Abstract
Carbon 13 nuclear magnetic resonance (NMR) isotopomer analysis was used to measure the rates of oxidation of long-chain fatty acids, ketones, and pyruvate to determine the minimum pyruvate concentration ([pyruvate]) needed to suppress oxidation of these alternative substrates. Substrate mixtures were chosen to represent either the fed or fasted state. At physiological [pyruvate], fatty acids and ketones supplied the overwhelming majority of acetyl-CoA. Under conditions mimicking the fed state, 3 mM pyruvate provided ∼80% of acetyl-CoA, but under fasting conditions 6 mM pyruvate contributed only 33% of acetyl-CoA. Higher [pyruvate], 10–25 mM, was associated with transient reduced cardiac output, but overall hemodynamic performance was unchanged after equilibration. These observations suggested that 3–6 mM pyruvate in the coronary arteries would be an appropriate target for studies with hyperpolarized [1-13C]pyruvate. However, the metabolic products of 3 mM hyperpolarized [1-13C]pyruvate could not be detected in the isolated heart during perfusion with a physiological mixture of substrates including 3% albumin. In the presence of albumin even at high concentrations of pyruvate, 20 mM, hyperpolarized H13CO3− could be detected only in the absence of competing substrates. Highly purified albumin (but not albumin from plasma) substantially reduced the longitudinal relaxation time of [1-13C]pyruvate. In conclusion, studies of cardiac metabolism using hyperpolarized [1-13C]pyruvate are sensitive to the effects of competing substrates on pyruvate oxidation.
Keywords: myocardium, pyruvate, dynamic nuclear polarization, substrate oxidation, isolated rat heart, carbon-13, nuclear magnetic resonance
fluxes in biochemical pathways are abnormal in many forms of heart disease. Metabolism in specific pathways, however, is difficult to measure with confidence in vivo by positron emission computed tomography (PET) or single photon emission computed tomography (SPECT) because the chemical fate of the tracer is not detected. Consequently, there is intense interest in the demonstration by Golman and colleagues (14, 15) that chemical information is retained in 13C images of the heart after injection of hyperpolarized (HP) [1-13C]pyruvate. Pyruvate is particularly attractive because products of its metabolism such as lactate and alanine provide information about enzyme activity in the cytosol, and the appearance of 13CO2 or H13CO3− is due to flux through a single mitochondrial enzyme, pyruvate dehydrogenase (PDH) (31). The ability to monitor 13CO2 and H13CO3− production is an important goal because the ratio 13CO2/H13CO3− is a direct measure of pH (12), and the ratio H13CO3−/[1-13C]lactate may be an index of myocardial recovery after ischemia (32). However, studies of 13CO2 appearance in the heart have been limited to isolated tissues where substrate concentrations do not mimic the physiological situation. In vivo, the heart is exposed to a complex mixture of compounds, including long-chain fatty acids, lactate, pyruvate, ketones, and glucose, and the myocardium readily switches among these energy sources (53). Furthermore, the concentration of each compound varies considerably as a consequence of disease and nutritional state. Depending on their concentration and other factors, any one of these molecules could suppress metabolism of HP [1-13C]pyruvate.
Translation of 13C hyperpolarization technology for physiological studies and clinical applications will require understanding the influence of circulating substrates on metabolism of injected HP pyruvate and specifically the minimum concentration of pyruvate necessary to overcome oxidation of competing substrates. At steady state, the rate of 13CO2 production is calculated as the rate of acetyl-CoA production × the fractional contribution of [1-13C]pyruvate to acetyl-CoA. Because the rate of acetyl-CoA production and consumption by the myocardium under baseline conditions is relatively constant, this relationship simply means that, other factors being equal, the appearance of 13CO2 or H13CO3− in the heart is proportional to the fractional contribution of pyruvate to acetyl-CoA. Although pyruvate carboxylation is known to occur within the heart, this contribution to overall TCA flux is small and is not affected by changes in pyruvate concentration ([pyruvate]) (8, 38, 39).
The objective of this study was to take advantage of this convenient relationship to determine the minimum concentration of 13C-enriched pyruvate required to compete effectively with physiological substrates for production of 13CO2 or, equivalently, acetyl-CoA. Intravenously administered pyruvate has been extensively examined as a therapeutic intervention for cardiac (17, 18, 35), neurological (10, 33), and acid-base disorders (28, 42). In these studies, the typical plasma concentration was 2–6 mM. In human subjects with congestive heart failure, [pyruvate] up to 6 mM in the coronary arteries was safe (17, 18), but adverse effects of pyruvate such as arrhythmias and fluctuations in blood pressure were reported at 9 mM pyruvate in dogs (24). The maximum efficacy of pyruvate in postischemic guinea pig hearts was 5–10 mM (5) perhaps suggesting that pyruvate metabolism cannot be further stimulated at higher concentrations. In the current study, the concentration range selected for detailed studies of pyruvate oxidation, 0.1–6 mM, was chosen based on a safe range described in these literature reports.
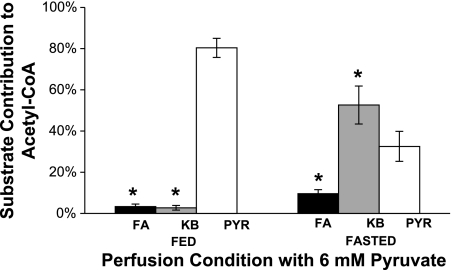
In this study, hearts were exposed to mixtures of ketones, long-chain fatty acids, lactate, pyruvate, and glucose. Although the isolated heart does not duplicate all features of the environment in vivo, it allows precise control of the concentration of each substrate as well as full hemodynamic monitoring, including cardiac output during changes in substrate concentrations. The concentration of each substrate was chosen to mimic plasma concentrations of these substrates in either the fed or fasted state (20) because fasting is common among patients, and increases in the concentration of fatty acids and ketones frequently occur among patients with diabetes or with heart disease. Although our fasting conditions do not completely model the complicated events of fasting, we are studying one component: how plasma substrate concentration will affect pyruvate utilization. Under conditions mimicking the fed state, 3 mM pyruvate was sufficient to produce ∼80% of the acetyl-CoA. At substrate concentrations present in fasting, pyruvate at 6 mM was oxidized at a significantly higher rate than at baseline, but oxidation of fats and ketones still provided the majority of acetyl-CoA. The hemodynamic effects of higher concentrations of pyruvate were also examined. Although transiently reduced cardiac output was observed at [pyruvate] >10 mM, hearts quickly recovered normal O2 consumption and function even at 25 mM pyruvate. Based on these studies, a target [pyruvate] for cardiac studies is ∼3–6 mM in the coronary arteries.
METHODS
Materials.
