Abstract
The mitogen-activated protein (MAP) kinases are involved in cellular responses to many stimuli, including hypoxia. MAP kinase signaling is regulated by a family of phosphatases that include MAP kinase phosphatase-1 (MKP-1). We hypothesized that mice lacking the Mkp-1 gene would have exaggerated chronic hypoxia-induced pulmonary hypertension. Wild-type (WT) and Mkp-1−/− mice were exposed to either 4 wk of normoxia or hypobaric hypoxia. Following chronic hypoxia, both genotypes demonstrated elevated right ventricular pressures, right ventricular hypertrophy as demonstrated by the ratio of the right ventricle to the left ventricle plus septum weights [RV(LV + S)], and greater vascular remodeling. However, the right ventricular systolic pressures, the RV/(LV + S), and the medial wall thickness of 100- to 300-μm vessels was significantly greater in the Mkp-1−/− mice than in the WT mice following 4 wk of hypobaric hypoxia. Chronic hypoxic exposure caused no detectable change in eNOS protein levels in the lungs in either genotype; however, Mkp-1−/− mice had lower levels of eNOS protein and lower lung NO production than did WT mice. No iNOS protein was detected in the lungs by Western blotting in any condition in either genotype. Both arginase I and arginase II protein levels were greater in the lungs of hypoxic Mkp-1−/− mice than those in hypoxic WT mice. Lung levels of proliferating cell nuclear antigen were greater in hypoxic Mkp-1−/− than in hypoxic WT mice. These data are consistent with the concept that MKP-1 acts to restrain hypoxia-induced arginase expression and thereby reduces vascular remodeling and the severity of pulmonary hypertension.
Keywords: nitric oxide synthase, arginase, pulmonary vascular remodeling
chronic hypoxia causes pulmonary vasoconstriction and structural remodeling of the pulmonary arteries (10, 33, 37). These changes result in decreased luminal diameter of the pulmonary arteries and thereby elevate vascular resistance leading to pulmonary hypertension (PH). In addition, chronic hypoxia stimulates erythropoiesis, causing polycythemia and an associated increase in blood viscosity that may also contribute to PH (43). PH eventually results in right ventricular hypertrophy and right ventricular dysfunction, which when severe is known as cor pulmonale (19).
Nitric oxide (NO) is a potent vasodilator and inhibits the proliferation of vascular smooth muscle cells. NO is produced from l-arginine via NO synthase (NOS), and there are three described isoforms of NOS [neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS)]. All three isoforms are expressed in the lung (5). l-Arginine can also be converted into urea and l-ornithine by arginase. There are two isoforms of arginase: arginase I and arginase II, both of which are expressed in the lung (36). l-Ornithine can be further metabolized to polyamines and proline, which are essential for cellular proliferation. Thus increases in arginase activity lead to greater proline and polyamine production, which would be expected to result in the cell proliferation and pulmonary vascular remodeling that underlie PH. Because arginase and NOS compete for their common substrate, l-arginine, increased arginase activity would result in decreased NO production, thereby favoring vasoconstriction (9). This concept is supported by the observation that pulmonary artery endothelial cells derived from the lungs of patients with PH had greater arginase II protein levels and lower NO production than did similar cells from the lungs of patients without PH (50).
Thus understanding the regulation of NOS and arginase in the lung is vital for developing new strategies for treating or preventing PH. The mitogen-activated protein (MAP) kinases are involved in the response to inflammation as well as in the regulation of cellular proliferation (28). We have demonstrated a central role for the dual-specificity phosphatase, MAP kinase phosphatase-1 (MKP-1), in limiting the inflammatory response by dephosphorylating and thereby deactivating the MAP kinases (53). It has been found in macrophages that inflammatory-mediated iNOS and arginase induction depends on the MAP kinases and that MKP-1 limits iNOS and arginase expression following inflammatory stimuli (26, 34, 53). Recently, it has been reported that the antiproliferative effects of sildenafil in pulmonary artery smooth muscle cells are due to induction of MKP-1 (25). Thus, given the role of MAP kinase signaling in arginase induction and cellular proliferation, we hypothesized that mice deficient in MKP-1 would have more severe chronic hypoxia-induced vascular remodeling and PH associated with greater arginase protein expression. To test this hypothesis, we exposed Mkp-1−/− mice and their wild-type (WT) littermates to hypobaric hypoxia or normoxia for 4 wk. The severity of the PH that these mice developed was determined by measuring right ventricular pressures in vivo and by measuring right ventricular weights and the wall thickness of pulmonary vessels. We also examined the protein expression of iNOS, eNOS, nNOS, arginase I, arginase II, and proliferating cell nuclear antigen (PCNA) in the lungs and right ventricle.
METHODS
Mice.
The generation of Mkp-1 knockout mice has been described previously (53). Cryopreserved embryos of Mkp-1 knockout mice (Mkp-1−/+ and Mkp-1−/−) on a C57BL6/129-mixed background were provided by Bristol-Myers Squibb Pharmaceutical Research Institute (Lawrenceville, NJ) and were regenerated into mice at the Jackson Laboratories (Bar Harbor, ME). These mice were bred in-house to yield both Mkp-1+/+ and Mkp-1−/− mice. All mice were maintained at 24°C with a relative humidity between 30 and 70% on a 12:12-h light-dark cycle. The mice were fed Harlan Teklad irradiated diet (Harlan Sprague-Dawley) ad libitum. All animals received humane care in accordance with the guidelines of the National Institutes of Health. The study protocol was approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children's Hospital.
Exposures.
Adult Mkp-1+/+ (n = 14) and Mkp-1−/− (n = 13) mice (17–28 g) were placed in a hypobaric hypoxic chamber with barometer pressure maintained at 380 ± 10 mmHg for a period of 4 wk. The chamber was opened three times each week to provide food and water, clean bedding, and to weigh the mice. Age- and weight-matched Mkp-1+/+ (n = 14) and Mkp-1−/− (n = 14) mice were kept under normoxia with ambient barometric pressure (∼760 mmHg) in similar conditions. An additional 12 adult Mkp-1+/+ and 12 Mkp-1−/− mice (17–28 g) were placed either in a hypobaric hypoxic chamber or normoxia for a period of 5 days.
In vivo measurement of right ventricular pressures.
On the day of study, animals (n = 19) were anesthetized with 50 mg/kg pentobarbital sodium intraperitoneally. An incision was made over the trachea, which was isolated and exposed. The trachea was cannulated using pulled polyethylene (PE)-90 tubing (BD Scientific, Franklin Lakes, NJ) and secured using 4–0 silk suture (Ethicon, Piscataway, NJ). The tracheal cannula was connected to a rodent ventilator (Hugo Sachs, March-Hugstetten, Germany) at a tidal volume of 5 ml/kg body wt and a respiratory rate of 50 breaths/min. A small incision was made above the xiphoid process, a 23-gauge needle connected to a blood pressure transducer (Columbus Instruments) was advanced in the right ventricular cavity through the incision, and placement was verified via the pressure waveform. The right ventricular pressure was continuously monitored using Cardiomax hardware and software (Columbus Instruments) and a Dell laptop personal computer. After a stable baseline pressure reading was established, the right ventricular pressures were measured for ∼5 min while the animals were ventilated with room air. Next, the ventilating gas mixture was changed from room air to 10% oxygen and balance nitrogen. Right ventricular pressures were then measured for an additional 10 min until a new stable pressure had been achieved.
Tissue preparation.
On the day of the study, the mice (n = 24) were killed with an intraperitoneal injection of pentobarbital sodium (100 mg/kg). The atria and major vessels were removed from the isolated heart and the right ventricle (RV) was dissected free from the left ventricle (LV) and septum (LV + S). The RV and the LV + S were weighed, frozen, and kept at −80°C for further study. The ratio of RV to LV + S weights was used to assess the degree of PH. The lungs and liver were removed, weighed, freeze-clamped in liquid nitrogen, and kept at −80°C for further analyses. The organs were homogenized, as previously described (10), in 0.8 ml of ice-cold Dulbecco's phosphate-buffered saline (DPBS, pH 7.4). Samples were centrifuged at 12,000 g for 15 min, and the supernatants were collected and analyzed for total protein content using the Bradford assay (Bio-Rad, Hercules, CA). The supernatants were stored at −80°C for further study.
Lung fixation.
