Abstract
The female sex has been associated with improved myocardial salvage after ischemia and reperfusion (I/R). Estrogen, specifically 17β-estradiol, has been demonstrated to mediate this phenomenon by limiting cardiomyocyte apoptosis. We sought to quantitatively assess the effect of sex, ovarian hormone loss, and I/R on myocardial Bax, Bcl-2, and apoptosis repressor with caspase recruitment domain (ARC) expression. Male (n = 48), female (n = 26), and oophorectomized female (n = 20) rabbits underwent 30 min of regional ischemia and 3 h of reperfusion. The myocardial area at risk and infarct size were determined using a double-staining technique and planimetry. In situ oligo ligation was used to assess apoptotic cell death. Western blot analysis was used to determine proapoptotic (Bax) and antiapoptotic (Bcl-2 and ARC) protein levels in all three ischemic groups and, additionally, in three nonischemic groups. Infarct size (43.7 ± 3.2%) and apoptotic cell death (0.51 ± 0.10%) were significantly attenuated in females compared with males (56.4 ± 1.6%, P < 0.01, and 4.29 ± 0.95%, P < 0.01) and oophorectomized females (55.7 ± 3.4%, P < 0.05, and 4.36 ± 0.51%, P < 0.01). Females expressed significantly higher baseline ARC levels (3.62 ± 0.29) compared with males (1.78 ± 0.18, P < 0.01) and oophorectomized females (1.08 ± 0.26, P < 0.01). Males expressed a significantly higher baseline Bax-to-Bcl-2 ratio (4.32 ± 0.99) compared with females (0.65 ± 0.13, P < 0.01) and oophorectomized females (0.42 ± 0.10, P < 0.01). I/R significantly reduced Bax-to-Bcl-2 ratios in males. In all other groups, ARC levels and Bax-to-Bcl-2 ratios did not significantly change. These results support the conclusion that in females, endogenous estrogen limits I/R-induced cardiomyocyte apoptosis by producing a baseline antiapoptotic profile, which is associated with estrogen-dependent high constitutive myocardial ARC expression.
Keywords: 17β-estradiol, cardiomyocyte apoptosis, apoptosis repressor with caspase recruitment domain, Bcl-2, Bax
a growing body of evidence over the past decade has implicated the female sex as a protective factor against myocardial ischemia-reperfusion (I/R) injury. Specifically, estrogen has been demonstrated to limit I/R-induced cardiomyocyte apoptosis. In humans, myocardial salvage achieved by primary percutaneous coronary intervention for acute myocardial infarction (MI) is greater in women compared with men (25). In perfused animal heart models of MI, (premenopausal) females showed smaller infarct sizes compared with their male counterparts (1, 14). Furthermore, different in vivo animal models of MI have shown that physiological replacement of or treatment with biologically active 17β-estradiol (E2) reduced infarct size (2–4, 12, 19, 31, 34, 37) and apoptosis (31, 37). The mechanisms underlying these cardioprotective effects remain to be fully elucidated.
Myocardial I/R injury is associated with an extensive loss of cardiomyocytes (5, 8, 33). Although acute myocardial I/R initiates cardiomyocyte cell death by both necrosis (uncontrolled cell death) and apoptosis (programmed cell death) (5, 8, 23, 33), the latter has been shown to play a more important role than previously recognized (5, 8, 33).
Injurious stimuli can trigger myocardial apoptosis by activating pathways intrinsic to the cell itself and/or via receptors extrinsic to the cell (5, 8, 33). Bax and Bcl-2 are two apoptotic proteins that are part of the intrinsic or mitochondrial apoptotic pathway (9, 33). They are both members of the Bcl-2 protein family, but they have opposite effects. Bax is proapoptotic, whereas Bcl-2 is antiapoptotic (9, 33). The apoptosis repressor with caspase recruitment domain (ARC) is a protein that is constitutively expressed in heart and skeletal muscle cells (18) and has been demonstrated to protect the heart against I/R injury by inhibiting both the intrinsic and extrinsic apoptotic pathways (10, 11, 33). ARC interferes with the activation of the mitochondrial death pathway through an interaction with Bax (11) and by blocking cytochrome c release from mitochondria (6). ARC interferes with the activation of the death receptor pathway through an interaction with caspase-2 and caspase-8 (18). Although estrogen is known to limit cardiomyocyte apoptosis, a relationship between sex or estrogen and myocardial expression of the powerful apoptosis inhibitor ARC has never been described.
The goals of this study were twofold. With our in vivo rabbit I/R protocol, we first sought to demonstrate the protective effect of the female sex on myocardial infarct size and cardiomyocyte apoptosis. Second, we sought to elucidate part of the antiapoptotic mechanism by quantatively assessing the influence of sex, ovarian hormone loss, and I/R on myocardial Bax, Bcl-2, and ARC expression.
MATERIALS AND METHODS
Animal care and use.
Animals were treated under experimental protocols approved by the University of Pennsylvania's Institutional Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Pub. No. 85-23, Revised 1996). A total of 119 mature male, female, and oophorectomized female New Zealand White rabbits (weighing 2.8–4.0 kg) were acquired (Covance, Denver, PA) and used in this study. Ninety-four rabbits (48 male, 26 female, and 20 oophorectomized females) were assigned to the ischemic group and underwent the I/R experimental protocol described below. Twenty-five additional rabbits (10 male, 10 female, and 5 oophorectomized females) were assigned to the nonischemic group and were used to assess constitutive (i.e., preischemia) apoptotic markers.
Oophorectomy protocol.
