Abstract
Though many consider the magnitude of respiratory sinus arrhythmia as an index of cardiac vagal control, its physiological origins remain unclear. One influential model postulates that the systolic pressure rise within a given beat stimulates the baroreflex arc to adjust the following heart period such that diastolic pressure is “stabilized” and hence displays lesser fluctuation. Accordingly, the magnitude of diastolic pressure fluctuations with respiration should change reciprocally after augmentation or inhibition of respiratory sinus arrhythmia. To test this, we augmented and subsequently inhibited respiratory sinus arrhythmia with vagotonic and vagolytic atropine administration in 19 healthy young volunteers to assess the relation between respiratory R-R interval and diastolic pressure fluctuations. Respiratory diastolic pressure fluctuations showed parallel rather than inverse changes in relation to those in respiratory sinus arrhythmia: they increased with augmented respiratory sinus arrhythmia (138 and 190% of baseline in the frequency and time domains, both P < 0.05) and tended to decrease with inhibited respiratory sinus arrhythmia (82 and 93% of baseline in frequency and time domains, P = 0.20 and P = 0.07). Furthermore, >60% of the change in diastolic pressure fluctuations was explained by the change in respiratory sinus arrhythmia (R2 = 0.62; P < 0.001), that is, an ∼50-ms increase or decrease in respiratory sinus arrhythmia resulted in a parallel ∼1-mmHg change in diastolic pressure fluctuations. Thus, in young healthy individuals during supine rest, respiratory fluctuations in R-R interval do not buffer against diastolic pressure fluctuations but actually cause diastolic pressure fluctuations. Therefore, our data provide little evidence for a predominant role of a baroreflex feedback mechanism underlying respiratory sinus arrhythmia during supine rest.
Keywords: R-R interval, baroreflex, diastolic blood pressure, atropine
since the first observation over 150 years ago that R-R interval shortened during inspiration and lengthened during expiration (23), respiratory sinus arrhythmia (RSA) has been widely studied. The cardiac vagal role in the genesis of RSA is known (1), and, although there may be some effect of sympathetic tone (39), many consider the magnitude of RSA as an index of cardiac vagal control (10, 16). However, the physiological origins of the waxing and waning vagal outflow responsible for RSA remain unclear and under some dispute (8, 15).
One influential model of RSA is based on the observation that changes in systolic pressure during respiration are relatively larger than those in diastolic pressure (6). This has been proposed to reflect the fact that the systolic pressure rise within a given beat stimulates the baroreflex arc to adjust the timing of the following R-wave such that diastolic pressure is “stabilized” and hence displays lesser fluctuation (15, 36). This conceptual model cites an obvious mechanism for the generation of RSA, the arterial baroreflex, and relies upon evidence for this hypothesis from animal experiments. Baroreceptor denervation substantially reduces RSA in conscious dogs (29) and substantially increases arterial pressure variability in dogs, baboons, and rats (5, 13, 31, 35). On the other hand, some evidence does suggest that respiratory fluctuations in arterial pressure might be secondary to RSA, contrary to a baroreflex mechanism. For example, in rats, vagal blockade abolishes respiratory fluctuations in R-R interval without any significant change in arterial pressure fluctuations (14).
Because of the limited experimental approaches available, the majority of work in humans pursuing the mechanism(s) underlying RSA has relied most upon noninvasive, inferential approaches, such as spectral analysis of blood pressure and R-R interval fluctuations (4, 12, 18, 25, 27). Results from these studies have been interpreted to suggest a prominent baroreflex role in the genesis and/or the modulation of RSA. For example, when sinusoidal stimulation is applied to the carotid baroreceptors in phase with respiration via neck suction, RSA spectral power is augmented, but when the same stimulus is applied out of phase, spectral power is reduced (although the intervention had no effect on diastolic pressure fluctuations) (18, 27). On the other hand, fixed-rate atrial pacing, which removes R-R interval variability, reduces diastolic pressure fluctuations at the respiratory frequency in supine subjects (38). This would suggest that RSA could augment rather than stabilize diastolic pressure fluctuations. However, these studies investigated the baroreflex role by opening the “closed-loop” system and engaging the reflex actively via neck suction or eliminating the reflex artificially via a “nonphysiological” intervention. By their very nature, these approaches could alter the intrinsic relation between blood pressure and R-R interval fluctuations.