Lungs were perfusion fixed as previously described (8, 10). Briefly, the mice (n = 12) were killed with intraperitoneal injections of pentobarbital sodium (100 mg/kg). The animal was secured to a heating block to maintain body temperature at 38°C. The trachea was cannulated via a tracheotomy with custom-fitted PE-90 tubing (BD Scientific) and secured using 4–0 silk suture (Ethicon). The tracheal cannula was connected to a rodent ventilator (Hugo Sachs) at a tidal volume of 5 ml/kg body wt and a respiratory rate of 50 breaths/min, using a gas mixture consisting of 5% CO2 in room air. The pulmonary artery and the LV were cannulated with custom-fitted PE-90 tubing secured using 4–0 silk suture. The preparation was immediately perfused in a nonrecirculating manner at a rate of 0.1 ml/min using a physiological saline solution containing 129.8 mM NaCl, 5.4 mM KCl, 0.83 mM MgSO4, 19 mM NaHCO3, 1.8 mM CaCl2, and 5.5 mM glucose with 4% albumin (wt/vol) and 300 μM meclofenamate added (all chemicals are from Sigma, St. Louis, MO) via a Masterflex roller pump (Cole-Parmer, Vernon Hills, IL). The perfusion flow rate was gradually increased to 15 ml·min−1·kg body wt−1 and maintained at this flow rate for the remainder of the perfusion period. After perfusion with a total volume of ∼5 ml, the perfusate was changed to fixative containing DPBS, 4% paraformaldehyde, 1 U/ml heparin, and 1 mM papaverine, and the lungs were perfused with ∼5 ml. During the last 10 min of fixation, the airways were filled with fixative and held at a constant airway pressure of 23 cmH2O. The heart and lungs were removed en bloc and placed in fixative for 30 min before cutting 2-mm sections that were left in fixative overnight at 4°C. The fixed tissues were washed and kept in PBS overnight and transferred to 70% ethanol.
Masson's trichrome stain.
The fixed tissue was serially dehydrated in increasing concentrations of ethanol overnight, paraffin embedded, cut, and deparaffinized for Masson's staining. Masson-stained vessel images from lung sections were used to determine pulmonary vascular remodeling using an Olympus optical BX-40 microscope. All images were captured under identical lighting conditions and optical settings. In addition, the images were segmented to eliminate background from analysis as previously described (10).
Elastin-stained vessel images from lung sections were used by personnel masked to the treatment to determine pulmonary vascular remodeling with MetaMorph Imaging System hardware and software (Universal Imaging) as previously described (8). The integrity and fullness (undulation free) of the internal elastic lamina identified artery from vein and indicated optimal lung fixation volume and pressure. The outer diameter of the vessel was assessed from the outer edge of the external elastic lamina, and the inner diameter of the vessel was assessed from the inner edge of the internal elastic lamina. Percent arterial wall thickness was determined by subtracting the inner diameter from the outer diameter and dividing by the outer diameter and expressing the resultant fraction as a percent. Vessels sectioned at oblique angles were excluded from analysis.
Western blot analysis.
The tissue homogenates were assayed for iNOS, eNOS, nNOS, arginase I, arginase II, PCNA, and β-actin protein by Western blot analysis as previously described (8, 10, 34). Lung tissue homogenates from mice exposed for 5 days were assayed for phosphorylated p38 (pp38) and total p38. Briefly, aliquots of tissue homogenate containing equal amounts of protein were diluted 1:1 with SDS sample buffer, heated to 95°C for 5 min, and then centrifuged at 10,000 g at room temperature for 2 min. The aliquots of the supernatant were used for SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes and blocked overnight in phosphate-buffered saline with 0.1% Tween (PBST) containing 5% nonfat dried milk. The membranes were then incubated with the primary antibody, iNOS (BD Transduction, San Diego, CA), eNOS (BD Transduction), nNOS (BD Transduction), arginase I (Santa Cruz Biotechnology, Santa Cruz, CA), arginase II (Santa Cruz Biotechnology), PCNA (Abcam, Cambridge, MA), pp38 (Cell Signaling Technologies, Beverly, MA), or p38 (BD Transduction) overnight at 4°C and then washed three times with PBST. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Bio-Rad) or HRP-conjugated goat anti-mouse IgG (Bio-Rad) for 1 h and washed three times with PBST. The protein bands were visualized using enhanced chemiluminescence (ECL reagent; Amersham Pharmacia Biotech, Piscataway, NJ) and quantified using densitometry (Sigma Gel; Jandel Scientific, San Rafael, CA). To control for protein loading, blots were stripped using a stripping buffer containing 62.5 mM Tris·HCl (pH 6.8), 2% SDS, and 100 mM 2-β-mercaptoethanol, and the blots were reprobed for β-actin (Abcam) as described above.
Nitrite/nitrate concentrations.
The lung and heart homogenates were assayed for the concentrations of nitrite/nitrate (NOx), using a chemiluminescence analyzer (Sievers, Boulder, CO), as previously described (10, 34). Briefly, 100 μl of sample were injected in a reaction chamber containing a mixture of vanadium (III) chloride in 2 M HCl heated to 90°C to reduce NOx to NO. The NO formed was evolved into the gas phase and carried into the analyzer using a constant flow of helium. The analyzer was calibrated using a standard curve derived from sodium nitrate.
Statistical analysis.
Values are shown as means ± SE. One-way ANOVA was used to compare the groups. A Student-Newman-Keuls post hoc test was used to identify differences between groups. Differences were considered significant when P < 0.05.
RESULTS
The effect of chronic hypoxia on right ventricular pressures.
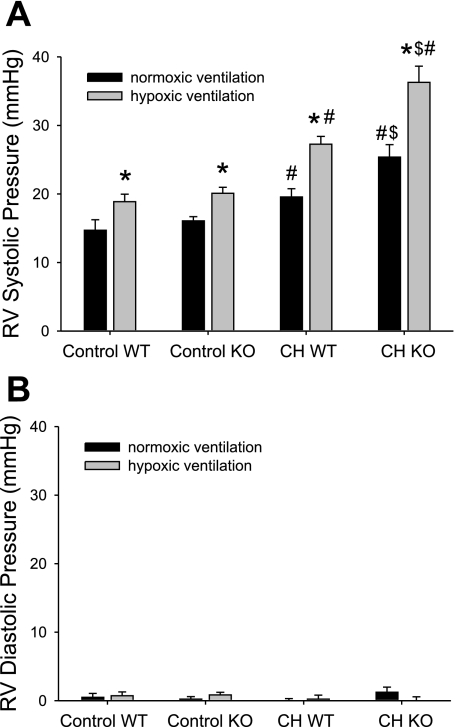
To determine if deficiency of MKP-1 would alter the physiological response to chronic hypoxia, we measured right ventricular pressures in anesthetized mice, both during ventilation with room air and during ventilation with a hypoxic gas mixture (FiO2 = 0.10). There was no significant difference in right ventricular systolic pressure (RVSP) during room air ventilation between WT and Mkp-1−/− mice exposed to normobaric room air (control) for 4 wk (Fig. 1). Both genotypes exposed to normoxia for 4 wk displayed a similar acute hypoxic pressor response, with a mean increase in RVSP of 4.2 ± 0.9 mmHg in the control WT animals and 4.0 ± 1.2 mmHg in the control Mkp-1−/− mice. Both the WT and the Mkp-1−/− mice exposed to hypobaric hypoxia for 4 wk had significantly higher RVSP during room air ventilation than did their respective controls (Fig. 1). Furthermore, the RVSP during room air ventilation were significantly greater in the Mkp-1−/− mice exposed to hypobaric hypoxia than in the WT mice exposed to hypobaric hypoxia (Fig. 1). In both genotypes of mice exposed to hypobaric hypoxia for 4 wk, there was an augmented response to acute hypoxic ventilation; WT had a mean acute hypoxic response of 7.7 ± 1.0 mmHg (P < 0.05 compared with control WT mice) while Mkp-1−/− mice had a mean acute hypoxic response of 10.9 ± 2.6 mmHg (P < 0.05 compared with control Mkp-1−/− mice). Indeed, the mean RVSP was significantly greater in the hypobaric hypoxia-exposed Mkp-1−/− mice during acute hypoxic ventilation than in the similarly exposed WT mice during acute hypoxic ventilation (Fig. 1). The right ventricular diastolic pressures ranged from 0 to 2 mmHg and were not different in any condition or group studied (Fig. 1B).
