Abstract
A Doppler signal converter has been developed to facilitate cardiovascular and exercise physiology research. This device directly converts audio signals from a clinical Doppler ultrasound imaging system into a real-time analog signal that accurately represents blood flow velocity and is easily recorded by any standard data acquisition system. This real-time flow velocity signal, when simultaneously recorded with other physiological signals of interest, permits the observation of transient flow response to experimental interventions in a manner not possible when using standard Doppler imaging devices. This converted flow velocity signal also permits a more robust and less subjective analysis of data in a fraction of the time required by previous analytic methods. This signal converter provides this capability inexpensively and requires no modification of either the imaging or data acquisition system.
Keywords: audio signal processing, flow measurement, analog circuit, frequency conversion
investigators studying cardiovascular physiology have long been interested in measuring arterial blood flow. Initially, this was done using strain-gauge plethysmography, a technique limited to intermittently measuring flow in distal extremities (5, 28, 30–32). In later studies, nonimaging Doppler ultrasound systems from Hokanson (DE Hokanson, Bellevue, WA) or Multigon (Multigon Industries, Yonkers, NY) provided a continuous arterial flow velocity signal that was recorded with ECG, blood pressure, and other physiological parameters by a PowerLab data acquisition system (ADInstruments, NSW, Australia) (15, 16, 19, 23, 27, 29). While nonimaging devices can measure flow velocity in vessels inaccessible to strain-gauge plethysmography, they cannot measure arterial diameter, an essential element in calculating arterial flow (20). An Interspec XL B-mode imaging system (Interspec, Conshohocken, PA) was subsequently acquired to obtain these diameter measurements (27, 29). At specified times during a study, the sonographer removed the nonimaging probe and applied the Interspec probe to image the artery. The repeated interchange of these probes required considerable skill to achieve a successful study and also raised the question of whether both probes consistently interrogated the same arterial site. In addition, since both nonimaging and imaging devices must be immediately available, one must provide space for both in a crowded clinical physiology laboratory.
A more desirable approach is to measure both arterial diameter and blood velocity with one machine without moving the probe from the selected arterial site. Toward this end, HDI 5000 Doppler ultrasound systems (ATL Ultrasound, Bothell, WA) were acquired as the standard imaging platforms used at our General Clinical Research Center (15, 16, 21–23). These machines provide excellent vascular images for selecting arterial sites and measuring arterial diameters and, in Doppler mode, can record flow velocity waveforms as video images. By the incorporation of both modalities, the HDI 5000 appeared to solve our blood flow measurement problem, but major operational issues emerged involving the intermittent character of data acquisition during long study paradigms and procedural bottlenecks in data management and analysis.
Operational issues aside, the primary limitation of measuring blood flow velocity with a standard Doppler imaging system such as the HDI 5000 is that it does not deliver a real-time velocity signal that can be simultaneously recorded with other physiological parameters. This seriously impedes an investigator's ability to continuously observe flow in response to infusions, exercise, or other experimental interventions.
Although the HDI 5000 lacks an analog flow velocity output, it does port two analog Doppler signals to a pair of RCA phono jacks (2). The goal of this project, therefore, was to accurately convert these Doppler audio signals to a real-time flow velocity signal, thereby enabling the HDI 5000 to provide flow velocity to a PowerLab data acquisition system. This capability would allow access to the analytic tools within Chart, the application software supplied with the PowerLab system.
METHODS
Concept Development
Designing the Doppler signal converter began with characterizing its input signals. The investigator verified that one audio output encodes for flow toward the transducer (positive velocity) and the other flow away from the transducer (negative velocity) by rapidly cycling an ultrasound probe by hand toward and away from the skin while listening to the Doppler signals through stereo headphones. The resulting sound abruptly shifted from one channel to the other as the probe changed direction.
To determine the bandwidth of the arterial flow velocity signals, the brachial artery flow velocity in a subject at rest was acquired with a Multigon and sampled with PowerLab. Fast Fourier transform (FFT) analysis revealed an effective bandwidth of 0–20 Hz, showing that arterial flow velocity signals reside well within the 100-Hz Nyquist frequency limit defined by the 200-Hz sampling rate used in our laboratory (24).
It was initially thought that Chart's data processing functions might implement a software-based Doppler signal converter requiring no hardware except cabling. This approach was abandoned, however, when FFT analysis of Doppler audio signals from brachial artery flow in a resting subject clearly showed frequencies exceeding the 2-kHz limit of Chart's frequency-to-voltage (F/V) conversion function (1), the crucial element in this concept.
