (both active and latent forms) in the medium of cultured fibroblasts (from 5.37 ± 0.50 pg/μg in the controls to 7.24 ± 0.40 in ET-1-treated cells; P < 0.05). Ac-SDKP blocked ET-1-stimulated TGF-β1 release in a dose-dependent manner (Fig. 9).
Fig. 9.
Effect of Ac-SDKP on endothelin-1 (ET-1)-stimulated TGF-β1 secretion by human cardiac fibroblasts. TGF-β1 was measured with a quantitative sandwich enzyme immunoassay. *P < 0.05, control vs. ET-1; †P < 0.05, Ac-SDKP + ET-1 vs. ET-1 alone; #P = 0.0021, Ac-SDKP + ET-1 vs. ET-1 alone (n = 4/group).
DISCUSSION
We found that, as expected, TGF-β1 induced robust differentiation of HCFs and increased collagen production. Treating the fibroblasts with Ac-SDKP resulted in inhibition of 1) TGF-β1-induced differentiation of fibroblasts into myofibroblasts, 2) TGF-β1-induced Smad2 and ERK1/2 phosphorylation, 3) collagen synthesis, 4) fibroblast proliferation, and 5) ET-1-induced TGF-β1 production. The anti-fibrotic effects of Ac-SDKP may be partially mediated by blocking differentiation of fibroblasts into myofibroblasts. The biological effects of TGF-β1 are mediated through a complex of type II and type I (TβRI) receptors characterized as serine-threonine kinases that transduce intracellular signals via both Smad and non-Smad pathways. The activated receptor complex phosphorylates R-Smads such as Smad2 and Smad3, which form homomeric and heteromeric complexes with a co-Smad (Smad4). These active Smad complexes enter the nucleus, where they bind to target genes and directly regulate their transcription (21, 35, 36). Thus phosphorylation of R-Smads followed by transfer to the nucleus is a critical step in activation of TGF-β signaling. As expected, our results showed that pSmad2 was significantly increased in TGF-β1-treated cells compared with controls. Ac-SDKP inhibited phosphorylation of Smad2 in HCFs, which would interrupt formation of the Smad2,3,4 complex and prevent it from entering the nucleus. Ac-SDKP also reportedly blocked phosphorylation of R-Smad in rat cardiac fibroblasts (29) and human mesangial cells treated with TGF-β1 (16). Indeed, transfecting HCFs with a silencing RNA against Smad2 almost completely blocked not only phosphorylation of Smad2 and tSmad2/3 but also differentiation of fibroblasts into myofibroblasts in response to TGF-β1 (9). Here we show that, in TGF-β1-stimulated HCFs, Ac-SDKP blocked R-Smad phosphorylation, suggesting that it suppresses transformation of HCFs to myofibroblasts and collagen synthesis in part via inhibition of Smad phosphorylation.
It is also known that Smad7 interrupts TGF-β1 signaling by competitive interaction with TβRI, thereby preventing TGF-β1 from binding to and activating R-Smads (12). Thus we questioned whether Ac-SDKP inhibits Smad2 phosphorylation of TGF-β1-stimulated HCFs by acting on the I-Smad Smad7. We found that, after exposure to TGF-β1 for 30 min, pSmad2 of HCFs was increased, but Smad7 levels remained unchanged compared with controls and were not affected by cotreatment with Ac-SDKP. Therefore, the inhibitory effects of Ac-SDKP on Smad2 activation after a 30-min TGF-β1 exposure are not mediated by Smad7 expression.
In human mesangial cells, TGF-β induces phosphorylation of R-Smad linker region serines directly via its own receptors and subsequent ERK activation, leading to increased collagen I synthesis. This supports previous evidence of positive cross talk between ERK and Smad pathways (13). Moreover, TGF-β1 has been shown to directly activate ERK mitogen-activated protein (MAP) kinase signaling, mediating those biological effects of TGF-β1 that are independent of Smad signaling (19). In the present study, Ac-SDKP suppressed ERK1/2 phosphorylation in TGF-β1-stimulated HCFs, suggesting that its inhibitory effects may be partially mediated through inhibition of the ERK MAP kinase pathway and/or ERK MAP kinase/Smad signaling.