Fig. 1.
Chronic hypoxia (CH) results in higher right ventricular (RV) pressures, and mitogen-activated protein (MAP) kinase phosphatase-1 (MKP)-1-deficient animals had higher RV pressures after chronic hypoxia than did wild-type (WT) animals. A: RV systolic pressures (RVSP) during 5 min of normoxia (black bars, FiO2 0.21), followed by 10 min of hypoxic (gray bars, FiO2 0.12), ventilation in anesthetized mice. Control animals were exposed to room air, whereas CH animals were exposed to hypobaric hypoxia (barometric pressure ≈ 380 mmHg) for 4 wk before study. Both WT and Mkp-1−/− [knockout (KO)] mice were studied. There were 5 animals in each study group, except for the CH KO, which had 4 animals. P < 0.05, hypoxic ventilation different from normoxic ventilation in the anesthetized animals in the same group (*), chronic hypoxia-exposed animals different from control animals in the same genotype and ventilation (#), and CH KO animals different from CH WT animals in the same ventilation condition ($). B: RV diastolic pressures from the same animals as in A. There were no differences in diastolic pressures with either ventilation, exposure, or genotype.
The effect of chronic hypoxia on the body weight.
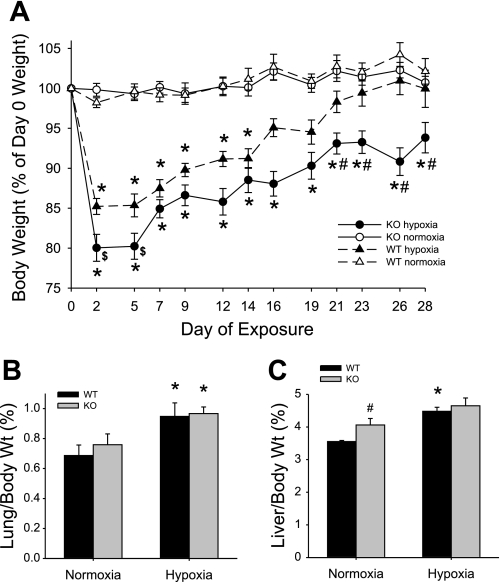
There was no significant change in body weight due to normoxic exposure in either the WT or Mkp-1−/− mice (Fig. 2A). Chronic hypoxic exposure caused both WT and Mkp-1−/− mice to initially lose weight, although the weight loss on days 2 and 5 was greater in the Mkp-1−/− mice than in the WT mice (Fig. 2A). The WT mice in chronic hypoxia slowly regained their body weight such that after 2 wk in chronic hypoxia there was no significant difference between the body weights of WT hypoxic and normoxic animals (Fig. 2A). On the other hand, the chronic hypoxia-exposed Mkp-1−/− mice did not regain their body weight such that their body weight was significantly less than the other three groups by 21 days in hypoxia, and remained different from the other three groups through 28 days of exposure (Fig. 2A).
Fig. 2.
MKP-1 deficiency results in sustained weight loss during chronic hypoxic exposure. A: body weight change (as a percent of the day 0 weight) during exposure in normoxic WT mice (▵), hypoxic WT mice (▲), normoxic Mkp-1−/− (KO) mice (○), and hypoxic Mkp-1−/− mice (●). Both genotypes lost weight initially during hypoxic exposure, but only Mkp-1−/− mice had significant weight loss by the end of the exposure period. P < 0.05, hypoxia different from normoxia same genotype (*), hypoxic Mkp-1−/− mice different from hypoxic WT mice ($), and hypoxic Mkp-1−/− mice different from 3 other study groups (#); n = 6 mice in each group. B: the ratio of lung-to-body weight in WT mice (black bars) or in Mkp-1−/− mice (gray bars, n = 6 in each group). Hypoxic exposure resulted in greater lung weights in both genotypes. *P < 0.05, hypoxia different from normoxia same genotype. C: the ratio of liver-to-body weight in WT mice (black bars) or in Mkp-1−/− mice (gray bars, n = 6 in each group). Liver weight was only increased in WT in hypoxia. P < 0.05, hypoxia different from normoxia same genotype (*) and Mkp-1−/− different from WT same condition (#).
There were no differences in the total lung weights or total liver weights between WT and Mkp-1−/− mice, and there were no differences in lung or liver weights between normoxic and chronically hypoxic animals, except that WT mice had a small but statistically significantly greater liver weight after 4 wk in chronic hypoxia than did normoxic WT mice (Table 1). The lung weight-to-body weight ratios of the chronic hypoxia-exposed animals were greater than those of the normoxia-exposed animals, although there was no difference in lung weight-to-body weight ratios between the two genotypes after 4 wk in hypoxia (Fig. 2B). The liver-to-body weight ratio was slightly but significantly greater in the normoxic Mkp-1−/− mice than in the normoxic WT mice. Hypoxic exposure caused a small but significant increase in liver weights only in the WT mice (Fig. 2C).
Table 1.
Lung and liver weights
| Lung Wt, g |
Liver Wt, g |
|||
|---|---|---|---|---|
| Wild type | Mkp-1−/− | Wild type | Mkp-1−/− | |
| Normoxia | 0.163 ± 0.022 | 0.168 ± 0.016 | 0.837 ± 0.028 | 0.912 ± 0.078 |
| Hypoxia | 0.210 ± 0.022 | 0.188 ± 0.022 | 0.990 ± 0.040* | 0.905 ± 0.041 |
Values are means ± SE, n = 6 mice for each group. Mkp-1−/−, mitogen-activated protein kinase phosphatase-1 deficient.
P < 0.05, normoxia different from hypoxia.
The effect of chronic hypoxia on right ventricular hypertrophy.
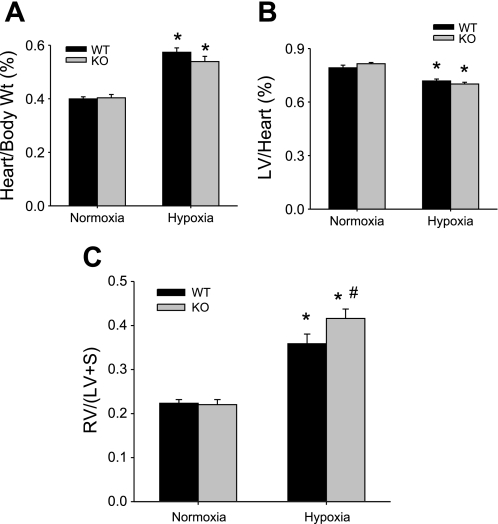
Given the effect of chronic hypoxia on right ventricular pressures and the differences between WT and Mkp-1−/− mice exposed to chronic hypoxia, we examined other markers of PH beginning with right ventricular hypertrophy. There was no difference in the total heart weight-to-body weight ratios between WT and Mkp-1−/− mice exposed to normobaric normoxia for 4 wk, and exposure to hypobaric hypoxia for 4 wk caused an increase in total heart weights in both WT and Mkp-1−/− mice (Fig. 3A). The left ventricular weights were not different between the normoxic-exposed WT and Mkp-1−/− mice. Chronic hypoxic exposure caused a small but statistically significant decrease in the left ventricular-to-heart weight ratios in both the WT and Mkp-1−/− mice (Fig. 3B). There was no difference between the genotypes in RV/(LV + S) after 4 wk of normoxia (Fig. 3C). Chronic hypoxic exposure resulted in a greater RV/(LV + S) in both genotypes; however, the RV/(LV + S) was significantly greater in the chronic hypoxia-exposed Mkp-1−/− mice than in the chronic hypoxia-exposed WT mice (Fig. 3C).
Fig. 3.
Chronic hypoxia-induced right ventricular hypertrophy was greater in Mkp-1−/− mice than in WT mice. A: the ratio of total heart-to-body weight in WT mice (black bars) and in Mkp-1−/− mice (gray bars, n = 6 in each group). Chronic hypoxia resulted in greater total heart weight in both genotypes. *P < 0.05, hypoxia different from normoxia same genotype. B: the ratio of left ventricular-to-total heart weight in WT mice (black bars) or in Mkp-1−/− mice (gray bars, n = 6 in each group). Chronic hypoxia resulted in lower left ventricle (LV) weights in both genotypes. *P < 0.05, hypoxia different from normoxia same genotype. C: the ratio of RV/[LV + septum (S)] in WT mice (black bars) or in Mkp-1−/− mice (gray bars, n = 6 in each group). RV/(LV + S) was increased by chronic hypoxia and was greater in the Mkp-1−/− mice than in the WT mice. P < 0.05, hypoxia different from normoxia same genotype (*) and Mkp-1−/− different from WT same condition (#).