The model of baroreflex-generated RSA (15, 36) suggests that, if the ability of the closed-loop system to generate RSA is either enhanced or depressed, the mechanism for RSA can be inferred from the resultant impact on diastolic pressure fluctuations. However, there are no studies that have manipulated the system without opening the closed loop. Because cardiac vagal tone is primarily responsible for genesis of RSA (1, 28), the closed-loop system can be manipulated by atropine administration. In fact, atropine has a primarily parasympathomimetic (vagotonic) effect at small doses (2), whereas, at larger doses, it has parasympatholytic (vagolytic) effects (20, 30) allowing both augmentation and inhibition of cardiac vagal tone, thus, in turn, augmenting and inhibiting RSA. Therefore, we tested the hypothesis that, if baroreflex control stabilizes diastolic pressure at the respiratory frequency, the magnitude of diastolic pressure fluctuations with respiration should change reciprocally after augmentation and inhibition of RSA with vagotonic and vagolytic atropine administration.
MATERIALS AND METHODS
Subjects.
Nineteen young healthy volunteers, aged 21–30 yr (mean age 24 ± 3 yr, 9 females) participated in this study. All volunteers were normal weight, free from overt cardiovascular disease, neurological disease, diabetes mellitus, obesity, and hypertension as determined by health history questionnaires, fasting blood chemistries, anthropometric measurements, and resting and maximal exercise hemodynamics and electrocardiograms (ECGs). No volunteer was taking any cardioactive medications or used tobacco products, and all volunteers were instructed to refrain from consuming caffeine and alcohol and from performing any vigorous physical activity for 24 h before testing. The study was approved by the Institutional Review Board at Spaulding Rehabilitation Hospital, and all subjects gave written informed consent.
Experimental protocol.
Subjects reported to the laboratory between 8:00 A.M. and 10:00 A.M. following a 12-h overnight fast. Following instrumentation and insertion of an antecubital venous catheter for atropine administration, subjects rested in the supine position for at least 15 min in a quiet room with dimmed lights. Subsequently, data were collected at baseline and after eight bolus doses of atropine sulfate (cumulative doses of 0.4, 0.8, 1.4, 2.2 3.2, 4.4, 5.8, and 7.2 μg/kg). After each atropine administration, 3 min were given for development of full drug effect followed by a 3-min data collection period in which subjects controlled their breathing frequency at a rate of 15 breaths/min (0.25 Hz) in response to an auditory cue. Breathing depth and frequency were monitored and recorded by a respiratory transducer band around the midchest. Inspiratory and expiratory durations were controlled and were equal (2 s each). Three minutes of data provide a sufficient number of breaths for reliable data analysis at this breathing frequency. R-R interval was recorded using ECG lead II. Beat-by-beat finger photoplethysmographic (Finapres; Omheda) arterial pressure was recorded in the left hand. To verify the accuracy of beat-by-beat blood pressure measurements, brachial arterial blood pressure was recorded from the right arm by an automated brachial cuff (Dinamap; Critikon) in the last minute of each 3-min data collection period. Atropine dosing was stopped if heart rate increased >10 beats/min from baseline or the subject reported symptoms of systemic cholinergic blockade (e.g., dry mouth).
Three sets of data were used for the subsequent analysis for each individual: data collected 1) before atropine administration (baseline), 2) following administration of the atropine dose that induced the highest respiratory frequency spectral power (peak RSA) for that subject, and 3) following the dose that induced the lowest respiratory frequency spectral power (minimum RSA) for that subject. Three individuals did not show any decrease in RSA below baseline and thus were excluded from comparisons between baseline and minimum RSA.
Data analysis.