The project focus then shifted to designing a hardware signal converter (Fig. 1) with two channels, each using a zero-crossing detector, a pulse conditioning circuit, and an F/V converter to process its Doppler signal (6, 23a, 36). The resulting positive and negative velocity signals would then be combined to produce the desired composite flow velocity signal.
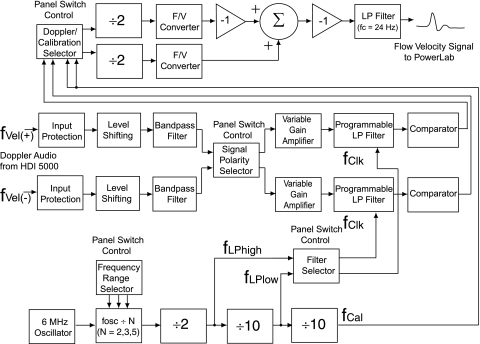
Fig. 1.
Doppler audio converter (front view). From left, controls are as follows: probe frequency range selector [“tailors” bandwidth of low-pass (LP) filters to Doppler frequency], channel selector for anti-reflection LP filter, calibration signal polarity selector, flow velocity signal polarity selector, positive velocity gain, and negative velocity gain (each gain control sets the signal detection threshold for its channel.).
Circuit Design
Bandwidth specification.
The HDI 5000 has recorded peak arterial flow velocities of ∼300 cm/s that correspond to Doppler frequency shifts of ∼12 kHz when using a maximum probe frequency of 6 MHz and insonation angle of 60°. Since the HDI 5000 also uses a high-pass filter with a corner frequency (fc) of 200 Hz to remove the artifact produced by low velocity arterial wall motion, the initial design specified a bandwidth of 200 Hz–15 kHz.
Component specification.
The converter's integrated circuits (ICs) include the following: LM358 op-amps for input and output signal conditioning (23b), LM393 comparators for signal detection (33), CD4013 flip-flops for pulse conditioning (8), CD4052 data selectors for signal switching (9), and LM2907 F/V converters for converting Doppler audio signals into flow velocity waveforms (23a). Additional ICs low-pass (LP) filter the Doppler signals and generate calibration signals as described in Testing and Refinement. The single-voltage operation of these ICs simplified selecting a power supply and facilitated packaging a compact unit (18 × 14 × 4 cm). To simplify the converter's wiring and reduce system noise, the signals were routed onboard by data selectors controlled by front panel switches (5a). The overall circuit was designed to optimize the performance of the F/V converters, the “linchpin” ICs in the converter.
Doppler signal processing.
Figure 2 diagrams the signal processing performed by the converter. An RCA-type stereo patch cable delivers two Doppler audio signals from the HDI 5000 to the input signal processors. Each input processor incorporates input protection, level shifting, and bandpass filtering. A pair of diodes limits signal amplitude to protect the input circuitry (7). Essential to a single-voltage analog circuit, level shifting establishes the signal's reference voltage (23b). The bandpass filter incorporates high-pass filtering to remove direct current signals that could saturate the input amplifier and LP filtering to limit high-frequency interference (35). Both Doppler signals pass through a data selector that, if needed, switches each signal to the other's pathway to invert the output flow velocity signal. A variable gain amplifier determines each signal's detection threshold. Each Doppler signal passes through a programmable LP filter and then a comparator where it is converted from a sinusoid into a 0–5-V square wave. Another data selector steers either the two Doppler signals or a calibration signal to a pair of flip-flop ICs. Each flip-flop halves the frequency of its signal, producing the necessary square wave clock signal for its F/V converter. Each F/V converter transforms the instantaneous frequency of its clock signal into a linearly proportional voltage representing flow velocity. At this point, both flow velocity signals have the same polarity; so, to recover directional information, one signal is inverted and combined with the other via a summing circuit. This composite flow velocity signal is LP filtered (fc = 24 Hz) en route to the converter output where a bayonet navy connector (BNC) coaxial cable carries it to PowerLab.
Fig. 2.
Functional schematic for Doppler audio converter. See methods for definitions of abbreviations.