The effect of chronic hypoxia on vascular remodeling.
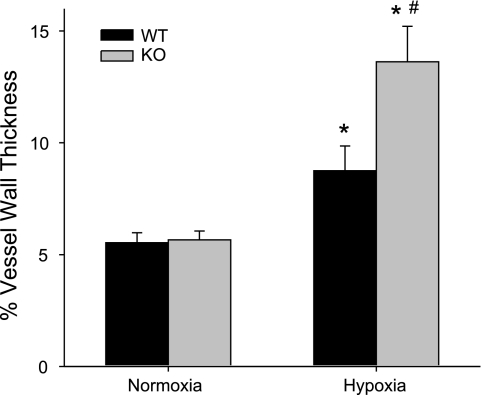
Another marker of PH is vascular remodeling; therefore, we determined the effects of 4 wk of hypoxic exposure on vascular remodeling by examining lung sections stained using Masson's trichrome stain as described in methods. The percent wall thickness was determined for vessels between 100 and 300 μm in outer diameter. There was no difference in vessel wall thickness between normoxia-exposed WT and Mkp-1−/− mice (Fig. 4). Chronic hypoxia caused significant vascular remodeling in both WT and Mkp-1−/− mice (Fig. 4). However, the vascular wall thickness was significantly greater in the chronic hypoxia-exposed Mkp-1−/− mice than in the chronic hypoxia-exposed WT mice (Fig. 4). Indeed, chronic hypoxia caused a 58 ± 20% increase in wall thickness in the WT mice, whereas in the Mkp-1−/− mice chronic hypoxia caused a 141 ± 28% increase in the wall thickness (P < 0.05).
Fig. 4.
Chronic hypoxia exposure resulted in greater vessel wall thickness in the lungs from Mkp-1−/− mice than in vessels from the lungs of WT mice. The percent vessel wall thickness from pulmonary arteries of 100 to 300 μm outer diameter from WT and Mkp-1−/− mice in normoxia and hypoxia. Hypoxia resulted in significant vascular remodeling in both genotypes; however, there was significantly greater wall thickness in the chronically hypoxic Mkp-1−/− mice than in the chronically hypoxic WT mice. The vessels were taken from lung sections from 3 animals in each group; the number of vessels is 31 for WT normoxia, 9 for Mkp-1−/− normoxia, 33 for WT hypoxia, and 43 for Mkp-1−/− hypoxia. P < 0.05, hypoxia different from normoxia same genotype (*) and Mkp-1−/− different from WT same condition (#).
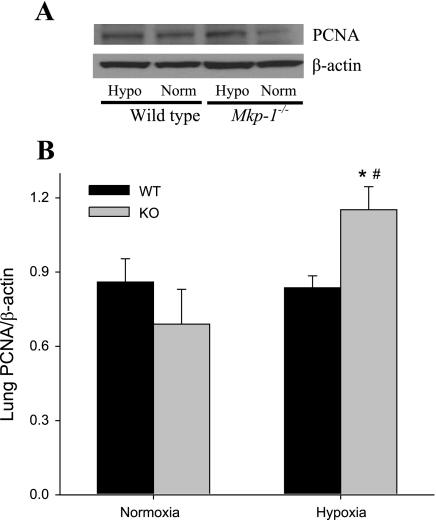
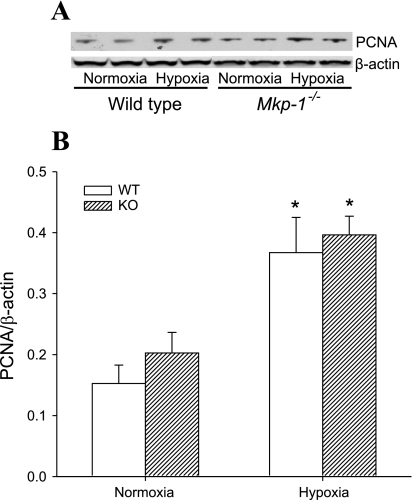
Because vascular remodeling is a proliferative response in the vessel wall, we investigated proliferation at 4 wk in these animals by examining PCNA expression in lung tissue using Western blotting. After 4 wk of exposure in the WT mice, there was no difference in PCNA levels between normoxia and chronic hypoxia (Fig. 5). On the other hand, the chronic hypoxia-exposed Mkp-1−/− mice had significantly greater levels of PCNA than did either the normoxic Mkp-1−/− mice or the chronic hypoxic WT mice (Fig. 5). These data suggest that only the Mkp-1−/− mice continued to have greater levels of proliferation after 4 wk of hypoxia.
Fig. 5.
Deficiency in MKP-1 resulted in higher proliferating cell nuclear antigen (PCNA) protein expression in the lungs following chronic hypoxia exposure. A: representative Western blot for PCNA in lung tissue. Note that the hypoxic (Hypo) tissues are in lanes 1 and 3, and the normoxic (Norm) tissues are in lanes 2 and 4. B: the mean densities for PCNA protein normalized to β-actin protein in the lung of WT (black bars) and Mkp-1−/− (gray bars) mice; n = 6 for each group. P < 0.05, hypoxia different from normoxia same genotype (*) and Mkp-1−/− different from WT same condition (#).
The effect of chronic hypoxia on NOS and arginase protein expression in the lungs.
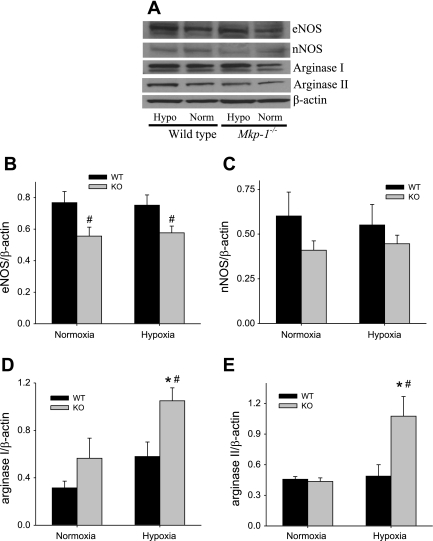
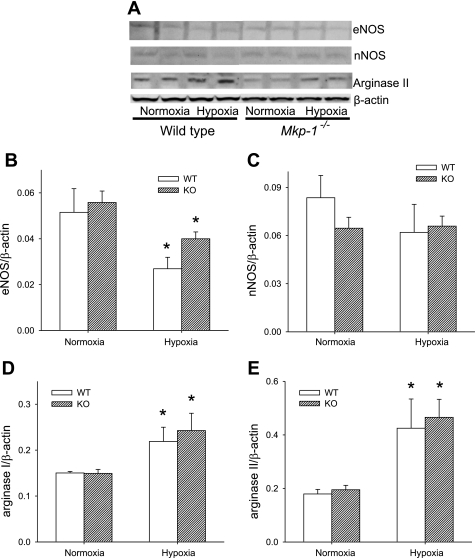
l-Arginine metabolism is central for both vascular constriction and vascular remodeling, with l-arginine metabolism by NOS favoring vasodilation but l-arginine metabolism by arginase favoring proliferation. Therefore, we examined the expression of NOS and arginase in the lungs after 4 wk exposure to either normobaric normoxia or hypobaric hypoxia using Western blot analysis. After 4 wk of exposure in these mice, we found no effect of chronic hypoxia exposure on either lung eNOS or lung nNOS protein levels (Fig. 6, B and C). Interestingly, we found that lung eNOS protein levels were lower (P < 0.05) in the Mkp-1−/− mice than in the WT mice in both normoxia and chronic hypoxia (Fig. 6B). Lung nNOS protein levels were not significantly different in the Mkp-1−/− mice than in the WT mice in either normoxia or chronic hypoxia (Fig. 6C). We could not detect any iNOS protein in the lungs from either Mkp-1−/− mice or WT mice after 4 wk in either normoxia or hypoxia.
Fig. 6.