Sexually mature 5- to 6-mo-old oophorectomized female New Zealand White rabbits (weighing 2.8–4.0 kg) were acquired from Covance. Under general anesthesia [buprenorphine (0.05 mg/kg sc), ketamine (50 mg/kg), and xylazine (10 mg/kg im)], rabbits underwent a laparotomy and bilateral oophorectomy. After the oophorectomy, rabbits were allowed to recover for 2 wk.
E2 plasma levels.
E2 plasma levels were measured in all animals before surgery. Plasma samples (1 ml) were analyzed by Anilytics (Gaithersburg, MD).
I/R surgical protocol.
Rabbits were sedated and induced with intramuscular ketamine (50 mg/kg), glycopyrrolate (0.2 mg/kg), and buprenorphine (0.05 mg/kg). After intubation with a 3-0 endotracheal tube, rabbits were ventilated with a mechanical respirator (model AWS, Hallowell EMC, Pittsfield, MA) using room air enriched with 0.6 l/min oxygen. Fluid-filled catheters were introduced into a small auricular artery and vein and into the right jugular vein for the continuous measurement of blood pressure and the administration of intravenous medications. Anesthesia was maintained with a continuous intravenous infusion of ketamine (0.02–0.04 mg·kg−1·min−1) and supplemental pentothal (2.5–5 mg/kg) as needed. Additionally, a high-fidelity pressure transducer (SPR-524, Millar Instruments, Houston, TX) was introduced through the right carotid artery into the left ventricle (LV).
After a left thoracotomy into the fourth intercostal space had been performed, the heart was exposed and the pericardium incised. A coronary snare was constructed by passing a pledgeted suture (4-0 silk, U.S. Surgical, Norwalk, CT) around a large branch of the circumflex coronary artery at ∼50% of the distance from the base to the apex of the heart and threaded through a small piece of polyethylene tubing. After this surgical preparation, animals were allowed to stabilize for 15 min.
A hyperthermia/hypothermia unit (Medi-Therm III, Gaymar Industries, Orchard Park, NY) was used to maintain the animal's core temperature between 39.0 and 40.5°C in rabbits. Arterial blood gases were measured in all animals, and pH was maintained between 7.40 and 7.45 throughout the protocol.
After instrumentation and baseline hemodynamic and echocardiographic data had been recorded, heparin (500 U/kg) and a prophylactic intravenous antiarrhythmic regimen of magnesium sulfate (100 mg bolus), lidocaine (2 mg bolus followed by 0.1 mg·kg−1·min−1), and amiodarone (6.7 μg/kg/min) were administered. Next, coronary snares were tightened to produce an ischemic region of the LV. Ischemia was confirmed by a visible color change in the ischemic myocardial region, ST elevations on the ECG, and regional wall motion abnormalities on the ECG. At the end of the 30-min ischemic period, the coronary snares were loosened, and the previously ischemic myocardium was reperfused for 3 h. The reperfused myocardium typically exhibited a visible hyperemic response. Hemodynamic and ECG measurements were again recorded after 15 min of ischemia and after 10 and 180 min of reperfusion.
Analysis of area at risk and infarct size.
At the end of the reperfusion period, the coronary snare was reapplied, vascular clamps were used to occlude the aorta, pulmonary artery, and inferior vena cava, and the right atrium was incised. Five milliliters of 1% Evans blue dye (Sigma-Aldrich, St. Louis, MO) was injected into the left atrium to delineate the myocardial area at risk (AAR). This was followed by a 20-meq intra-atrial bolus of potassium chloride to arrest the heart. The heart was explanted, and the LV was isolated and fixed in a 20% gelatin solution (laboratory grade 275 bloom, Fisher, Fair Lawn, NJ) for 20 min. After fixation, the LV was sectioned perpendicular to its long axis into eight 2- to 3-mm transverse slices. The thickness of each slice was measured with a digital micrometer, and all slices were photographed. The infarct area was delineated by photographing and measuring the slices after 20 min of incubation in 2% triphenyltetrazolium chloride (TTC; Sigma-Aldrich) at 37°C. All photographs were imported into an image-analysis program (Image Pro Plus, MediaCybernetics, Silver Spring, MD), and computer-assisted planimetry was performed. The AAR is expressed as a percentage of the entire LV, and the infarct size is expressed as a percentage of the AAR.
No-ischemia surgical protocol.
Nonischemic animals were prepared and anesthetized as described in the I/R surgical protocol. After a left thoracotomy into the fourth intercostal space had been performed, the heart was exposed and the pericardium was incised. Vascular clamps were used to occlude the aorta, pulmonary artery, and inferior vena cava, and the right atrium was incised. This was followed by a 20-meq intra-atrial bolus of potassium chloride to arrest the heart. The heart was explanted, and the LV was isolated and fixed in a 20% gelatin solution (laboratory grade 275 bloom, Fisher) for 20 min. A 1- to 2-mm transmural specimen was removed from the LV, snap frozen in liquid nitrogen, and stored at −80°C.
Temperature and hemodynamic measurements.
Arterial blood pressure, LV pressure (LVP), heart rate (HR), surface ECG, and core temperature were continuously monitored (model 78534C, Hewlett-Packard, Palo Alto, CA) throughout the protocol in all animals. Hemodynamics, HR, and core temperature were recorded at baseline, after 15 min of ischemia, and after 10 and 180 min of reperfusion (Sonometrics, London, ON, Canada). From these data, the maximal rate of LVP increase over time (dP/dtmax), rate-pressure product (RPP), and LV end-diastolic pressure (LVEDP) were calculated for each time point in all rabbits. The RPP is a sensitive index of myocardial O2 consumption and was calculated by multiplying HR by systolic blood pressure at all time points (40).
ECG measurements.