[3-13C]sodium pyruvate (99% enriched) was purchased from Isotec Laboratories. U-13C long-chain fatty acids, [1-13C]sodium pyruvate, and [1,3-13C2]ethylacetoacetate (99% enriched) were purchased from Cambridge Isotope Laboratories (Andover, MA). The trityl radical tris[8-carboxyl-2,2,6,6-tetra-[2-(1-hydroxyethyl)]-benzo-(1,2-d:4,5-d)-bis-(1,3)-dithiole-4-yl]methyl sodium salt was purchased from Oxford Molecular Biotools (Abingdon, Oxfordshire, UK). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) at the highest quality available. BSA was fatty acid free and was purchased in the anhydrous form. Male Sprague-Dawley rats (260–330 g) were obtained from Charles River Laboratories (Wilmington, MA) and given free access to standard rat chow and water until the day of death. The studies were performed under a protocol approved by the University of Texas Southwestern Medical Center Animal Care and Use Committee.
Working heart preparation.
Hearts were rapidly excised from rats under general anesthesia. The aorta was immediately cannulated for retrograde Langendorff perfusion at 100 cm of hydrostatic pressure and later converted to a working preparation as described below. The initial perfusate was a modified Krebs-Henseleit medium maintained at 37°C that contained (in mM): 25 NaHCO3, 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 10 glucose, and 1.25 CaCl2. The buffer was saturated with a 95:5 mixture of O2-CO2. Polyethylene tubing attached to a pressure transducer was inserted in the left ventricle through the mitral valve. This enabled the continuous measurement of left ventricular developed pressure and heart rate throughout the experiment. Myocardial O2 consumption (MV̇o2) was calculated from the difference in O2 tension (measured with a blood gas analyzer) between the perfusion medium in the arterial supply line and the coronary effluent, which was collected via a cannula placed in the pulmonary artery. Coronary flow and aortic output were measured using a stopwatch and a graduated cylinder. After ∼5–10 min of perfusion in the Langendorff mode, the hearts were switched to a working mode at a left atrial pressure of 15 cmH2O and supplied with a buffered medium containing the same mixture of electrolytes but with one of the following mixtures of oxidizable substrates. The “fed” mixture contained glucose (10 mM, unlabeled), lactate (1.2 mM, unlabeled), pyruvate (0.12 mM, unlabeled), [1,3-13C2]acetoacetate (0.17 mM), long-chain fatty acids [total 0.4 mM, a mixture of palmitate, palmitoleic, stearic, oleate, and linoleic in physiological concentrations, all U-13C (see Refs. 19 and 20 for details)], and 3% BSA. The “fasted” mixture contained glucose (4.9 mM, unlabeled), lactate (0.89 mM, unlabeled), pyruvate (0.08 mM, unlabeled), [1,3-13C2]acetoacetate (1.2 mM), long-chain fatty acids (total 0.85 mM, a mixture of palmitate, palmitoleic, stearic, oleate, and linoleic in physiological concentrations, all U-13C), and 3% BSA. These substrate concentrations duplicate the plasma concentrations in a fed or fasted rat. In all experiments, the medium was oxygenated with a thin-film multibulb oxygenator, filtered, and recirculated (34).
Six groups of hearts (n = 3–5 in each group) were studied. Each group was exposed to a different concentration of [3-13C]pyruvate, in addition to the other substrates described above: group 1, fed mixture plus 0.12 mM [3-13C]pyruvate; group 2, fed mixture plus 1 mM [3-13C]pyruvate; group 3, fed mixture plus 3 mM [3-13C]pyruvate; group 4, fed mixture plus 6 mM [3-13C]pyruvate; group 5, fasted mixture plus 3 mM [3-13C]pyruvate; and group 6, fasted mixture plus 6 mM [3-13C]pyruvate. The lower concentrations of pyruvate were not studied with the fasted mixture because ketones and long-chain fatty acids are already known to be the dominant energy source at low concentrations of pyruvate (20).
The hearts were perfused for 15 min with the fed or fasted medium without [3-13C]pyruvate. After collection of baseline hemodynamic data and O2 consumption, an appropriate amount of 500 mM [3-13C]pyruvate was added at a rate of 0.04 ml/s to the left atrial reservoir. The hearts were perfused for an additional 40 min. O2 consumption and hemodynamics were measured every 15 min. At the end of the perfusion period, the heart was freeze-clamped using aluminum tongs precooled in liquid N2 (LN2). A small portion of the frozen tissue was used for determination of the wet-to-dry ratio by weighing the tissue before and after slow oven drying. The remainder of the frozen tissue was pulverized into a fine powder under LN2, extracted with 6% perchloric acid, centrifuged at 15,000 g for 15 min at 4°C, neutralized with KOH, and centrifuged a second time. The supernatant was freeze-dried, and the lyophilized heart extract was dissolved in 0.60 ml of 2H2O and pH corrected to 7.1 for nuclear magnetic resonance (NMR) analysis.
In a separate set of experiments, working hearts were studied as described above using the fed mixture of substrates except that the substrates were not 13C enriched. In these experiments, hearts were exposed to graded concentrations of pyruvate (10, 15, and 25 mM). One group of hearts was exposed to 25 mM NaCl in addition to the normal concentration of 143 mM sodium to observe the effect of excess sodium from the pyruvate salt on the heart. The hearts were perfused for 15 min before the slow addition, 0.04 ml/s, of 2.5 M unlabeled pyruvate. The hearts were perfused for an additional 40 min. O2 consumption and hemodynamics were measured every 15 min. At the end of the perfusion period, the heart was freeze-clamped using aluminum tongs precooled in LN2. A small portion of the frozen tissue was used for determination of the wet-to-dry ratio by weighing the tissue before and after slow oven drying.
HP 13C NMR spectroscopy of the isolated heart.
Hearts were rapidly excised from rats under general anesthesia and perfused retrograde through the aorta at 37°C and 100 cmH2O using standard Langendorff methods. Heart rate and developed pressure were monitored through a catheter in the left ventricle. The heart was supplied with a nonrecirculating medium and placed in a 20-mm NMR tube. The perfusing medium was a modified Krebs-Henseleit buffer containing the electrolytes described above plus 10 mM glucose and bubbled continuously with a 95:5 mixture of O2-CO2. The water-jacketed glass perfusion apparatus was placed in the bore of a Varian Inova 9.4 T magnet. During a preparation and stabilization time of ∼20 min, the NMR probe was tuned, and the field homogeneity was optimized using the 23Na free induction decay (FID). A line width of 15 Hz was typically obtained. Shimming on 23Na approximates the 13C frequency and shimmed volume, whereas the short spin-spin relaxation time (T2) values of 23Na allow for quicker pulsing. After stable mechanical performance was established, the perfusate was switched to the fed mixture described above (long-chain fatty acids bound to albumin, lactate, pyruvate, ketones, and glucose, all not 13C enriched), filtered through a 5-μm pore cellulose acetate filter, and recirculated. 13C NMR spectra were acquired at 100 MHz in a 20-mm Varian broadband probe using 66° pulses. The first FIDs were acquired a few seconds before injection of the HP solution, described below. Serial, single FIDs were acquired with 16 K complex data points over a ± 21,000 Hz bandwidth with proton decoupling using GARP (47). Acquisition time was ∼1 s, giving a time to repeat of 1 s for each scan. Typically 20–30 separate FIDs were collected. These data were zero-filled before Fourier transformation. The relative peak areas were measured by integration using the VNMR software.
