Abstract
Several anticonvulsant agents, including topiramate and valproate, have been found to reduce alcohol consumption in rodent models of drinking. The question of whether the novel anticonvulsant agent, zonisamide, shares similar actions in either mice or rats was investigated in the present experiments. In an initial experiment, the consumption of a 10% ethanol/5 % sucrose solution, available for one hour, by Wistar rats treated with lactose, topiramate, or zonisamide was determined. In a second experiment, the intake of a 10% ethanol/water solution, accessible for two hours, by C57BL/B6N mice treated with either zonisamide or vehicle was assessed. In the rat, 50-mg/kg (PO) doses of either topiramate or zonisamide produced significant, but moderate decreases in ethanol/sucrose intake. The administration of a 50-mg/kg (IP) dose of zonisamide to mice resulted in a marked lowering in ethanol consumption. These results provide evidence that zonisamide administration will decrease ethanol consumption by both mice and rats in limited access models of drinking, and might, like topiramate, be useful as a medication for alcoholism.
Keywords: Alcohol, Alcoholism, Novel Anticonvulsants, Sulfamates, Sulfonamides, Topiramate, Zonisamide
1. Introduction
Anticonvulsant agents have been evaluated in both pre-clinical and clinical studies as medications for the treatment of alcohol dependence. Certain older anticonvulsant agents have been found to decrease ethanol consumption in rodent models of drinking. These agents include carbamazepine (Messiha et al., 1986) and valproate (Gardell et al., 1998). In several clinical trials, carbamazepine (Mueller et al., 1997), the carbamazepine metabolite, oxcarbazepine (Croissant et al., 2004), and several valproate-related derivatives have been found to reduce ethanol consumption in alcoholic individuals (Longo et al., 2002; Salloum et al., 2005). However, in other clinical trials neither carbamazepine (Kranzler et al., 1995) nor divalproex, a valproate derivative (Brady et al., 2002) significantly altered alcohol intake in alcohol dependent subjects. Also, valproate has not been found to reduce the desire of alcoholic individuals to drink (Minuk et al., 1995). Thus, questions remain concerning the efficacy of older anticonvulsants as medications for the treatment of alcohol dependence.
Newer anticonvulsant medications, including gabapentin (Bisaga and Evans, 2006), lamotrigine (Rubio et al., 2006), tiagabine (Nguyen et al., 2005; Rimondini et al., 2002; Schmitt et al., 2002), and topiramate (Gabriel and Cunningham, 2005; Johnson et al., 2003; Nguyen et al., 2007; Rubio et al., 2004) also have been evaluated in preclinical and clinical studies as agents for the treatment of alcoholism. At present, only topiramate has been shown to reduce ethanol consumption in alcohol dependent subjects in a double-blind placebo-controlled trial (Johnson et al., 2003).
Topiramate, a derivative of sulfamate fructopyranose, is a representative of a new class of sulfamate anticonvulsants (Marynoff et al., 1998). These agents bear some structural resemblance to the sulfonamide anticonvulsants. The novel anticonvulsant zonisamide (1,2-benzisoxazole-3-methanesulfonamide) is a member of this class of anticonvulsants (Mayanoff et al., 1998), however, whether treatment with zonisamide or other related sulfonamide anticonvulsants would modify ethanol consumption has not been previously examined.
Both topiramate (Astrup et al., 2004; Brown et al., 2002; Carpenter et al., 2002; Chengappa et al., 2002; Tonsad et al., 2005; Wilding et al., 2004) and zonisamide (Gadde et al., 2003; McElroy et al., 2004) may promote weight loss in obese individuals suggesting that these agents may share an ability to influence one form of appetitive behavior. One mechanism that might mediate the effects of topiramate, and possibly zonisamide, on weight is suggested by the findings that the chronic administration of topiramate has been shown to increase neuropeptide Y messenger ribonucleic acid (mRNA) in the hypothalamus while decreasing hypothalamic mRNA for neuropeptide Y1 and Y5 receptors in Osborne-Mendel rats (York et, al., 2000). Topiramate also has been shown to elevate hypothalamic neuropeptide Y in Flinders rat lines (Husum et al., 2003). These findings are of interest because neuropeptide Y has been implicated in the regulation of ethanol consumption in rodents (Gilpin et al., 2003; Gilpin et al., 2004; Gilpin et al., 2005; Hayes et al., 2005; Schroeder et al., 2005; Sparta et al., 2004). Whether zonisamide has similar actions on neuropeptide Y activity remains to be determined.
Topiramate and zonisamide have some common pharmacological effects. Both agents may block voltage-sensitive sodium channels (Rock et al, 1989; Schauf et al., 1987; Taverna et al., 1999; Zona et al., 1997) and are low potency inhibitors of carbonic anhydrase (Maryanoff et al., 1998; Maryanoff et al., 2005; Masuda et al., 1980; Masuda and Karasawa, 1993). Both topiramate and zonisamide inhibit calcium channel activity, however, topiramate acts selectively on L-type calcium channels (Zhang et al., 2000) while zonisamide actions are directed towards T-type calcium channels (Kito et al., 1996; Suzuki et al., 1992). Topiramate may inhibit the activity of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA)/kainate receptors (Angehagen et al., 2004; Gibbs et al., 2000; Gryder and Rogawski, 2003; Qian and Noebels, 2003; Skradski and White, 2000). Zonisamide administration inhibits excitatory post-synaptic potentials in frontal cortical tissues in a manner consistent with the blockade of AMPA receptors (Huang et al., 2005). Whether topiramate and zonisamide act on the same AMPA/kainate receptor populations is open to question. There is clear evidence that topiramate may have selective actions on the kainate-type of receptors that contain Glu-R5 subunits (Gryder and Rogawski, 2003), while studies of the interaction of zonisamide with specific AMPA/kainate receptor subunits have not been conducted.
