Abstract
Toll-like receptor 2 (TLR2), a key component of the innate immune system, is linked to inflammation and myocardial dysfunction after ischemia-reperfusion injury (I/R). Treatment of the heart with mesenchymal stem cells (MSCs) is known to improve myocardial recovery after I/R in part by paracrine factors such as VEGF. However, it is unknown whether TLR2 activation on the MSCs affects MSC-mediated myocardial recovery and VEGF production. We hypothesized that the knockout of TLR2 on the MSCs (TLR2KO MSCs) would 1) improve MSC-mediated myocardial recovery and 2) increase myocardial and MSC VEGF release. With the isolated heart perfusion system, Sprague-Dawley rat hearts were subjected to I/R and received one of three intracoronary treatments: vehicle, male wild-type MSCs (MWT MSCs), or TL2KO MSCs. All treatments were performed immediately before ischemia, and heart function was measured continuously. Postreperfusion, heart homogenates were analyzed for myocardial VEGF production. Contrary to our hypothesis, only MWT MSC treatment significantly improved the recovery of left ventricular developed pressure and the maximal positive and negative values of the first derivative of pressure. In addition, VEGF production was greatest in hearts treated with MWT MSCs. To investigate MSC production of VEGF, MSCs were activated with TNF in vitro and the supernatants collected for ELISA. In vitro basal levels of MSC VEGF production were similar. However, with TNF activation, MWT MSCs produced significantly more VEGF, whereas activated TLR2KO MSC production of VEGF was unchanged. Finally, we observed that MWT MSCs proliferated more rapidly than TLR2KO MSCs. These data indicate that TLR2 may be essential to MSC-mediated myocardial recovery and VEGF production.
Keywords: innate immunity, inflammation, stem cell therapy, paracrine signaling, vascular endothelial growth factor
stem cell treatment and bone-marrow derived mesenchymal stem cell (MSC) treatment specifically has been shown to minimize myocardial functional impairment and inflammation associated with ischemia-reperfusion injury (I/R) (1). It is unlikely that MSC-derived cardioprotection results solely from stem cells differentiating into cardiac myocytes since researchers report modest rates of stem cell survival after transplantation (7, 37). In addition, MSC treatment in the acute setting improves myocardial functional recovery without evidence of differentiation (42). Therefore, researchers have investigated the possibility that the improved cardiac contractile performance seen with MSC treatment is in part due to paracrine signaling. We and others have reported that paracrine signaling is indeed involved with stem cell-mediated cardioprotection (38, 42). Supporting this paracrine hypothesis are the observations that treatment with cell-free, MSC-conditioned media minimizes myocardial infarct size and improves myocardial function (16, 36). While the particular growth factors that contribute to the beneficial paracrine effects continue to be defined, vascular endothelial growth factor (VEGF) is one of the central growth and survival factors for the injured heart (18, 23, 35).
Toll-like receptors (TLRs) play a critical role in the innate immune system recognition of microbial pathogens (27). TLRs are also associated with myocardial survival and recovery following ischemic injury (8). This may be in part due to TLR recognition of host-derived molecules that are released in response to injury leading to subsequent TLR-induced inflammation (13). While 10 TLRs are known to exist in humans, the activation of myocardial TLR2 in particular is associated with proinflammatory signaling (6), endothelial dysfunction, increased infarct size (11), and impaired contractile function after I/R (33).
TLR2 is also expressed by MSCs (29), but it is unknown whether the presence of this receptor affects MSC-mediated myocardial protection. Given the published literature documenting the deleterious role of TLR2 on cardiomyocytes, we hypothesized that the knockout of TLR2 on the MSCs (TLR2KO MSCs) would improve acute myocardial recovery after global I/R, a scenario clinically encountered when arresting the heart for cardiac surgery. In addition, this effect would correlate with increased myocardial and MSC VEGF production. The purposes of the present study were to investigate the effects of TLR2 on MSC-mediated myocardial recovery after acute I/R as measured by 1) functional indexes, 2) myocardial growth factor production, and 3) in vitro MSC growth factor production.
MATERIALS AND METHODS
Animals.
Male wild-type mice (MWT; C57BL/6J, Jackson Laboratory, Bar Harbor, ME), male TLR2KO mice (B6.129-Tlr2tm1Kir/J, Jackson Laboratory) and normal male (250–350 g, 9 to 10 wk old) Sprague-Dawley rats (Harlan, Indianapolis, IN) were fed a standard diet and acclimated in a quiet quarantine room for 1 wk before the experiments. TLR2KO mice are viable animals and phenotypically similar to MWT mice but possess a targeted deletion for TLR2. The presence or absence of TLR2 was verified using deidentified samples sent to an independent genotyping facility (Transnetyx, Cordova, TN). The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Indiana University. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Preparation of mouse bone marrow MSCs.
