Abstract
Previous animal and human studies have suggested that a muscle reflex engaged during contraction leads to heightened levels of sympathetic activity in congestive heart failure (CHF). The present experiment was designed to test the role for bradykinin, which is produced within contracting skeletal muscle and contributes to the muscle reflex through its action on kinin B2 receptors located on the endings of thin fiber muscle afferents. CHF was induced in rats by myocardial infarction (MI) after coronary artery ligation. Echocardiography was performed to determine fractional shortening (FS), an index of the left ventricular function. In the decerebrate rats, we examined renal sympathetic nerve activity (RSNA) during 1 min intermittent (1 to 4 s stimulation to relaxation) contraction of left triceps surae muscles. RSNA responded synchronously as tension was developed, and the response was significantly (P < 0.05) greater in MI rats [+39 ± 9% s−1 (integrated RSNA over time); n = 16] with 20 ± 2% of FS than that in control healthy rats (+19 ± 2% s−1; n = 16) with 49 ± 2% of FS. Tension development did not differ significantly between the two groups of rats. Thirty minutes after intra-arterial injection into the hindlimb circulation of the kinin B2 receptor antagonist, HOE-140 (2 μg/kg), the RSNA response to contraction was significantly reduced in the MI rats (+26 ± 7% s−1) but not in the control rats (+17 ± 2% s−1). These data suggest that bradykinin within contracting muscle is part of the exaggerated muscle reflex seen in CHF.
Keywords: kinin B2 receptor, myocardial infarction, exercise
in patients with congestive heart failure (CHF), augmented sympathetic nerve activity is observed at rest (16) as well as during exercise (25, 26, 34). The augmented sympathetic nerve activity during exercise is a possible cause of exercise intolerance in patients of this disease (36). A reflex originating in contracting muscle has been suggested to lead to the augmented sympathetic nerve activity seen during exercise in CHF (12, 17, 21, 34, 35, 37–39). The muscle reflex is a sympathoexcitatory mechanism, which is activated by the increase in discharge of mechanically and chemically sensitive afferents in active muscle due to contraction and stimulates the medulla responsible for cardiovascular and respiratory regulation (5, 7). The mechanisms by which the muscle reflex responses become exaggerated in CHF are not fully understood.
One possible mechanism responsible for the exaggerated muscle reflex responses observed in CHF is that muscle afferents become sensitized during contraction by the metabolites produced (35). In CHF, skeletal muscle morphology and metabolism are altered (2, 18). A number of metabolites produced by muscle contraction, such as ATP, bradykinin, cyclooxygenase products, lactic acid, potassium, and so on, have been suggested as potential stimulants and/or sensitizers of muscle afferents responding to contraction (5, 7, 35). The present report focuses on the role for bradykinin, which is an autacoid produced within the interstitium of most tissues and is synthesized from its precursor kininogen after activation of the enzyme, kallikrein. Scott et al. (32) have examined the effect of ketoprofen infusion, which inhibits the synthesis of prostaglandins and bradykinin activity on an increase in ventilation during handgrip exercise followed by postexercise ischemia in CHF patients. They found that the increased prostaglandins and bradykinin products during exercise in CHF were reduced after ketroprofen infusion, associated with the reduction of the ventilatory response during postexercise ischemia. The exact role for bradykinin in the muscle reflex regulation of the sympathetic nervous system during contraction in CHF is unknown.
We hypothesize that the activation of bradykinin receptors located on muscle afferents during contraction is part of the exaggerated muscle reflex responses in CHF. The purpose of this report is to determine whether the blockade of bradykinin receptors in skeletal muscle reduces muscle contraction-induced sympathetic nerve responses in CHF more than those in healthy individuals. In decerebrate rats, we examined the responses of renal sympathetic nerve activity (RSNA) to muscle contraction before and after intra-arterial injection into the hindlimb circulation of a kinin B2 receptor antagonist [d-Arg,Hyp3Thi5,d-Tic7,Oic8]bradykinin (HOE-140). We then compared the effect of this injection on the reflex RSNA response between rats with CHF and healthy rats. In a previous report, kinin B2, but not B1, receptors were shown to mediate the cardiovascular effects of bradykinin on skeletal muscle afferents to cause the muscle reflex in anesthetized cats (28). Therefore, a kinin B2 receptor blocker was employed to examine its effect on the sympathetic nerve response to muscle contraction in the present experiments.