The present experiments were conducted to assess whether zonisamide might have actions on ethanol consumption that are similar to those produced by topiramate administration. The effects of zonisamide administration on ethanol consumption in both rats and mice were determined in limited access single bottle choice experiments. In an initial experiment, the actions of zonisamide on the consumption of an alcohol-sucrose solution by Wistar rats were compared with those of topiramate. Topiramate and zonisamide were orally administered in this experiment. The presence of sucrose in the solution used to assess drinking behavior introduces the confounding factors of sugary taste and the ready availability of energy from sugar. To circumvent this problem, a second experiment was conducted using C57BL/B6 mice, a strain of mice that will readily learn to consume ethanol without the use of sucrose fading procedures (Le et al., 1994). In the second experiment, the effects of zonisamide were tested on the consumption of a 10% ethanol-water solution by C57BL/BN mice. Also, in contrast to the first experiment, animals were injected in the mouse experiment with test agents to avoid the stresses associated with the oral administration of drugs, which include restraining the animals, the forced placement of a tube into the mouth, and possible residual unpleasant drug tastes.
2. Methods
The Boston University Institutional Animal Care and Use Committee approved all of the procedures used in the experiments described below.
2.1. Experiment 1
2.1.1. Animals
Animals: Thirty male Wistar rats (Charles River Laboratories, Inc., Wilmington, MA) were used in this experiment. Animals were randomly assigned to lactose (n=10), topiramate (n=10), or zonisamide (n=10) treatment groups. They were individually housed in a facility separate from the testing environment and maintained on a 12-hour light: 12-hour dark cycle. Training and testing were performed during the light cycle. Animals had free access to food and water.
2.1.2 Training and Testing
Rats were given access to ethanol solutions in acrylic chambers with a 3/8-inch hole drilled in the side, located about an inch above the bottom of the chamber. Forty-milliliter glass tubes with rubber stoppers and metal sipper tubes placed through the holes were mounted to the side of each box.
Rats were given unrestricted access to ethanol-sucrose solutions during two-hour sessions. The concentration of ethanol in these solutions was systematically increased, while the concentration of sucrose was decreased over a 38-day period. On days 1-11 animals were given access to a 2% ethanol-10% sucrose solution, on days 12-24 to a 5% ethanol-5% sucrose solution, on days 25 to 38 an 8% ethanol-5% sucrose solution, and then to a 10% ethanol-5% sucrose solution, which was continued for 18 days prior to the start of the treatment phase.
The drug treatment phase began after animals had completed 8 weeks of drinking sessions. During this phase and during the post-treatment phase animals continued to be given access to a 10% ethanol-5% sucrose solution during test sessions. Rats in the three treatment groups were treated with 25-mg/kg doses of either topiramate or zonisamide or with 25-mg/kg of lactose for five days, respectively. The dose of the two test anticonvulsant medications was then increased to 50-mg/kg daily for 5 days. Immediately after the drug treatment phase, the daily consumption of ethanol-sucrose solution was monitored in the post-treatment phase for one week to determine if any drug-induced changes in drinking would persist after the discontinuation of drug. Before and during the treatment phase rats' weights were recorded daily to allow monitoring of drug-induced weight changes.
2.1.3 Drug Preparation and Administration
In this experiment (Experiment #1) all test agents were administered orally. These agents were delivered in two mini-marshmallows (Kraft®) during the first four days of the drug treatment phase in an effort to reduce the stress of forced oral administration of test agents. Drugs were then administered orally in a saccharin-sweetened solution, by a syringe connected to a soft plastic tube. Only commercially available oral forms of topiramate and zonisamide could be obtained for this first experiment. Topiramate (Topamax®-Ortho-McNeil Pharmaceutical, Raritan, New Jersey) (200 mg), zonisamide (Zonegran®-Elan Biopharmaceuticals, San Diego, California) (100 mg), or lactose (100 mg) were each suspended in powdered form in distilled water brought up to a volume of 1 milliliter. Two saccharin tablets were added to each milliliter of the prepared suspensions to mask drug tastes. Test agents were administered two hours prior to the placement of animals into drinking chambers with the exception of topiramate, which was administered one hour before testing during days 9 and 10 of the treatment phase. Times of topiramate administration were varied because of uncertainties concerning the pharmacokinetics of orally administered topiramate in the rat. Data concerning topiramate plasma concentrations appear to be available only for times at 1, 6 and later hours (up to 24 hours) following oral dosing of this drug (Shank et al., 2000).
2.1.4 Statistical Analysis
Mean ethanol consumption during the baseline phase in g/kg for each animal was calculated by taking the mean of the last five drinking days prior to drug administration. The g/kg of ethanol consumed each day during drug administration minus the baseline phase value was then divided by the baseline value for each animal to determine the percent change from baseline. For days on which the 50 mg/kg doses were administered, separate comparisons for percent change in ethanol intake were conducted between the lactose group and corresponding results for each anticonvulsant group. These comparisons entailed the use of a two-way repeated measures analysis of variance (ANOVA), with treatment as the between group factor and treatment day (time) as the within subject factor. Pair-wise comparisons for each treatment day were performed using Fisher's least-significant difference test.
One-way ANOVA's were used to compare mean baseline volume of ethanol intake, percent change in ethanol intake on treatment Day 5 (25 mg/kg dose in suspension) and mean animals' weights amongst groups. Repeated measures one-way ANOVA's were used for within group comparisons of mean baseline ethanol consumption with mean intake during the marshmallow and post-treatment phases groups, and of group weights across the treatment period. Two-way repeated measures ANOVA's with group and time as factors were performed to determine if weight were altered by drug administration during the treatment phase.
2.2 Experiment 2
2.2.1. Animals
Male C57BL/B6NHsd mice (Harlan Sprague Dawley, Indianapolis, IN), derived from an NIH colony, were used in this experiment. These mice are putatively alcohol preferring. They were assigned to either vehicle (n=10) or zonisamide (n=9) treatment groups. Mice weighed approximately 25 g when they arrived and were 5- 6 weeks old. Mice were handled daily for a minimum of five days prior to the induction of drinking and were maintained on a 12-hour light/dark cycle with all testing conducted during the light cycle. They were housed individually with free access to food and water.
2.2.2. Training and testing
Drinking sessions were one hour in length. These sessions were conducted in plastic cages that were identical to home cages except that they did not contain bedding. A 15-milliliter graduated wide mouth centrifuge tube was suspended in the cage from an overhead wire screen cover. A metal sipper tube was placed into a stopper in the mouth of the centrifuge tube. Mice were induced to drink by the initial presentation of 3% ethanol in tap water during drinking sessions over a 7-day period. Animals were then given access to a 5% ethanol solution for 7 days. The concentration of the ethanol solution made available was then increased to 10%. Mice were then allowed to drink a 10% ethanol solution for 13 days prior to the administration of zonisamide or vehicle injections.