Data were digitized and stored on computer at a sampling rate of 1,000 Hz (Power Lab; ADInstruments). R-R intervals were determined from ECG recordings using a peak detection algorithm custom written in Matlab (version 7.4; Mathworks, Natick, MA) and were visually inspected for artifacts and errors. R-R interval rather than heart rate was used to assess responses, since it most linearly represents changes in parasympathetic chronotropic effect (19, 26). Paced breathing segments were used for all data analysis. The R-R interval power spectral densities were calculated with fast-Fourier transforms based on Welch's periodogram algorithm on 4-Hz resampled R-R interval time series. RSA was assessed as the spectral power at the breathing frequency (0.25 Hz). This measure has been proposed as a standard and valid measure of cardiac vagal effects (37).
Mean, systolic, and diastolic pressures were obtained from the continuous beat-by-beat blood pressure waveform over the 3-min data collection period. To assess the variability in arterial pressure and R-R intervals, we used both spectral characterization and a time-domain signal-averaging approach. Spectral powers at the breathing frequency in diastolic and systolic pressure were calculated as described above. For time-domain signal-averaging, each respiratory cycle was determined from the respiratory waveform, and R-R intervals and diastolic pressures within each respiratory cycle were aligned relative to inspiration onset. As mentioned above, the ratio of inspiration to expiration time was equal; thus, aligning the waveforms relative to expiration would produce the same results. Because heart beats (therefore, R-waves and nadirs in blood pressure) can occur at different times relative to inspiration onset, tachograms of R-R interval and diastolic pressure were constructed for each respiratory cycle. Tachograms rather than linear interpolation were used since the latter can result in superfluous values, especially when unusually long or short R-R intervals occur. Subsequently, the tachograms were averaged across all respiratory cycles to obtain an average respiratory excursion in R-R interval and diastolic pressure. Respiration-induced R-R interval and diastolic pressure fluctuations were calculated as the range (maximum-minimum) in the average tachogram.
Statistics.
Respiratory powers were log-transformed (to ensure normality) and compared via a two-tailed paired t-test. Average R-R interval, mean, systolic, and diastolic pressure, and respiratory fluctuations in systolic and diastolic pressure and in R-R interval were similarly compared with a two-tailed paired t-test. The relations between the changes in respiratory fluctuations in R-R intervals and in diastolic pressure from baseline to peak RSA and from baseline to minimum RSA were assessed via a linear regression. Because each individual was included in the regression two times, the assumption of independence for a linear regression could be violated, so we performed a chi-square test of independence to determine whether changes from baseline to peak RSA and from baseline to minimum RSA were independent. The result demonstrated that they were (degrees of freedom = 288, X2 = 304, P = 0.25) and so a simple linear regression was performed on all data. Values are presented as means ± SD, and differences were considered statistically significant when P < 0.05.
RESULTS
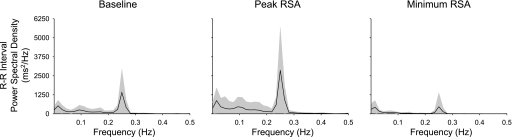
The cumulative atropine dose that resulted in peak RSA ranged from 0.4 to 3.2 μg/kg (mean 1.37 μg/kg, 1.4 μg/kg in half of the subjects, and 3.2 μg/kg in only one subject), and the cumulative dose that resulted in minimum RSA ranged from 3.2 to 7.2 μg/kg (mean 6.17 μg/kg, 7.2 μg/kg in 59% of the subjects). For all subjects, the atropine dose causing peak RSA was always less than the dose causing minimum RSA. Although we did not measure ventilation directly, we compared the spectral power of respiration for each subject during baseline and during peak and minimum RSA. Administration of vagotonic or vagolytic doses of atropine did not alter respiratory depth or frequency compared with baseline (2-tailed paired t-test, P > 0.40). As expected, the spectral estimate of RSA increased from baseline to peak, almost doubling (t = 6.42; P < 0.05), and decreased from baseline to minimum, reducing by ∼40% (t = 6.15, P < 0.05; Fig. 1 and Table 1). Mean R-R interval increased during peak RSA and decreased during minimum RSA in all subjects (t = 8.30 and t = 2.16, P < 0.05; see Table 1). Diastolic, systolic, and mean pressures did not change significantly at peak RSA but were increased at minimum RSA (see Table 1).
Fig. 1.
Average power spectral density of R-R interval at baseline (left) and during peak (middle) and minimum (right) respiratory sinus arrhythmias (RSA). Shaded areas show SDs.
Table 1.