A single-step purification method using plastic adherence was used as previously described (28), with the following modifications: 9- to 10-wk-old male MWT and TLR2KO mice were euthanized, and bone marrow cells were collected from bilateral femurs and tibias by removing the epiphyses and flushing the shafts with Iscove's modified Dulbecco's medium (IMDM; GIBCO Invitrogen, Carlsbad, CA) and 10% FBS (GIBCO Invitrogen) using a syringe with a 23-gauge needle. The cells were disaggregated by vigorous pipetting. The remaining clumps of tissue were removed by filtering the cells through a 30-μm nylon mesh. The filtered cells were washed with IMDM and centrifuged for 5 min at 300 g at 24°C, and the resulting cell pellet was resuspended and cultured in 25-cm2 culture flasks (Corning, Corning, NY) with IMDM and 10% FBS at 37°C. MSCs preferentially attached to the plastic surface of the flask. After 48 h, nonadherent cells in suspension were discarded. Complete medium was added (IMDM, 10% FBS and 1% penicillin-streptomycin) and replaced every 3 or 4 days thereafter. When the cultures reached 80–90% of confluence, the MSC was passaged. Cells were recovered by the addition of a solution of 0.25% trypsin-EDTA (GIBCO Invitrogen) and replated in 75-cm2 culture flasks. MSCs were restricted to passages 3–9 for all experiments. MSC cultures and experiments were maintained at 37°C in 5% CO2-95% room air.
Assessment of cell surface markers.
To assess the cell surface markers of our stem cell preparations, flow cytometry was used as previously described (29). The following antibodies were used: anti-CD45 (30-F11)-FITC, anti-CD90-phycoerythrin (PE), anti-stem cell antigen-1-PE (Sca-1; Ly6A/E), and anti-CD44-PE and the recommended isotype control for each fluorochrome (BD Biosciences Pharmingen, San Jose, CA). MSCs were harvested and incubated with the specific antibodies (1 μg/1 × 105 cells) for 30 min at 4°C in the dark. After incubation, the cells were washed with PBS and fixed in 1% formalin overnight. The cells were analyzed the next day using a FACSCalibur cytometer (BD Biosciences).
Differentiation experiments.
To investigate the ability of the MSCs to differentiate, a differentiation kit was used to induce adipogenesis and osteogenesis (R&D Systems, Minneapolis, MN). Per the manufacturer's instructions, MSCs were incubated with differentiation media and, after the designated incubation period, stained with anti-fatty acid-binding protein 4 (FABP4) for the adipogenesis group and anti-osteopontin for the osteogenesis experiment. MSCs were then incubated with a Northern Lights 557 (NL557; R&D)-conjugated secondary detection antibody. In addition to the negative control recommended by the kit, an additional negative control/group was added (MSCs with complete media, incubated with the primary and secondary antibodies from the kit). The nuclei of both groups were counterstained with 100 μl of Vectashield 4,6-diamidino-2-phenylindole (DAPI; Vector, Burlingame, CA). With the use of a Nikon TE2000U microscope (Nikon, Melville, NY), cell morphology and fluorescence were assessed at ×200 magnification. Images were digitized with QCapture (QImaging, Surrey, BC, Canada) and transferred to Adobe Creative Suite 4 (Adobe Systems, San Jose, CA).
Isolated heart (Langendorff) experiments.
All isolated rat hearts were subjected to the same I/R protocol: 15 min of equilibration, 1 min of an infusion treatment, 25 min of warm global ischemia (37°C), and 40 min of reperfusion. The rats were randomly assigned to one of three infusion treatments: 1) vehicle (n = 8), 2) one million MWT MSCs (n = 12), and 3) one million TLR2KO MSCs (n = 13). After recovery from the cell culture flask, MSCs were washed with PBS, centrifuged at 300 g, resuspended in warm (37°C) Krebs-Henseleit (KH) solution, and infused over 1 min through the aortic cannula into the coronaries. The total volume of each infusion was 1 ml. We have previously reported that MSCs are present in the myocardium using this protocol (42). Heart function was continuously recorded throughout the experiment.