During the treatment phase of this experiment mice were treated daily with 25 mg/kg of zonisamide or vehicle for 5 days followed by a daily dose of 50-mg/kg daily for 5 days. Animals were given access to ethanol on the first three days on which the 25-mg/kg dose of zonisamide was administered, and during the 5 days in which the 50-mg/kg dose of this drug was administered. After the completion of the treatment period, animals were given access to 10% ethanol solutions daily for 3 days to allow an assessment of ethanol consumption after discontinuation of zonisamide administration. Before and during the treatment phase, the weights of mice were recorded daily to allow monitoring of drug-induced changes in the animals' weights.
2.2.3 Drug Preparation and Administration
Zonisamide (ChemAgis, Mountain Lakes, New Jersey) was dissolved in a 40% propylene glycol solution by first suspending this drug in propylene glycol, followed by the addition of hot distilled water. A 40% propylene glycol solution was used as the control vehicle in this experiment. Both zonisamide and the control vehicle were administered by IP injection in a volume of 10-ml/kg 45 minutes prior to the placement of mice into the drinking chambers.
2.2.4 Statistical Analysis
Mean ethanol consumption in g/kg for the three days prior to start of the drug treatment phase was used as the baseline level of alcohol intake in this experiment. The mean ethanol intake for the first three days of post-treatment phase was used as a measure of ethanol intake after treatment discontinuation. One-way ANOVA's were used to compare baseline values. Within group comparisons of alcohol intake between the pre- and post-treatment phases were performed using one-way repeated measures ANOVA's. Separate comparisons were conducted between vehicle and each dose of anticonvulsant for percent change in ethanol intake. Two-way repeated measures ANOVA's were used for these comparisons with treatment as a between group factor and time as a within subject factor. Post-hoc testing was conducted using the Fisher's least-significant difference test. Two-way repeated measures ANOVA analysis was also used for comparisons for weight over the treatment phase.
3.0 Results
3.1 Experiment 1(Rats)
The first 38 pre-drug days allowed enough time for the rats to escalate to the final concentration of 10% ethanol, 5% sucrose. Animals then continued drinking at this concentration for 18 days prior to the start of the treatment phase.
The mean daily intake of ethanol in g/kg for each treatment group for the baseline phase, the administration of drugs in marshmallows (treatment phase Days 1-4), and the post-treatment phase of this experiment are presented in Figure 1. Mean baseline ethanol consumption did not differ amongst the three treatment groups. There was also no significant difference amongst the groups in the amount of ethanol consumed during the 4 days (treatment phase Days1-4) during which test agents were administered in marshmallows. The mean amount of ethanol consumed during days on which marshmallows were eaten was significantly less than for the baseline phase for the topiramate [F(1,9)=37.6; p=0.0002] and zonisamide [F(1,9)=11.7; p=0.008], and approached being significantly different for the lactose [F(1,9)=4.5; p= 0.06] group.
Fig 1.
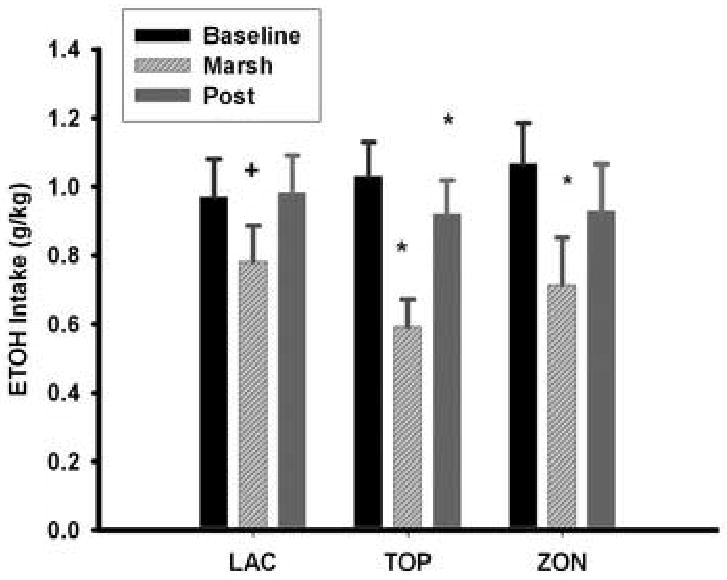
Mean (± S.E.) g/kg daily intake of ethanol for the 5 day baseline phase [Baseline], the drug in marshmallow treatment phase (treatment phase Days 1-4) [Marsh], and for the post-treatment phase [Post] by the lactose [LAC] (n=10), topiramate [TOP] (n=10) and zonisamide [ZON] (n=10) treatment groups. * p <0.05 for comparison with baseline for the respective group. + = 0.06 for comparison with baseline for the lactose group.
For treatment Days 5-10, when drugs were administered in suspension form, the mean daily percent change from baseline in ethanol consumption over the drug treatment phase for each group is shown in Figure 2. For treatment phase Day 5, when the 25-mg/kg dose of anticonvulsant drugs was administered in suspension form, the treatment effect was not significant for mean percent in ethanol solution consumed for each active agent compared to lactose. It should be noted, however, that values for mean percent change from baseline were lower for the anticonvulsant treatment groups than values for the lactose group. For treatment phase Days 6-10, when 50-mg/kg doses of drugs were administered in suspension form, the treatment effect was significant for both the topiramate [F(1,18)=11.2; p=0.004] and zonisamide groups [F(1,18)=10.2; p=0.005] when each group was compared to the lactose group. Pair-wise comparisons of topiramate and lactose groups indicate a significant between group difference only for Day 10. Significant or near significant differences between zonisamide and lactose groups were detected for several treatment days (see Figure 2).
Fig 2.