Quantitative, two-dimensional, open-chest ECGs were performed at baseline, after 15 min of ischemia, and after 10 and 180 min of reperfusion in all animals. Images were recorded on a Sonos 7500 ultrasound system using a 12-MHz transducer (Philips, Andover, MA) with a custom-made offset device and recorded on a 0.5-in. VHS videotape at 30 Hz (Panasonic AG-6300 VHS Recorder). The transducer was placed at the cardiac apex, and two orthogonal long-axis views were recorded. LV end-systolic volume (LVESV) and LV end-diastolic volume (LVEDV) were calculated using Simpson's rule. The ejection fraction and stroke volume were calculated with LVESV and LVEDV. Cardiac output was calculated with HR and stroke volume.
Tissue preparation.
The entire AAR from each LV slice was excised. A 1- to 2-mm transmural specimen was removed from the AAR, snap frozen in liquid nitrogen, and stored at −80°C. The remainder of the AAR was fixed for 24 h in 10% formalin and subsequently embedded in paraffin.
Preliminary work in our laboratory has shown that TTC staining influenced subsequent protein expression measurements (Western blot analysis), but in situ oligo ligation (ISOL) assays were unaffected (unpublished observations). Evans blue staining was not found to affect either measurements. TTC staining after I/R was avoided in 10 males, 10 females, and 10 oophorectomized females. The corresponding segment of the AAR of these rabbits was used for protein expression measurements.
ISOL assay.
For the identification of apoptotic cells, an ISOL assay (Intergen, Purchase, NY) with a high specificity for staining the specific DNA fragmentation characteristic of apoptosis was selected (20). The ISOL assay produces significantly fewer false positive results compared with the more commonly used TUNEL assay (20). The ISOL assay uses T4 DNA ligase to bind synthetic biotinylated oligonucleotides to 3′-dT overhangs. Paraffin-embedded tissue was sectioned into 5-μm slices and deparaffinized by three changes of xylene followed by three changes of absolute ethanol. Subsequently, endogenous peroxidase was quenched in 3% hydrogen peroxide in PBS. After the tissue sections had been washed, they were treated with 20 μg/ml proteinase K in PBS, washed again, and placed in an equilibration buffer. Next, a solution of T4 DNA ligase and oligonucleotides was applied to the slides and incubated overnight at 16–22°C. ApopTag detection of ligated oligonucleotides was accomplished by applying a streptavidin-peroxidase conjugate that was developed with diaminobenzidine. Finally, tissue sections were counterstained in hematoxylin.
Entire tissue sections were digitalized using a scanning microscope and analyzed using an image-analysis software package (Image Pro Plus, MediaCybernetics). ISOL-positive and ISOL-negative nuclei were counted in the AAR. Results are expressed as an apoptotic index, which is defined as the percentage of ISOL-positive cells per total number of cells in the entire AAR.
Western blot analysis.
Myocardial Bax, Bcl-2, and ARC expression levels were determined using the Western blot technique. The levels of these apoptotic proteins were determined in the myocardium from the AAR of animals subjected to I/R and in the corresponding segment of the LV of animals not subjected to I/R.
Frozen tissue sections (0.5–1.0 g) were minced on dry ice before being placed in ice-cold lysis buffer (10 mM HEPES, 150 mM NaCl, 1% CHAPS, and 0.2 mM DTT) supplemented with Halt protease inhibitor cocktail (PI-78410, Fisher Scientific, Pierce, Rockford, IL, 1:100 dilution), Halt phosphatase inhibitor cocktail (PI-78420, Fisher Scientific, Pierce, 1:100 dilution), and PMSF (P7626, Sigma-Aldrich). The minced tissue was homogenized for 20 s and placed on dry ice for 15 s; the process was repeated as necessary. After being mixed at 4°C for 20 min, the homogenate was centrifuged at 1,000 g at 4°C for 20 min. The supernatant was collected, and the protein concentration was determined using the bicinchoninic acid protein assay kit (Fisher Scientific, Pierce).
Equal amounts of protein (40 μg) were separated on a 10% bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene fluoride membrane (Invitrogen). Membranes were blocked in 5% nonfat dried milk in Tween 20-Tris-buffered saline (TTBS; 25 mM Tris·Cl, 137 mM NaCl, 2.7 mM KCl, and 0.05% Tween 20) for 1–2 h at room temperature with constant agitation. Primary antibody was then diluted in fresh blocking buffer and allowed to incubate overnight at 4°C with shaking. The following primary polyclonal antibodies were used: Bax (no. 2772, Cell Signaling Technology, Danvers, MA, 1:500 dilution), Bcl-2 (N-19, SC-492, Santa Cruz Biotechnology, Santa Cruz, CA, 1:300 dilution), and ARC (H-150, SC-11435, Santa Cruz Biotechnology, 1:200 dilution); GAPDH (L-20, SC-31915, Santa Cruz Biotechnology, 1:200 dilution) was used as a loading control. The membrane was then washed four times in TTBS. Membranes were then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (NA934, Amersham Biosciences, Piscataway, NJ, 1:5,000 dilution), washed four times, and developed with electrochemiluminescence Western blot detection reagents (Amersham Biosciences). The integrated optical density (IOD) of each band was determined using Image Pro Plus (MediaCybernetics). The IOD of each apoptotic protein band was normalized to the IOD of the loading control band and expressed in arbitrary units.
Statistics.
Measurements are reported as means ± SE. One-way ANOVA with a post hoc Tukey “honestly significantly different” test was used for all comparisons among groups. Repeated-measures ANOVA with a post hoc Bonferroni correction was used for all comparisons within groups. The software package that was used for statistical analysis was SPSS version 16.0 (SPSS, Chicago, IL). Statistically significant differences were established at P values of <0.05.