Hearts were isolated as previously described (42). Rats were anesthetized (pentobarbital sodium, 60 mg/kg ip) and heparinized (500 units ip). The hearts were rapidly excised via median sternotomy and placed in an ice-cold modified KH solution containing (in mM) 11 dextrose, 110 NaCl, 1.2 CaCl2, 4.7 KCl, 20.8 NaHCO3, 1.18 KHPO4, and 1.17 MgSO4. The aorta was cannulated, and the heart was perfused in the constant-pressure, isovolumetric Langendorff mode with 37°C KH solution. The total ischemic time was <45 s. The perfusate was bubbled with 95% O2-5% CO2 and continuously filtered through a 0.45-μm filter. A pulmonary arteriotomy and left atrial appendage resection were performed, allowing the insertion of a water-filled latex balloon through the left atrium into the left ventricle. The left ventricular preload volume (balloon volume) was held constant during the entire experiment to allow for continuous recordings of the left ventricular developed pressure (LVDP). The balloon was adjusted to a mean left ventricular end-diastolic pressure of 8 mmHg (range 6–10 mmHg) during the equilibration period. Pacing wires were fixed to the right atrium and left ventricle, and hearts were paced at 6 Hz, 3 V, and 8 ms (∼350 beats/min) during equilibrium and reperfusion. A three-way stopcock above the aortic root was used to create global ischemia, during which the heart was placed in a 37°C organ bath. Coronary flow was measured by collecting pulmonary artery effluent. Data were continuously recorded with a PowerLab 8 preamplifier/digitizer (AD Instruments, Milford, MA) and a Mini Mac computer (Apple Computer, Cupertino, CA). Maximal positive and negative values of the first derivative of pressure (±dP/dt) were calculated with PowerLab software. After reperfusion, the heart was removed from the apparatus, sectioned, and snap frozen in liquid nitrogen.
Myocardial VEGF expression.
Six to eight hearts/group were homogenized for 2 min in a cold buffer solution consisting of 80% radioimmunoprecipitation assay buffer, 10% proteinase inhibitor cocktail, and 10% phosphatase inhibitors (Sigma Aldrich, St. Louis, MO). The homogenate was then centrifuged at 12,000 rpm for 5 min, and the total protein concentration was determined by the Bradford method using an Eppendorf biophotometer (Eppendorf, Westbury, NY). The production of VEGF (in pg/ml) was determined using a rat ELISA kit (R&D) according to the manufacturer's instructions. VEGF values were then normalized to protein concentration (in pg/mg of myocardial protein). All samples and standards were measured in duplicate.
MSC VEGF expression.
MWT and TLR2KO MSCs were plated in 12-well plates (Corning) at 0.1 × 106 cells per well in 1 ml of complete media. After 24 h of incubation, four wells were assigned to each treatment group: 1) complete media and 2) complete media + TNF (50 ng/ml). The cells were incubated for an additional 24 h. MSC-secreted VEGF was measured in the supernatant using an ELISA kit per the manufacturer's instructions (R&D). The concentrations of VEGF obtained were in picograms/milliliter of supernatant and normalized to folds of control. All samples and standards were measured in duplicate.
MSC proliferation assay.
Proliferation was measured using the 5-bromo-2′-deoxy-uridine labeling and detection kit III (BrdU; Roche Applied Science, Indianapolis, IN). The cells were plated at 5,000 cells/well on a 96-well culture plate (Corning) in 100 μl of complete medium. The cells were incubated at 37°C in 5% CO2-95% room air for 48 h with one media change after 24 h. The cells were then assayed for their ability to incorporate BrdU according to the manufacturer's instructions.
Presentation of data and statistical analysis.
Reported values represent means ± SE or folds of control. All measures of myocardial function were compared using ANOVA with post hoc Holms-Sidak test. Measures of protein expression were compared using the Student's t-test or ANOVA as appropriate. A probability value of <0.05 was considered statistically significant.
RESULTS
MWT and TLR2KO MSC cell surface marker expression.
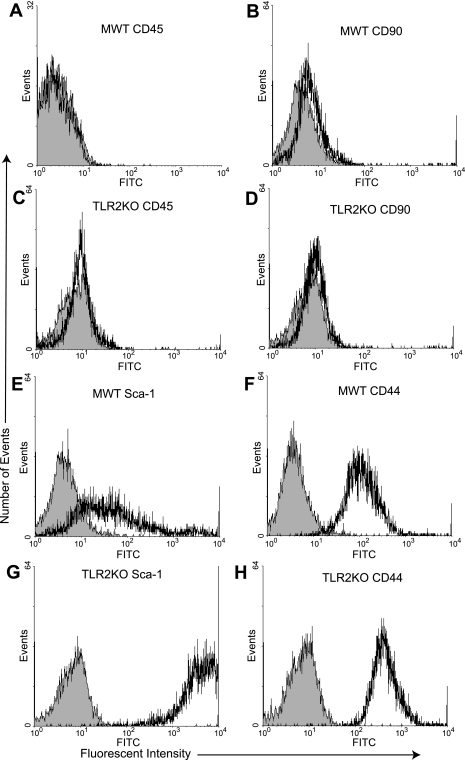
Flow cytometry was used to confirm that the isolated stem cell populations were not contaminated with hematopoietic stem cells and possessed markers consistent with MSCs. MWT and TLR2KO MSCs were >95% negative for CD45 and CD90, which are known to be negative in murine MSCs (28, 29) (Fig. 1, A–D). In contrast, MWT MSCs were positive for the stem cell markers Sca-1 and CD44 (Fig. 1, E and F). Similarly, TLR2KO MSCs were positive for Sca-1 and CD44 (Fig. 1, G and H) (5, 28, 29).