After 4 wk of hypoxia, there was no difference in lung nitric oxide synthase (NOS) protein levels, but lung arginase levels were increased only in hypoxic Mkp-1−/− mice. A: representative Western blots for endothelial NOS (eNOS), neuronal NOS (nNOS), arginase I, arginase II, and β-actin for lung tissue from WT and Mkp-1−/− mice after 4 wk in normoxia or hypoxia. Note that the hypoxic tissues are in lanes 1 and 3, and the normoxic tissues are in lanes 2 and 4. B: the mean densities for eNOS protein normalized to β-actin protein in the lung of WT (black bars) and Mkp-1−/− (gray bars) mice. The levels of eNOS protein were significantly lower in the Mkp-1−/− mice than in the WT mice. C: the mean densities for nNOS protein normalized to β-actin protein in the lung of WT (black bars) and Mkp-1−/− (gray bars) mice. The levels of nNOS protein were not significantly different between genotypes. We were unable to detect inducible NOS (iNOS) in the lungs from any of the groups of mice studied. D: the mean densities for arginase I protein normalized to β-actin protein in the lungs of WT (black bars) and Mkp-1−/− (gray bars) mice. E: the mean densities for arginase II protein normalized to β-actin protein in the lungs of WT (black bars) and Mkp-1−/− (gray bars) mice; n = 5–6. P < 0.05, hypoxia different from normoxia same genotype (*) and Mkp-1−/− different from WT same condition (#).
There were no significant differences in lung arginase I or lung arginase II protein levels between the genotypes exposed to normoxia (Fig. 6, D and E). There was no significant effect of the exposure on either lung arginase I or lung arginase II protein levels after 4 wk exposure to hypoxia in WT mice (Fig. 6, D and E). Interestingly, we found that 4 wk of hypoxic exposure resulted in significantly greater lung arginase I protein levels in the Mkp-1−/− mice than in the WT mice (Fig. 6D). Similarly, we found that lung arginase II protein levels were also significantly elevated after chronic hypoxic exposure only in the Mkp-1−/− mice (Fig. 6E). Thus, 4 wk of chronic hypoxic exposure resulted in greater levels of arginase I and arginase II only in the Mkp-1−/− mice.
Lung NO production after 4 wk of exposure.
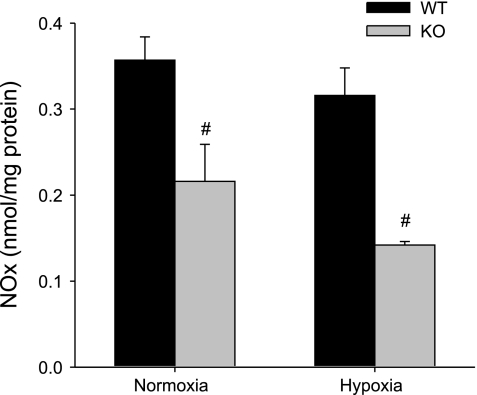
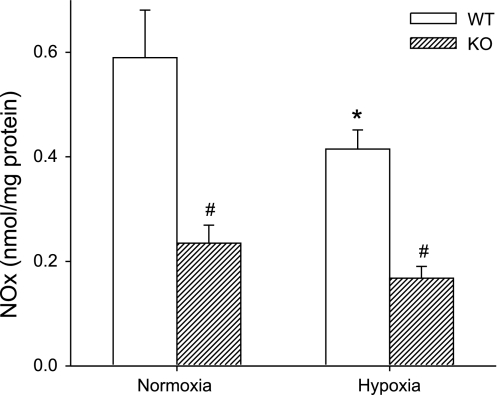
The lung tissue levels of NOx were assayed as a measure of NO production rates in the lungs at the time of death. Consistent with the observed changes in eNOS and nNOS protein levels in WT and Mkp-1−/− mice, the NOx levels were lower in the normoxic Mkp-1−/− mice than in the normoxic WT mice (Fig. 7). Chronic hypoxic exposure had no significant effect on the lung tissue levels of NOx in either WT or Mkp-1−/− mice, although the NOx levels were significantly lower in the chronic hypoxic Mkp-1−/− mice than in the chronic hypoxic WT mice (Fig. 7).
Fig. 7.
Deficiency in MKP-1 decreased NO production in the lungs. Accumulation of nitrite/nitrate (NOx) in the lungs from WT (black bars) and Mkp-1−/− (gray bars) mice under normoxia or hypoxia was measured using a chemiluminescence NO analyzer and normalized to lung protein concentration (nmol/mg protein); n = 5–6 in each group. #P < 0.05, Mkp-1−/− different from WT same condition.
The effect of chronic hypoxia on p38 activation in the lung.
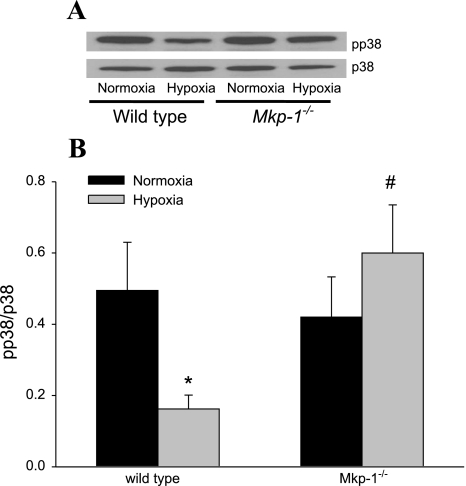
The lung tissue levels of pp38 and total p38 were examined using Western blotting after 4 wk of exposure to chronic hypoxia or normoxia. pp38 levels after 4 wk of exposure were relatively low with no differences detectable between the genotypes or exposures (data not shown). Therefore, we examined lung tissue from an earlier time point, after 5 days of exposure to either chronic hypoxia or normoxia, for pp38 and total p38. Exposure to 5 days of chronic hypoxia resulted in a significant reduction in pp38 in the lungs from WT mice (Fig. 8). On the other hand, there was no reduction in pp38 in the Mkp-1−/− mice (Fig. 8). These data confirm that deficiency in MKP-1 in these mice resulted in greater p38 activation in chronic hypoxia.
Fig. 8.
Deficiency of MKP-1 resulted in greater phosphorylated p38 (pp38) expression after 5 days in hypoxia. A: representative Western blot for pp38, which was then stripped and reprobed for p38. B: the mean densities of pp38 normalized to total p38 in the lung of WT and Mkp-1−/− mice after 5 days in normoxia (black bars) or hypoxia (gray bars). P < 0.05, hypoxia different from normoxia same genotype (*) and hypoxia-exposed Mkp-1−/− different from hypoxia-exposed WT (#).
The effect of chronic hypoxia on PCNA in the RV.
Given that the RV weights were greater after exposure to chronic hypoxia, and there was a difference between the two genotypes, we investigated cellular proliferation in RVs by assaying for PCNA by Western blotting. There was no difference in right ventricular PCNA levels between WT and the Mkp-1−/− mice exposed to normoxia (Fig. 9). On the other hand, the hypoxia-exposed WT and Mkp-1−/− mice had significantly greater levels of PCNA in the RVs than did the respective normobaric normoxia-exposed mice (Fig. 9). These data suggest that, unlike what was observed in the lung tissue, greater levels of cellular proliferation remain in the RVs of both genotypes after 4 wk of exposure to hypoxia.
Fig. 9.
Chronic hypoxia resulted in higher right ventricular PCNA protein expression at 4 wk in both WT and Mkp-1−/− mice. A: representative Western blot for PCNA in right ventricular tissue. B: the mean densities for PCNA protein normalized to β-actin protein in the right ventricles of WT (open bars) and Mkp-1−/− (cross-hatched bars) mice. *P < 0.05, hypoxia different from normoxia same genotype.
The effect of hypoxia on NOS and arginase expression in the RV.
After 4 wk exposure to either normobaric normoxia or hypobaric hypoxia, the hearts were harvested, and protein was isolated from the RVs for Western blot analysis. The right ventricular levels of eNOS protein were significantly lower in the animals exposed to chronic hypoxia than in those animals exposed to normoxia for 4 wk, although there was no difference in the right ventricular levels of eNOS protein between the chronic hypoxia-exposed WT and Mkp-1−/− mice (Fig. 10B). We found no effect of chronic hypoxia or genotype on right ventricular nNOS protein levels (Fig. 10C). Similar to the lungs, we could not detect any iNOS in these RVs after 4 wk of normoxic or hypoxic exposure in either genotype.
Fig. 10.