MATERIALS AND METHODS
All procedures of this study were approved by the Animal Care Committee of this institution.
Coronary artery ligation.
Coronary artery ligation surgery was performed to induce CHF after myocardial infarction (MI), as performed in our previous experiments (12, 17). Male Sprague-Dawley rats (190 to 250 g) were anesthetized by the inhalation of an isoflurane-oxygen mixture (2% to 5% isoflurane in oxygen), intubated, and artificially ventilated. An incision between the fourth and fifth ribs was made, and the left ventricular wall was exposed through a thoracotomy. The left coronary artery was then ligated. The survival rate after the ligation surgery was about 85%.
Echocardiography.
More than 8 wk after the ligation surgery, transthoracic echocardiography (Sequoia C256, Acuson/Siemens Corp) was performed to assess the cardiac structure and function (12, 17). On the basis of the fractional shortening (FS) determined by echocardiography, 16 rats with CHF induced by MI, whose FS was ≤35% (mean ± SE: 20 ± 2%), and the age and body weight matched 16 control healthy rats, whose FS was ≥40% (mean ± SE: 49 ± 2%), were employed in the experiment. The echocardiographic data as well as the morphometric characteristics of those rats are presented in Table 1.
Table 1.
Morphometric and echocardiographic characteristics
| Healthy Control | MI | |
|---|---|---|
| n | 16 | 16 |
| Body weight, g | 510 ± 11 | 553 ± 20 |
| Heart weight/body weight, mg/g | 2.7 ± 0.1 | 3.1 ± 0.1* |
| LVEDP, mmHg | 2 ± 2 | 15 ± 3* |
| LVDD, mm | 0.82 ± 0.02 | 1.09 ± 0.02* |
| LVSD, mm | 0.42 ± 0.02 | 0.87 ± 0.04* |
| FS, % | 49 ± 2 | 20 ± 2* |
Values are means ± SE; n, number of animals. MI, myocardial infarction; LVEDP, left ventricular end-diastolic pressure; LVDD, left ventricular end-diastolic diameter; LVSD, left ventricular end-systolic diameter; FS, fractional shortening [=(LVDD − LVSD)/LVDD × 100].
P < 0.05 vs. control with the unpaired t-test.
Experimental preparation.
The rats with CHF more than 8 wk after the ligation surgery and the control rats were studied in the experimental protocol. Rats were anesthetized with a mixture of isoflurane (<4%) and oxygen. The trachea was cannulated, and the lungs were then artificially ventilated with a respirator (model 683, Harvard). The left jugular vein and common carotid artery were cannulated to administer drugs and to record arterial pressure, respectively. The arterial catheter was attached to a pressure transducer (MLT0380/D, AD Instruments). Needle electrodes were placed on the chest of the rat to record the electrocardiogram (ECG). The ECG signal was amplified with an AC Preamplifier (P55, Grass Instruments). Heart rate (HR) was calculated beat to beat with the detection of the time between successive R waves in the ECG. Arterial pH was measured with a pH meter (B-212, Horiba) during the surgery and experiment and was maintained within normal limits (pH = 7.4) with an intravenous infusion of a sodium bicarbonate solution (8.4%). Body temperature was adequately maintained with a heating pad. The rat was held in a stereotaxic apparatus (900LS, David KOPF Instruments).
To administer drugs into the arterial supply of the skeletal muscle of the left hindlimb, a catheter was inserted within the right common iliac artery and its tip was placed at the junction of the iliac arteries (39). As a result, substances injected from this catheter first enter the left hindlimb circulation via the left common iliac artery. To trap the injectate within the left hindlimb circulation, a reversible ligature was placed around the left common iliac vein.