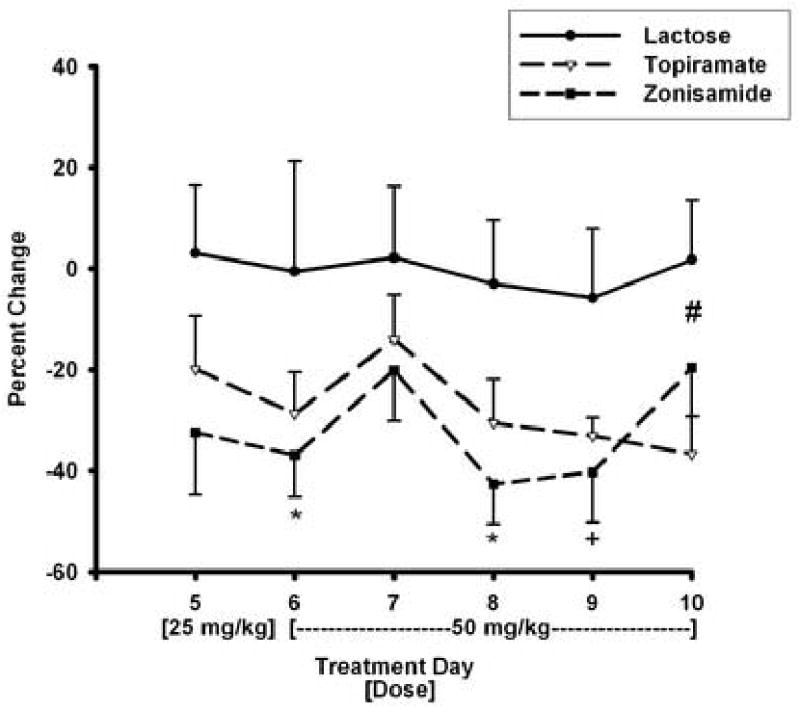
Ethanol intake during treatment phase Days 5-10, when test agents were administered in oral suspensions is presented. Intake is shown as the mean (± S.E.) percent change from baseline in 10% ethanol-5% sucrose solution consumption by the lactose, topiramate, and zonisamide treatment groups. Anticonvulsant agents were administered as 25-mg/kg dose on Day 5 and as a 50-mg/kg dose on Days 6-10. * p < 0.05 for comparison between zonisamide and with lactose groups. + p= 0.057 for comparison between zonisamide and lactose groups. # p < 0.05 for comparison between topiramate and lactose groups.
The mean daily amount of ethanol consumed during the post-treatment phase was not significantly different from the mean daily amount consumed during the baseline period for either the lactose or zonisamide groups (Figure 1). The mean daily intake of ethanol during the post-treatment phase was significantly reduced [F(1,9)=6.75; p=0.03], albeit to a small extent, from the level consumed at baseline for the topiramate group.
Mean weights for the three treatment groups did not differ significantly from one another for the day prior to the start of the treatment phase. For daily weights obtained for the period of time ranging from the day prior to the start of treatment to the last day of the treatment phase, group × time interactions were not significant for weights between the lactose and topiramate groups or the lactose and zonisamide groups. There was a modest increase in weight over the treatment phase for all of the treatment groups. Mean weights changed from the day before treatment to the last day treatment from 425.6 (SE ± 13.6) g to 438.6 (SE ± 14.5) g, from 449 (SE ± 14.2) g to 459 (SE ± 14.7) g, and from 427.5 (SE ± 12.9) to 440.0 (SE ± 12.7) for lactose, topiramate, and zonisamide groups respectively. For each group, the time effect was significant (p< 0.001) for mean weights obtained from the day before treatment to the last day of treatment.
3.2 Experiment 2 (Mice)
Although the mean baseline ethanol intake in the mice was higher for the zonisamide group than for vehicle group, these values did not differ significantly [F(1,17)= 2.5; p= 0.13] between these two groups (Figure 3). For percent change from baseline in ethanol intake there was a significant treatment effect for the 50-mg/kg [F(1,17)=11.3; p=0.004], but not for the 25-mg/kg zonisamide dose. Significant or near significant between group differences were found for Days 7, 8, and 9. It should be noted that a significant between group difference was not detected on Day 10 due, in part, to an increase in drinking from Day 9 by mice in the zonisamide group. This suggests the possibility that tolerance may develop to the effects of zonisamide on drinking. However, because of the reduction of drinking in the vehicle group for this day, the failure to find a significant between group difference may reflect a Type II error.
Fig 3.
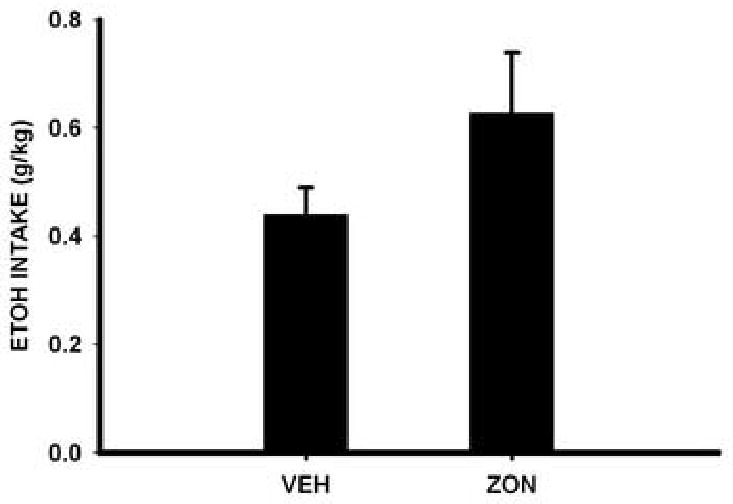
Mean (± S.E.) g/kg ethanol intake by mice for the vehicle [VEH] (n=10) and zonisamide [ZON] (n=9) treatment groups for the baseline phase.
When mean ethanol intake for the baseline period and the post-treatment phase were compared, no significant difference between values obtained for these phases were found for either the vehicle [F(1,9)= 1.14; p=0.31] or the zonisamide [F(1,8)=2.96; p=0.12] groups. Mean weights of mice did not differ between the treatment groups the day prior to the administration of test agents. The time × group interaction was significant [F(10,170)=2.78; p=0.003] for weights obtained during the time period ranging between the day before the treatment phase to the end of the treatment phase. This interaction appears to reflect a trend for slightly greater rate in weight loss over the treatment phase in the zonisamide group as compared to the vehicle group. The mean weight for mice in the zonisamide group changed from a mean of 32.4 (SE ± 1.0) g to a mean of 30.8 (± 0.9) g, while mean weights for the vehicle-treated animals went from 32.05 (SE ± 1.0) g to 31.3 (SE ± 0.9) g.