Hyperpolarization and delivery of 13C-enriched pyruvate to hearts.
The polarization process was started ∼90 min before each experiment. The details have been described previously (31, 32). Briefly, ∼20 μl of 1.5 M [1-13C]pyruvate and 16.6 mM trityl radical prepared in 50:50 glycerol-deionized H2O was polarized using a HyperSense instrument (Oxford Instruments Molecular Biotools, Abingdon, UK). ∼90 min of microwave irradiation at 1.4 K typically yielded ∼15% polarization. The polarization of 15% was measured by separate dissolution experiments in the liquid state. The SD of these measurements is ∼1% on a day-to-day basis. The frozen, polarized sample was rapidly thawed by dissolution in 4 ml of hot water (∼180°C) containing 0.85 mM Na2EDTA. The resulting solution (3 ml) was further diluted in 20 ml of perfusing medium containing BSA, long-chain fatty acids, etc., as described above (all substrates not 13C enriched) to achieve a final concentration of 3 mM HP [1-13C]pyruvate. The total time required for dissolution, ejection, and further dilution was ∼10 s. The solution was injected gently and continuously by catheter in the perfusion column directly above the heart at a rate of ∼0.3 ml/s. The temperature of all solutions entering the heart was 37°C, and the pH was 7.3–7.4. The NMR console was triggered to start acquisition at the end of the dissolution process. To inject a 20 mM HP [1-13C]pyruvate solution to the heart, 100 μl of the [1-13C]pyruvate/trityl radical solution was polarized. Following dissolution with hot (∼180°C) 0.85 mM Na2EDTA (aqueous) solution, 3 ml of the resulting solution were diluted with 3 ml of the perfusion medium described above and gently injected to the heart at a rate of ∼0.3 ml/s.
13C NMR spectroscopy of solutions.
Proton-decoupled 13C spectra of heart extracts at 25°C were acquired at 150 MHz on a Varian VNMRS spectrometer (Varian Instruments, Palo Alto, CA) using a 5-mm broadband NMR probe, a 45° observe pulse, and a 3-s delay between pulses. Broadband proton decoupling was achieved using WALTZ (48). Relative peak areas for each multiplet component were determined by deconvolution using ACDLabs SpecManager (Advanced Chemistry Development, Toronto, Canada). Substrate 13C fractional enrichments and 13C multiplet data were used in a metabolic model (tcaCALC) to measure the relative rates of oxidation of each substrate, and anaplerosis (29). To measure the effects of albumin on 13C relaxation times, [1-13C]pyruvate (2.5 mM in PBS + D2O for a lock) was added to eight different concentrations of BSA (0–3%) with and without long-chain physiological fatty acids at a fixed ratio of 0.4 mM fatty acids/3% BSA. 13C spin-lattice relaxation times (T1) were measured at 150 MHz using standard inversion-recovery methods at 37°C.
Statistical analysis.
Data are presented as means ± 1 SD. Two-tailed t-tests were performed assuming unequal variance using Microsoft Excel for Figs. 4 and 5, A and C. For Fig. 3, the pyruvate, fatty acid, ketone body oxidation, and PDH flux, response to increased [pyruvate] was assessed with one-way ANOVA followed by Dunnett's procedure for comparing 1, 3, and 6 mM [pyruvate] with the 0.1 concentration while adjusting for multiple testing. For Fig. 5B, the developed pressure was compared using a mixed-model repeated-measures analysis that included concentration and pre vs. post comparisons in the model. Within each concentration, pre vs. post changes were made with least-squared means contrasts that were derived from the mixed model and adjusted for multiple testing using the Bonferroni-Holm method. Statistical analyses for Figs. 3 and 5B were performed with SAS version 9.2 (SAS Institute, Cary, NC).
Fig. 4.
Effect of 6 mM pyruvate on the contribution of ketones (KB), long-chain fatty acids (FA), and pyruvate (Pyr) to acetyl-CoA. Hearts exposed to the fed mixture derived ∼80% of acetyl-CoA from 6 mM pyruvate (open bar); the contribution of ketones (gray bar) or fatty acids (solid bar) was negligible. However, hearts exposed to the “fasted” mixture preferentially oxidized ketones. Pyruvate contributed only 33% of the acetyl-CoA. Data are means ± SD. *P < 0.05 vs. respective Pyr.
Fig. 5.
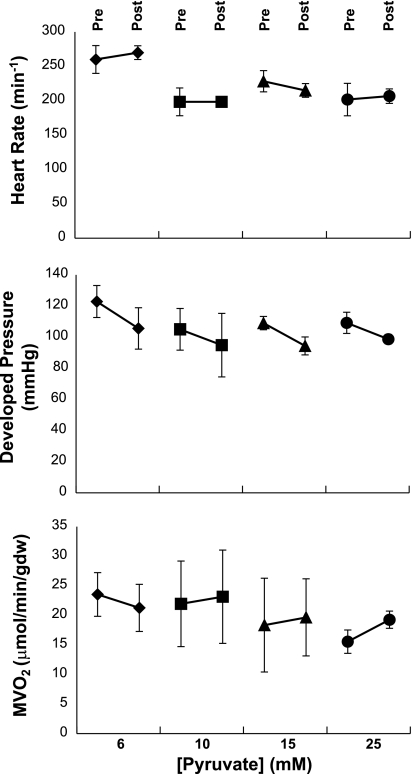
Influence of [pyruvate] on hemodynamic performance of the isolated working heart. “Pre” and “Post” refer to measurements immediately before and 5 min after switching to 6, 10, 15, or 25 mM pyruvate, respectively. Within the 1st min of switchover, there was a variable decrease in developed pressure that recovered quickly. Heart rate, developed pressure, and O2 consumption were not altered substantially by exposure to graded [pyruvate]. Data are means ± SD. All pre vs. post data sets were not significantly different from each other for heart rate and myocardial O2 consumption (MV̇o2). Developed pressure decreased significantly with all concentrations included in the repeated-measures model (pre vs. post difference 13.3 mmHg, 95% confidence interval, 8.8–17.9 mmHg, P < 0.0001). However, no significant pre vs. post differences were observed within any concentration, analyzed with Bonferroni-Holm adjusted multiple comparisons.
Fig. 3.