Chronic hypoxia decreased eNOS protein expression in the right ventricle and increased arginase protein expression in both genotypes. A: representative Western blots for eNOS, nNOS, arginase II, and β-actin for right ventricular tissue from WT and Mkp-1−/− mice after 4 wk in normoxia or hypoxia. B: the mean densities for eNOS protein normalized to β-actin protein in the right ventricle of WT (open bars) and Mkp-1−/− (cross-hatched bars) mice. After chronic hypoxia, the levels of eNOS protein in the lung were lower than in respective control-exposed animals for both genotypes. C: the mean densities for nNOS protein normalized to β-actin protein in the right ventricle of WT (open bars) and Mkp-1−/− (cross-hatched bars) mice. We were unable to detect iNOS in the right ventricle from any of the groups of mice studied. D: the mean densities for arginase I protein normalized to β-actin protein in the right ventricle of WT (open bars) and Mkp-1−/− (cross-hatched bars) mice. After 4 wk of chronic hypoxic exposure, the protein levels of arginase were increased in both genotypes. E: the mean densities for arginase II protein normalized to β-actin protein in the right ventricle of WT (open bars) and Mkp-1−/− (cross-hatched bars) mice. After 4 wk of chronic hypoxia, the arginase II protein levels were greater in both genotypes than in their respective normoxic controls; n = 5–6 in each group. *P < 0.05, hypoxia different from normoxia same genotype.
There were no differences in either arginase I or arginase II protein levels in the RVs between WT and Mkp-1−/− mice exposed to normoxia (Fig. 10, D and E). Both WT and Mkp-1−/− mice exhibited significantly greater right ventricular arginase I protein levels after 4 wk of exposure to hypobaric hypoxia than did the comparable genotype exposed to normoxia for 4 wk (Fig. 10D). Similarly, we found that right ventricular arginase II protein levels were significantly greater after chronic hypoxic exposure in both the WT and Mkp-1−/− mice than in the respective normoxia-exposed WT and Mkp-1−/− mice (Fig. 10E). Thus, 4 wk of hypoxia resulted in greater levels of arginase I and arginase II in the RV of both the WT and the Mkp-1−/− mice.
NO production in the right heart after 4 wk of exposure.
The right ventricular tissue levels of NOx were assayed as a measure of NO production rates in the RV at the time of death. Despite no detectable differences in the protein levels of eNOS and nNOS in WT and Mkp-1−/− mice, the NOx levels were lower in the Mkp-1−/− mice than in the WT mice following 4 wk of exposure to either normoxia or hypoxia (Fig. 11). Interestingly, chronic hypoxic exposure in the WT mice resulted in significantly lower right ventricular tissue levels of NOx than those seen in normoxic WT mice (Fig. 11).
Fig. 11.
Deficiency in MKP-1 decreased NO production in the right ventricle, whereas chronic hypoxia decreased NO production only in WT mice. Accumulation of NOx in the right ventricles from WT (open bars) and Mkp-1−/− (cross-hatched bars) mice under normoxia or hypoxia was measured using a chemiluminescence NO analyzer and normalized to right ventricular protein concentration (nmol/mg protein); n = 5–6 in each group. P < 0.05, Mkp-1−/− different from WT same condition (#) and hypoxia different from normoxia same genotype (*).
DISCUSSION
The major findings of this study were that chronic hypoxic exposure in mice deficient in MKP-1 resulted in more severe PH as evidenced by 1) higher right ventricular pressures, 2) greater RV/(LV + S), and 3) greater wall thickness of pulmonary vessels than in chronically hypoxic WT mice. Furthermore, hypoxic Mkp-1−/− mice had lower NOS protein expression, greater lung arginase I and arginase II protein expression, and greater lung PCNA protein expression than did hypoxic WT mice. Taken together, these findings demonstrate that mice deficient in MKP-1 develop more severe PH with lower NOS levels and greater arginase levels after 4 wk in hypoxia than do WT mice. These data suggest that MKP-1 is involved in the restraint of hypoxia-induced arginase expression in the lung.
Chronic hypoxic exposure has been extensively used as an animal model of PH. We found that WT mice housed in chronic hypoxia lost weight initially, but, despite continued hypoxic exposure, the WT mice regained their initial body weights by the end of the exposure period. A similar pattern of weight loss has been reported for CD1 mice (14), SV129 mice (29), and FVB mice (32). Interestingly, the mice deficient in MKP-1 did not regain their starting body weights by the end of the exposure. This suggests that the mice were more stressed by the exposure, and either did not take in adequate calories to regain body weight or had increased metabolic demand. Wu et al. (48) have recently shown that Mkp-1−/− mice are resistant to weight gain on a chow diet. Thus, under the stress of chronic hypoxia, it may be that mice deficient in MKP-1 cannot regain body weight, even if chow intake were comparable to the WT mice.
We found that the RVSP were greater following chronic hypoxic exposure and that the hypoxic Mkp-1−/− mice had greater RVSP than did the WT hypoxic mice. All of the mice had a significant increase in RVSP during ventilation with a hypoxic gas mixture. In the normoxia-exposed mice, the hypoxic pressor response was similar between the two groups, despite the fact that lung tissue and right ventricular tissue levels of NO were lower in the Mkp-1−/− mice than in the WT mice. In eNOS−/− mice, Fagan et al. (13) reported that ventilation with the equivalent of 10% oxygen at sea level resulted in RVSP values that were not different from those seen in WT mice, whereas exposure to milder hypoxia resulted in higher RVSP levels in eNOS−/− mice than in WT mice. Thus, it may be that acute exposure to severe hypoxia results in hypoxic pulmonary vasoconstriction that is not significantly modulated by endogenous NO production in mice.
A wide variety of genes have been implicated in the regulation of right ventricular hypertrophy and vascular remodeling in response to chronic hypoxia. In human studies, polymorphisms in the type 2 bone morphogenetic protein receptor (Bmpr2) and the serotonin transporter genes have been implicated in familial PH (37). Our data clearly identify Mkp-1 as a gene involved in the pulmonary hypertensive response to chronic hypoxia. We found that deficiency of MKP-1 leads to greater right ventricular pressures, and an exacerbation of both right ventricular hypertrophy and vascular remodeling. Mice deficient in the genes for atrial natriuretic peptide (6), soluble guanylate cyclase (47), cyclooxygenase 2, or Bmpr2 (15) also have been shown to have greater hypoxia-induced right ventricular hypertrophy and/or vascular remodeling than do WT mice. Louzier et al. (29) found that vascular endothelial growth factor-B knockout mice developed similar degrees of chronic hypoxia-induced PH as did WT mice. It has also been reported that mice deficient in the galectin-1 gene (4) or the tryptophan hydroxylase gene (21) developed less severe PH following chronic hypoxic exposure than did their respective WT controls. These studies demonstrate that there are many genes regulating the pulmonary hypertensive response to hypoxia, which may represent novel therapeutic targets in treating PH. Furthermore, some of these genetic pathways will certainly converge through similar signaling pathways. For example, both Mkp-1 and Bmpr2 influence p38 phosphorylation (15, 28).
The role of MKP-1 in hypoxia-induced PH is poorly understood. Although MKP-1 has been recognized as a hypoxia-responsive gene, its role in the response to hypoxia remains unclear (27, 41). A recent study (7) demonstrated that hypoxia results in a relatively rapid activation of both JNK and p38, the preferred targets of MKP-1, in bovine aortic endothelial cells. This led us to examine p38 phosphorylation in the lungs from our mice. We found significant differences in pp38 at 5 days of hypoxic exposure between WT and Mkp-1−/− mice, but little differences between genotypes by 4 wk. It is of interest to note that pp38 levels in the lungs of animals exposed to hypoxia at 4 wk were not different among genotypes or compared with animals exposed to normoxia. This is consistent with a study in rats by Jin et al. (22) that found pp38 levels had returned to basal levels by 14 days of hypoxic exposure. In vascular fibroblasts, hypoxia induced atypical protein kinase Cζ-mediated MKP-1 expression, leading to inactivation of ERK and suppression of cell proliferation (42). Thus, our findings of increased cellular proliferation in the lungs of hypoxic Mkp-1−/− mice compared with that in hypoxic WT mice are consistent with the notion that MKP-1 plays a role in suppressing cellular proliferation in hypoxia-induced PH. A role for MKP-1 in suppressing cellular proliferation is also consistent with results from other models of vascular remodeling (23, 30, 52). Indeed, Li et al. (25) have found that sidenafil, a phosphodiesterase 5 inhibitor, increased MKP-1 protein expression and activity in pulmonary vascular smooth muscle cells, resulting in decreased cellular proliferation. In addition to a role for the MAP kinases in vascular remodeling, there may be a role for the MAP kinases in vasoconstriction. For example, Han et al. (20) found that 4-aminopyridine-induced contraction of pulmonary arterial rings was associated with ERK activation and that the contraction was attenuated by ERK inhibitors. In canine pulmonary artery smooth muscle cells, p38 has been shown to modulate contraction (51). Morrell et al. (31) found that stimulation of p38 activity using ansiomysin augmented hypoxic force generation in isolated rat pulmonary artery rings. Thus, it is possible that the greater p38 phosphorylation we found in the chronically hypoxic MKP-1-deficient mice compared with the chronically hypoxic WT mice resulted in augmented hypoxic pulmonary vasoconstriction. Thus, MKP-1 may serve a dual role in hypoxia-induced PH; by restraining MAP kinase phosphorylation, MKP-1 may modulate both vasoconstriction and vascular remodeling.