The left RSNA was measured as performed previously (11, 12). The left kidney was exposed retroperitoneally through a left flank incision. A bundle of the renal nerves was carefully dissected from other connective tissues. A piece of laboratory film was placed under the isolated nerves, and two tips of a bipolar electrode to record the activity were placed between the nerves and the film. They were embedded in a silicon gel (Kwik-Sil, WPI). The RSNA signal was amplified with a differential amplifier (P511, Grass Instruments) with a band-pass filter of 100 Hz in low-cut frequency and of 3 kHz in high-cut frequency and made audible. The left Achilles tendon was isolated by cutting the calcaneus bone, and the left triceps surae muscles were isolated. The hindlimb was fixed in space with a patellar precision clamp to prevent limb movement. All visible branches of the left sciatic nerve except for those innervating the triceps surae muscles were cut. The common tibial nerve was carefully dissected and then placed on a shielded bipolar electrode for electrically evoking contraction of the left triceps surae muscles. They were embedded in the silicone gel. The electrode was connected to a stimulator (S88, Grass Instruments). The tension generated by the triceps surae muscles was measured with a force transducer (FT03, Grass Instruments) connected to the Achilles tendon.
Decerebration was carried out as previously performed (11, 12). After the withdrawal of the anesthesia, a recovery period > 90 min was allowed before beginning the experimental protocols.
Experimental protocol.
The rats were mechanically ventilated at 70 min−1 frequency with 5.5–6.0 ml/kg tidal volume. The left triceps surae muscles were stretched to create a baseline tension of 50–100 g. The motor threshold, which is the minimum current intensity necessary to evoke twitching of the triceps surae muscles, was determined by the electrical stimulation of the tibial nerve with 0.1 ms pulse duration. After collecting 30 s of baseline data, 1 min repetitive contraction of the left triceps surae muscles was induced. The duty cycle was 1 to 4 s stimulation to relaxation so that the muscle was stimulated 12 times for 1 min (11, 12). Contraction was evoked by the electrical stimulation of the tibial nerve (40 Hz of frequency; 0.1 ms of a pulse duration; <2× motor threshold of intensity). This contraction protocol was performed before and 30 and 60 min after intra-arterial injection of HOE-140 (2.0 μg/kg). The reversible suture placed around the left common iliac vein was tightened to trap the injected HOE-140 within the hindlimb and then released 10 min after the injection.
At the end of data collection, the rat was paralyzed with an intravenous infusion of pancuronium bromide (0.5 mg/kg). The left tibial nerve was then continuously (30 s) stimulated at an intensity that induced contraction in the experiment. This procedure was done to confirm that the observed responses to contraction were not due to the direct stimulation of muscle afferents. We reasoned that if sympathetic nerve activity, blood pressure, or HR rose during muscle paralysis, a direct stimulatory effect on sensory afferents would then seem likely. None of the rats showed any increases in those parameters during this stimulation.
After all protocols, the renal nerve was cut between the electrode and the neural axis to record the background noise of RSNA. A polyethylene catheter was inserted into the right carotid artery and was threaded into the left ventricle for the measurement of left ventricular end-diastolic pressure. At the conclusion of the experiment, the rats were humanely euthanized with an intravenous injection of pentobarbital sodium (75 mg/kg), followed by an intravenous injection of potassium chloride (2 mol/l, 1 ml).
In a subset of control rats (n = 3), we tested whether the dose used for HOE-140 (2.0 μg/kg) would be sufficient to block the activities of the kinin B2 receptors in the hindlimb. We observed that when bradykinin (20 μg/kg) was injected intra-arterially into the circulation, the blood pressure decreased in the isoflurane-anesthetized rats. An injection of 2.0 μg/kg of HOE-140 into the left hindlimb circulation abolished the decrease in blood pressure seen when bradykinin was injected. This suggests that kinin B2 receptors were blocked by this dosage of HOE-140 in the present experiment.
Data acquisition and statistical analyses.
All measured variables were displayed continuously on a computer monitor and stored on a hard disk through analog-digital conversion (PowerLab/8s, AD Instruments) at a 1-kHz sampling rate. RSNA signals were analyzed using methods previously employed (11, 12). We obtained full-wave rectified signals of RSNA as well as the background noise signals. The noise component was subtracted by the rectified RSNA signal. A moving average collected over 50 ms was then obtained. To quantify the sympathetic response to muscle contraction, basal values were obtained by taking mean values for 30 s of baseline and considered as 100% by evaluating the mean, and then relative changes from baseline were evaluated (ΔRSNA). The obtained values were averaged every 100 ms. The data after this normalization of each rat were used to calculate the tension-time index (TTI), integrated ΔRSNA during tension development, and the integrated ΔRSNA per TTI. The TTI and the integrated ΔRSNA were calculated by integrating tension development and time during the development of muscle tension and by integrating the increases in RSNA during and following tension development and its time, respectively.