4. Discussion
The results of this investigation support the hypothesis that zonisamide may reduce ethanol consumption in rodents. In both mice and rats, significant reductions in ethanol consumption were observed after administration of the 50-mg/kg dose, but not after the 25-mg/kg dose. Following the discontinuation of zonisamide administration, both rats and mice resumed consumption of ethanol solutions at levels that were equivalent to baseline intake levels, suggesting that the effects on drinking of this anticonvulsant were readily reversible. While the 50-mg/kg dose of zonisamide reduced ethanol intake, it did not alter daily weight in the rats over the treatment period, and produced only modest weight loss in mice. This suggests that the effects of this drug on ethanol consumption are not associated with a marked influence on the neuronal systems that are involved in the regulation of food consumption.
The oral administration of topiramate to rats resulted in a moderate reduction in the consumption of an ethanol-sucrose solution. The overall treatment effect was significant for the 50-mg/kg dose of topiramate. However, while ethanol consumption tended to be lower for the topiramate than for the lactose group when the 50-mg/kg dose of this drug was administered, between group comparisons for individual treatment days indicated a significant group difference for the last treatment day only. Other investigators have shown that the administration of topiramate will decrease alcohol consumption in C57BL/B6J mice (Gabriel and Cunningham, 2005; Nguyen et al., 2007). It is of interest to note that topiramate administration has not been found to alter ethanol-induced place preference in C57Bl/6J mice (Gremel et al., 2006).
Drugs were administered to rats in marshmallows during the first four treatment days to minimize the stresses associated with forced oral administration. The consumption of marshmallows two-hours before drinking sessions, however, tended to reduce the amount of ethanol/sucrose solution consumed, although this effect only approached significance for the rats treated with lactose. Consequently, drugs were administered in suspension form during the last 6 days of treatment.
The reduction in the intake of ethanol-sucrose solutions seen after the consumption of marshmallow suggests that the presence of sugar may have been a major factor in motivating rats to drink these solutions, because the motivation for consuming sucrose may have been satiated by the marshmallows. The presence of sugars was clearly not a factor, however, in motivating mice to drink ethanol-water solutions in the second experiment. This leaves open the question as to whether zonisamide altered ethanol consumption by mice and rats through similar mechanisms involving a selective action on the motivation to consume alcohol, or if its influence on the consumption of sugars was a major factor for rats and not mice. In recent work in a collaborating laboratory, a 50 mg/kg IP dose of zonisamide was found to reduce the consumption of an ethanol/water solution in one group of Long Evans rats, but not that of a sucrose solution in a second group of animals (Leite-Morris and Ciraulo, 2007). Thus, zonisamide administration may have a selective inhibitory effect on ethanol intake while not influencing sucrose consumption. The lack of effect of zonisamide administration on sucrose solution intake also suggests that this drug does not have a non-specific suppressant effect on liquid consumption.
In mice, the administration of topiramate, at doses that reduced ethanol consumption in C57BL/6 mice, did not significantly alter motor activity (Nguyen et al., 2007). Locomotor activity is not significantly decreased by the IP administration of topiramate at doses ranging between 30 and 50-mg/kg in rats (Ciraulo et al., 2006; Shannon et al., 2005). Performance on the rotorod is not impaired by the administration of doses of topiramate at a dose of 200-mg/kg IP to mice (Tutka et al., 2005). These results suggest that topiramate-induced reductions in ethanol consumption observed in the present study also are unrelated to effects of this agent on motor activity.
When used clinically, anticonvulsant doses are increased gradually to enhance the extent to which they are tolerated. An attempt was made to parallel this practice in the present study with the dose of topiramate and zonisamide being raised to the 50-mg/kg level only after five days of administration of the 25-mg/kg dose. This approach should have reduced the likelihood that non-specific drug effects such as sedation had an influence on the consumption of ethanol by the experimental animals when the 50-mg/kg dose was administered.
The doses of zonisamide tested in animals in the present study were comparable to those found to be effective in the prevention or suppression seizure activity in mice (Borowicz et al., 2006; Masuda et al., 1979; Masuda et al., 1980; Nakamura et al., 1994) and rats (Hamada et al., 2001; Kitano et al., 2005; Masuda et al., 1979; Nakamura et al., 1994). These doses are markedly lower than those of zonisamide reported to produce motor impairing or sedative/hypnotic effects in most studies. The ratio for plasma concentrations of anti-seizure to motor impairing (as assessed by the rotorod test) effects of zonisamide have been reported to be 7.6 and 8.8 for STD-ddy strain mice and Wistar HLA strain rats, respectively (Masuda et al., 1979). In Sprague-Dawley rats, locomotor activity was significantly suppressed by a minimum effective dose of 300-mg/kg (IP) of zonisamide (Shannon et al., 2005). Oral doses of zonisamide below 300-mg/kg (PO) did not alter the spontaneous movement of mice or rats (Masuda et al., 1980). The ED50 for impairment of Wistar rats on the rotorod for zonisamide has been reported to be 160 mg/kg (PO) (Kitano et al., 2005). Impairment of performance of mice on the rotorod test has been produced by zonisamide with ED50's ranging between 100-292 mg/kg (PO) (Kitano et al., 2005; Masuda et al., 1980; Uno et al., 1979). A 50 mg/kg dose of zonisamide when administered IP did not significantly alter latency for escape by Long Evans rats from the water in a Morris Water Maze (Ciraulo et al., 2006). The ED50 for the hypnotic effects of zonisamide in mice was found to be 934-mg/kg (PO) (Masuda et al., 1980). These findings suggest that zonisamide, at the doses used in the present study, may not have hypnotic or motor impairing effects in either mice or rats.
Higher concentrations of zonisamide may be attained in the plasma after IP injection of this drug than if this drug was administered orally when the same mg/kg dose of the drug is administered (Masuda et al., 1979; Nagatomo et al., 1996). This may be one explanation as to why the effects of zonisamide on ethanol intake were greater in mice treated IP with this anticonvulsant than they were in rats who received zonisamide orally. This may also explain why zonisamide had an effect on weight loss in the present study in mice, but not in rats. Finally, the possibility needs to be considered that non-specific effects of zonisamide resulting in reduced ethanol consumption would be more likely to occur in the present experiment in mice after IP injection of this agent than it would in rats following the oral administration of this drug.