The effects of TGF-β1 on cell proliferation are cell type-dependent. Although TGF-β1 reportedly stimulates proliferation of both mouse and rat cardiac fibroblasts (21, 29), its effects on proliferation of HCFs are still unknown. We found that proliferation of fibroblasts stimulated with TGF-β1 increased 2.5-fold, and this increase doubled to 5-fold with 5% FGS present in the medium. Ac-SDKP inhibited these increases, but it had less effect when cells were treated with TGF-β1 rather than serum. This phenomenon was also observed in neonatal rat cardiac fibroblasts (29), but the mechanisms involved are unclear. It is noteworthy that the anti-proliferative effect of Ac-SDKP seems to be less pronounced than its effect on human cardiac fibroblast transformation or collagen synthesis when cells are stimulated with TGF-β1, which suggests that inhibition of fibroblast proliferation may not play a very important role in Ac-SDKP inhibition of fibrosis.
Profibrotic and progrowth factors such as ANG II, ET-1, and TGF-β1 may act in either an autocrine or paracrine manner to stimulate collagen production and induce cardiomyocyte hypertrophy under physiological and/or pathophysiological conditions (43). ANG II released by cardiomyocytes and ET-1 released by cardiac fibroblasts and endothelial cells may act as paracrine hormones, stimulating TGF-β1 expression in cardiomyocytes (43). It is not known whether TGF-β1 is produced and released by human cardiac fibroblasts stimulated with ET-1. We previously reported that Ac-SDKP reduced cardiac TGF-β1 expression and fibrosis in rats with ANG II-induced hypertension or heart failure (26, 28, 32, 45) and inhibited ET-1-stimulated collagen production in rat cardiac fibroblasts (33). Therefore, we hypothesized that Ac-SDKP inhibits ET-1-stimulated TGF-β1 production, causing fibroblasts to become myofibroblasts (which are potent producers of collagen). Our findings revealed that significant total TGF-β1 (both active and latent forms) was released by quiescent fibroblasts and that ET-1 significantly increased total TGF-β1 production, which was reduced to normal levels by simultaneous treatment with Ac-SDKP. The anti-fibrotic effects of Ac-SDKP are most likely due in part to inhibition of TGF-β1 production, thus reducing TGF-β1-induced fibroblast proliferation and differentiation and hence collagen production.
In summary, we believe our study shows for the first time that, in cultured human cardiac fibroblasts, Ac-SDKP inhibits both TGF-β1-induced transformation of fibroblasts into myofibroblasts and TGF-β1 signaling Smad/ERK1/2 activation. In this way, Ac-SDKP might decrease transformation of cardiac fibroblasts into myofibroblasts, which could decrease net collagen production.
Perspectives
These findings provide evidence that Ac-SDKP is a potent inhibitor of myofibroblast transformation and collagen production in human cardiac fibroblasts. When plasma and/or tissue levels of Ac-SDKP are elevated in patients treated with ACEi, it may act as an endogenous anti-fibrotic hormone. More importantly, design and synthesis of a new analog of Ac-SDKP that is resistant to ACE or endopeptidases should lead to improved methods of treating or preventing fibrosis in patients with diabetes, hypertension, and other cardiovascular diseases.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-071806-01 (N.-E. Rhaleb) and HL-028982 (O. A. Carretero).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 97: 839–844, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor β(1)heterozygous mice. J Mol Cell Cardiol 32: 187–195, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderone A, Murphy RJ, Lavoie J, Colombo F, Beliveau L. TGF-β1 and prepro-ANP mRNAs are differentially regulated in exercise-induced cardiac hypertrophy. J Appl Physiol 91: 771–776, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Campbell SE, Janicki JS, Weber KT. Temporal differences in fibroblast proliferation and phenotype expression in response to chronic administration of angiotensin II or aldosterone. J Mol Cell Cardiol 27: 1545–1560, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol 18: 283–290, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 147: 325–338, 1995 [PMC free article] [PubMed] [Google Scholar]