Hemodynamic variables at baseline and during peak and minimum RSA
| Baseline | Peak RSA | Minimum RSA | |
|---|---|---|---|
| Heart rate, beats/min | 60.7 ± 11.0 | 54.4 ± 8.13* | 67.0 ± 14.7* |
| Diastolic BP, mmHg | 61.9 ± 8.3 | 63.5 ± 9.3 | 74.8 ± 13.7* |
| Systolic BP, mmHg | 111.5 ± 11.5 | 113.0 ± 14.9 | 125.2 ± 17.5* |
| Mean BP, mmHg | 78.7 ± 8.7 | 80.4 ± 11.0 | 92.8 ± 14.8* |
| RSA power, ms2 | 1,347 ± 1,474 | 2,877 ± 2,664* | 492 ± 951* |
| Respiratory R-R interval fluctuations, ms | 106.8 ± 59.3 | 158.4 ± 76.5* | 56.2 ± 51.2* |
| Respiratory diastolic BP power, mmHg2 | 0.40 ± 0.45 | 0.80 ± 1.03* | 0.33 ± 0.45 |
| Respiratory diastolic BP fluctuations, mmHg | 2.68 ± 0.94 | 3.69 ± 1.52* | 2.51 ± 0.82† |
| Respiratory systolic BP power, mmHg2 | 1.74 ± 1.76 | 1.61 ± 1.42 | 1.81 ± 2.04 |
| Respiratory systolic BP fluctuations, mmHg | 4.75 ± 2.11 | 4.33 ± 1.58 | 5.16 ± 2.66 |
Values are means ± SD. RSA, respiratory sinus arrhythmia; BP, blood pressure.
P < 0.05 and
P = 0.07 compared with baseline.
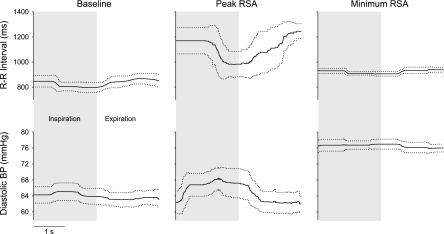
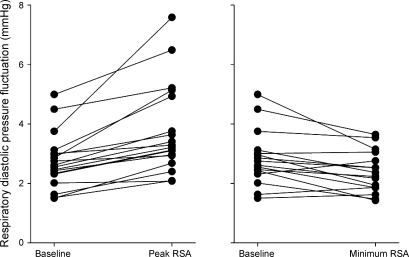
Figure 2 shows an example of the time domain-averaged RSA and the related diastolic pressure fluctuations for a representative subject. As assessed in the time domain, arterial pressure fluctuations preceded R-R interval fluctuations in all individuals at baseline, in all but one individual during peak RSA and in 13 out of 16 individuals during minimum RSA. Similar to the spectral power, the time domain estimate of RSA significantly increased (t = 5.66; P < 0.05) and subsequently decreased (t = 5.47, P < 0.05 compared with baseline; Table 1) with atropine. Respiratory-mediated fluctuations in diastolic pressure showed parallel rather than inverse changes in relation to those in RSA. Diastolic pressure fluctuations increased with augmented RSA in all 19 subjects, assessed in either the time domain (138% of the baseline; t = 5.01; P < 0.05; Fig. 3 and Table 1) or the frequency domain (190% of the baseline; t = 4.01, P < 0.05; Table 1). Similarly, diastolic pressure fluctuations were reduced with the inhibited RSA in all but five subjects when assessed in the time domain (93% of the baseline; t = 1.92, P = 0.07; Fig. 3 and Table 1) and tended to be reduced when assessed in the frequency domain (82% of the baseline; t =1.34, P = 0.20; Table 1). Despite the change in diastolic pressure fluctuations, there were no statistically significant changes in systolic pressure fluctuations (P > 0.36) or in their spectral power (baseline vs. peak RSA: t = 0.20, P = 0.84; baseline vs. minimum RSA: t = 1.00, P = 0.33; Table 1). Furthermore, changes in systolic pressure fluctuations were not consistent across individuals, with roughly half of the individuals demonstrating consistent changes after vagotonic or vagolytic atropine.