The limited access model of drinking used in the present study fails to incorporate many features of drinking seen in the clinical manifestations of alcohol dependence. For example, mice or rats, when given limited access to ethanol, do not typically consume sufficient quantities of ethanol over a long enough period of time to produce the sustained high blood alcohol concentrations needed to produce physical dependence such as is seen in severe alcoholism. Ethanol consumption by animals in the experiments described here can be characterized as being modest. This level of consumption would not be expected to produce elevations in brain or blood alcohol concentrations that exceed those observed in previously reported limited access model studies of alcohol consumption (Finn et al., 2004; Nurmi et al., 1999; O'Callaghan et al., 2002). Despite the limitations associated with the use of limited access models, drugs such as naltrexone (Kim et al., 2004; Stromberg et al., 1998; Stromberg et al., 2002), that have reduced ethanol consumption in limited access sessions, have been found to be effective medications for the treatment of alcoholism. Therefore, the finding in the present study that zonisamide decreases ethanol intake in both rats and mice may indicate that zonisamide, like topiramate, will reduce alcohol consumption in humans.
Fig 4.
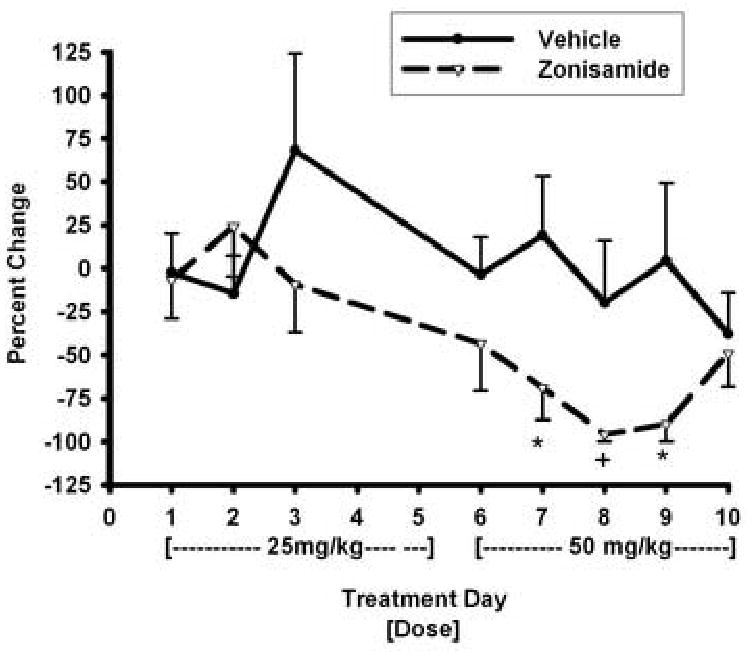
Mean (± S.E.) percent change from baseline in intake of 10% ethanol solution by mice over treatment phase Days 1-10 for the zonisamide and vehicle groups. A dose of 25 mg/kg was administered over treatment phase Days 1-5 and 50 mg/kg during days 6-10. Alcohol consumption was not assessed on Days 4 and 5. * p < 0.05 for comparison with lactose group. + p= 0.054 for comparison with lactose group.
Acknowledgments
This research was supported, in part, by KO5-DA00099 awarded to CK and RA01-AAB727 awarded to DAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angehagen M, Ben-Menachem E, Shank R, Ronnback L, Hansson E. Topiramate modulation of kainate-induced calcium currents is inversely related to channel phosphorylation level. J Neurochem. 2004;88:320–5. doi: 10.1046/j.1471-4159.2003.02186.x. [DOI] [PubMed] [Google Scholar]
- Astrup A, Caterson I, Zelissen P, Guy-Grand B, Carruba M, Levy B, Sun X, Fitchet M. Topiramate: long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obes Res. 2004;12:1658–69. doi: 10.1038/oby.2004.206. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend. 2006;83:25–32. doi: 10.1016/j.drugalcdep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Borowicz KK, Luszczki JJ, Sobieszek G, Ratnaraj N, Patsalos PN, Czuczwar SA. Interactions between zonisamide and conventional antiepileptic drugs in the mouse maximal electroshock test model. Eur Neuropsychopharmacol. 2006 Jul 27; doi: 10.1016/j.euroneuro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Brady KT, Myrick H, Henderson S, Coffey SF. The use of divalproex in alcohol relapse prevention: a pilot study. Drug Alcohol Depend. 2002;67:323–30. doi: 10.1016/s0376-8716(02)00105-9. [DOI] [PubMed] [Google Scholar]
- Brown RO, Orr CD, Hanna DL, Williams JE, Dickerson RN. Topiramate and weight loss in patients with neurodevelopmental disabilities. Pharmacotherapy. 2002;22:831–5. doi: 10.1592/phco.22.11.831.33620. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Leon Z, Yasmin S, Price LH. Do obese depressed patients respond to topiramate? A retrospective chart review. J Affect Disord. 2002;69:251–5. doi: 10.1016/s0165-0327(01)00337-8. [DOI] [PubMed] [Google Scholar]
- Chengappa KN, Chalasani L, Brar JS, Parepally H, Houck P, Levine J. Changes in body weight and body mass index among psychiatric patients receiving lithium, valproate, or topiramate: an open-label, nonrandomized chart review. Clin Ther. 2002;24:1576–84. doi: 10.1016/s0149-2918(02)80061-3. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Knapp CM, Crosby S, Kornetsky C. The Effects of topiramate and zonisamide in combination with alcohol on spatial memory. Neurospsychopharmacol. 2006;31 1:S79–90. [Google Scholar]
- Croissant B, Scherle T, Diehl A, Heinz A, Mann K. Oxcarbazepine in alcohol relapse prevention--a case series. Pharmacopsychiatry. 2004;37:306–7. doi: 10.1055/s-2004-832691. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–9. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:75–80. doi: 10.1097/01.alc.0000150014.79657.64. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Franciscy DM, Wagner HR, 2nd, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289:1820–5. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Whalen CA, Chambers MD, Boswell KJ, Hubbell CL, Reid LD. Valproate reduces intake of alcoholic beverage among rats. Behav Pharmacol. 1998;9:683–9. doi: 10.1097/00008877-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000 1:41. S10–6. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Neuropeptide Y in the paraventricular nucleus of the hypothalamus increases ethanol intake in high- and low-alcohol-drinking rats. Alcohol Clin Exp Res. 2004;28:1492–8. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Sensitized effects of neuropeptide Y on multiple ingestive behaviors in P rats following ethanol abstinence. Pharmacol Biochem Behav. 2005;81:740–9. doi: 10.1016/j.pbb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–94. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Gabriel KI, Cunningham CL. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:783–90. doi: 10.1111/j.1530-0277.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–74. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Song HK, Ishida S, Yagi K, Seino M. Contrasting effects of zonisamide and acetazolamide on amygdaloid kindling in rats. Epilepsia. 2001;42:1379–86. doi: 10.1046/j.1528-1157.2001.26800.x. [DOI] [PubMed] [Google Scholar]