RESULTS
E2 plasma levels.
The E2 plasma level was significantly higher in females (15.6 ± 2.6 pg/ml) compared with males (2.1 ± 0.8 pg/ml, P < 0.01) and oophorectomized females (5.9 ± 1.2 pg/ml, P < 0.05). The difference between males and oophorectomized females was nonsignificant.
Arterial blood gas analysis.
Baseline arterial blood gas values were equivalent in all groups. pH was 7.40–7.45, arterial Pco2 was 40.0–45.0 mmHg, arterial Po2 was 475–490 mmHg, HCO3− was 23–26 mmol/l, base excess was −3.0 to 3.0, and O2 saturation was 98–100%. Measurements remained within these ranges in all groups during ischemia and subsequent reperfusion.
AAR and infarct size measurements.
The procedural mortality for each group that underwent I/R was 0%. The size of the AAR expressed as a percentage of the LV mass was similar among males, females, and oophorectomized females (Fig. 1). Myocardial infarct size expressed as a percentage of the AAR was significantly smaller in females compared with males and oophorectomized females. There was no significant difference in infarct size between males and oophorectomized females.
Fig. 1.
Area at risk (AAR) expressed as a percentage of the left ventricle (LV) and infarct size (I) expressed as a percentage of the AAR in all ischemic groups (males: n = 38, females: n = 16, and oophorectomized females: n = 10). Values are means ± SE. Error bars represent SE. *P < 0.01 vs. males and P < 0.05 vs. oophorectomized females.
Temperature and hemodynamic parameters.
Core temperatures were equivalent in all groups throughout the protocol (39.0–40.5°C). At baseline and after 3 h of reperfusion, HRs were significantly lower and mean arterial pressure (MAP) was significantly higher in females and oophorectomized females compared with males (Table 1). I/R lowered HRs and MAP in all groups. Compared with baseline, males and females showed significantly lower HRs and MAP after 3 h of reperfusion, whereas the difference was nonsignificant in oophorectomized females. At baseline, LV systolic function (contractility; +dP/dtmax) was significantly higher in females compared with males and oophorectomized females. Differences in contractility between males and females persisted throughout I/R. I/R decreased contractility in all groups. Males showed a significantly lower contractility after 3 h of reperfusion compared with baseline. LVEDP and RPP (a sensitive index of myocardial O2 consumption) did not differ among groups at any time point. I/R increased LVEDP and decreased RPP in all groups. Males showed a significantly higher LVEDP after 3 h of reperfusion compared with baseline, and females showed a significantly lower RPP after 3 h of reperfusion compared with baseline.
Table 1.
Hemodynamic parameters
| Reperfusion |
||||
|---|---|---|---|---|
| Parameter | Baseline | Ischemia (15 min) | 10 min | 180 min |
| HR, beats/min | ||||
| Males | 268 ± 4 | 259 ± 4 | 250 ± 4‡ | 246 ± 3‡ |
| Females | 243 ± 6* | 253 ± 7 | 250 ± 9 | 224 ± 3*‡ |
| Oophorectomized females | 245 ± 10* | 236 ± 5* | 241 ± 8 | 227 ± 7* |
| MAP, mmHg | ||||
| Males | 63 ± 2 | 54 ± 2‡ | 54 ± 2‡ | 49 ± 2‡ |
| Females | 77 ± 3* | 67 ± 3*‡ | 62 ± 4‡ | 58 ± 2*‡ |
| Oophorectomized females | 75 ± 4* | 61 ± 3 | 64 ± 3‡ | 63 ± 3* |
| LVEDP, mmHg | ||||
| Males | 2.2 ± 0.4 | 5.9 ± 0.9‡ | 5.2 ± 0.6‡ | 4.9 ± 0.8‡ |
| Females | 2.7 ± 0.5 | 3.8 ± 0.8 | 4.2 ± 0.8 | 3.8 ± 0.9 |
| Oophorectomized females | 2.4 ± 0.5 | 5.6 ± 0.9‡ | 3.8 ± 0.4 | 3.6 ± 0.5 |
| +dP/dtmax, mmHg/s | ||||
| Males | 1977 ± 63 | 1636 ± 69‡ | 1601 ± 84‡ | 1415 ± 72‡ |
| Females | 2367 ± 137* | 2235 ± 89* | 2087 ± 186* | 1986 ± 168* |
| Oophorectomized females | 1734 ± 68† | 1544 ± 70† | 1593 ± 95 | 1583 ± 142 |
| RPP/1,000, mmHg·beats·min−1 | ||||
| Males | 20.5 ± 0.6 | 17.0 ± 0.6‡ | 16.7 ± 0.6‡ | 18.2 ± 2.6 |
| Females | 19.7 ± 0.9 | 17.7 ± 1.0‡ | 16.6 ± 1.3‡ | 14.6 ± 0.8‡ |
| Oophorectomized females | 19.6 ± 2.1 | 15.9 ± 0.7 | 16.2 ± 1.2 | 14.9 ± 0.8 |
Values are means ± SE. HR, heart rate; MAP, mean arterial pressure; LVEDP, left ventricular (LV) end-diastolic pressure; +dP/dtmax, maximal rate of LV pressure increase over time; RPP, rate-pressure product.
P < 0.05 vs. males at that time point;
P < 0.05 vs. females at that time point;
P < 0.05 vs. baseline.
ECG parameters.