Calibration
Doppler frequency (fD) is typically related to flow velocity (u) by Eq. 1, where fP is the excitation frequency of the ultrasound probe, θ is the insonation angle, and c is the average velocity of sound in tissue (36).
| (1) |
Solving Eq. 1 for u yields Eq. 2, a more useful calibration tool.
| (2) |
Since c = 1,540 m/s, a value first determined by Ludwig (17) and adopted as a standard by ultrasound device manufacturers, and fP and θ are determined and displayed by the HDI 5000, assigning a known calibration frequency (fcal) to fD establishes the relationship between fD and u.
The converter provides a 6-, 10-, or 15-kHz calibration signal that represents the Doppler frequency shift associated with a flow velocity of ∼380 cm/s for probes operating at 2.5, 4.0, or 6.0 MHz, respectively, at a maximum θ of 60°. A data selector directs the selected fcal to either of the F/V converters after which it is processed and presented to PowerLab as a direct current voltage whose polarity depends on the switch position.
With the use of Eq. 2, where fD = fcal, an Excel spreadsheet (Microsoft, Redmond, WA) calculated u for θ between 0° and 60° in 1° increments for these three probe frequencies and presented u, fP, and θ in a lookup table. During a study, when a satisfactory arterial site has been located, the HDI 5000 displays fP and θ, which are then used to select the associated value of u from the table. Chart's two-point calibration function uses this flow velocity to rescale the recorded calibration signal and subsequent flow velocity signals, thus providing direct velocity measurements from the recorded waveforms.
Testing and Refinement
Engineering data for refining the converter design were obtained by identifying experiments using an HDI 5000 to measure arterial flow, installing the Doppler signal converter between the HDI 5000 and PowerLab units and recording flow velocity with one additional PowerLab channel.
During early observations of flow in brachial and femoral arteries when using the converter, abrupt signal drops or “spikes” frequently appeared within the systolic peak of the flow velocity signal displayed by PowerLab. Many of these spikes resulted from Doppler signal aliasing within the HDI 5000 whenever arterial flow velocity exceeded the velocity range selected by the operator. This aliasing caused the peak flow velocity to “wrap around” into the low velocity part of the range and was remedied by selecting an appropriate velocity range.
Even when a nonaliasing velocity range was selected, however, low or even negative velocity spikes continued to appear within systolic peak flow signals. These artifacts were attributed to the reflection of rapidly transmitted systolic pressure waves from downstream arterial structures such as bifurcations, stenoses, or vessel tapering (4). This idea was supported by a study where a SphygmaCor arterial tonometry system (AtCor Medical, Sydney, Australia) measured the augmentation component of systolic pressure caused by a reflected pulse wave that arrived just after peak systolic pressure, i.e., at the same time as low or negative velocity artifacts appeared in the systolic flow velocity signal (18).
To investigate these artifacts, brachial artery Doppler audio signals from a resting subject were directly sampled by PowerLab at 20 kHz. FFT analysis was performed on coincident short (50–100 ms) segments of both signals. Repeating this analysis on adjacent segments revealed a narrowband negative velocity signal (4 kHz ± 100 Hz) that occurred at or just after the systolic peak on the positive velocity channel. This 4-kHz Doppler signal corresponded to a negative flow velocity of ∼100 cm/s, a velocity frequently sufficient to cancel out the peak systolic flow velocity at that instant. Contrast this with the expected negative velocity signal due to transient retrograde flow as the distended artery relaxes during diastole. FFT analysis of diastolic relaxation signals showed Doppler frequencies ≤ 0.5 kHz.
After Chart's digital LP filter (fc = 1.0 kHz) was applied to the negative velocity Doppler signal, FFT analysis showed that the 4-kHz artifact was removed without affecting the diastolic relaxation signal.
Following this experiment, a two-channel Krohn-Hite variable LP filter was inserted between the HDI 5000 and the converter and each filter's fc was initially set at 10 kHz (Krohn-Hite, Cambridge, MA). During subsequent brachial and femoral flow studies, reflection artifacts appearing on the flow velocity signals could be reproducibly observed or removed by switching fc of the negative velocity filter between 10 and 1.0 kHz. These studies also showed the need to scale fc of both filters in proportion to the Doppler probe frequency to ensure that only the reflection artifact was removed from the negative flow velocity signal. Filter fc values were then defined for each probe frequency: 15 and 1.5 kHz at 6 MHz, 10 and 1.0 kHz at 4 MHz, and 6 and 0.6 kHz at 2.5 MHz.