- Hayes DM, Knapp DJ, Breese GR, Thiele TE. Comparison of basal neuropeptide Y and corticotropin releasing factor levels between the high ethanol drinking C57BL/6J and low ethanol drinking DBA/2J inbred mouse strains. Alcohol Clin Exp Res. 2005;29:721–9. doi: 10.1097/01.ALC.0000164375.16838.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Ueno S, Okada M, Kaneko S. Zonisamide at clinically relevant concentrations inhibits field EPSP but not presynaptic fiber volley in rat frontal cortex. Epilepsy Res. 2005;67:51–60. doi: 10.1016/j.eplepsyres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Husum H, Van Kammen D, Termeer E, Bolwig G, Mathe A. Topiramate normalizes hippocampal NPY-LI in flinders sensitive line ‘depressed’ rats and upregulates NPY, galanin, and CRH-LI in the hypothalamus: implications for mood-stabilizing and weight loss-inducing effects. Neuropsychopharmacology. 2003;28:1292–9. doi: 10.1038/sj.npp.1300178. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Kim SG, Han BD, Park JM, Kim MJ, Stromberg MF. Effect of the combination of naltrexone and acamprosate on alcohol intake in mice. Psychiatry Clin Neurosci. 2004 Feb;58:30–6. doi: 10.1111/j.1440-1819.2004.01189.x. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Komiyama C, Makino M, Takasuna K, Takazawa A, Sakurada S. Anticonvulsant properties of the novel nootropic agent nefiracetam in seizure models of mice and rats. Epilepsia. 2005;46:811–8. doi: 10.1111/j.1528-1167.2005.66504.x. [DOI] [PubMed] [Google Scholar]
- Kito M, Maehara M, Watanabe K. Mechanisms of T-type calcium channel blockade by zonisamide. Seizure. 1996;5:115–9. doi: 10.1016/s1059-1311(96)80104-x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Bauer LO, Klinghoffer V. Carbamazepine treatment of cocaine dependence: a placebo-controlled trial. Drug Alcohol Depend. 1995;38(3):203–11. doi: 10.1016/0376-8716(95)01100-d. [DOI] [PubMed] [Google Scholar]
- Le AD, Ko J, Chow S, Quan B. Alcohol consumption by C57BL/6, BALB/c, and DBA/2 mice in a limited access paradigm. Pharmacol Biochem Behav. 1994;47:375–8. doi: 10.1016/0091-3057(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Leite-Morris K, Ciraulo DA. Novel anticonvulsant zonisamide reduces ethanol seeking and consumption in rats. Research Society on Alcoholism. 2007 Abstract. [Google Scholar]
- Longo LP, Campbell T, Hubatch S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;2:55–64. doi: 10.1300/J069v21n02_05. [DOI] [PubMed] [Google Scholar]
- Maryanoff BE, Costanzo MJ, Nortey SO, Greco MN, Shank RP, Schupsky JJ, Ortegon MP, Vaught JL. Structure-activity studies on anticonvulsant sugar sulfamates related to topiramate. Enhanced potency with cyclic sulfate derivatives. J Med Chem. 1998;41:1315–43. doi: 10.1021/jm970790w. [DOI] [PubMed] [Google Scholar]
- Maryanoff BE, McComsey DF, Costanzo MJ, Hochman C, Smith-Swintosky V, Shank RP. Comparison of sulfamate and sulfamide groups for the inhibition of carbonic anhydrase-II by using topiramate as a structural platform. J Med Chem. 2005;48:1941–7. doi: 10.1021/jm040124c. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Karasawa T. Inhibitory effect of zonisamide on human carbonic anhydrase in vitro. Arzneimittelforschung. 1993;43:416–8. [PubMed] [Google Scholar]
- Masuda Y, Karasawa T, Shiraishi Y, Hori M, Yoshida K, Shimizu M. 3-Sulfamoylmethyl-1,2-benzisoxazole, a new type of anticonvulsant drug. Pharmacological profile. Arzneimittelforschung. 1980;30:477–83. [PubMed] [Google Scholar]
- Masuada Y, Utsui Y, Shiraishi Y, Karasawa T, Yoshida K, Shimizu M. Relationships between plasma concentrations of diphenylhydantoin, phenobarbital, carbamazepine, and 3-sulfamoylmethyl-1,2-benzisoxazole (AD-810), a new anticonvulsant agent, and their anticonvulsant or neurotoxic effects in experimental animals. Epilepsia. 1979 Dec;20:623–33. doi: 10.1111/j.1528-1157.1979.tb04846.x. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Hudson JI, Nelson EB, Keck PE. Zonisamide in the treatment of binge-eating disorder: an open-label, prospective trial. J Clin Psychiatry. 2004;65:50–6. doi: 10.4088/jcp.v65n0108. [DOI] [PubMed] [Google Scholar]
- Messiha FS, Butler D, Adams MK. Carbamazepine and ethanol elicited responses in rodents. Alcohol. 1986;3:131–3. doi: 10.1016/0741-8329(86)90022-4. [DOI] [PubMed] [Google Scholar]
- Minuk GY, Rockman GE, German GB, Duerksen DR, Borrett G, Hoeschen L. The use of sodium valproate in the treatment of alcoholism. J Addict Dis. 1995;14:67–74. doi: 10.1300/J069v14n02_07. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Stout RL, Rudden S, Brown RA, Gordon A, Solomon DA, Recupero PR. A double-blind, placebo-controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcohol Clin Exp Res. 1997;21:86–92. [PubMed] [Google Scholar]
- Nagatomo I, Yasuaki A, Nagase F, Nomaguchi M, Takigawa M. Relationships between convulsive seizures and brain serum concentrations of phenobarbital and zonisamide in mutant inbred EL mouse. Brain Res. 1996;731:190–8. doi: 10.1016/0006-8993(96)82386-9. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Tamura S, Kanda T, Ishii A, Ishihara K, Serikawa T, Yamada J, Sasa M. Inhibition by topiramate of seizures in spontaneously epileptic rats and DBA/2 mice. Eur J Pharmacol. 1994 Mar 11;254:83–9. doi: 10.1016/0014-2999(94)90373-5. [DOI] [PubMed] [Google Scholar]