We found that MKP-1-deficient mice had greater arginase expression in their lungs than did WT mice after 4 wk in hypoxia. Arginase is the first step in polyamine and proline synthesis, which are critical for cellular proliferation. We found greater PCNA levels after 4 wk in the chronically hypoxic Mkp-1−/− mice than in hypoxic WT mice, consistent with the concept that the mice deficient in MKP-1 were in a proproliferative state. A role for arginase in the development of PH has been suggested by a study in endothelial cells harvested from lung biopsy samples either from patients with PH or without PH, in which the endothelial cells from patients with PH had greater arginase II expression and activity and lower NO production than did the endothelial cells from patients without PH (50). The regulation of arginase expression in the lung is poorly understood. Albina et al. (1) found that hypoxia-induced arginase I expression involved the CCAAT/enhancer-binding protein β in macrophages. In inflammatory models, it has been shown that MAP kinase activity is associated with upregulation of arginase expression (44). Our data taken in light of these studies is consistent with the concept that hypoxia leads to dysregulated MAP kinase activation and greater arginase upregulation, resulting in a greater degree of PH. Considering that NOS and arginase share a common substrate, l-arginine, upregulation of arginase may limit NOS activity and NO production (9, 11, 24, 34). The decreased NO production that we found may in part be due to increased arginase protein expression, which results in increased l-arginine utilization by arginase at the expense of NOS. Thus, the greater degree of chronic hypoxia-induced PH that we found in the Mkp-1−/− mice compared with the WT mice involves greater arginase expression. It has long been postulated that PH is initially caused by vasoconstriction and that over time vasoconstriction becomes less important and vascular remodeling becomes the dominant cause of PH. However, recent data suggest that vasoconstriction may remain the dominant cause of PH in chronically hypoxic animals. For example, Nagaoka et al. (33) found that infusion of a Rho kinase inhibitor, Y-27632, in rats exposed to chronic hypoxia for 3–4 wk resulted in a decrease in pulmonary vascular resistance to levels seen in control rats. Furthermore, vasoconstriction may be the dominant contributor to PH even in the face of significant pulmonary vascular remodeling. Indeed, in an animal model of severe PH induced by injection of a vascular endothelial growth factor receptor antagonist followed by chronic hypoxic exposure, which results in the formation of occlusive vascular lesions, treatment with the rho kinase inhibitor, fasudil, resulted in significant pulmonary vasodilation (40). Thus, there is likely a component of both vasoconstriction and vascular remodeling underlying the chronic hypoxia-induced PH found in our mice, and arginase induction in Mkp-1−/− mice likely contributes to both through decreased NO production and increased ornithine production.
It is interesting to consider the decrease in the constitutively expressed eNOS protein levels in the lungs from the Mkp-1−/− mice compared with the WT mice. The regulation of the constitutively expressed eNOS gene is complex. There are some studies that suggest that the activation of the MAP kinase pathway results in decreased eNOS protein expression. For example, p38 activation suppressed eNOS promoter activity in bovine aortic endothelial cells (49), whereas inhibiting IL-1β-induced activation of p38 prevented the IL-1β-induced decrease in eNOS expression (38). In rats treated with lipopolysaccharide, there was a significant increase in pp38 and a significant decrease in eNOS protein levels in the thoracic aorta (17). Taken together, these studies support the notion that deficiency of MKP-1, by allowing for more sustained MAP kinase activation, may result in lower levels of eNOS protein following stimuli such as hypoxia that activate the MAP kinases.
We found greater right ventricular hypertrophy, as measured by the RV/(LV + S), in the Mkp-1−/− mice than in the WT mice. Strnisková et al. (45) found that p38 and JNK were activated in the RVs of rats exposed to hypoxia. Ohki et al. (39) found that, in the right atrial appendages of patients undergoing cardiac surgery, those with pressure overload had significantly greater expression of the MKP-1 gene than did patients from the control group. Furthermore, it has been found that MKP-1 overexpression prevented cardiac hypertrophy due to phenylephrine (46) or aortic banding (2). In a recent report on gene expression in rats given either low-dose or high-dose monocrotaline (3), the low-dose animals had significantly greater expression of the Mkp-1 gene and developed less right ventricular hypertrophy than did the animals receiving high-dose monocrotaline. These data taken together support the notion that MKP-1 in the heart acts to attenuate right ventricular hypertrophy in response to hypoxia. However, unlike the data from the lung, it is not clear how MKP-1 in the heart acts to attenuate right ventricular hypertrophy. Both arginase I and arginase II are greater in the RVs of the chronically hypoxic mice of both genotypes than in those mice exposed to normoxia. This chronic hypoxia-induced increase in arginase expression is associated with greater PCNA protein in the RV, but again there is no difference between the genotypes. Finally, NO production in the RVs is lower in the Mkp-1−/− mice than in the WT mice, but there is little effect of chronic hypoxic exposure on NO production in the RVs. These findings suggest that the mechanisms underlying the greater right ventricular hypertrophy are different from the mechanisms underlying the alterations in pulmonary vascular tone. Further studies will be needed to elucidate these mechanisms.
In terms of weakness of this study, it should be remembered that when using knockout animals, which have a gene deletion from embryogenesis, there is always a chance that compensatory responses will impact the outcome of any study. It should be pointed out that Mkp-1−/− mice have no readily discernable phenotype, are fertile, and have a normal life expectancy. An easily discernable phenotype is only found when Mkp-1−/− mice are stressed; for example, we have shown that Mkp-1−/− mice have a profound response to endotoxin (5, 28, 34, 53). It should also be pointed out that the Mkp-1−/− mice used in these studies are global knockout mice, and therefore the responses are not lung specific.
In conclusion, we found that mice deficient in MKP-1 had higher right ventricular pressures and developed more severe right ventricular hypertrophy and vascular remodeling after 4 wk in hypoxia than did WT mice. This was associated with greater protein levels of arginase and decreased levels of NOS protein and NO production in the lungs of hypoxic Mkp-1−/− mice than in the lungs of hypoxic WT mice. These data are consistent with the concept that MKP-1 acts to restrain hypoxia-induced arginase expression and thereby reduces vascular remodeling and the severity of PH.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-075261 (L. D. Nelin) and grants from the National Institute of Allergy and Infectious Diseases (AI-057798 and AI-068956, Y. Liu).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Bristol-Myers Squibb Pharmaceutical Research Institute for providing Mkp-1 knockout mice.