Although it performed well, this variable filter-converter configuration was cumbersome to use, so the converter circuitry was redesigned to incorporate two programmable MAX293 LP filter ICs (20). A square-wave clock signal sets each filter's fc where the ratio of clock frequency (fclock) to fc is 100:1, so one simply changes fclock to change fc. A 6.0-MHz oscillator generates the master clock signal used to derive both filters' clock signals and the calibration signal (Fig. 2) (10). A switch-programmed 74HC161 “divide by N” IC initially divides the oscillator's frequency by 2, 3, or 5 (11). A flip-flop halves this frequency, producing the 1.5, 1.0, or 0.6 MHz clock signal, fLPhigh, that sets fc of the positive velocity channel's LP filter to 15, 10, or 6 kHz. A CD4017 IC divides fLPhigh by 10, concurrently producing a 150, 100, or 60 kHz clock signal, fLPlow, that sets fc of the negative velocity channel's LP filter to 1.5, 1.0, or 0.6 kHz. A second CD4017 divides fLPlow by 10 to yield the 15, 10, or 6 kHz calibration signal. The circuit schematic is available with the online version of this article.
Validation
Two validation studies, one in vitro and one in vivo, acquired flow velocity data simultaneously with both the HDI 5000 and Doppler audioconverter PowerLab systems. Since time-averaged mean (TAM) flow velocity is the most frequently reported quantity in physiological studies using Doppler ultrasound (15, 16, 20–23, 27, 29), it was selected as the basis of comparison of the two systems.
In Vitro Study
Protocol.
The in vitro study employed a mock circulatory loop adapted from a system developed by Tschakovsky and colleagues (26). A dilute solution of cornstarch, by providing scattering particles that ultrasonically resemble red blood cells, served as the “blood” in this system. This solution, typically filling a 3-liter reservoir, was magnetically stirred to maintain the cornstarch in suspension. A Master-Flex roller pump (Masterflex, Vernon Hills, IL) circulated this solution to provide quasi-steady flow within the loop. A test chamber was fabricated from a transparent rectangular plastic container where lengths of silastic tubing (internal diameter = 2.8–8.9 mm) were passed through the container's sides and across the container to simulate arteries. Water was added to the test chamber to cover the tubing to a depth of 5 cm. The solution was drawn from the reservoir through a loop of Tygon tubing and pumped through a compliance chamber to filter out high-frequency pulses from the pump rollers just before passing through the silastic “artery” and returning to the reservoir.
In each experiment, one of the silastic arteries was selected for study and the HDI 5000 probe, operated at 4 MHz, was clamped in place over the selected artery. The Doppler signal converter was installed between the HDI 5000 and PowerLab and configured. The initial pump speed was set, and the flow in the loop was allowed to stabilize. The HDI 5000 and probe were checked and adjusted to display the cleanest image and Doppler flow velocity signal. PowerLab then recorded a calibration signal and a sample of the flow velocity signal. Each experiment comprised 10 trials, where the pump speed was increased between trials to increase flow rate in a series of steps. During each trial, as soon as the HDI 5000 displayed a Doppler flow velocity signal, PowerLab began data sampling, continuing for 30 s. During that time, the HDI 5000 recorded one 15-s Doppler video clip. Three such recordings were obtained at each pump speed. Each PowerLab data record was annotated with timing marks coinciding with the beginning and end of each video clip to enable the same flow velocity signals to be identified for poststudy analysis by both HDI 5000 and PowerLab.
Data analysis.
HDI 5000.
Doppler video clips were loaded into the HDI 5000, and a video clip was selected and displayed. The operator identified a region of interest on the flow velocity waveform and manually traced it using a trackball. A program within the HDI 5000 automatically calculated and displayed peak, time-averaged peak, and TAM velocity for the traced waveform, and the operator manually logged the TAM velocity. This process was repeated for each clip recorded during the study.
POWERLAB.
The annotated timing marks were used to identify each flow velocity data segment coincident with an HDI 5000 video clip. The cursor within Chart was used to select all data within that segment. Chart then computed the mean (TAM) velocity of the selected data and automatically logged it into the Data Pad, a user-configured table within Chart that was saved as a text file. This was repeated for all three runs at each flow rate within each experiment.
Summary.
From eight experiments, 202 TAM flow velocities were independently measured with the HDI 5000 and PowerLab systems over the range of pump speeds and vessel diameters. Both sets of TAM velocity measurements were then imported into an Excel spreadsheet for further analysis.
In Vivo Study
Protocol.