The data are expressed as means ± SE. Baseline data were obtained from the averaged values for 30 s immediately before the intervention of muscle contraction. The data were analyzed with unpaired t-tests and one- or two-way repeated ANOVA. After the ANOVA procedure, if appropriate, Dunnett's or Tukey's post hoc tests were used to assess significant differences. The criterion of the significance was set at P < 0.05.
RESULTS
Intra-arterial injection of HOE-140 did not affect basal mean arterial pressure, HR, or RSNA in either of the two rat groups studied (Table 2). Furthermore, there were no significant differences in these values in the healthy controls and the MI rats.
Table 2.
Basal MAP, HR, and signal-to-noise ratio for the basal RSNA before and 30 and 60 (recovery) min after intra-arterial injection of HOE-140 into the hindlimb circulation
| Control |
MI |
|||||
|---|---|---|---|---|---|---|
| Before HOE-140 | 30 min After HOE-140 | Recovery | Before HOE-140 | 30 min After HOE-140 | Recovery | |
| MAP, mmHg | 93 ± 3 | 88 ± 2 | 87 ± 2 | 89 ± 2 | 87 ± 3 | 89 ± 3 |
| HR, beats/min | 446 ± 15 | 444 ± 14 | 447 ± 14 | 426 ± 8 | 423 ± 10 | 426 ± 10 |
| Signal-to-noise ratio for RSNA | 3.9 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.8 ± 0.3 | 3.9 ± 0.4 | 3.9 ± 0.5 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate; RSNA, renal sympathetic nerve activity. There were no significant differences between those values (P > 0.05).
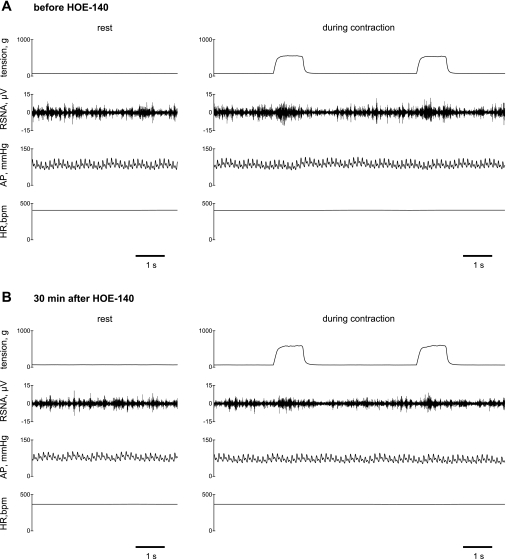
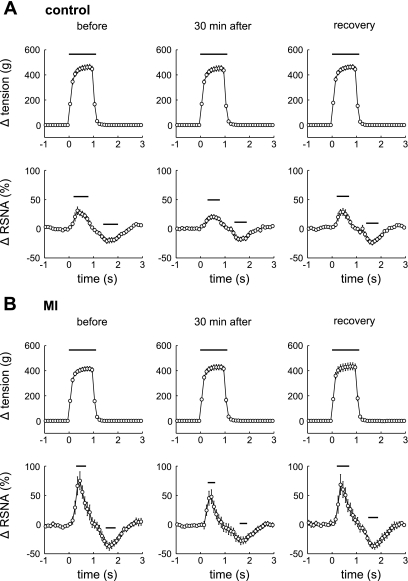
The representative recording for a MI rat (FS = 24%) before and 30 min after injection of HOE-140 presented in Fig. 1 shows that the RSNA increased as tension was developed during 1 min of intermittent contractions. The results after the normalization of RSNA during a stimulation-relaxation cycle averaged over 12 interventions of muscle (Fig. 2) show that muscle contraction significantly increased RSNA in both control healthy and MI groups, irrespective of the administration of HOE-140. The RSNA responses to contraction occurred rapidly. The increase in RSNA was then inhibited rapidly. Significant differences between the basal value and the overshoot of the decrease in the RSNA followed by muscle contraction were observed. During 4-s bouts of muscle relaxations between contractions, the RSNA returned toward baseline levels in both control healthy and MI groups (Fig. 2). The relative changes in arterial pressure and HR during contractions were of small magnitude (Fig. 1). An intermittent contraction did not significantly affect the average mean arterial pressure or HR because this contraction paradigm induced variable patterns of the cardiovascular changes in different animals. The characteristics of these neural and cardiovascular dynamics seen during the intermittent contraction are consistent with previous reports in rats and cats (11, 12, 43).