Fig. 1.
Male wild-type (MWT) and Toll-like receptor 2 knockout (TLR2KO) mesenchymal stem cell (MSC) expression of cell surface markers. Plastic-adherent MSCs isolated from the bone marrow of adult MWT and TLR2KO mice are >95% negative for CD45 (FITC) and CD90 (phycoerythrin), 2 non-MSC markers by flow cytometry (A and B, and C and D). MSCs isolated from MWT and TLR2KO mice expressed both stem cell antigen-1 (Sca-1; E and G) and CD44 (F and H). The shaded areas denote the isotype controls. The intensity of Sca-1 staining (x-axis) was greater in the TLR2KO MSC group compared with the MWT MSC group.
MSC adipogenic and osteogenic differentiation potential.
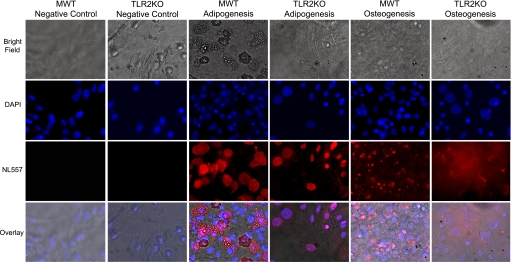
After incubation with either adipogenic or osteogenic differentiation media, MWT and TLR2KO MSCs expressed FABP4 and osteopontin, indicating that both cell types are able to differentiate into adipocytes and osteocytes (Fig. 2). There were no visible vacuoles present in the TLR2KO adipogenesis group.
Fig. 2.
Multipotency of MWT and TLR2KO MSCs. MWT and TLR2KO MSCs after incubation with adipogenic or osteogenic differentiation media expressed fatty acid-binding protein 4 or osteopontin markers of adipogenesis and osteogenesis respectively [Northern Lights 577 (NL557)-positive red staining]. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). There were no visible vacuoles present in the TLR2KO adipogenesis group. Representative fields at ×200 magnification are shown. (Per APS Ethical Policy on figure reproduction, contrast adjustment was applied to the entire image in Adobe Photoshop because of interface issues with QCapture and Adobe Photoshop software.)
Effect of TLR2 on MSC-mediated myocardial recovery after I/R.
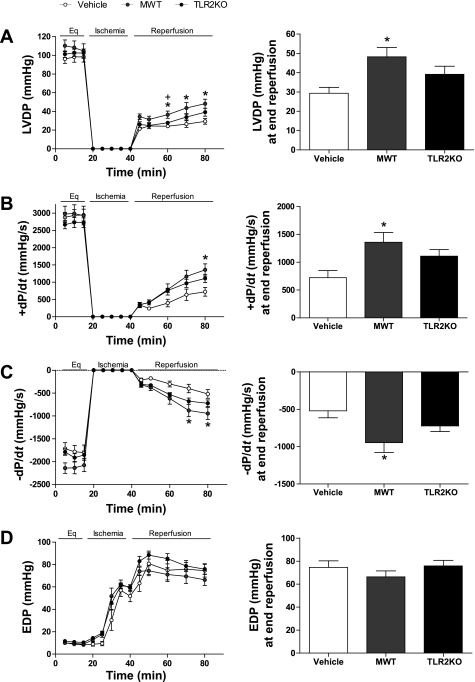
I/R resulted in markedly decreased LVDP in all groups compared with the baseline LVDP. However, postischemic recovery of LVDP was significantly higher in hearts infused with preischemic MWT MSCs compared with vehicle hearts at end reperfusion (48.21 ± 4.81 vs. 29.31 ± 3.1 mmHg at end reperfusion; 46% vs. 31% recovery of the baseline equilibration LVDP). The infusion of TLR2KO MSCs did increase LVDP recovery (36.66 ± 3.66 mmHg at end reperfusion; 37% recovery of baseline); however, this trend was not statistically different from the vehicle (Fig. 3A). Postischemic recovery of +dP/dt was significantly greater in the MWT MSC group compared with vehicle at end reperfusion (1357.21 ± 117.76 vs. 721.80 ± 127.8 mmHg/s: 48% vs. 25% recovery of baseline, respectively) (Fig. 3B). The infusion of TLR2KO increased +dP/dt but not to a statistically significant degree (1109.65 ± 118.14 mmHg/s; 41% recovery of baseline) (Fig. 4B). Similar to +dP/dt, MWT MSCs improved the recovery of −dP/dt at end reperfusion compared with the vehicle (−946.68 ± 129.47 vs. −519.10 ± 93.50 mmHg/s; 45% vs. 30% of baseline, respectively). TLR2KO MSCs also improved the recovery of −dP/dt but not significantly (−722.69 ± 74.30 mmHg; 38% recovery of baseline) (Fig. 3C). There were no significant differences in the mean end-diastolic pressure among the groups (Fig. 3D).