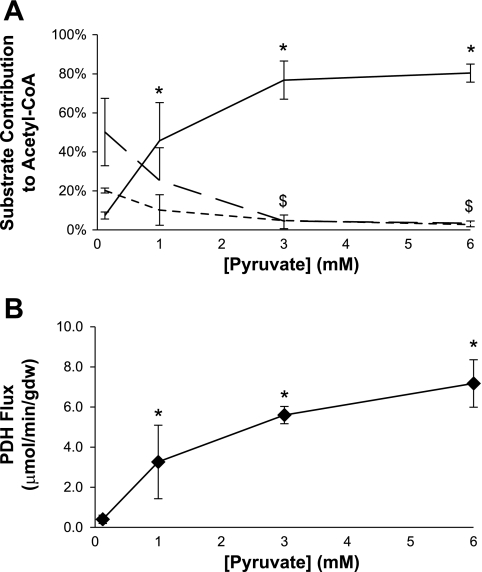
Influence of [pyruvate] on sources of acetyl-CoA and flux through pyruvate dehydrogenase (PDH) using a fed buffer. A: relative rates of oxidation of ketones (dotted line), long-chain fatty acids (broken line), or pyruvate (solid line) are shown. At 3 mM pyruvate, oxidation of competing substrates is largely suppressed. B: flux through PDH. Flux through PDH is maximal at 3–6 mM pyruvate. Data are means ± SD. Analysis of variance revealed statistically significant responses to increased [pyruvate] for pyruvate (P < 0.0001), fatty acid (P = 0.004), ketone body oxidation (P = 0.006), and PDH flux (P = 0.0004). *P < 0.05 [pyruvate] vs. [pyruvate] = 0.12 mM. $P < 0.05 for fatty acids and ketone bodies vs. respective 0.12 mM data point.
RESULTS
Pyruvate oxidation in the fed vs. fasted substrate mixtures.
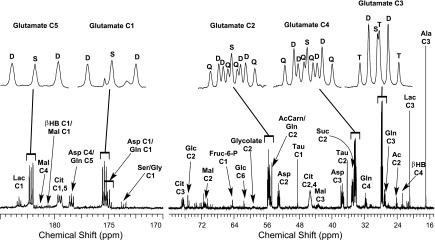
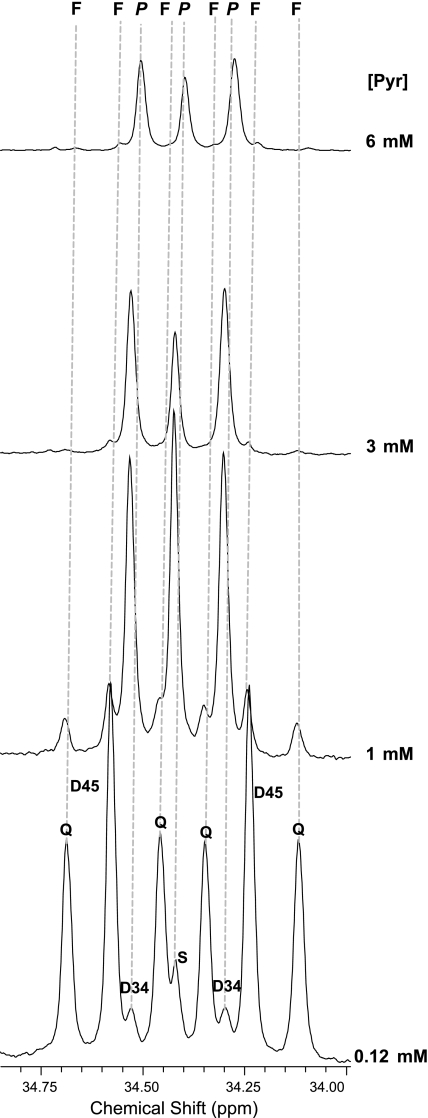
The glutamate multiplets observed in 13C NMR spectra were used to determine the labeling pattern of acetyl-CoA entering the TCA cycle (26–30, 50). A typical spectrum of a heart extract is shown in Fig. 1, with the insets displaying the 13C-13C spin-spin coupling in glutamate. In the glutamate carbon-4 resonance (Fig. 2), the quartet (Q, due to glutamate labeled in positions 3, 4, and 5) and the doublet D45 (due to glutamate labeled in positions 4 and 5 but not 3) are due to oxidation of U-13C long-chain fatty acids, whereas the doublet, D34, and the singlet, S, are due to oxidation of [3-13C]pyruvate. The effects of graded concentrations of [3-13C]pyruvate on the glutamate spectrum are shown in Fig. 2. Because the ratio of long-chain fatty acid oxidation relative to pyruvate oxidation is simply the ratio of multiple areas (Q + D45)/(S + D34) (50), it is evident that, at physiological [pyruvate], fatty acids overwhelm pyruvate as an energy source. At higher [pyruvate], the situation is reversed: pyruvate is by far the preferred substrate for oxidation.
Fig. 1.
1H-decoupled 13C nuclear magnetic resonance (NMR) spectrum of a heart extract. The working heart was supplied with a mixture of 13C-enriched long-chain fatty acids, ketones, lactate, pyruvate, and glucose in concentrations typical of a fed, rested animal. The multiplets of glutamate carbons 1–5, shown as insets, are the result of 13C-13C spin-spin coupling and reflect the relative rates of oxidation of these substrates. Ala, alanine; βHB, β-hydroxybutyrate; Lac, lactate; Ac, acetate; Suc, succinate; Asp, aspartate; Mal, malate; Cit, citrate; D, doublet; Q, quartet; S, singlet; T, triplet.
Fig. 2.
Influence of pyruvate concentration ([pyruvate]; [Pyr]) on the carbon-4 resonance of glutamate. These 13C spectra were acquired from hearts exposed to the “fed” mixture of substrates at graded [pyruvate], 0.12 mM (bottom) or 1.0, 3.0, and 6 mM (top). The fraction of acetyl-CoA derived from pyruvate relative to the fraction from long-chain fatty acids is indicated by the ratio (S + D34)/(Q + D45). At 3 mM pyruvate, the oxidation of long-chain fatty acids is inhibited. F, resonances arising from oxidation of 13C-enriched long-chain fatty acids; P, resonances arising from oxidation of 13C-enriched pyruvate, Q, quartet due to J45 and J34 coupling; D34, doublet due to J34 coupling; D45, doublet due to J45; S, singlet due to glutamate with 13C in position 4 but not position 3 or 5. J is standard notation for the spin-spin coupling constant.
When perfused with the fed buffer, the fraction of acetyl-CoA from pyruvate, ketones, and fatty acids, determined from a full isotopomer analysis (29, 30), are shown in Fig. 3A, and the effect of increasing [pyruvate] on flux through PDH determined using 13C isotopomer analysis and MV̇o2 is shown in Fig. 3B. At a physiological concentration of pyruvate, 0.12 mM, ∼50% of the heart's energy is supplied by fatty acids, ∼20% from ketones, and the remainder from lactate, pyruvate, glucose, and stored sources. At 1 mM [pyruvate] (∼10× physiological), both fatty acid and ketone oxidation were reduced and pyruvate oxidation increased to supply ∼45% of acetyl-CoA. At 3 mM pyruvate and 6 mM pyruvate, the contribution of fatty acids and ketones to acetyl-CoA was small, and pyruvate provided ∼80% of acetyl-CoA. Anaplerosis relative to TCA cycle flux was ∼8–10% for all concentrations of pyruvate.