The converter was evaluated during a clinical research study that measured brachial artery flow velocity over a wide range of flow rates in 10 normal volunteers (5 men and 5 women). Each volunteer provided written, informed consent. This protocol was reviewed and approved by the Institutional Review Board within the Penn State College of Medicine.
In each volunteer, three different arm elevations (+45°, 0°, and −45°) established three different baseline flow rates. At each elevation, a standard reactive hyperemia procedure was performed, where blood flow into the arm was occluded for 10 min via a pneumatic cuff, inducing a transient ischemia. Following the abrupt release of this occlusion, arterial flow in the arm rapidly approached a maximum and steadily diminished toward baseline over the next 3 min as perfusion was reestablished. During these 3 min, the HDI 5000 recorded 13 Doppler video clips lasting 5 to 6 s each at 15-s intervals while PowerLab continuously recorded flow velocity. All PowerLab recordings were annotated with timing marks coinciding with the beginning and end of each video clip.
Data analysis.
HDI 5000.
All 13 Doppler video clips from a selected arm elevation trial within a given experiment were retrieved, and a clip was selected and displayed. Guided by the ECG, the analyst located the first complete cardiac cycle and traced its flow velocity signal. As previously described, peak, time-averaged peak, and TAM velocities for that cycle were automatically computed and displayed and TAM velocity was manually logged. This process was repeated for successive cardiac cycles during the clip. The average of all TAM velocities measured within the clip was computed and reported as the representative TAM velocity for that clip (time point). This analysis was performed on the remaining 12 video clips recorded within the selected arm elevation trial and repeated for the other two trials.
POWERLAB.
The flow velocity waveform from a selected arm elevation trial was displayed. The analyst then used the annotated timing marks to locate flow velocity data segments coincident with the associated HDI 5000 video clips. Within each velocity data segment, the region spanning the largest number of complete cardiac cycles was selected. Chart then computed the mean (TAM) value of the selected velocity data and logged it into its Data Pad, a user-configured table. This procedure was repeated on the velocity data associated with each remaining time point within the selected trial. All three arm elevation trials were processed in like manner, and when all TAM values were determined, the Data Pad was saved as a text file for further processing by Excel.
Summary.
The HDI 5000 and PowerLab systems independently measured TAM velocity from flow velocity data recorded at 13 time points during each of three arm elevation trials performed during each of the 10 experiments. The resulting 390 TAM velocity measurements from each device were imported into an Excel spreadsheet for analysis.
RESULTS
Does the Doppler signal converter provide a sufficiently accurate flow velocity signal to replace a laborious HDI 5000-based analysis with a faster and less subjective PowerLab-based analysis? To answer this crucial question, the agreement between velocity measurements made with the PowerLab and HDI 5000 systems was assessed as follows.
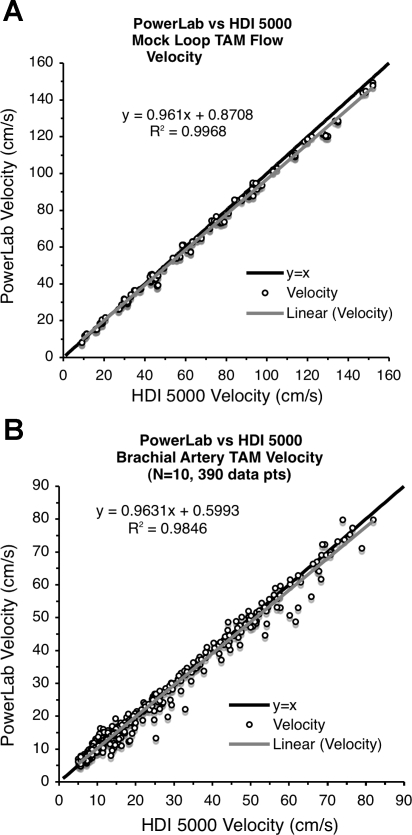
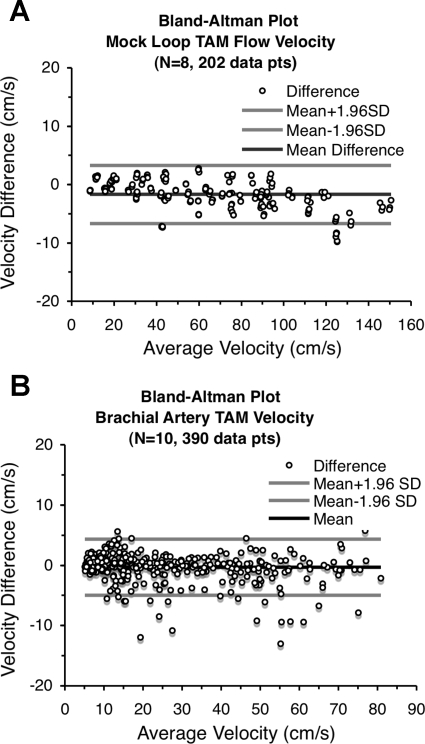
The scatterplots in Fig. 3 visually compare the TAM flow velocities from the two devices for both the in vitro (Fig. 3A) and in vivo (Fig. 3B) validation studies. While each scatterplot closely approaches its ideal “y = x” line, the high correlation alone suggests but does not signify agreement, so these two data sets were examined using the Bland-Altman procedure (3). The mean difference line within each Bland-Altman plot in Fig. 4 shows minimal deviation from the ideal “zero difference” line, and the small range of the 95% limits of agreement support the claim of agreement between these two measurement systems. These data sets were also tested with the concordance correlation method developed by Lin (12) since this metric quantifies both precision and accuracy when comparing measurement systems. The resulting concordance correlation coefficients for the in vitro data, 0.997 (95% confidence interval: 0.996 and 0.997, P < 0.001) and in vivo data, 0.992 (95% confidence interval: 0.990 and 0.993, P < 0.001), confirm the Bland-Altman results and further support the claim that flow velocity signals produced by the Doppler signal converter are comparable with those produced by the HDI 5000 and can be reliably used to measure flow velocity.