Baseline ECGs demonstrated normal wall motion in all animals and similar LVEDV and LVESV between groups. Images obtained after coronary occlusion demonstrated a loss of anteroapical contractility in the AAR. Elevations in LVESV and LVEDV were observed in all animals during the period of coronary occlusion and subsequent reperfusion (Table 2). After 15 min of ischemia, LVEDV and LVESV were significantly higher in females and oophorectomized females compared with males. Differences in LVEDV and LVESV between males and females persisted throughout reperfusion. Fractional shortening was equivalent in all groups throughout the protocol except after 10 min of reperfusion. However, this difference did not persist after 180 min of reperfusion. Stroke volume and cardiac output were equivalent in all groups throughout the protocol. Ejection fraction was also similar among groups at each time point. Males showed a significantly lower ejection fraction after 3 h of reperfusion compared with baseline.
Table 2.
Echocardiographic parameters
| Reperfusion |
||||
|---|---|---|---|---|
| Parameter | Baseline | Ischemia (15 min) | 10 min | 180 min |
| FS, % | ||||
| Males | 29.4 ± 1.1 | 26.5 ± 1.2 | 34.0 ± 1.7 | 27.6 ± 1.5 |
| Females | 27.4 ± 1.7 | 22.3 ± 2.1 | 24.9 ± 2.2* | 22.6 ± 2.2 |
| Oophorectomized females | 28.5 ± 2.8 | 27.2 ± 3.0 | 31.2 ± 1.5 | 28.2 ± 4.3 |
| LVEDV, ml | ||||
| Males | 3.4 ± 0.1 | 4.2 ± 0.2† | 4.1 ± 0.2† | 4.1 ± 0.1† |
| Females | 3.9 ± 0.2 | 4.9 ± 0.2*† | 4.8 ± 0.2*† | 4.7 ± 0.2*† |
| Oophorectomized females | 3.7 ± 0.2 | 4.9 ± 0.2*† | 4.4 ± 0.2† | 4.3 ± 0.2† |
| LVESV, ml | ||||
| Males | 2.2 ± 0.1 | 2.9 ± 0.1† | 2.8 ± 0.1† | 2.8 ± 0.1† |
| Females | 2.5 ± 0.2 | 3.5 ± 0.1*† | 3.3 ± 0.2*† | 3.3 ± 0.1*† |
| Oophorectomized females | 2.3 ± 0.1 | 3.4 ± 0.2*† | 2.9 ± 0.1† | 2.9 ± 0.2† |
| SV, ml | ||||
| Males | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Females | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Oophorectomized females | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| EF, % | ||||
| Males | 34.0 ± 1.0 | 30.7 ± 1.1 | 30.3 ± 1.2 | 28.6 ± 0.9† |
| Females | 34.3 ± 1.3 | 28.1 ± 1.3† | 30.4 ± 1.5 | 29.6 ± 1.0 |
| Oophorectomized females | 36.4 ± 1.5 | 31.1 ± 1.4 | 34.6 ± 1.7 | 31.9 ± 2.1 |
| CO, l/min | ||||
| Males | 3.1 ± 0.2 | 3.4 ± 0.3 | 3.1 ± 0.3 | 3.0 ± 0.2 |
| Females | 3.2 ± 0.3 | 3.3 ± 0.3 | 3.5 ± 0.3 | 3.1 ± 0.2 |
| Oophorectomized females | 3.3 ± 0.2 | 3.6 ± 0.2 | 3.7 ± 0.2 | 3.1 ± 0.2 |
Values are means ± SE. FS, fractional shortening; LVEDV, LV end-diastolic volume; LVESV, LV end-systolic volume; SV, stroke volume; EF, ejection fraction; CO, cardiac output.
P < 0.05 vs. males at that time point;
P < 0.05 vs. baseline.
Myocyte apoptosis.
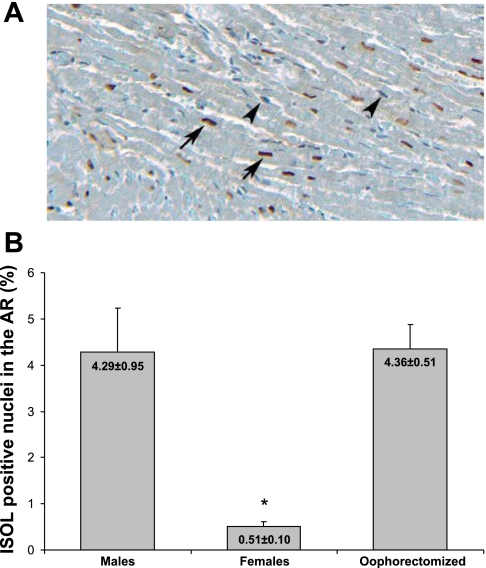
The percentage of ISOL-positive myocytes in the AAR was significantly lower in females compared with males and oophorectomized females (Fig. 2, A and B). The difference between males and oophorectomized females was nonsignificant.
Fig. 2.
In situ oligo ligation (ISOL)-positive nuclei in the AAR. A: ISOL reaction in the myocardial AAR (specimen taken from the AAR of an oophorectomized female). Original magnification: ×10. Arrows indicate dark red ISOL-positive nuclei of apoptotic cells. Arrowheads indicate blue ISOL-negative nuclei. B: comparison of apoptosis (expressed as the percentage of ISOL-positive nuclei in the myocardial AAR) among ischemic groups (n = 8 for all groups) after 30 min of myocardial ischemia and 180 min of reperfusion. Values are means ± SE. Error bars represent SE. *P < 0.01 vs. males and oophorectomized females.
Apoptotic proteins.