- Nguyen SA, DeLeon CP, Malcolm RJ, Middaugh LD. Tiagabine reduces ethanol reward in C57BL/6 mice under acute and chronic administration regimens. Synapse. 2005;56:135–46. doi: 10.1002/syn.20138. [DOI] [PubMed] [Google Scholar]
- Nguyen SA, Malcolm R, Middaugh LD. Topiramate reduces ethanol consumption in C57BL/6 mice. Synapse. 2007;61:150–156. doi: 10.1002/syn.20350. [DOI] [PubMed] [Google Scholar]
- Nurmi M, Kiianmaa K, Sinclair JD. Brain ethanol levels after voluntary ethanol drinking in AA and Wistar rats. Alcohol. 1999;19:113–8. doi: 10.1016/s0741-8329(99)00022-1. [DOI] [PubMed] [Google Scholar]
- O'Callaghan MJ, Croft AP, Watson WP, Brooks SP, Little HJ. Low alcohol preference among the “high alcohol preference” C57/BL10 mice; factors affecting such preference. Pharmacol Biochem Behav. 2002;72:475–81. doi: 10.1016/s0091-3057(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Topiramate alters excitatory synaptic transmission in mouse hippocampus. Epilepsy Res. 2003;55:225–33. doi: 10.1016/s0920-1211(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. Effects of tiagabine and diazepam on operant ethanol self-administration in the rat. J Stud Alcohol. 2002;63:100–6. [PubMed] [Google Scholar]
- Rock DM, Macdonald RL, Taylor CP. Blockade of sustained repetitive action potentials in cultured spinal cord neurons by zonisamide (AD 810, CI 912), a novel anticonvulsant. Epilepsy Res. 1989;3:138–43. doi: 10.1016/0920-1211(89)90041-7. [DOI] [PubMed] [Google Scholar]
- Rubio G, Lopez-Munoz F, Alamo C. Effects of lamotrigine in patients with bipolar disorder and alcohol dependence. Bipolar Disord. 2006;8:289–93. doi: 10.1111/j.1399-5618.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Jimenez-Arriero MA, Palomo T, Manzanares J, Ferre F. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37:37–40. doi: 10.1055/s-2004-815473. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Cornelius JR, Daley DC, Kirisci L, Himmelhoch JM, Thase ME. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2005;62:37–45. doi: 10.1001/archpsyc.62.1.37. [DOI] [PubMed] [Google Scholar]
- Schauf CL. Zonisamide enhances slow sodium inactivation in Myxicola. Brain Res. 1987 Jun 9;413:185–8. doi: 10.1016/0006-8993(87)90168-5. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Waldhofer S, Weigelt T, Hiemke C. Free-choice ethanol consumption under the influence of GABAergic drugs in rats. Alcohol Clin Exp Res. 2002;26:457–62. [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The neuropeptide-Y Y5 receptor antagonist L-152,804 decreases alcohol self-administration in inbred alcohol-preferring (iP) rats. Alcohol. 2005;36:179–86. doi: 10.1016/j.alcohol.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon HE, Eberle EL, Peters SC. Comparison of the effects of anticonvulsant drugs with diverse mechanisms of action in the formalin test in rats. Neuropharmacology. 2005;48:1012–20. doi: 10.1016/j.neuropharm.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultured neurons. Epilepsia. 2000;41 1:S45–7. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Fee JR, Hayes DM, Knapp DJ, MacNeil DJ, Thiele TE. Peripheral and central administration of a selective neuropeptide Y Y1 receptor antagonist suppresses ethanol intake by C57BL/6J mice. Alcohol Clin Exp Res. 2004;28:1324–30. doi: 10.1097/01.ALC.0000139829.67958.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998 May;15:281–9. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Rukstalis MR, Mackler SA, Volpicelli JR, O'Brien CP. A comparison of the effects of 6-beta naltrexol and naltrexone on the consumption of ethanol or sucrose using a limited-access procedure in rats. Pharmacol Biochem Behav. 2002 May;72:483–90. doi: 10.1016/s0091-3057(02)00721-9. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kawakami K, Nakamura F, Nishimura S, Yagi K, Seino M. Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 1992;12:21–7. doi: 10.1016/0920-1211(92)90087-a. [DOI] [PubMed] [Google Scholar]
- Taverna S, Sancini G, Mantegazza M, Franceschetti S, Avanzini G. Inhibition of transient and persistent Na+ current fractions by the new anticonvulsant topiramate. J Pharmacol Exp Ther. 1999;288:960–8. [PubMed] [Google Scholar]
- Tonstad S, Tykarski A, Weissgarten J, Ivleva A, Levy B, Kumar A, Fitchet M. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96:243–51. doi: 10.1016/j.amjcard.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Tutka P, Mroz T, Klucha K, Piekarczyk M, Wielosz M. Bupropion-induced convulsions: preclinical evaluation of antiepileptic drugs. Epilepsy Res. 2005;64:13–22. doi: 10.1016/j.eplepsyres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Uno H, Kurokawa M, Masuda Y, Nishimura H. Studies on 3-substituted 1,2-benzisoxazole derivatives. 6. Syntheses of 3-(sulfamoylmethyl)-1,2-benzisoxazole derivatives and their anticonvulsant activities. J Med Chem. 1979 Feb;22:180–3. doi: 10.1021/jm00188a011. [DOI] [PubMed] [Google Scholar]
- Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M, OBES-002 Study Group A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28:1399–410. doi: 10.1038/sj.ijo.0802783. [DOI] [PubMed] [Google Scholar]
- York DA, Singer L, Thomas S, Bray GA. Effect of topiramate on body weight and body composition of Osborne-Mendel rats fed a high-fat diet: alterations in hormones, neuropeptide, and uncoupling-protein mRNAs. Nutrition. 2000;16:967–75. doi: 10.1016/s0899-9007(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia. 2000;41 1:S52–60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neurosci Lett. 1997;231:123–6. doi: 10.1016/s0304-3940(97)00543-0. [DOI] [PubMed] [Google Scholar]