Fig. 3.
Effects of preischemic MSC infusion on myocardial function. Ischemia-reperfusion injury resulted in markedly decreased left ventricular developed pressure (LVDP) in all groups. Postischemic recovery of LVDP was significantly greater from 20 min postischemia to end reperfusion in hearts infused with MWT MSCs compared with vehicle hearts (A). Recovery of maximal positive and negative values of the first derivative of pressure (±dP/dt) was also significantly increased in the MWT MSC-treated hearts compared with the vehicle (B and C). Infusion of TLR2KO MSCs did increase LVDP and ±dP/dt, however, this trend did not reach significance (A–C). The end-diastolic pressure (EDP; D) was not significantly different among the groups. There was no significant difference in the end equilibrium (Eq) measurements of the groups (n = 8 vehicle, 12 MWT, and 13 TLR2KO-infused hearts). *P < 0.02, MWT MSC-treated hearts vs. vehicle hearts; +P < 0.03, MWT vs. TLR2KO MSC-treated hearts (ANOVA with post hoc Holms-Sidak).
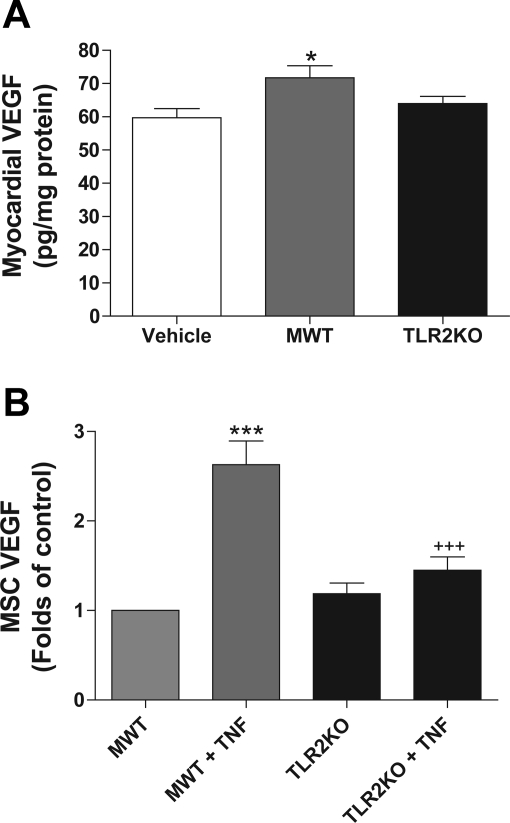
Fig. 4.
TLR2 effects on VEGF production. Preischemic treatment of the heart with MWT MSCs increased production of myocardial VEGF following ischemia-reperfusion injury (A). VEGF results are expressed as pg/mg of protein of myocardial homogenate. *P < 0.05, MWT vs. vehicle-treated hearts; n = 6–8 hearts/group. In culture, basal production of VEGF is similar between MWT and TLR2KO MSCs (B). However, with TNF activation, MWT MSCs produced significantly more VEGF (∼2.6 × control), whereas activation of TLR2KO MSCs did not significantly change VEGF production (∼1.3 × control). ***P < 0.001, MWT + TNF vs. MWT; +++P < 0.001, MWT + TNF vs. TLR2KO + TNF (ANOVA with post hoc Holm-Sidak).
Myocardial VEGF response to stem cell infusion and I/R.
MWT MSC-treated hearts produced significantly more VEGF (71.6 ± 3.7 pg/mg of myocardial protein) compared with the vehicle group (59.7 ± 2.7 pg/mg of myocardial protein) (Fig. 4A) (P < 0.04). There was a trend toward an increased myocardial VEGF production in the TLR2KO MSC group (65.1 ± 2.3 pg/mg of myocardial protein); however, this trend was not significant compared with that in the vehicle or MWT group.