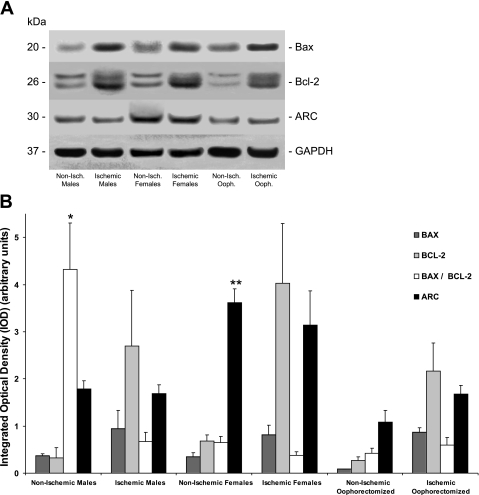
Apoptotic protein levels were determined using the Western blot technique (Fig. 3, A and B). Nonischemic females showed significantly higher constitutive myocardial ARC expression (3.62 ± 0.29) than nonischemic males (1.78 ± 0.18, P < 0.01) and nonischemic oophorectomized females (1.08 ± 0.26, P < 0.01). Both Bax and Bcl-2 levels did not significantly differ between all nonischemic groups. Nonischemic males expressed a significantly higher Bax-to-Bcl-2 ratio (4.32 ± 0.99) than nonischemic females (0.65 ± 0.13, P < 0.01) and nonischemic oophorectomized females (0.42 ± 0.10, P < 0.01).
Fig. 3.
Western blot analysis. A: representative immunoblots. B: Bax, Bcl-2, Bax/Bcl-2, and apoptosis repressor with caspase recruitment domain (ARC) levels in the AAR of the ischemic groups (n = 10 for all groups) and in the corresponding segment of the LV of the nonischemic groups (n = 10 for males and females and n = 5 for oophorectomized females) as determined by the Western blot technique. Bax-to-Bcl-2 ratios were determined for individual rabbits followed by the calculation of group means. Integrated optical density values are reported as means ± SE. Error bars represent SE. *P < 0.01 vs. Bax-to-Bcl-2 ratios in ischemic males, nonischemic females, and nonischemic oophorectomized females; **P < 0.01 vs. ARC levels in nonischemic males and nonischemic oophorectomized females.
I/R did not significantly change ARC, Bax, and Bcl-2 expression in the AAR compared with constitutive levels in all groups. I/R significantly reduced the Bax-to-Bcl-2 ratio compared with baseline in males (4.32 ± 0.99 vs. 0.67 ± 0.19, P < 0.01). Bax-to-Bcl-2 ratios did not significantly change compared with baseline in females (0.65 ± 0.13 vs. 0.38 ± 0.08, P > 0.05) and oophorectomized females (0.42 ± 0.10 vs. 0.60 ± 0.16, P > 0.05). After I/R, ARC, Bax, Bcl-2, and Bax-to-Bcl-2 levels did not differ significantly among ischemic males, females, and oophorectomized females.
DISCUSSION
Previous studies have implicated that the female sex or, more specifically, estrogen attenuates myocardial infarct size and cardiomyocyte apoptosis after I/R (1–4, 12, 14, 19, 25, 31, 34, 37). This study confirms that the female sex significantly attenuates myocardial infarct size and apoptotic cell death. Removing the main source of endogenous estrogen production in females by oophorectomy effectively reduces E2 plasma levels and abrogates the sex difference in infarct size and apoptosis. These findings strongly suggest that endogenous estrogen contributes to myocardial salvage after I/R injury in females.
The novel finding of this study is the significantly higher constitutive myocardial expression of the powerful apoptosis inhibitor ARC in females compared with males and oophorectomized females. Oophorectomy reduced E2 plasma levels and eliminated the sex difference in both constitutive ARC expression and myocardial salvage after I/R. These findings strongly suggest an association between E2 plasma levels, ARC expression, and myocardial salvage. This study was, however, not designed to definitively determine the mechanism by which E2 upregulates ARC. Future studies are necessary to fully elucidate this potentially important mechanism.
We also demonstrated that females constitutively expressed a significantly lower proapoptotic Bax-to-Bcl-2 ratio compared with males, which could have theoretically contributed to the sex-related improvement in myocardial salvage (23). However, the importance of this ratio with regard to myocardial protection seems to be limited, since it was not affected by oophorectomy and was not associated with improved myocardial salvage in the absence of the high constitutive ARC levels demonstrated in the intact female.
Taken together, our data supports the conclusion that in females, endogenous estrogen limits I/R-induced cardiomyocyte apoptosis by producing a baseline antiapoptotic profile, which is associated with an estrogen-dependent high constitutive myocardial expression of ARC.
Although baseline HR was significantly lower and baseline MAP significantly higher in ischemic females and ischemic oophorectomized females compared with ischemic males, the RPP, a sensitive index of myocardial O2 consumption (40) that incorporates HR and arterial blood pressure, did not differ among groups at baseline and throughout I/R. Therefore, differences in HR are unlikely to account for the observed differences in infarct size.
Apart from their antiarrhythmic actions, the three components of the prophylactic antiarrhythmic regimen used in this study, i.e., magnesium sulfate, lidocaine, and amiodarone, have all been shown to protect against myocardial I/R injury (15, 17, 22). This may have influenced infarct size. However, since all three ischemic groups received the same regimen, comparisons between these groups are still justified. Nonischemic groups did not receive the antiarrhythmic regimen, and, since the influence of this regimen on apoptotic protein expression was not investigated, differences in protein expression between ischemic and nonischemic groups should be interpreted cautiously.
In this study, the sububcellular localization of Bax, Bcl-2, and ARC was not measured, which is a limitation, since Bcl-2 mainly resides in the outer mitochondrial membrane (9, 33) and Bax translocates from the cytosol to mitochondria after death signaling (9, 33). ARC is predominantly located in the cytosol but has also been proposed to translocate to the mitochondria after death signaling (6). Thus, measurement of the subcellular localization of these apoptotic proteins would be an interesting subject for future research.