- 9.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 48: 89–100, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Gabbiani G. Evolution and clinical implications of the myofibroblast concept. Cardiovasc Res 38: 545–548, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89: 1165–1173, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-β-dependent responses in human mesangial cells. FASEB J 17: 1576–1578, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells; a possible role in collagen expression. Kidney Int 56: 1710–1720, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Jelaska A, Korn JH. Role of apoptosis and transforming growth factor β1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum 43: 2230–2239, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-β-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol 14: 863–872, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension 25: 1252–1259, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Kupfahl C, Pink D, Friedrich K, Zurbrügg HR, Neuss M, Warnecke C, Fielitz J, Graf K, Fleck E, Regitz-Zagrosek V. Angiotensin II directly increases transforming growth factor β1 and osteopontin and indirectly affects collagen mRNA expression in the human heart. Cardiovasc Res 46: 463–475, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 26: 3957–3967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci USA 86: 779–782, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, Novak L, Renfrow MB, Chen YF. Atrial natriuretic peptide inhibits transforming growth factor β-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res 102: 185–192, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol 70: 1992–2003, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Majesky MW, Daemen MJ, Schwartz SM, Dilley RJ, Gordon D, Benditt EP, Wilcox JN. Alpha 1-adrenergic stimulation of platelet-derived growth factor A-chain gene expression in rat aorta vascular remodeling in the growth hormone transgenic mouse growth factors and cell proliferation in human atherosclerosis. J Biol Chem Circ Res Transplant Proc 21: 3692–3694, 1989 [Google Scholar]
- 24.Massagué J. TGFβ signal transduction. Annu Rev Biochem 67: 753–791, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 389: 631–635, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Peng H, Carretero OA, Brigstock DR, Oja-Tebbe N, Rhaleb NE. Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension 42: 1164–1170, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. Antifibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 37: 794–800, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, Rhaleb NE. Angiotensin-converting enzyme inhibitors. A new mechanism of action. Circulation 112: 2436–2445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pokharel S, Rasoul S, Roks AJM, van Leeuwen REW, van Luyn MJA, Deelman LE, Smits JF, Carretero OA, van Gilst WH, Pinto YM. N-acetyl-Ser-Asp-Lys-Pro inhibits phosphorylation of Smad2 in cardiac fibroblasts. Hypertension 40: 155–161, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Pradelles P, Frobert Y, Créminon C, Ivonine H, Frindel E. Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin β4 in mouse tissues. FEBS Lett 289: 171–175, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Pradelles P, Frobert Y, Créminon C, Liozon E, Massé A, Frindel E. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analysed by enzyme immunoassay. Biochem Biophys Res Commun 170: 986–993, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, Sanchez-Mendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens 22: 593–603, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhaleb NE, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension 37: 827–832, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhaleb NE, Peng H, Yang XP, Liu YH, Mehta D, Ezan E, Carretero OA. Long-term effect of N-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation 103: 3136–3141, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Silverman HS, Pfeifer MP. Relation between use of anti-inflammatory agents and left ventricular free wall rupture during acute myocardial infarction. Am J Cardiol 59: 363–364, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Weber KT. Cells expressing angiotensin II receptors in fibrous tissue of rat heart. Cardiovasc Res 31: 518–525, 1996 [PubMed] [Google Scholar]
- 39.Sun Y, Zhang JQ, Zhang J, Lamparter S. Cardiac remodeling by fibrous tissue after infarction in rats. J Lab Clin Med 135: 316–323, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Zhang JQ, Zhang J, Ramires FJA. Angiotensin II, transforming growth factor-β1 and repair in the infarcted heart. J Mol Cell Cardiol 30: 1559–1569, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA 102: 437–442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomita H, Egashira K, Ohara Y, Takemoto M, Koyanagi M, Katoh M, Yamamoto H, Tamaki K, Shimokawa H, Takeshita A. Early induction of transforming growth factor-β via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 32: 273–279, 1998 [DOI] [PubMed] [Google Scholar]
- 43.van Wamel AJ, Ruwhof C, van der Valk-Kokshoorn LJ, Schrier PI, van der Laarse A. Stretch-induced paracrine hypertrophic stimuli increase TGF-beta1 expression in cardiomyocytes. Mol Cell Biochem 236: 147–153, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Weber KT, Sun Y, Katwa LC. Myofibroblasts and local angiotensin II in rat cardiac tissue repair. Int J Biochem Cell Biol 29: 31–42, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension 43: 229–236, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]