Fig. 2.
A representative example of respiratory fluctuations, averaged over all respiratory cycles for one individual, in R-R interval (top) and diastolic blood pressure (bottom) at baseline (left) and during the peak (middle) and minimum (right) RSA. Dotted lines show SDs.
Fig. 3.
The change in respiratory fluctuations in diastolic blood pressure after augmentation and inhibition of vagally mediated oscillations in R-R interval.
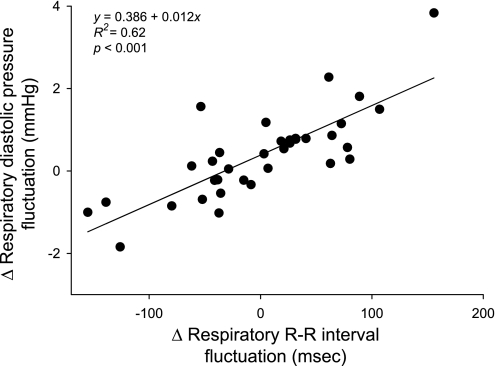
Linear regression showed a strong relationship between changes in RSA and in diastolic pressure fluctuations across subjects. In fact, >60% of the change in diastolic pressure fluctuations could be explained by the change in RSA (R2 = 0.62; P < 0.001; Fig. 4). Furthermore, regression analysis revealed that a 50-ms change in RSA resulted in an ∼1-mmHg change in diastolic pressure fluctuations.
Fig. 4.
Linear relation between the change in respiratory R-R interval and diastolic blood pressure fluctuations across subjects.
DISCUSSION
Presumably, a primary mechanism for the genesis of RSA is baroreflex-mediated modulation of cardiac vagal efferent traffic. Respiratory changes in intrathoracic pressure result in stroke volume fluctuations (11) that are transmitted into arterial pressure fluctuations. Consequently, it has been suggested that, during respiration, baroreflex-mediated lengthening and shortening of R-R interval follow fluctuations in stroke volume and systolic pressure and dampen those in diastolic pressure (6). Therefore, lesser spectral power in diastolic pressure (relative to that in R-R interval or in systolic pressure) at the respiratory frequency could be cited as evidence of a predominant baroreflex-mediated mechanism [e.g., Karemaker (15)]. Consistent with this mechanism, we observed that arterial pressure fluctuations preceded R-R interval fluctuations in all individuals at baseline, and in a majority of individuals during peak and minimum RSA. This phase relation between arterial pressure and R-R interval fluctuations is, in fact, compatible with a baroreflex-mediated phenomenon. However, this observation alone does not necessarily indicate a causal link but provides only inferential evidence, at best. Indeed, in contrast to the expected consequence of this temporal relation (that baroreflex-mediated lengthening and shortening of R-R interval will follow fluctuations in systolic pressure and dampen those in diastolic pressure), our data show that, during supine rest, diastolic pressure fluctuations change in parallel with RSA when the ability of the closed-loop system to generate vagally mediated fluctuations is either enhanced or depressed. Therefore, our findings contradict a dominant baroreflex mechanism for RSA during supine rest in young healthy individuals.
Our results are in contrast to prior studies of baroreceptor denervation and arterial pressure control in animals. Several studies have documented an increase in arterial pressure variability following selective carotid baroreceptor denervation (5, 13, 31). However, it should noted that, in all these studies, resting arterial pressure variability returned to normal levels within a few days after surgical denervation. This suggests that more prominent mechanisms may play a role in long-term control of resting arterial pressure after recovery, and the arterial baroreflex may not have a consistent role in respiratory blood pressure variability. Furthermore, these experiments were necessarily done under anesthesia, and there are interspecies differences between humans (bipedal) and dogs (quadripedal).