To date, much work with in vivo rabbit I/R protocols has focused on the positive effects of exogenous estrogen treatment on infarct size. According to Sbarouni et al. (34), the intramuscular administration of conjugated estrogens at a dose of 1 mg/day for 4 wk reduced myocardial infarct size in oophorectomized female rabbits. Booth et al. (2, 3) obtained similar results in rabbits with a single dose of 20 μg E2 administered intravenously 30 min before coronary occlusion. Hale et al. (12) reported that acute treatment of male and female rabbits with 1 mg E2 administered intravenously 15 min before I/R reduced infarct size in females and males, and Das et al. (4) reported that pretreatment of male rabbits with E2 (10 μg/kg iv) before I/R significantly reduced infarct size. In all these in vivo rabbit I/R protocols, the quantitative effect of estrogen on cardiomyocyte apoptosis and myocardial apoptotic protein expression was not a subject of investigation.
This study strongly supports the association between plasma estrogen levels, constitutive myocardial ARC expression, I/R-induced myocyte apoptosis, and myocardial salvage. In future studies, additional experimental cohorts in which estrogen is pharmacologically administered to intact females, oophorectomized females, and males will likely further our understanding of the effect of estrogen dose on the phenomenon that we report here.
The exact underlying molecular mechanisms by which estrogen exerts its antiapoptotic effects in cardiomyocytes are still largely unknown and require further research. This study revealed an important part of the underlying mechanism but has to been seen as a small part of a bigger and much more complex picture. Previous studies have shown numerous other broad-range interrelated genomic and nongenomic antiapoptotic effects of estrogen that may provide opportunities for future therapeutic manipulation. These include estrogen receptor (ER)-α and -β and phosphatidylinositol 3-kinase (PI3K)-mediated Akt activation (16, 31, 39), PI3K-mediated PKC-δ and -ε activation (1, 29), decreased NF-κB activation (16), inhibition of NF-κB DNA binding by ER-α and -β (32), decreased p38 MAPK-mediated acute myocardial inflammation with decreased TNF-α levels (38), free radical scavenging and antioxidant effects (16, 28), increased nitric oxide (NO) synthase and NO production (16, 28), modulation of Ca2+ influx and release (16, 28), activation of (mitochondrial) ATP-sensitive K+ channels (4, 16, 28), increased Bcl-2 levels (39), and decreased caspase 3 and caspase 8 activity (38, 39). In our study, Bcl-2 levels were slightly higher in females compared with males and oophorectomized females. However, these differences were nonsignificant.
The concept of early therapeutic interference with pro- and antiapoptotic stimuli to limit infarct size and prevent (ischemic) heart failure is extremely attractive. However, the development of a realistic strategy to accomplish such a goal has suffered not only from a lack of understanding of the importance of the different apoptotic mechanisms in cardiomyocytes and the interactions between them but also from the lack of organ specificity of the involved apoptotic proteins. This explains why the identification and characterization of a powerful cardiomyocyte-specific endogenous antiapoptotic factor, such as ARC, represents a promising therapeutic target.
It is clear that the protective mechanisms of estrogen on a molecular and cellular level are yet to be fully elucidated, but the same is true for the effects on a larger (cardiovascular) scale. A number of short- and long-term protective cardiovascular mechanisms have been discovered, including endothelium-dependent vasodilatation (26), endothelium-dependent dilatation of atherosclerotic coronary arteries (41), inhibition of the renin-angiotensin system (7), neovascularization resulting in collateral blood flow (13), a beneficial influence on lipoprotein metabolism (26), prevention of atherosclerosis by various mechanisms (30, 41), protection against vascular injury by inhibition of vascular smooth muscle proliferation (26, 27), acceleration of endothelial cell growth (26), attenuation of myocardial inflammation (35), increased HR, stroke volume, and cardiac output (21), attenuation of cardiac hypertrophy in response to pressure overload (36), normalization of wall tension, and inhibition of LV dilatation (35). Estrogen is also thought to have antiarrhythmic properties (4, 24).
Interestingly, potential harmful effects of estrogen have also been described. Van Eickels et al. (37) described a number of harmful long-term cardiovascular effects in mice, including increased LV and cardiomyocyte hypertrophy and ventricular remodeling 6 wk after MI with associated increased mortality. A possible explanation is that adverse effects of estrogen on a larger (cardiovascular) scale may mitigate the beneficial effects on a cellular level. This teaches us that the cardiovascular effects of estrogen are complex and that we are only beginning to understand them.