Fig. 3.
Scatterplot comparison of Doppler signal converter-PowerLab with HDI 5000 time-averaged mean (TAM) flow velocities. A: in vitro study (mock circulatory loop). B: in vivo study (arm elevation with reactive hyperemia).
Fig. 4.
Bland-Altman plots of TAM flow velocity data. A: in vitro study (mock circulatory loop). B: in vivo study (arm elevation with reactive hyperemia).
DISCUSSION
Historically, to measure blood flow noninvasively, clinical investigators have had to accommodate their experimental designs to the limitations of the available technology such as strain-gauge plethysmography and nonimaging Doppler ultrasound. It is not surprising, therefore, that Doppler ultrasound imaging technology has revolutionized the noninvasive assessment of blood flow by incorporating high-quality vascular imaging for estimating lumenal area and accurate flow velocity measurements within the same machine. Present Doppler imaging systems efficiently gather, analyze, and report clinical data, typically by using a menu of preconfigured and optimized study protocols. However, these protocols may not be readily applied to clinical research studies where biomedical signals from a variety of instruments are recorded simultaneously to observe the body's response to an experimental intervention. These machines provide limited capacity to incorporate additional signals, and data management is geared toward archiving images and generating standardized reports rather than providing Doppler information in a format easily integrated into a multichannel data stream. While older Doppler imaging systems, e.g., Flo-Map (Cardiometrics, Mountain View, CA), provide an analog flow velocity signal for external recording, using such legacy devices for clinical research may not be a viable solution. Assuming working devices are even available, their imaging technology is much less capable than that of newer digital systems.
Given the excellent imaging and flow measurement capabilities of a system such as the HDI 5000, why develop a Doppler signal converter? To address this, consider how a standard HDI 5000 is typically used in clinical research. Initially, it is operated in imaging mode to locate an arterial site of interest. During study paradigms lasting ≥30 min, while ECG, blood pressure, and other signals are continuously monitored, the HDI 5000 is switched between Doppler mode to record flow velocity waveforms and imaging mode to record arterial images for diameter measurements. These waveforms and images are saved as short (<20 s) video clips. During a long study paradigm, therefore, flow velocity is not continuously recorded but rather sporadically sampled, leaving gaps in the data. This forces the investigator to guess which are the most fruitful time points in the study to record these video clips and complicates the process of synchronizing flow velocity data with other biomedical signals. (21–23). Poststudy, the analyst measures flow velocity from individual video clips by tracing Doppler flow velocity waveforms beat by beat and manually logging the data, a subjective process requiring up to 10 h/study. Data analysis with the HDI 5000 is frequently delayed, however, by the need to archive video clips as soon as possible after each study to recover space on the HDI 5000's small 17-Gb hard disk before the next study. This archiving requires 2 to 3 h to transfer data from the hard disk to the magneto-optical disk to CD-R, and processing an archived study typically requires a day to reload and analyze the video data. Since several research groups share the HDI 5000, the mutually exclusive nature of the data acquisition and analysis frequently creates scheduling issues affecting the efficient use of these machines.