Nevertheless, similar controlled experiments have not been done in humans for obvious reasons. Most reports rely on retrospective studies with only a small number of patients following surgical interventions or jugular radiotherapy (32, 40–42), and results from these studies are hard to reconcile. For example, on the one hand, there appears to be an increase in mean pressure variability following radiotherapy of the neck compared with preoperation levels (40). However, on the other hand, ambulatory pressure variability does not appear to change after neck radiation compared with age-matched controls, despite a substantial decrease in baroreflex sensitivity (41). Indeed, the long-term effect of baroreceptor denervation appears to be highly heterogenous among individuals [see Timmers et al. (42) for a review]. Part of this discrepancy may be because most studies report the changes in arterial pressure variability only with reference to “normal population levels” or to healthy age-matched controls. However, substantial interindividual variation in hemodynamic responses precludes reliable population-level comparison of differences in arterial pressure variability. Therefore, the implications of these studies for elucidating the role of the arterial baroreflex in RSA are unclear.
Other studies have investigated the baroreflex contribution to RSA in healthy individuals using maneuvers that actively engage carotid baroreceptors [e.g., neck suction (18, 27)]. However, the role of the carotid baroreflex in modulating cardiac vagal traffic during supine rest cannot be inferred from observations when the reflex is actively engaged. For example, it is possible that the respiratory gating of central vagal-cardiac motorneurons may regulate cardiac vagal activity at rest (7, 8), but peripheral baroreflex activation may contribute or interrupt feedforward central modulation of vagal activity. In addition, at rest, peripheral cardiopulmonary afferents may play some role (3). Furthermore, the peripheral carotid chemoreflex may partake in regulation of RSA; alterations in central and peripheral chemoreceptor traffic, in response to oxygen, carbon dioxide, and hydrogen levels, can modulate respiratory patterns (24), and active chemoreceptor-mediated modulation of RSA has been described in dogs (33, 45, 46) and in humans (43). Thus it is likely that active engagement of the baroreflex overrides other mechanisms that may also be responsible for RSA during supine rest. Therefore, these data are not necessarily counter to our conclusion that baroreflex-mediated mechanisms are not the primary contributor to RSA during supine rest, but merely demonstrate the prominent role for the baroreflex during hemodynamic perturbations.
Studies of anesthetized dogs (17) and humans with heart transplants (9) suggest that low-dose atropine exerts its effects via a central neural mechanism. On the other hand, there is also evidence indicating a peripheral receptor effect of low-dose atropine; doses that result in no measurable atropine in human cerebral spinal fluid produce bradycardia (44) and an atropine derivative that cannot cross the blood-brain barrier also induces bradycardia (21). Nonetheless, whether or not the change in RSA is mediated via central (altered vagal outflow) or peripheral (altered end-organ responses) pathways does not impact the interpretation of our results. The end-organ responses to inhibition and disinhibition of vagal activity should still reflect a baroreflex modulatory mechanism regardless of the site at which vagal effects are enhanced; by enhancing outflow from vagal neurons and/or enhancing the chronotropic response to vagal outflow, the closed-loop baroreflex response to spontaneous pressure oscillations presumably should be enhanced.
An immediate implication of our results is that “spontaneous indexes” of baroreflex control assessed using breathing-induced changes in R-R interval and blood pressure during supine rest are not likely to be reliable surrogates for direct measures of baroreflex gain. Spontaneous indexes, especially the “sequence” technique, encompass the relation between systolic arterial pressure and R-R intervals during inspiration and/or expiration, based on the implicit assumption that respiratory-mediated arterial pressure fluctuations are sensed and buffered via baroreflex mechanisms. This assumption, in turn, is based on the model that the systolic pressure rise within a given beat stimulates the baroreflex arc to adjust the timing of the following R-wave such that diastolic pressure is stabilized and hence displays lesser fluctuation (6). However, the robust linear relation between changes in respiratory-mediated R-R interval and diastolic pressure fluctuations undermines the presumed model upon which spontaneous indexes are constructed. This mismatch between the assumptions behind spontaneous baroreflex indexes and the system's responses to alterations in RSA may explain why estimates of baroreflex function using spontaneous indexes are inconsistent with those from pharmacological interventions (22).