Fig. 1.
Typical recordings of muscle tension developed within the triceps surae muscles, renal sympathetic nerve activity (RSNA), arterial pressure (AP), and heart rate [HR, in beats/min (bpm)] at rest (for 5 s) and during 1 min of intermittent (1 to 4 s stimulation to relaxation) static muscle contraction (for 10 s) in a myocardial infarction (MI) rat [fractional shortening (FS) = 24%] before (A) and 30 min after (B) intra-arterial injection of HOE-140 into the hindlimb circulation. Data during 3rd and 4th of 12 repeated contractions are presented in A and B. RSNA increased as tension was developed.
Fig. 2.
Changes from baseline in muscle tension and RSNA (averaged over every 100 ms) during a cycle averaged over 12 interventions of muscle contraction in 16 controls with 49 ± 2% of FS (A) and 16 MI rats with 20 ± 2% of FS (B) before and 30 min after injection of HOE-140 (2 μg/kg) as well as recovery (60 min after injection). Values are means ± SE. Time = 0 indicates the onset of tension development. The changes in these values from baseline were evaluated with each basal value averaged for 30 s just before the first contraction. Horizontal bars indicate significant differences in the responses from baseline, detected by Dunnett post hoc test following 1-way repeated ANOVA.
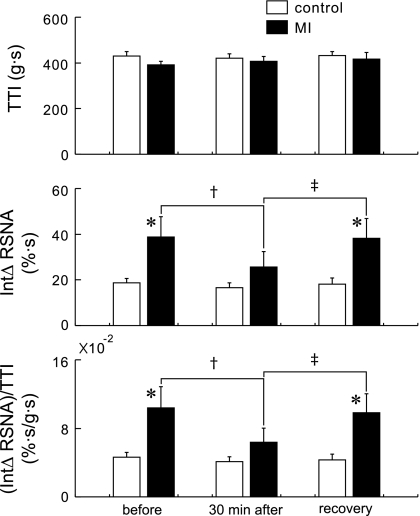
There was no significant difference in the TTI between the groups or between the protocols (+430 ± 20 vs. +391 ± 15 g·s, control vs. MI rats before injection; +420 ± 20 vs. +408 ± 20 g·s, 30 min after injection; and +432 ± 17 vs. +417 ± 29 g·s, 60 min after injection) (Fig. 3). Before the injection of HOE-140, the integrated ΔRSNA in the MI rats [+39 ± 9% s−1 (integrated RSNA over time)] was significantly larger than that in the controls (+19 ± 2% s−1). Thirty minutes after injection of HOE-140, the integrated ΔRSNA in the MI rats (+26 ± 7% s−1) was significantly reduced compared with that before injection; on the other hand, this reduction was not observed in the controls (+17 ± 2% s−1). Sixty minutes after injection, the integrated ΔRSNA were similar to the ones noted before injection (controls, +18 ± 3% s−1; and MIs, +38 ± 9% s−1). The integrated ΔRSNA per TTI was significantly larger in the MI rats [+10 ± 2 (×10−2)% s−1/g·s] than that in the controls before injection of HOE-140 [+5 ± 1 (×10−2)% s−1/g·s], and this injection (30 min after) significantly attenuated the integrated ΔRSNA per TTI in the MIs [+6 ± 2 (×10−2)% s−1/g·s] but not in the healthy controls [+4 ± 1 (×10−2)% s−1/g·s]. Sixty minutes after injection, the integrated ΔRSNA per TTI in the MIs recovered to the level seen before injection [10 ± 2 (×10−2)% s−1/g·s]. In the control rats, the integrated ΔRSNA per TTI was +4 ± 1 (×10−2)% s−1/g·s 60 min after injection.