In summary, this study adds to a growing body of evidence that suggests that endogenous estrogen produces cardioprotective effects. The novel finding reported here is the estrogen-dependent high constitutive myocardial expression of antiapoptotic ARC in females. This finding adds to our overall understanding of the underlying cellular mechanism of myocardial resistance to I/R-induced injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-63954, HL-71137, and HL-76560. R. C. Gorman and J. H. Gorman, 3rd, are supported by individual American Heart Association Established Investigator awards.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315: 1125–1135, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Booth EA, Marchesi M, Kilbourne EJ, Lucchesi BR. 17β-Estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther 307: 395–401, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Booth EA, Marchesi M, Knittel AK, Kilbourne EJ, Lucchesi BR. The pathway-selective estrogen receptor ligand WAY-169916 reduces infarct size after myocardial ischemia and reperfusion by an estrogen receptor dependent mechanism. J Cardiovasc Pharmacol 49: 401–407, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Das B, Sarkar C. Similarities between ischemic preconditioning and 17β-estradiol mediated cardiomyocyte KATP channel activation leading to cardioprotective and antiarrhythmic effects during ischemia/reperfusion in the intact rabbit heart. J Cardiovasc Pharmacol 47: 277–286, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res 61: 414–426, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ekhterae D, Lin Z, Lundberg MS, Crow MT, Brosius FC, 3rd, Núñez G. ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ Res 85: e70-–e77., 1999 [DOI] [PubMed] [Google Scholar]
- 7.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Gill C, Mestril R, Samali A. Losing heart: the role of apoposis in heart disease–a novel therapeutic target? FASEB J 16: 135–146, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Green DR, Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson AB, Sayen MR, Williams SD, Crow MT, Gottlieb RA. TAT protein transduction into isolated perfused hearts: TAT-apoptosis repressor with caspase recruitment domain is cardioprotective. Circulation 106: 735–739, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson AB, Tsai JG, Logue SE, Crow MT, Gottlieb RA. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J Biol Chem 279: 21233–21238, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hale SL, Birnbaum Y, Kloner RA. Estradiol, administered acutely, protects ischemic myocardium in both female and male rabbits. J Cardiovasc Pharmacol Ther 2: 47–52, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hamada H, Kim MK, Iwakura A, Li M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors α and β mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114: 2261–2270, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Johnson MS, Moore RL, Brown DA. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol 290: H2644–H2647, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kaczmarek DJ, Herzog C, Larmann J, Gillmann HJ, Hildebrand R, Schmitz M, Westermann A, Harendza T, Werdehausen R, Osthaus AW, Echtermeyer F, Hahnenkamp K, Wollert KC, Theilmeier G. Lidocaine protects from myocardial damage due to ischemia and reperfusion in mice by its antiapoptotic effects. Anesthesiology 110: 1041–1049, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock 23: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Koo EH, Park YC, Lim SH, Kim HZ. Amiodarone offsets the cardioprotective effects of ischaemic preconditioning against ischemia/reperfusion injury. J Int Med Res 34: 140–151, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Koseki T, Inohara N, Chen S, Núñez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA 95: 5156–5160, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TM, Lin MS, Chou TF, Tsai CH, Chang NC. Adjunctive 17β-estradiol administration reduces infarct size by altered expression of canine myocardial connexin43 protein. Cardiovasc Res 63: 109–117, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Lesauskaite V, Epistolato MC, Ivanoviene L, Tanganelli P. Apoptosis of cardiomyocytes in explanted and transplanted hearts. Comparison of results from in situ TUNEL, ISEL, and ISOL reactions. Am J Clin Pathol 121: 108–116, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Magness RR, Parker CR, Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17β. Am J Physiol Endocrinol Metab 265: E690–E698, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Matsusaka T, Hasebe N, Jin YT, Kawabe J, Kikuchi K. Magnesium reduces myocardial infarct size via enhancement of adenosine mechanism in rabbits. Cardiovasc Res 54: 568–575, 2002 [DOI] [PubMed] [Google Scholar]
- 23.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 286: H1923–H1935, 2004 [DOI] [PubMed] [Google Scholar]
- 24.McHugh NA, Cook SM, Schairer JL, Bidgoli MM, Merrill GF. Ischemia- and reperfusion-induced ventricular arrhythmias in dogs: effects of estrogen. Am J Physiol Heart Circ Physiol 268: H2569–H2573, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Mehilli J, Ndrepepa G, Kastrati A, Nekolla SG, Markwardt C, Bollwein H, Pache J, Martinoff S, Dirschinger J, Schwaiger M, Schomig A. Gender and myocardial salvage after reperfusion treatment in acute myocardial infarction. J Am Coll Cardiol 45: 828–831, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology 138: 3330–3339, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res 75: 478–486, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Murriel CL, Mochly-Rosen D. Opposing roles of δ and εPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys 420: 246–254, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nascimento CA, Kauser K, Rubanyi GM. Effect of 17β-estradiol in hypercholesterolemic rabbits with severe endothelial dysfunction. Am J Physiol Heart Circ Physiol 276: H1788–H1794, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res 95: 692–699, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Pelzer T, Neumann M, de Jager T, Jazbutyte V, Neyses L. Estrogen effects in the myocardium: inhibition of NF-κB DNA binding by estrogen receptor-α and -β. Biochem Biophys Res Commun 286: 1153–1157, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Reeve JL, Duffy AM, O'Brien T, Samali A. Don't lose heart–therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med 9: 609–622, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sbarouni E, Iliodromitis EK, Bofilis E, Kyriakides ZS, Kremastinos DT. Short-term estrogen reduces myocardial infarct size in oophorectomized female rabbits in a dose-dependent manner. Cardiovasc Drugs Ther 12: 457–462, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Smith PJ, Ornatsky O, Stewart DJ, Picard P, Dawood F, Wen WH, Liu PP, Webb DJ, Monge JC. Effects of estrogen replacement on infarct size, cardiac remodeling, and the endothelin system after myocardial infarction in ovariectomized rats. Circulation 102: 2983–2989, 2000 [DOI] [PubMed] [Google Scholar]
- 36.van Eickels M, Grohé C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17β-Estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104: 1419–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 37.van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, Grohe C, Mendelsohn ME, Karas RH. 17β-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol 41: 2084–2092, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17β-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 40: 205–212, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, Kelly M, Meldrum DR. Estrogen receptor (β) mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol 296: R972–R978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson PL, Moyers JR, Ports T, Chatterjee K, Ullyott D, Hamilton WK. Rate-pressure product and myocardial oxygen consumption during surgery for coronary artery bypass. Circulation 60: 170–173, 1979 [DOI] [PubMed] [Google Scholar]
- 41.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation 81: 1680–1687, 1990 [DOI] [PubMed] [Google Scholar]