The sources of energy in the heart were quite different when exposed to the fasted substrate mixtures (Fig. 4). At 3 mM pyruvate under fasted conditions, ketones supplied most of the acetyl-CoA, ∼65%, and fatty acids and pyruvate supplied ∼23% and ∼7%, respectively. Doubling the concentration of pyruvate to 6 mM inhibited fatty acid and ketone oxidation somewhat, but ketones remained the preferred substrate for production of acetyl-CoA even at 6 mM pyruvate (Fig. 4). Flux through PDH when pyruvate was present at 6 mM in the fasted mixture was roughly equal to PDH flux at 1 mM pyruvate in the fed mixture.
Effect of graded [pyruvate] on cardiac function.
The effects of adding high concentrations of pyruvate to the perfusing medium (6, 10, 15, or 25 mM) in the presence of a fed mixture of fatty acids, ketones, lactate, and glucose is shown in Fig. 5. There was no effect of pyruvate in this concentration range on heart rate (Fig. 5, top), developed pressure (Fig. 5, middle), or MV̇o2 (Fig. 5, bottom) when measured 10–15 min after administration of pyruvate. However, at [pyruvate] >10 mM, there was a variable decrease in aortic flow during the first 2–3 min after exposure to pyruvate without a change in developed pressure or heart rate (data not shown). All hearts recovered baseline function within 5 min. Hearts exposed to 25 mM NaCl did not show any variation in heart rate, developed pressure, or MV̇o2 (data not shown).
13C NMR spectra of the isolated heart.
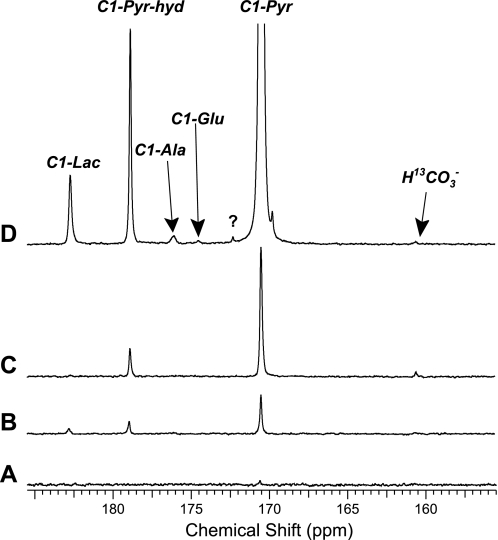
13C NMR spectra of the isolated rat heart collected after injection of HP [1-13C]pyruvate are shown in Fig. 6. In the presence of fatty acid-free BSA and no other substrates, only a very small signal from HP [1-13C]pyruvate could be detected after administration of 3 mM HP [1-13C]pyruvate (Fig. 6A). In the presence of ketones and long-chain fatty acids bound to albumin, HP [1-13C]lactate and HP [1-13C]pyruvate hydrate, in addition to HP [1-13C]pyruvate, could be detected after administration of 3 mM HP [1-13C]pyruvate (Fig. 6B). Because the concentrations of pyruvate and BSA are the same in Fig. 6, A and B, the differences between the spectra must be because of an effect of fatty acids or ketones on pyruvate metabolism, an interaction among substrates and BSA to reduce polarization of HP [1-13C]pyruvate, or some combination of the two. The effect of a higher concentration of HP [1-13C]pyruvate in the presence of BSA but no other substrates was tested, and the results are shown in Fig. 6C. A product of oxidation of HP [1-13C]pyruvate, HP H13CO3−, can be detected at ∼161 ppm. However, in the presence of competing substrates, fatty acids and ketones, this HP H13CO3− signal disappears as would be expected if fatty acids or ketones are being oxidized (Fig. 6D) in preference to HP [1-13C]pyruvate. Interestingly, in the presence of fatty acids and BSA (Fig. 6, B and D), both HP [1-13C]lactate and HP [1-13C]pyruvate hydrate were detected in approximately the same ratio, regardless of the concentration of administered pyruvate, but, in the absence of fatty acids, HP [1-13C]lactate could not be detected (Fig. 6, A and C). In all studies, the HP signals were lost in the presence of albumin within ∼30 s, and the overall signal to noise was attenuated compared with previous studies (31, 32).
Fig. 6.
13C Spectra of isolated hearts exposed to HP [1-13C]pyruvate. A: 3 mM HP [1-13C]pyruvate injection in heart in the presence of 3% BSA and no fatty acids. B: 3 mM HP [1-13C]pyruvate injection in heart in the presence of 3% BSA and 0.4 mM fatty acids. C: 20 mM HP [1-13C]pyruvate injection in heart in the presence of 3% BSA and no fatty acids. D: 20 mM HP [1-13C]pyruvate injection in heart in the presence of 3% BSA and 0.4 mM fatty acids.
Together, these experiments demonstrate that, in the presence of albumin, there is a complex interaction among alternative physiological substrates (ketones and fatty acids) and the 13C NMR spectrum of HP pyruvate and its metabolic products. Based on the spectrum in Fig. 6D, it appears that HP [1-13C]pyruvate is not being oxidized since the HP H13CO3− signal could not be detected. Common illnesses and brief fasting cause variation of plasma ketones and fatty acids, as wells as plasma albumin, and for this reason the effective concentration of pyruvate for 13C cardiac imaging will be sensitive to the condition of the subject. In principle, sufficiently high concentrations of pyruvate in the coronary arteries should overcome competing substrates, but high concentrations of pyruvate may have adverse effects (24, 25).
Effect of albumin on the longitudinal relaxation time of [1-13C]pyruvate.
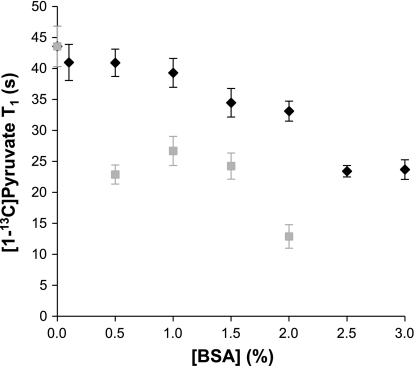
The T1 of carbon-1 of pyruvate was ∼44 s at 2.5 mM, 37°C, and 150 MHz in aqueous solution in the absence of albumin. In the presence of albumin mixed with long-chain fatty acids, there was a graded and approximately linear effect of albumin concentration ([albumin]) on T1 of carbon-1 of pyruvate = 44.7 − 6.6[albumin], where T1 is in seconds, [albumin] is in percent, r2 = 0.91 (Fig. 7). At 3% albumin, the T1 of pyruvate carbon-1 was 25 ± 2 s. In the presence of fatty acid-free albumin, the T1 of pyruvate carbon-1 was further reduced to ∼10 s at 3% [albumin]. Numerous small molecules competitively bind albumin, and these studies with fatty acid-free albumin or with albumin bound to fatty acids do not duplicate conditions in vivo. Therefore, plasma was prepared from fed rats pretreated with heparin. The T1 of carbon-1 of [1-13C]pyruvate, 25 mM, in rat plasma was ∼38 s at 37°C and 150 MHz.