REFERENCES
- 1.Albina JE, Mahoney EJ, Daley JM, Wesche DE, Morris SM, Jr, Reichner JS. Macrophage arginase regulation by CCAAT/enhancer-binding protein beta. Shock 23: 168–72, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bueno OF, De Windt LJ, Lim HW, Tymitz KM, Witt SA, Kimball TR, Molkentin JD. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res 88: 88–96, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Buermans HPJ, Redout EM, Schiel AE, Musters RJP, Zuidwijk M, Eijk PP, van Hardeveld C, Kasanmoentalib S, Visser FC, Ylstra B, Simonides WS. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 21: 314–323, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Case D, Irwin D, Ivester C, Harral J, Morris K, Imamura M, Roedersheimer M, Paterson A, Carr M, Hagen M, Saavedra M, Crossno J, Young KA, Dempsey EC, Poirier F, West J, Majka S. Mice deficient in galectin-1 exhibit attenuated physiological responses to chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 292: L154–L164, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Calvert TJ, Chicoine LG, Liu Y, Nelin LD. Deficiency of mitogen-activated protein kinase phosphotase-1 results in iNOS-mediated hypotension in response to low-dose endotoxin. Am J Physiol Heart Circ Physiol 294: H1621–H1629, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chen YF, Feng JA, Li P, Xing D, Ambalavanan N, Oparil S. Atrial natriuretic peptide-dependent modulation of hypoxia-induced pulmonary vascular remodeling. Life Sci 79: 1357–1365, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chen SC, Liu YC, Shyu KG, Wang DL. Acute hypoxia to endothelial cells induces activating transcription factor 3 (ATF3) expression that is mediated via nitric oxide. Atherosclerosis 201: 281–288, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chicoine LG, Avitia JW, Deen C, Nelin LD, Earley S, Walker BR. Developmental differences in pulmonary eNOS expression in response to chronic hypoxia in the rat. J Appl Physiol 93: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 287: L60–L68, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chicoine LG, Paffett ML, Girton MR, Metropoulus MJ, Joshi MS, Bauer JA, Nelin LD, Resta TC, Walker BR. Maturational changes in the regulation of pulmonary vascular tone by nitric oxide in neonatal rats. Am J Physiol Lung Cell Mol Physiol 293: L1261–L1270, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagan KA, Morrissey B, Fouty BW, Sato K, Harral JW, Morris KG, Jr, Hoedt-Miller M, Vidmar S, McMurtry IF, Rodman DM. Upregulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir Res 2: 306–313, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan KA, Fouty BW, Tyler RC, Morris KG, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics 22: 292–307, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DB, Lowery J, Anderson L, Brink M, Reese J, de Caestecker M. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 294: L98–L109, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, Kourembanas S, Perrella MA. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation 117: 2114–22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Sharma AC. Despite minimal hemodynamic alterations endotoxemia modulates NOS and p38-MAPK phosphorylation via metalloendopeptidases. Mol Cell Biochem 265: 47–56, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev 80: 1337–1372, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation 116: 2992–3005, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Han W, Tang X, Wu H, Liu Y, Zhu D. Role of ERK1/2 signaling pathways in 4-aminopyridine-induced rat pulmonary vasoconstriction. Eur J Pharmacol 569: 138–144, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, Saurini F, Eddahibi S, Hamon M, Adnot S. Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 293: L1045–L1052, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jin N, Hatton N, Swartz DR, Xia X, Harrington MA, Larsen SH, Rhoades RA. Hypoxia activates Jun-N-terminal kinase, extracellular signal-regulated protein kinase and p38 kinase in pulmonary arteries. Am J Respir Cell Mol Biol 23: 593–601, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Lai K, Wang H, Lee WS, Jain MK, Lee ME, Haber E. Mitogen-activated protein kinase phosphatase-1 in rat arterial smooth muscle cell proliferation. J Clin Invest 98: 1560–1567, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Meininger CJ, Hawker JR, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline synthesis in endothelial cells. Am J Physiol Regul Integr Comp Physiol 282: R64–R69, 2002. 11742824 [Google Scholar]
- 25.Li B, Yang L, Shen J, Wang C, Jiang Z. The antiproliferative effect of sildenafil on pulmonary artery smooth muscle cells is mediated via upregulation of mitogen-activated protein kinase phosphatase-1 and degradation of extracellular signal-regulated kinase 1/2 phosphorylation. Anesth Analg 105: 1034–1041, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Liscovsky MV, Ranocchia RP, Gorlino CV, Alignani DO, Moron G, Maletto BA, Pistoresi-Palencia MC. Interferon-γ priming is involved in the activation of arginase by oligodeoxinucleotides containing CpG motifs in murine macrophages. Immunology 128: e159–e169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Shi Y, Han Z, Pan Y, Liu N, Han S, Chen Y, Lan M, Qiao T, Fan D. Suppression of the dual-specificity phosphatase MKP-1 enhances HIF-1 trans-activation and increases expression of EPO. Biochem Biophys Res Commun 312: 780–786, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases–regulating the immune response. Nat Rev Immunol 7: 202–212, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Louzier V, Raffestin B, Leroux A, Branellec D, Caillaud JM, Levame M, Eddahibi S, Adnot S. Role of VEGF-B in the lung during development of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 284: L926–L937, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res 97: 434–42, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Morrell ED, Tsai BM, Wang M, Crisostomo PR, Meldrum DR. p38 mitogen-activated protein kinase mediates the sustained phase of hypoxic pulmonary vasoconstriction and plays a role in phase I vasodilation. J Surg Res 134: 335–341, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Mühling J, Paddenberg R, Hempelmann G, Kummer W. Hypobaric hypoxia affects endogenous levels of α-keto acids in murine heart ventricles. Biochem Biophys Res Comm 342: 935–939, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Nagaoka T, Morio Y, Casanova N, Nauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol 293: C632–C640, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Nelin LD, Nash HE, Chicoine LG. Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1232–L1239, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am J Respir Cell Mol Biol 33: 394–401, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman JH, Phillips JA, 3rd, Loyd JE. Narrative review: the enigma of pulmonary arterial hypertension: new insights from genetic studies. Ann Intern Med 148: 278–283, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Niwano K, Arai M, Koitabashi N, Hara S, Watanabe A, Sekiguchi K, Tanaka T, Iso T, Kurabayashi M. Competitive binding of CREB and ATF2 to cAMP/ATF responsive element regulates eNOS gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 26: 1036–1042, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ohki R, Yamamoto K, Ueno S, Mano H, Misawa Y, Fuse K, Ikeda U, Shimada K. Transcriptional profile of genes induced in human atrial myocardium with pressure overload. Int J Cardiol 96: 381–387, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Seta KA, Kim R, Kim H, Millhorn DE, Beitner-Johnson D. Hypoxia-induced regulation of MAPK phosphotase-1 as identified by subtractive suppression hybridization and cDNA microarray analysis. J Biol Chem 276: 44405–44412, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Short MD, Fox SM, Lam CF, Stenmark KR, Das M. Protein kinase Cζ attenuates hypoxia-induced proliferation of fibroblasts by regulating MAP kinase phosphotase-1 expression. Mol Biol Cell 17: 1995–2008, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104: 110–118, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Stempin CC, Garrido VV, Dulgerian LR, Cerbán FM. Cruzipain and SP600125 induce p38 activation, alter NO/arginase balance and favor the survival of Trypanosoma cruzi in macrophages. Acta Trop 106: 119–127, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Strnisková M, Ravingerová T, Neckár J, Kolár F, Pastoreková S, Barancík M. Changes in the expression and/or activation of regulatory proteins in rat hearts adapted to chronic hypoxia. Gen Physiol Biophys 25: 25–41, 2006 [PubMed] [Google Scholar]
- 46.Thorburn J, Carlson M, Mansour SJ, Chien KR, Ahn NG, Thorburn A. Inhibition of a signaling pathway in cardiac muscle cells by active mitogen-activated protein kinase kinase. Mol Biol Cell 6: 1479–1490, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, Pellens M, Gillijns H, Swinnen M, Graveline A, Collen D, Dewerchin M, Brouckaert P, Bloch KD, Janssens S. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation 116: 936–943, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab 4: 61–73, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Xing F, Jiang Y, Liu J, Zhao K, Mo Y, Liu Z, Zeng Y. Downregulation of human endothelial nitric oxide synthase promoter activity by p38 mitogen-activated protein kinase activation. Biochem Cell Biol 84: 780–788, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Xu W, Kaneko FT, Zheng S, Comhair SAA, Janocha AJ, Goggans T, Thunnissen FBJM, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurun SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Yamboliev IA, Hedges JC, Mutnick JLM, Adam LP, Gerthoffer WT. Evidence for modulation of smooth muscle force by the p38 MAP kinase/HSP 27 pathway. Am J Physiol Heart Circ Physiol 278: H1899–H1907, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Yang YB, Yang YX, Su B, Tang YL, Zhu BY, Hu ZW, Li GY, Li YJ, Liao DF. Probucol mediates vascular remodeling after percutaneous transluminal angioplasty via down-regulation of the ERK1/2 signaling pathway. Eur J Pharmacol 570: 125–134, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203: 131–40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]