Fig. 3.
Tension-time index (TTI), integrated (Int) ΔRSNA, and the integrated ΔRSNA per TTI before and 30 min after intra-arterial injection of HOE-140 as well as recovery (60 min after injection). Values are means ± SE. *P < 0.05 between 16 controls and 16 MI rats. †P < 0.05 between before and 30 min after injection of HOE 140. ‡P < 0.05 between 30 min after injection and recovery. The significances were detected by Tukey post hoc test following 2-way repeated ANOVA.
DISCUSSION
This study demonstrates that intra-arterial injection of the kinin B2 receptor blocker, HOE-140, into the hindlimb circulation reduced RSNA responses to muscle contraction in rats with CHF but not in control rats. The data presented in this report suggest that the activation of bradykinin receptors on muscle afferents during contraction is part of the exaggerated muscle reflex seen in CHF.
An increase in bradykinin during muscle contraction (15, 32, 33, 40) stimulates muscle afferents and sensitizes afferents responding to contraction, thereby contributing to autonomic function during exercise (3, 8, 19, 28, 41, 42). The muscle reflex engaged during contraction comprises the muscle mechanoreflex and metaboreflex (5, 7). Intermittent muscle contraction employed in the present experiment is considered to mainly stimulate muscle mechanoreceptors because 1) the RSNA response was synchronized with tension during the bouts of contraction and 2) RSNA responded rapidly at the onset of muscle tension development (11, 12, 43). Based on these data, it is well reasoned that the sensitizing effect of bradykinin on mechanosensitive muscle afferents was prevented by HOE-140 injection. In turn, the exaggerated RSNA responses induced by the mechanoreflex were significantly attenuated in the MI rats.
Important issues regarding the muscle reflex regulation of the cardiorespiratory system during exercise in CHF include whether muscle mechanoreceptors and/or metaboreceptors stimulation exaggerate the muscle reflex responses in this disease [see the debate between Middlekauff and Sinoway (23) and Piepoli and Coats (30)]. Our standpoint is that the muscle mechanoreflex, but not metaboreflex, explains the exaggerated muscle reflex. Data collected in our and other's laboratories suggest that muscle mechanoreflex is sensitized, whereas muscle metaboreflex is desensitized (17, 21, 22, 24, 35, 37–39). Thus results of the present report suggest that HOE-140 is capable of reducing the muscle reflex responses in CHF through an effect on mechanosensitive muscle afferents. The present experiments showed that the RSNA during bouts of muscle relaxations between contractions were at the baseline level in both the control and MI rats (Fig. 2), suggesting that the effects of muscle metaboreceptor activation are unlikely to result in the excess increase in sympathetic nerve activity in response to contraction in CHF. Nevertheless, the effects of metaboreflex activation during contraction are difficult to be ruled out from those of mechanoreflex activation. Regarding the muscle metaboreflex regulation in CHF, it is noted that a work by Piepoli and coworkers (29, 33) shows that the ventilatory as well as pressor responses mediated by metaboreflex activation are exaggerated in CHF.
The possible factors by which bradykinin sensitizes muscle afferents responding to contraction more in CHF may include the upregulation of kinin B2 receptors located on muscle afferents in CHF. However, previous reports provide data that may refute this hypothesis. In heart tissues obtained from CHF patients (13) and rats with heart failure induced after pressure overload hypertrophy (14), a downregulation of B2 receptors has been observed. Nevertheless, it is not clear whether these findings observed in the heart tissues are applicable to plastic changes in kinin receptors on muscle afferents of CHF. This issue will need to be tested in future investigations. Another possible factor is that more bradykinin accumulates during muscle contraction in CHF. The decrease in intracellular pH in the exercising limb and the increase in venous lactic acid levels during exercise are greater in CHF patients than in healthy subjects (33, 34). Of note, the extent of the release of bradykinin from contacting skeletal muscle in cats has been shown to be correlated with the magnitude of the decrease in venous pH and the increase in venous lactate, respectively (40). In human studies (32, 33), it was reported that bradykinin concentration in the blood draining skeletal muscle is higher during exercise as well as at rest in CHF patients than that in healthy subjects.