Fig. 7.
Effect of albumin on the spin-lattice relaxation time (T1) of 2.5 mM [1-13C]pyruvate. ♦, Fatty acids present; ■, no fatty acids present. When fatty acids were not present, T1 times were not able to be determined at [albumin] >2%. The two data sets were significantly different from each other at [albumin] ≥0.5%.
DISCUSSION
In the presence of long-chain fatty acids bound to albumin plus other substrates in physiological concentrations and under steady-state conditions, the contribution of pyruvate to acetyl-CoA and therefore to CO2 and bicarbonate is very sensitive to the concentration of pyruvate. At 3 mM pyruvate, ∼80% of acetyl-CoA was derived from pyruvate under conditions mimicking the fed state. In the presence of competing substrates mimicking the fasted state, only ∼33% of acetyl-CoA was derived from pyruvate even at 6 mM. These results are consistent with the known regulation of flux through PDH by both end-product inhibition and phosphorylation-dephosphorylation. PDH kinase phosphorylates PDH, thereby inactivating the enzyme, and, since pyruvate inhibits PDH kinase (9, 21, 40, 44), the presence of pyruvate would be expected to stimulate pyruvate oxidation. Earlier reports have shown that oxidation of pyruvate is suppressed by fatty acids and ketones and that the effects of fatty acids on pyruvate oxidation are overcome by increasing [pyruvate] (2, 4, 7, 8, 11, 20, 21, 23, 24, 36, 39, 43, 49, 54, 55). However, competition between pyruvate and physiological mixtures of competing substrates for oxidation in the heart during graded changes in [pyruvate] has not been carefully examined previously.
The current results demonstrate that, even at 1 mM, pyruvate begins to suppress oxidation of both fatty acid and ketones. At or above 3 mM, pyruvate is the dominant oxidative fuel for the heart when the competing substrates are typical of the fed state. These findings confirm those of Schroeder et al. (45), which showed H13CO3− production leveling off with an infusion of HP [1-13C]pyruvate in a rat heart between 40 and 80 mM. The authors calculated that an 80 mM pyruvate infusion equated a 5.3 mM myocardial [pyruvate] based on a 10-s infusion time and 15 ml of blood passing through the heart. Drake et al. (11) and Laughlin et al. (24) have shown similar findings that increased lactate concentration or [pyruvate] can be the preferred substrate for the heart over fatty acids or glucose. However, the effect of 6 mM pyruvate on ketone and fatty acid oxidation is blunted under conditions mimicking the fasting state, which is similar to in vivo results obtained by Schroeder et al. (46). The authors report an ∼74% decrease in H13CO3− production in fasted animals following an 80 mM HP [1-13C]pyruvate injection (46). Similarly, the current studies show an ∼60% reduction in the utilization of pyruvate in isolated rat hearts perfused under fasted conditions. These results relate well to studies by Olson (37) and Bassenge et al. (3) that showed elevated ketone body concentration during fasting will suppress carbohydrate utilization in the heart. This concentration of pyruvate, 6 mM, is at the upper limit of steady-state plasma [pyruvate] used in many previous studies (10, 16–18, 22, 24, 45, 56).
The isolated heart preparation is well suited to studies of substrate competition because all concentrations can be precisely controlled. Furthermore, the general features of substrate utilization measured in the isolated heart match results in vivo. For example, long-chain fatty acids are preferred over carbohydrates in vivo and in isolated hearts (2, 4, 43, 51). Ketone bodies inhibit fatty acid and glucose oxidation in both preparations during fasting (3, 37), and elevated pyruvate or lactate effectively competes with both fatty acids and glucose in both preparations as well (11, 24). Although there is a good general agreement between in vivo studies and results from isolated hearts, the relevance of results in isolated tissues must be confirmed in vivo. From the current results, we anticipate that intravenously administered HP pyruvate may not provide the same quality images under conditions of hyperketonemia compared with control.
The nutritional state of the heart is not the only factor that affects substrate utilization, but the disease state will as well. Several studies have shown decreased H13CO3− production in a diseased state after administration of HP [1-13C]pyruvate (15, 32, 46). Schroeder et al. (46) demonstrated that streptozotocin-treated rats have decreased PDH flux compared with controls. Golman et al. (15) and Merritt et al. (32) determined that PDH flux is near zero after ischemia. All three studies fall within the optimal range of myocardial [pyruvate] determined by Schroeder et al. (45) and this work for imaging.
These experiments illustrate a fundamental difference between studies of cardiac metabolism by HP [1-13C]pyruvate compared with SPECT or PET. Radiotracers are administered in such low concentration that the effect of the labeled substrate on metabolism is negligible. However, for detection of HP H13CO3−, a significant mass of HP [1-13C]pyruvate must be administered to overcome the presence of competing substrates, and the mass of administered pyruvate itself influences metabolism. Consequently, metabolic exams with [1-13C]pyruvate are not tracer studies in the conventional sense.
Furthermore, the potential toxicity of pyruvate must be considered. The metabolic consequences of high-dose pyruvate are likely to be complex in the heart, since the cytosol will become more oxidized (decreased NADH/NAD+) because of flux of pyruvate through lactate dehydrogenase. Pyruvate oxidation in the mitochondria will have the opposite effect, an increase in NADH/NAD+. In spite of these metabolic effects, in the current experiments, there was no evidence for adverse hemodynamic events up to ∼10 mM, and even at higher concentrations there was only a transient decrease in cardiac output and no change in developed pressure. It is not possible to extrapolate these data to in vivo conditions, especially in the setting of heart disease or depressed cardiac output, and the isolated heart model by definition excludes possible effects of pyruvate on the peripheral circulation. Nevertheless, these results are consistent with earlier studies that emphasized a role for pyruvate as a therapeutic agent. Typically, the target concentration was between 2 and 6 mM, but a significant cardioprotective effect was reported among patients exposed to a 10 mM pyruvate-fortified cardioplegia solution during coronary artery bypass surgery (35). A preliminary conclusion is that a reasonable target concentration in the coronary arteries is 3–6 mM to suppress oxidation of competing substrates and that the heart may tolerate exposure to significantly higher concentrations.
These observations from 13C isotopomer analysis of tissue extracts suggested that HP 13CO2 and H13CO3− should be readily observed in isolated hearts supplied with the same mixture of substrates. This anticipated result would be consistent with the finding by Golman and colleagues (13–15) that imaging of HP H13CO3− in the heart is feasible in vivo. Because the bolus dose of HP [1-13C]pyruvate was small in those studies (0.3 mmol/kg) compared with earlier studies of therapeutic pyruvate [0.9 mmol/kg (22) or 1.5 mmol/kg (56)], one might anticipate that the concentration of HP pyruvate was modest in the coronary arteries in those studies. Consequently, it was not anticipated that H13CO3− could not be detected from the isolated heart supplied with albumin and competing substrates. Because the concentration of pyruvate was clearly sufficient to provide much of the acetyl-CoA oxidized in the TCA cycle, this finding cannot be the result of simply inhibition of pyruvate oxidation by competing substrates. Two factors likely play a role.