MSC expression of VEGF.
Stem cell production of paracrine factors including VEGF is an important component of stem cell-mediated repair. To determine whether differences in VEGF production exist between MWT and TLR2KO MSCs, basal and stimulated VEGF production were measured (Fig. 4B). The basal production of VEGF was not significantly different between the two groups. However, TNF activation of MWT MSCs resulted in a 2.6× increase in VEGF production compared with baseline, whereas TNF activation of TLR2KO MSCs did not result in a significant increase in VEGF production.
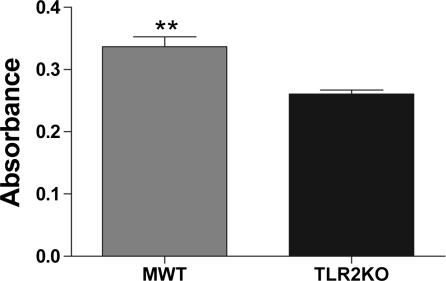
TLR2-deficiency results in decreased MSC proliferation.
In culture, MWT MSCs appeared to proliferate more rapidly than TLR2KO MSCs. To quantify this, we used a BrdU assay and observed that MWT MSC incorporated more BrdU than TLR2KO MSCs, supporting our observation that MWT proliferate more rapidly than TLR2KO MSCs (Fig. 5).
Fig. 5.
TLR2 effects on MSC proliferation. TLR2KO MSCs proliferated less rapidly compared with MWT MSCs after 48 h of incubation in complete media as measured by 5-bromo-2′-deoxy-uridine (BrdU) incorporation. Absorbance is measured at a wavelength of 405 nm with a reference wavelength of 490 nm. **P < 0.01, MWT vs. TLR2KO MSCs (Student's t-test).
DISCUSSION
Stem cell therapy is an active area of research for numerous clinical conditions such as ischemic heart disease, peripheral arterial disease, kidney disease, and sepsis (21, 26, 31, 34). The acute benefits of stem cells in response to injury have been increasingly linked to the paracrine actions of these cells (20, 41). We and others have reported that hearts treated with MSCs display improved functional recovery in association with increased paracrine growth factor production (e.g., VEGF) (9, 15, 23). Thus one important task for stem cell researchers is to elucidate those factors that negatively or positively affect stem cell paracrine function. In other words, can the paracrine effects of a stem cell be improved?
TLRs are key components of the innate immune response and play a significant role in modulating inflammation (8, 12, 13, 39). The activation of TLR2, in particular, has been linked to endothelial dysfunction, decreased infarct size (11), cardiomyocyte inflammation, and TNF-mediated contractile dysfunction (33, 45). However, TLR2 has also been implicated in protecting cardiomyocytes from apoptosis (14). TLR2 is present on the surface of MSCs (29); however, the role of TLR2 with respect to MSC-derived cardioprotection and paracrine signaling is unknown. Given the preponderance of data linking TLR2 to endothelial and cardiomyocyte dysfunction, we hypothesized that TLR2KO would increase MSC-derived cardioprotection and paracrine production of VEGF.
To determine how TLR2 might affect MSC function, we used MWT and TLR2KO MSCs. One of the challenges of using bone marrow-derived stem cells is the heterogeneity of the stem cells that are isolated from the bone marrow. In addition, MSCs are rare, comprising <0.01% of the nucleated cells in the bone marrow (30). As such it is important to confirm that the cells from the bone marrow are multipotent and not contaminated with other stem cells. Before performing our experiments, we verified the phenotype of our MSC isolates using a combination of positive and negative cell surface markers based on previously published reports. Both the MWT and TLR2KO cells were >95% negative for CD11b (data not shown) and CD45, which are two known markers of hematopoietic stem cells (29). In addition, <5% of the isolates expressed CD90 (5). However, both cell lines expressed Sca-1 and CD44, consistent with prior published results (5, 29). The multipotency of both MWT and TLR2KO MSCs was confirmed when the cell lines were induced to express cell surface markers consistent with adipocytes and osteocytes.
After the MSC phenotype of these cells was confirmed, their cardioprotective abilities were evaluated in a model of isolated heart perfusion. We chose this model because of its unique ability to investigate the effects of MSC treatment on the heart after global I/R, a scenario that is clinically applicable to cardiac surgery. Our myocardial functional findings constitute the initial observation that TLR2KO on MSCs impaired stem cell-mediated protection of the heart after acute global I/R, which was contrary to our hypothesis. Similar to our prior results, the preischemic treatment of hearts with MWT MSCs improved the functional recovery after I/R as measured by LVDP and ±dP/dt (42, 44). While treatment with TLR2KO MSCs did improve myocardial recovery, this trend was not statistically different from the vehicle hearts. This suggests that contrary to the literature describing the deleterious role of TLR2 on endothelial cells and cardiomyocytes, TLR2 is needed for the functional recovery mediated by MSCs after acute global I/R injury.