Wang and colleagues (44–46) have investigated the role for bradykinin in the activation of cardiac sympathetic afferent reflex in CHF. They have shown that RSNA responses as well as of cardiac sympathetic afferent responses to the epicardial application of bradykinin are greater in the CHF dog model than they are in healthy control dogs. The exaggerated reflex response to bradykinin in CHF was reduced by indomethacin, which inhibits the cyclooxygenase enzyme and reduces the production of prostaglandins. Prostaglandins increase bradykinin responses (19, 27). Of note, we (10) as well as others (20) have demonstrated that cyclooxygenase inhibition reduces the exaggerated muscle reflex response during contraction in CHF. Prostaglandins are produced by muscle contraction (5, 7). Prostaglandins might mediate the bradykinin-mediated muscle reflex response in CHF observed in the present experiment.
Exercise training therapy in CHF patients can relieve its symptoms such as fatigue and dyspnea, improve exercise capacity and quality of life, as well as reduce hospitalization and, to some extent, risk of mortality (1). Improved cardiovascular regulation seen in endothelial function (4) and sympathetic activation (31) by exercise training in CHF has been reported. Furthermore, the muscle reflex regulation of the cardiorespiratory system during exercise was shown to be altered by training in CHF patients (29). It will be of importance to determine whether the mechanisms found in the present experiments, namely, kinin receptor-exaggerated muscle reflex in heart failure, can be improved by exercise training.
Limitations.
Several issues need to be considered as a study limitation. First, although we attempted to trap HOE-140 within the left hindlimb by occluding the left femoral vein during and after infusion of the injectate, it was difficult to conclude that the effect of the injectate was local on the muscle afferents but not systemic. Nevertheless, the effect of this procedure, intra-arterial injection, on the muscle reflex response has been shown to be different from that of intravenous injection which is systemic (9). It is suggested that the effect of the injectate is mainly localized within the hindlimb muscles with the procedure used in the previous (9, 39) and present studies. Second, it is not clear whether bradykinin in the muscle was more in the MI rats than that in the control rats. Nevertheless, it has been shown that bradykinin in resting and contracting skeletal muscle was more in CHF patients than that in healthy subjects (32, 33). Third, HOE-140 did not abolish the RSNA response to contraction in rats, suggesting that substances besides bradykinin, such as lactic acid, ATP, and arachidonic acid metabolites (5, 7, 35), were also likely to play a role in activating the muscle reflex. It is considered that these substances work synergistically on populations of muscle afferents (6).
In conclusion, the data presented in this report show that kinin B2 receptor blockade reduces the muscle contraction-induced sympathetic nerve response in rats with CHF but not in healthy control rats. We suggest that bradykinin is part of the exaggerated muscle reflex in CHF, thereby augmenting the sympathoexcitation seen during exercise in this disease.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-075533 and HL-078866 (to J. Li) and HL-060800 (to L. I. Sinoway) and American Heart Association Beginning Grant-in-Aid 0865416D (to S. Koba).
DISCLOSURES
There are no conflicts of interest to disclosure.
ACKNOWLEDGMENTS
We thank Dr. Marc Kaufman for encouragement of this project and Jennie Stoner for secretarial help.
Present affiliation of S. Koba: Division of Integrative Physiology, Tottori University Faculty of Medicine.