Attenuation of the pyruvate signal (Fig. 6A) in the presence of fatty acid-free albumin demonstrated that the longitudinal relaxation rate of pyruvate was significantly increased by albumin, an observation confirmed in separate experiments with graded concentrations of fatty acid-free albumin (Fig. 7). The mechanism of the adverse effect of albumin on pyruvate T1 has not been investigated extensively. Albumin has an affinity for numerous small molecules (41). The albumin typically used in isolated tissue experiments is extensively processed with the specific objective of removing most of these ligands. In cardiac studies, albumin is generally used to deliver physiological long-chain fatty acids to the heart since these substrates are otherwise insoluble. However, these experiments illustrate that highly purified albumin may adversely affect experiments with HP pyruvate. Chatham and Forder (6) reported disappearance of the 1H NMR signal of pyruvate with the addition of BSA, and Stepuro et al. (52) described an albumin-catalyzed exchange of pyruvate methyl protons with the solvent. These earlier experiments demonstrate that, under some conditions, pyruvate binds to albumin. The current results demonstrate that the magnitude of the effect of albumin on pyruvate T1 is sensitive to the presence of albumin ligands. It is likely that the effect is less significant in vivo where numerous other ligands such as bilirubin, porphyrins, and metal ions are present, and it is conceivable that the concentration of fatty acids is often higher in vivo than used in the current study.
A second factor is the duration of exposure of the heart to high concentrations of pyruvate. A stepped but transient increase in [pyruvate] as would be expected from a bolus injection may produce a different set of metabolic conditions compared with a sustained change in concentration of pyruvate over 20–30 min. Under the latter conditions, designed to produce metabolic steady state, fluxes through all reactions feeding the TCA cycle are fixed. Although the rate of phosphorylation-dephosphorylation of PDH is very rapid (21, 36), the input function of a bolus of pyruvate may have a complex effect on the concentration of the injectate in the coronary arteries (1) and on fluxes through PDH during the first few seconds as the [pyruvate] changes.
Together, these experiments plus earlier studies with isolated hearts demonstrate that the appearance of HP 13CO2 and HP H13CO3− after exposure to HP [1-13C]pyruvate is reduced by competing substrates and by exposure to albumin. Both effects are concentration dependent. These results suggest a conceptual framework for designing studies in isolated hearts with HP [1-13C]pyruvate. At low concentration of competing substrates, most of the CO2 production will be the result of oxidation of pyruvate, and the rate of 13CO2 appearance will be near-maximal even at relatively low [pyruvate], ∼3 mM. As the concentration of competing substrates increases or as the neurohumoral environment changes, the rate of CO2 production from pyruvate will be reduced but can be recovered at higher [pyruvate] in the coronary arteries. Eventually, however, the delivery of high concentrations of pyruvate will be limited by practicalities such as osmolality or toxicity. Technical factors such as the effects of highly purified albumin on the T1 of pyruvate must also be considered. In vivo, the effects of albumin are likely to be less significant, but the concentration of competing substrates will significantly influence the appearance of HP 13CO2 and HP H13CO3−.
GRANTS
This study was supported by National Institutes of Health Grants RR-02584 and HL-34557.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
Angela Milde and Charles Storey provided outstanding technical support. We appreciate expert advice by William Mander from Oxford Instruments Molecular Biotools and Beverley Huet from the Division of Biostatistics at the University of Texas Southwestern Medical Center.
REFERENCES
- 1.Bae Kyongtae T, Tran Huy Q, Heiken Jay P. Uniform vascular contrast enhancement and reduced contrast medium volume achieved by using exponentially decelerated contrast material injection method. Radiology 231: 732–736, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ballard FB, Danforth WH, Naegle S, Bing RJ. Myocardial metabolism of fatty acids. J Clin Invest 39: 717–723, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassenge E, Wendt VE, Schollmeyer P, Bluemchen G, Gudbjarnason S, Bing RJ. Effect of ketone bodies on cardiac metabolism. Am J Physiol 208: 162–168, 1965 [DOI] [PubMed] [Google Scholar]
- 4.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 16: 504–515, 1954 [DOI] [PubMed] [Google Scholar]
- 5.Bunger R, Mallet RT, Hartman DA. Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile failure. Eur J Biochem 180: 221–233, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Chatham JC, Forder JR. Lactic acid and protein interactions: implications for the NMR visibility of lactate in biological systems. Biochim Biophys Acta 1426: 177–184, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Comte B, Vincent G, Bouchard B, Des Rosiers C. Probing the origin of acetyl-CoA and oxaloacetate entering the citric acid cycle from the 13C labeling of citrate released by perfused rat hearts. J Biol Chem 272: 26117–26124, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Comte B, Vincent G, Bouchard B, Jette M, Cordeau S, Des Rosiers C. A 13C mass isotopomer study of anaplerotic pyruvate carboxylation in perfused rat hearts. J Biol Chem 272: 26125–26131, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Cooper RH, Randle PJ, Denton RM. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J 143: 625–641, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkstra U, Gabreels F, Joosten E, Wevers R, Lamers K, Doesburg W, Renier W. Friedreich's ataxia: intravenous pyruvate load to demonstrate a defect in pyruvate metabolism. Neurology 34: 1493–1497, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Drake AJ, Haines JR, Noble MIM. Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc Res 14: 65–72, 1980 [DOI] [PubMed] [Google Scholar]
- 12.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, in't Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature Lond 453: 940–943, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Golman K, in't Zandt R, Thaning M. Real-time metabolic imaging. Proc Natl Acad Sci USA 103: 11270–11275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golman K, Petersson JS. Metabolic imaging and other applications of hyperpolarized 13C. Acad Radiol 13: 932–942, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Golman K, Petersson JS, Magnusson P, Johansson E, Aakeson P, Chai CM, Hansson G, Maansson S. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Mag Res Med 59: 1005–1013, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hasenfuss G, Maier LS, Hermann HP, Luers C, Huenlich M, Zeitz O, Janssen PML, Pieske B. Influence of pyruvate on contractile performance and Ca2+ cycling in isolated failing human myocardium. Circulation 105: 194–199, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hermann HP, Arp J, Pieske B, Koegler H, Baron S, Janssen PML, Hasenfuss G. Improved systolic and diastolic myocardial function with intracoronary pyruvate in patients with congestive heart failure. Eur J Heart Fail 6: 213–218, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hermann HP, Pieske B, Schwarzmuller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet 353: 1321–1323, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey FMH, Alvarez L, Diczku V, Sherry AD, Malloy CR. Direct evidence that perhexiline modifies myocardial substrate utilization from fatty acids to lactate. J Cardiovasc Pharmacol 25: 469–472, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Jeffrey FMH, Diczku V, Sherry AD, Malloy CR. Substrate selection in the isolated working rat heart: effects of reperfusion, afterload, and concentration. Basic Res Cardiol 90: 388–396, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A, and reduced and oxidized NAD. Biochem J 154: 327–348, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristo G, Yoshimura Y, Niu J, Keith Byron J, Mentzer Robert M, Jr, Bunger R, Lasley Robert D. The intermediary metabolite pyruvate attenuates stunning and reduces infarct size in in vivo porcine myocardium. Am J Physiol Heart Circ Physiol 286: H517–H524, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Latipaa PM, Peuhkurinen KJ, Hiltunen JK, Hassinen IE. Regulation of pyruvate dehydrogenase during infusion of fatty acids of varying chain lengths in the perfused rat heart. J Mol Cell Cardiol 17: 1161–1171, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Laughlin MR, Taylor J, Chesnick AS, DeGroot M, Balaban RS. Pyruvate and lactate metabolism in the in vivo dog heart. Am J Physiol Heart Circ Physiol 264: H2068–H2079, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Mallet RT. Pyruvate: metabolic protector of cardiac performance. Proc Soc Exp Biol Med 223: 136–148, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Malloy CR, Jones JG, Jeffrey FM, Jessen ME, Sherry AD. Contribution of various substrates to total citric acid cycle flux and anaplerosis as determined by 13C isotopomer analysis and O2 consumption in the heart. Mag Res Mat Phys Biol Med 4: 35–46, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Malloy CR, Sherry AD, Jeffrey FMH. Analysis of tricarboxylic acid cycle of the heart using carbon-13 isotope isomers. Am J Physiol Heart Circ Physiol 259: H987–H995, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Malloy CR, Sherry AD, Jeffrey FMH. Carbon flux through citric acid cycle pathways in perfused heart by carbon-13 NMR spectroscopy. FEBS Lett 212: 58–62, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Malloy CR, Sherry AD, Jeffrey FMH. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by carbon-13 NMR spectroscopy. J Biol Chem 263: 6964–6971, 1988 [PubMed] [Google Scholar]
- 30.Malloy CR, Thompson JR, Jeffrey FMH, Sherry AD. Contribution of exogenous substrates to acetyl coenzyme A: measurement by carbon-13 NMR under non-steady-state conditions. Biochemistry 29: 6756–6761, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci USA 104: 19773–19777, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merritt ME, Harrison C, Storey C, Sherry AD, Malloy CR. Inhibition of carbohydrate oxidation during the first minute of reperfusion after brief ischemia: NMR detection of hyperpolarized 13CO2 and H13CO3−. Magn Reson Med 60: 1029–1036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mongan PD, Capacchione J, Fontana JL, West S, Bunger R. Pyruvate improves cerebral metabolism during hemorrhagic shock. Am J Physiol Heart Circ Physiol 281: H854–H864, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Neely JR, Liebermeister H, Morgan HE. Effect of pressure development on membrane transport of glucose in isolated rat heart. Am J Physiol 212: 815–822, 1967 [DOI] [PubMed] [Google Scholar]
- 35.Olivencia-Yurvati AH, Blair JL, Baig M, Mallet RT. Pyruvate-enhanced cardioprotection during surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesthesia 17: 715–720, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Olson MS, Dennis SC, DeBuysere MS, Padma A. The regulation of pyruvate dehydrogenase in the isolated perfused rat heart. J Biol Chem 253: 7369–7375, 1978 [PubMed] [Google Scholar]
- 37.Olson RE. Effect of pyruvate and acetoacetate on the metabolism of fatty acids by the perfused rat heart. Nature Lond 195: 597–599, 1962 [DOI] [PubMed] [Google Scholar]
- 38.Panchal AR, Comte B, Huang H, Dudar B, Roth B, Chandler M, Des Rosiers C, Brunengraber H, Stanley WC. Acute hibernation decreases myocardial pyruvate carboxylation and citrate release. Am J Physiol Heart Circ Physiol 281: H1613–H1620, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Panchal AR, Comte B, Huang H, Kerwin T, Darvish A, Des Rosiers C, Brunengraber H, Stanley WC. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol 279: H2390–H2398, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Pettit FH, Pelley JW, Reed LJ. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun 65: 575–582, 1975 [DOI] [PubMed] [Google Scholar]
- 41.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology (Baltimore, Md) 41: 1211–1219, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Reinhart WH, Gaudenz R, Walter R. Acidosis induced by lactate, pyruvate, or HCl increases blood viscosity. J Crit Care 17: 68–73, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Rothlin ME, Bing RJ. Extraction and release of individual free fatty acids by the heart and fat depots. J Clin Invest 40: 1380–1386, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saiki Y, Lopaschuk GD, Dodge K, Yamaya K, Morgan C, Rebeyka IM. Pyruvate augments mechanical function via activation of the pyruvate dehydrogenase complex in reperfused ischemic immature rabbit hearts. J Surg Res 79: 164–169, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Schroeder MA, Atherton HJ, Cochlin LE, Clarke K, Radda GK, Tyler DJ. The effect of hyperpolarized tracer concentration on myocardial uptake and metabolism. Magn Reson Med 61: 1007–1014, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci USA 105: 12051–12056, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaka AJ, Barker PB, Freeman R. Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson 64: 547–552, 1985 [Google Scholar]
- 48.Shaka AJ, Keeler J, Freeman R. Evaluation of a new broadband decoupling sequence: WALTZ-16. J Magn Reson 53: 313–340, 1983 [Google Scholar]
- 49.Sherry AD, Malloy CR, Roby RE, Rajagopal A, Jeffrey FMH. Propionate metabolism in the rat heart by carbon-13 nmr. Spectrosc Biochem J 254: 593–598, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherry AD, Malloy CR, Zhao P, Thompson JR. Alterations in substrate utilization in the reperfused myocardium: a direct analysis by carbon-13 NMR. Biochemistry 31: 4833–4837, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Shipp JC, Opie LH, Challoner D. Fatty acid and glucose metabolism in the perfused heart. Nature Lond 189: 1018–1019, 1961 [Google Scholar]
- 52.Stepuro II, Moroz AR, Piletskaya TP. Studies of deuterium exchange catalyzed by human serum albumin. Biokhimiya (Moscow) 51: 729–736, 1986 [PubMed] [Google Scholar]
- 53.Taegtmeyer H. Six blind men explore an elephant: aspects of fuel metabolism and the control of tricarboxylic acid cycle activity in heart muscle. Basic Res Cardiol 79: 322–336, 1984 [DOI] [PubMed] [Google Scholar]
- 54.Weiss RG, Chacko VP, Gerstenblith G. Fatty acid regulation of glucose metabolism in the intact beating rat heart assessed by carbon-13 NMR spectroscopy: the critical role of pyruvate dehydrogenase. J Mol Cell Cardiol 21: 469–478, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Wieland O, Von Funcke H, Loeffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett 15: 295–298, 1971 [DOI] [PubMed] [Google Scholar]
- 56.Yanos J, Patti MJ, Stanko RT. Hemodynamic effects of intravenous pyruvate in the intact, anesthetized dog. Crit Care Med 22: 844–850, 1994 [DOI] [PubMed] [Google Scholar]