We then directed our attention to determining the role of VEGF in mediating the observed functional results. The critical role of VEGF in MSC-mediated myocardial protection was previously indicated by the impairment of MSC-mediated cardioprotection following the knockdown of VEGF in these cells (23). In addition, TNF, a proinflammatory cytokine, is released in response to myocardial ischemia and has been shown to induce the MSC production of VEGF (9, 24, 25). In the present experiments, we again observed that TNF augmented VEGF release in MWT MSCs but not TLR2KO MSCs. Thus the inability of TLR2KO MSCs to increase VEGF production in response to TNF may partially explain the impaired functional recovery observed compared with MWT MSC-treated hearts. A similar trend was observed with respect to myocardial VEGF production in that hearts treated with MWT MSCs produced more VEGF than the vehicle hearts similar to previous results (9). Taken together, the decreased levels of VEGF production by TLR2KO MSCs and by hearts treated with TLR2KO may partially explain the decreased functional recovery. The mechanism linking TLR2 and VEGF production in MSCs is unknown. However, it has been reported that TLR2 mediates VEGF production via ERK- and activator protein-1-dependent pathways (40), which needs further focus in future investigations. The increased levels of VEGF may lead to increased activation of VEGF receptor 1 (VEGFR1) on the heart. VEGFR1 has been shown to be critical in knockout studies of myocardial VEGFR1 where knockout has resulted in decreased myocardial recovery after global I/R (2). However, further mechanistic studies are needed to fully elaborate this pathway and whether or not it is a factor in stem cell-mediated myocardial protection.
While the impairment of TLR2KO MSC-mediated cardioprotection may be partly due to decreased VEGF, another explanation may be related to an alteration in multipotency. TL2KO MSCs expressed FABP4, consistent with differentiation into adipocytes; however, intracellular lipid vacuoles were not seen as in the MWT adipocyte group. It is possible that TLR2KO alters as yet undiscovered signaling pathways that lead to this phenotypic alteration. Supporting this hypothesis is a report that a deficiency of TLR2 in neural progenitor cells impaired terminal differentiation (32). The role of TLR2 with respect to stem cell differentiation potential is not clear-cut, however, since the activation of TLR2 has also been reported to inhibit MSC differentiation (29). Future experiments using the coronary artery ligation model of chronic myocardial ischemia would be better suited to investigate these aspects.
One additional piece of information supporting the important role of TLR2 for MSC function is our observation that the TLR2KO MSCs proliferate less rapidly than their MWT counterpart in culture. Our results are consistent with a previous report that the activation of TLR2 increases MSC proliferation (29). MSC survival in vivo may be related to the ability of the stem cells to proliferate after transplantation in the heart. This hypothesis is supported by the observation that an increased proliferation of MSCs is associated with an improved myocardial function following chronic ischemia/infarction after stem cell treatment (10). While it is unlikely that proliferation occurs in the present model of acute I/R, the modulation of the TLR2 signaling pathway may be a potential strategy for optimizing the benefits of stem cell therapy for chronic myocardial ischemia.
Our results add to the growing body of knowledge surrounding MSC-mediated cardioprotection. At present, MSCs may be a lead candidate for stem cell-based therapy as they are thought to possess unique properties such as immunoprivilege, which may allow them to modulate the recipient's immune response (4). Given the increasing evidence supporting the link of exogenous treatment with paracrine growth factors with improved myocardial function (3, 18, 22), it is reasonable to investigate the manipulation of MSCs to alter their production of paracrine factors. We and others have reported that MSCs can be altered to produce increased paracrine factors with associated improved myocardial function (10, 19, 41, 43). We now add our observation that MSC TLR2 is beneficial in the acute setting for preischemic stem cell treatment of myocardial I/R and VEGF production. This observation was contrary to our hypothesis that the presence of TLR2 on the MSCs would impair MSC paracrine function. The preischemic infusion or injection of MSCs may yield acute benefits in myocardial VEGF production as well as function that may hold clinical significance for procedures that use periods of ischemia such as cardiac surgery, angioplasty, or transplantation. Therefore, improving our understanding of the receptors involved in MSC signaling may lead to novel strategies that optimize the beneficial effects of MSCs. A continued investigation into the downstream signaling involved with TLR2 and the effects of other TLRs is needed to delineate the complex pathways involved with MSC-derived cardioprotection.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-GM-070628 and R01-HL-085595 (to D. R. Meldrum), 1F32-HL-092718 (to A. M. Abarbanell), 1F32-HL-092719 (to J. L. Herrmann), and 1F32-HL-093987 (to B. R. Weil).