One limitation to interpretation of our findings is that there was a significant rise in arterial pressure during minimum RSA. It is feasible that this increased afferent baroreflex activity and further reduced RSA while also decreasing sympathetic outflow to the vasculature. Lesser sympathetic outflow might well alter the magnitude of respiratory diastolic pressure fluctuations (34). However, the significant change in diastolic pressure fluctuations at peak RSA, without any significant change in average arterial pressures, suggests that the increase in the blood pressure at minimum RSA alone cannot explain our results. Furthermore, we assume that the blood pressure response is due to the tachycardia (∼6 beats/min) that accompanies mildly vagolytic atropine. However, if we assume that the responses to vagolytic atropine were purely baroreflex-mediated phenomena, we cannot explain an increase in pressure causing a tachycardia, a reduction in RSA, and a tendency to decrease diastolic pressure fluctuations. It should also be noted that we tested our hypothesis in healthy young individuals during supine rest, and our conclusion may not hold for different populations and under different conditions. Last, our study was designed to test a model of RSA that is concerned with only one of the possible mechanisms (baroreflex) that may partake in the genesis of RSA. Therefore, although our data provide little evidence for a predominant role of baroreflex-mediated mechanism underlying RSA during supine rest, we cannot speculate on the relative roles of other potential mechanisms.
In summary, our results show that, in young healthy individuals during supine rest, respiratory fluctuations in diastolic pressure are not buffered against by R-R interval fluctuations and that the change in R-R interval fluctuations when RSA is augmented or inhibited are closely tracked by the changes in diastolic pressure fluctuations. Therefore, our data provide little evidence for a predominant role of a baroreflex mechanism underlying RSA during supine rest, and this may have implications for the use of spontaneous baroreflex indexes.
GRANTS
This work was supported by National Institute on Ageing Grant AG-014376.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol Heart Circ Physiol 249: H867–H875, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Averill KH, Lamb LE. Less commonly recognized actions of atropine on cardiac rhythm. Am J Med Sci 237: 304–318, 1959 [DOI] [PubMed] [Google Scholar]
- 3.Baselli G, Cerutti S, Civardi S, Malliani A, Pagani M. Cardiovascular variability signals: towards the identification of a closed-loop model of the neural control mechanisms. IEEE Trans Biomed Eng 35: 1033–1046, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol 517: 617–628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowley AW, Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res 32: 564–576, 1973 [DOI] [PubMed] [Google Scholar]
- 6.deBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol Heart Circ Physiol 253: H680–H689, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Eckberg DL. The human respiratory gate. J Physiol 548: 339–352, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckberg DL. Point:Counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 106: 1740–1742, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Epstein AE, Hirschowitz BI, Kirklin JK, Kirk KA, Kay GN, Plumb VJ. Evidence for a central site of action to explain the negative chronotropic effect of atropine: studies on the human transplanted heart. J Am Coll Cardiol 15: 1610–1617, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. Am J Physiol Heart Circ Physiol 246: H838–H842, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Guz A, Innes JA, Murphy K. Respiratory modulation of left ventricular stroke volume in man measured using pulsed Doppler ultrasound. J Physiol 393: 499–512, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedman AE, Hartikainen JE, Tahvanainen KU, Hakumaki MO. Power spectral analysis of heart rate and blood pressure variability in anaesthetized dogs. Acta Physiol Scand 146: 155–164, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Ito CS, Scher AM. Regulation of arterial blood pressure by aortic baroreceptors in the unanesthetized dog. Circ Res 42: 230–236, 1978 [DOI] [PubMed] [Google Scholar]
- 14.Japundzic N, Grichois ML, Zitoun P, Laude D, Elghozi JL. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst 30: 91–100, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 106: 1742–1743, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol 39: 801–805, 1975 [DOI] [PubMed] [Google Scholar]
- 17.Katona PG, Lipson D, Dauchot PJ. Opposing central and peripheral effects of atropine on parasympathetic cardiac control. Am J Physiol Heart Circ Physiol 232: H146–H151, 1977 [DOI] [PubMed] [Google Scholar]