REFERENCES
- 1.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6: 292–300, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alteration of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Franz M, Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res 92: 369–383, 1975 [DOI] [PubMed] [Google Scholar]
- 4.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation 93: 210–214, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447 [Google Scholar]
- 6.Kaufman MP, Hayes SG. Receptor synergy from thin fiber muscle afferents. Focus on “Dorsak root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1”. J Neurophysiol 100: 1169–1170, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibres with endings in skeletal muscle. Circ Res 50: 133–139, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Koba S, Gao Z, Sinoway LI. Oxidative stress and the muscle reflex in heart failure. J Physiol 587: 5227–5237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koba S, Xing J, Sinoway LI, Li J. Cyclooxygenase products contribute to enhanced muscle reflex in heart failure (Abstract). FASEB J 22: 952.–16., 2008 [Google Scholar]
- 11.Koba S, Xing J, Sinoway LI, Li J. Differential sympathetic outflow elicited by active muscle in rats. Am J Physiol Heart Circ Physiol 293: H2335–H2343, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H311–H321, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kuoppala A, Shiota N, Kokkonen JO, Liesmaa I, Mäyränpää M, Kovanen PT, Lindstedt KA. Down-regulation of cardiprotective bradykinin type-2 receptors in the left ventricle of patients with end-stage heart failure. J Am Coll Cardiol 40: 119–125, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kuoppala A, Shiota N, Lindstedt KA, Rysa J, Leskinen HK, Luodonpaa M, Liesmaa I, Ruskoaho H, Kaaja R, Kovanen PT, Kokkonen JO. Expression of bradykinin receptors in the left ventricles of rats with pressure overload hypertrophy and heart failure. J Hypertens 21: 1729–1736, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Langberg H, Bjørn C, Boushel R, Hellsten Y, Kjær M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol 542: 977–983, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and mataboreflex responses after myocardial infarctions in rats. Circulation 110: 3049–3054, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol 18: 187–195, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E2. Brain Res 225: 95–105, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, MacLellan WR, Hage A, Moriguchi J, Patel J. Cyclooxygenase products sensitize muscle mechanoreceptors in humans with heart failure. Am J Physiol Heart Circ Physiol 294: H1956–H1962, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, MacLellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol 287: H1937–H1943, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Middlekauff HR, Sinoway LI. Increased mechanoreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol 102: 492–494, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Momen A, Bower D, Boehmer J, Kunselman AR, Leuenberger UA, Sinoway LI. Renal blood flow in heart failure patients during exercise. Am J Physiol Heart Circ Physiol 287: H2834–H2839, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Murai H, Takamura M, Maruyama M, Nakano M, Ikeda T, Kobayashi D, Otowa K, Ootsuji H, Okajima M, Furusho H, Takata S, Kaneko S. Altered firing pattern of single-unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J Physiol 587: 2613–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negrão CE, Rondon MU, Tinucci T, Alves MJ, Roveda F, Braga AM, Reis SF, Nastari L, Barretto AC, Krieger EM, Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure sensitivity. Am J Physiol Heart Circ Physiol 280: H1286–H1292, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Nerdrum T, Baker DG, Coleridge HM, Coleiridge JC. Interaction of bradykinin and prostaglandin E1 on cardiac pressor reflex and sympathetic afferents. Am J Physiol Regul Integr Comp Physiol 250: R815–R822, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75: 2061–2068, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilator responses to exercise in patients with chronic heart failure: effect of physical training. Circulation 93: 940–952, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Piepoli MF, Coats AJ. Increased metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol 102: 494–497, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Scott AC, Wensel R, Davos CH, Georgiadou P, Ceri Davies L, Coats AJ, Francis DP, Piepoli MF. Putative contribution of prostaglandin and bradykinin to muscle reflex hyperactivity in patients on Ace-inhibitor therapy for chronic heart failure. Eur Heart J 25: 1806–1813, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ, Piepoli MF. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation 106: 214–220, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol 84: 1551–1559, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Sinoway LI, Zelis R. The peripheral circulation. Cardiol Clin 7: 63–71, 1989 [PubMed] [Google Scholar]
- 37.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Smith SA, Mammen PPA, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Smith SA, Mitchell JH, Naseem H, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 69: 1225–1230, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Stebbins CL, Longhurst JC. Bradykinin in reflex cardiovascular responses to static muscular contraction. J Appl Physiol 61: 271–279, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Stebbins CL, Longhurst JC. Bradykinin-induced chemoreflexes from skeletal muscle: implications for the exercise pressor reflex. J Appl Physiol 59: 56–63, 1985 [DOI] [PubMed] [Google Scholar]
- 43.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic nerve activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64: 592–599, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Wang W. Cardiac sympathetic afferent stimulation by bradykinin in heart failure: role of NO and prostaglandins. Am J Physiol Heart Circ Physiol 275: H783–H788, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Schultz HD, Ma R. Cardiac sympathetic afferent sensitivity is enhanced in heart failure. Am J Physiol Heart Circ Physiol 277: H812–H817, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Zucker IH. Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol Regul Integr Comp Physiol 271: R751–R756, 1996. [DOI] [PubMed] [Google Scholar]