DISCLOSURES
None.
REFERENCES
- 1.Abarbanell AM, Coffey AC, Fehrenbacher JW, Beckman DJ, Herrmann JL, Weil B, Meldrum DR. Proinflammatory cytokine effects on mesenchymal stem cell therapy for the ischemic heart. Ann Thorac Surg 88: 1036–1043, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Addya S, Shiroto K, Turoczi T, Zhan L, Kaga S, Fukuda S, Surrey S, Duan LJ, Fong GH, Yamamoto F, Maulik N. Ischemic preconditioning-mediated cardioprotection is disrupted in heterozygous Flt-1 (VEGFR-1) knockout mice. J Mol Cell Cardiol 38: 345–351, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Askari A, Unzek S, Goldman CK, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Cellular, but not direct, adenoviral delivery of vascular endothelial growth factor results in improved left ventricular function and neovascularization in dilated ischemic cardiomyopathy. J Am Coll Cardiol 43: 1908–1914, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Atoui R, Shum-Tim D, Chiu RC. Myocardial regenerative therapy: immunologic basis for the potential “universal donor cells”. Ann Thorac Surg 86: 327–334, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89: 1235–1249, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyocytes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res 72: 384–393, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Carr CA, Stuckey DJ, Tatton L, Tyler DJ, Hale SJ, Sweeney D, Schneider JE, Martin-Rendon E, Radda GK, Harding SE, Watt SM, Clarke K. Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: an in vivo cine-MRI study. Am J Physiol Heart Circ Physiol 295: H533–H542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crisostomo PR, Abarbanell AM, Wang M, Lahm T, Wang Y, Meldrum DR. Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. Am J Physiol Heart Circ Physiol 295: H1726–H1735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC, Robbins RC, Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation 120: S247–S254, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol 27: 1064–1071, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med 4: 444–454, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Frantz S, Ertl G, Bauersachs J. Toll-like receptor signaling in the ischemic heart. Front Biosci 13: 5772–5779, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem 276: 5197–5203, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata J, Umezawa A. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res 288: 51–59, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Guzman MJ, Crisostomo PR, Wang M, Markel TA, Wang Y, Meldrum DR. Vascular endothelial growth factor improves myocardial functional recovery following ischemia/reperfusion injury. J Surg Res 150: 286–292, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Haider HK, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ Res 103: 1300–1308, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109: 1543–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation 109: 2692–2697, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Luo Z, Diaco M, Murohara T, Ferrara N, Isner JM, Symes JF. Vascular endothelial growth factor attenuates myocardial ischemia-reperfusion injury. Ann Thorac Surg 64: 993–998, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol 295: H2308–H2314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol 274: R577–R595, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Meldrum DR, Meng X, Dinarello CA, Ayala A, Cain BS, Shames BD, Ao L, Banerjee A, Harken AH. Human myocardial tissue TNFalpha expression following acute global ischemia in vivo. J Mol Cell Cardiol 30: 1683–1689, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol 84: 333–341, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109: 1422–1432, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95: 9–20, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Raeburn CD, Zimmerman MA, Arya J, Banerjee A, Harken AH. Stem cells and myocardial repair. J Am Coll Surg 195: 686–693, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9: 1081–1088, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H503–H509, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J 24: 514–525, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept 117: 3–10, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1: 129–137, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98: 1414–1421, 2006 [DOI] [PubMed] [Google Scholar]
- 39.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31: 280–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varoga D, Paulsen F, Mentlein R, Fay J, Kurz B, Schutz R, Wruck C, Goldring MB, Pufe T. TLR-2-mediated induction of vascular endothelial growth factor (VEGF) in cartilage in septic joint disease. J Pathol 210: 315–324, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Tan J, Wang Y, Meldrum KK, Dinarello CA, Meldrum DR. IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc Natl Acad Sci USA 106: 17499–17504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock 25: 454–459, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Zeller CN, Wang Y, Markel TA, Weil B, Abarbanell A, Herrmann JL, Kelly ML, Coffey A, Meldrum DR. Role of tumor necrosis factor receptor 1 in sex differences of stem cell mediated cardioprotection. Ann Thorac Surg 87: 812–819, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, Chao W, Hellman J, Schmidt U. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med 35: 886–892, 2007 [DOI] [PubMed] [Google Scholar]