The signal converter described here expands the research potential of the HDI 5000 by accurately processing two Doppler audio signals into a continuous flow velocity signal that can be seamlessly sampled with other low-frequency physiological signals. Thus flow velocity can be recorded during an entire experimental intervention and explored at length later, rather than forcing the investigator to rely on a relatively few video “snapshots.” Recording flow velocity with PowerLab enables one to use Chart's signal processing functions to rapidly analyze this signal instead of using the HDI 5000 to perform a tedious and less complete analysis of video clips. To measure blood flow, one need only use the HDI 5000 to record a few images for measuring arterial diameters rather than additional video clips of velocity waveforms. Diameters can be quickly measured immediately following the study without requiring that these arterial images be archived, although doing so would require much less disk space, time, and effort. In a recent study, Parker et al. (25) used an HDI 5000 with the Doppler signal converter and PowerLab to measure flow velocity and achieved a 95% reduction in the time required to measure TAM blood flow, from ∼100 min to ∼5 min/experiment, when compared with the same data recorded and analyzed using the HDI 5000 alone. Finally, flow velocity data recorded by PowerLab via the converter can be analyzed at any time on any computer equipped with Chart, thus completely eliminating scheduling issues created when analyzing data with the HDI 5000.
As the signal converter processes the audio output of a Doppler imaging system, its performance is necessarily dependent on the quality of that output; i.e., the stronger the Doppler signal, the clearer are both the video display and audio signals from the HDI 5000 and the more reliable the signal detection and processing by the converter. At present, since the HDI 5000 most easily and reliably detects Doppler signals originating from the brachial, femoral, and carotid arteries, the Doppler signal converter most easily and reliably converts these signals as well. Doppler signals from renal and coronary arteries are more technically challenging to detect and acquire by both the Doppler imaging system and signal converter. Continued efforts are needed to improve both the technique of acquiring Doppler signals from these arteries as well as the signal detection capability of the Doppler signal converter. Although the HDI 5000 was exclusively used during the development of the signal converter, the converter is currently transitioning to service with the newer Philips iE33 and Acuson Sequoia 512 systems (Siemens Medical Systems, Iselin, NJ).
GRANTS
This project was supported by the National Heart, Lung, and Blood Institute Grant P01-HL-077670.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge and thank Dr. Lawrence Sinoway for encouragement and continued support of this project.
REFERENCES
- 1.ADInstruments Cycle variables. In: Chart Extensions User's Guide Castle Hill NSW, Australia: ADInstruments, 1995, p. 9–16 [Google Scholar]
- 2.ATL Ultrasound Theory of operation. In: HDI 5000 Ultrasound System Field Service Manual Bothell, WA: ATL Ultrasound, 2000, p. 4-11–4-13 [Google Scholar]
- 3.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986. [PubMed] [Google Scholar]
- 4.Caro CG, Pedley TJ. The systemic arteries: physiological evidence of wave reflections. In: The Mechanics of the Circulation Oxford, UK: Oxford University Press, 1978, p. 291–294 [Google Scholar]
- 5.Crawford P, Good PA, Gutierrez E, Feinberg JH, Boehmer JP, Silber DH, Sinoway LI. Effects of supplemental oxygen on forearm vasodilation in humans. J Appl Physiol 82: 1601–1606, 1997. [DOI] [PubMed] [Google Scholar]
- 5a.Horowitz P, Hill W. Electronic construction techniques: cold switching philosophy. In: The Art of Electronics Cambridge, UK: Cambridge University Press, 1980, p. 551–553 [Google Scholar]
- 6.Horowitz P, Hill W. Feedback and operational amplifiers: comparators and Schmitt trigger. In: The Art of Electronics Cambridge, UK: Cambridge University Press, 1980, p. 124–126 [Google Scholar]
- 7.Horowitz P, Hill W. Foundations: diodes and diode circuits. In: The Art of Electronics Cambridge, UK: Cambridge University Press, 1980, p. 35–44 [Google Scholar]
- 8.Lancaster D. Clocked logic—the JK and D flip-flops. In: CMOS Cookbook Indianapolis, IN: Howard W. Sams, 1977, p. 259–294 [Google Scholar]