- 18.Keyl C, Dambacher M, Schneider A, Passino C, Wegenhorst U, Bernardi L. Cardiocirculatory coupling during sinusoidal baroreceptor stimulation and fixed-frequency breathing. Clin Sci (Lond) 99: 113–124, 2000 [PubMed] [Google Scholar]
- 19.Koizumi K, Terui N, Kollai M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. J Auton Nerv Syst 12: 251–259, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Kollai M, Jokkel G, Bonyhay I, Tomcsanyi J, Naszlady A. Relation between baroreflex sensitivity and cardiac vagal tone in humans. Am J Physiol Heart Circ Physiol 266: H21–H27, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Kottmeier CA, Gravenstein JS. The parasympathomimetic activity of atropine and atropine methylbromide. Anesthesiology 29: 1125–1133, 1968 [DOI] [PubMed] [Google Scholar]
- 22.Lipman RD, Salisbury JK, Taylor JA. Spontaneous indices are inconsistent with arterial baroreflex gain. Hypertension 42: 481–487, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Ludwig C. zur Kenntniss des Einflusses der Respirations bewegungen auf den Blutlauf im Aortensysteme. Arch Anat Physiol Leipzig 13: 242–302, 1847 [Google Scholar]
- 24.O'Regan RG, Majcherczyk S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol 100: 23–40, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Parker P, Celler BG, Potter EK, McCloskey DI. Vagal stimulation and cardiac slowing. J Auton Nerv Syst 11: 226–231, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Piepoli M, Sleight P, Leuzzi S, Valle F, Spadacini G, Passino C, Johnston J, Bernardi L. Origin of respiratory sinus arrhythmia in conscious humans. An important role for arterial carotid baroreceptors. Circulation 95: 1813–1821, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Raczkowska M, Eckberg DL, Ebert TJ. Muscarinic cholinergic receptors modulate vagal cardiac responses in man. J Auton Nerv Syst 7: 271–278, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Rimoldi O, Pierini S, Ferrari A, Cerutti S, Pagani M, Malliani A. Analysis of short-term oscillations of R-R and arterial pressure in conscious dogs. Am J Physiol Heart Circ Physiol 258: H967–H976, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res 19: 400–411, 1966 [DOI] [PubMed] [Google Scholar]
- 31.Shade RE, Bishop VS, Haywood JR, Hamm CK. Cardiovascular and neuroendocrine responses to baroreceptor denervation in baboons. Am J Physiol Regul Integr Comp Physiol 258: R930–R938, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension 42: 110–116, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Shykoff BE, Naqvi SS, Menon AS, Slutsky AS. Respiratory sinus arrhythmia in dogs. Effects of phasic afferents and chemostimulation. J Clin Invest 87: 1621–1627, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St. Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res 85: 457–469, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Tang X, Dworkin BR. The dmNTS is not the source of increased blood pressure variability in baroreflex denervated rats. Auton Neurosci 148: 21–27, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tank J, Baevski RM, Fender A, Baevski AR, Graves KF, Ploewka K, Weck M. Reference values of indices of spontaneous baroreceptor reflex sensitivity. Am J Hypertens 13: 268–275, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Task Force Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 38.Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation 93: 1527–1532, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol 280: H2804–H2814, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Timmers HJ, Karemaker JM, Lenders JW, Wieling W. Baroreflex failure following radiation therapy for nasopharyngeal carcinoma. Clin Auton Res 9: 317–324, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Timmers HJ, Karemaker JM, Wieling W, Kaanders JH, Folgering HT, Marres HA, Lenders JW. Arterial baroreflex and peripheral chemoreflex function after radiotherapy for laryngeal or pharyngeal cancer. Int J Radiat Oncol Biol Phys 53: 1203–1210, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol 553: 3–11, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzeng YC, Larsen PD, Galletly DC. Effects of hypercapnia and hypoxemia on respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Am J Physiol Heart Circ Physiol 292: H2397–H2407, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Virtanen R, Kanto J, Iisalo E, Iisalo EU, Salo M, Sjovall S. Pharmacokinetic studies on atropine with special reference to age. Acta Anaesthesiol Scand 26: 297–300, 1982 [DOI] [PubMed] [Google Scholar]
- 45.Yasuma F, Hayano J. Augmentation of respiratory sinus arrhythmia in response to progressive hypercapnia in conscious dogs. Am J Physiol Heart Circ Physiol 280: H2336–H2341, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Yasuma F, Hayano JI. Impact of acute hypoxia on heart rate and blood pressure variability in conscious dogs. Am J Physiol Heart Circ Physiol 279: H2344–H2349, 2000. [DOI] [PubMed] [Google Scholar]