- 9.Lancaster D. CMOS analog switches. In: CMOS Cookbook Indianapolis, IN: Howard W. Sams, 1977, p. 349–355 [Google Scholar]
- 10.Lancaster D. Crystal oscillators. In: CMOS Cookbook. Indianapolis, IN: Howard W. Sams, 1977, p. 235–238 [Google Scholar]
- 11.Lancaster D. Divide-by-N counters. In: TTL Cookbook Indianapolis IN: Howard W. Sams, 1974, p. 217–257 [Google Scholar]
- 12.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45: 255–268, 1989 [PubMed] [Google Scholar]
- 15.Lott ME, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol 287: R586–R591, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Lott ME, Herr MD, Sinoway LI. Effects of transmural pressure on brachial artery mean blood velocity dynamics in humans. J Appl Physiol 93: 2137–2146, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ludwig GD. The velocity of sound through tissues and the acoustic impedance of tissues. J Acoust Soc Am 22: 862–866, 1950 [Google Scholar]
- 18.Lydakis C, Momen A, Blaha C, Herr MD, Leuenberger UA, Sinoway LI. Changes of elastic properties of central arteries during acute resistance exercise and lower body negative pressure. Eur J Appl Physiol 102: 633–641, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Markel TA, Daley JC, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI. Aging and the exercise pressor reflex in humans. Circulation 107: 675–678, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Maxim Integrated Products Eighth-order low-pass, elliptic, switched-capacitor filters. In: MAX293/MAX294/MAX297 Application Note 19-0020 Sunnyvale, CA: Maxim Integrated Products, 2005, p. 1–8 [Google Scholar]
- 21.Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: role of muscle reflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Momen A, Gahremanpour A, Mansoor A, Kunselman AR, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol 102: 735–739, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: role of muscle reflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003. [DOI] [PubMed] [Google Scholar]
- 23a.National Seminconductor LM2907/LM2917 frequency-to-voltage converter. In: Application Note DS007942 Arlington, TX: National Semiconductor, 2001, p. 1–20 [Google Scholar]
- 23b.National Semiconductor Use the LM158/LM258/LM358 dual, single supply op-amp. In: Application Note AN-116 Arlington, TX: National Semiconductor, 1980, p. 1–4 [Google Scholar]
- 24.Oppenheim AV, Schafer RW. Sampling of continuous-time signals. In: Digital Signal Processing. Englewood Cliffs, NJ: Prentice-Hall, 1975, p. 26–30 [Google Scholar]
- 25.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol 103: 1583–1591, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Pyke KE, Hartnett JA, Tschakovsky ME. Are the dynamic response characteristics of brachial artery flow-mediated dilation sensitive to the magnitude of increase in shear stimulus? J Appl Physiol 105: 282–292, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Shoemaker JK, Hogeman CS, Khan MH, Kimmerley DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Shoemaker JK, Hogeman CS, Leuenberger UA, Herr MD, Gray KS, Silber DH, Sinoway LI. Sympathetic discharge and vascular resistance after bed rest. J Appl Physiol 84: 612–617, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker JK, Naylor HL, Hogeman CS, Sinoway LI. Blood flow dynamics in heart failure. Circulation 99: 3002–3008, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Silber D, McLaughlin D, Sinoway LI. Leg exercise conditioning increases peak forearm blood flow. J Appl Physiol 71: 1568–1573, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Sinoway LI, Hendrickson C, Davidson WR, Prophet S, Zelis R. Characteristics of flow-mediated vasodilation in human subjects. Circ Res 64: 32–42, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Sinoway LI, Musch TI, Minotti JR, Zelis R. Enhanced maximal metabolic vasodilation in the dominant forearms of tennis players. J Appl Physiol 61: 673–678, 1986. [DOI] [PubMed] [Google Scholar]
- 33.Texas Instruments M193, LM293, LM393, LM193A, LM293A, LM393A, LM2903, LM2903Q dual differential comparators. In: Linear Circuits: Data Book Dallas, TX: Texas Instruments, 1992, vol. 3, p. 3-27–3-32 [Google Scholar]
- 35.Webster JG. Amplifiers and signal processing: active filters. In: Medical Instrumentation: Application and Design. Boston, MA: Houghton-Mifflin, 1978, p. 121–124 [Google Scholar]
- 36.Webster JG. Measurement of flow and volume of blood. In: Medical Instrumentation: Application and Design Boston, MA: Houghton-Mifflin, 1978, p. 399–414 [Google Scholar]