Abstract
Although models of emotion have focused on the relationship between anger and approach motivation associated with aggression, anger is also related to withdrawal motivation. Anger-out and anger-in styles are associated with psychopathology and may disrupt the control of attention within the context of negatively valenced information. The present study used event-related brain potentials (ERPs) to examine whether anger styles uniquely predict attentional bias to negative stimuli during an emotion-word Stroop task. High anger-out predicted larger N200, P300, and N400 to negative words, suggesting that aggressive individuals exert more effort to override attention to negative information. In contrast, high anger-in predicted smaller N400 amplitude to negative words, indicating that negative information may be readily available (primed) for anger suppressors, requiring fewer resources. Individuals with an anger-out style might benefit from being directed away from provocative stimuli that might otherwise consume their attention and foster overt aggression. Findings indicating that anger-out and anger-in were associated with divergent patterns of brain activity provide support for distinguishing approach- and withdrawal-related anger styles.
Keywords: Anger, Emotion, Motivation, Cognitive Resources, Event-Related Potentials
Researchers have postulated approach- and withdrawal-related motivational systems that are implemented in several brain regions and that play a crucial role in the experience and expression of emotion. Anger, a feeling evoked when individuals believe that they or others are treated badly or unfairly (Averill, 2001), involves approach and/or withdrawal behavior depending on context (e.g., Berkowitz, 1990; Watson, 2009; though see Carver & Harmon-Jones, 2009). Spielberger (1988, 1999) developed the State-Trait Anger Expression Inventory (STAXI), which conceptualizes anger expression styles that occur in connection with angry feelings. The STAXI anger-out scale reflects aggression, defined as the expression of angry verbal or motor behavior directed toward people or objects, whereas the STAXI anger-in scale is conceptualized as measuring suppression or inhibition of outward signs of anger and/or withdrawing from an anger-inducing situation.
Approach (anger-out) and withdrawal (anger-in) anger styles may disrupt the control of attention in the context of negatively valenced information, interfering with successful emotion regulation. Several behavioral studies have indicated that angry individuals display an attentional bias toward negatively valenced stimuli (e.g., Cohen et al., 1998; Eckhardt & Cohen, 1997; Kirsch et al., 2005; Smith & Waterman, 2003, 2004; van Honk et al., 2001) that could underlie the potential for angry individuals to perceive ambiguous situations as hostile and/or threatening (e.g., Hazebrook, et al., 2001; Wenzel & Lystad, 2005). Furthermore, approach and withdrawal anger styles may differ in the timing and activation of attentional bias to negative stimuli.
Unlike behavioral measures such as reaction time (RT), event-related brain potentials (ERPs) offer multiple, millisecond measurements of attentional processes. However, little research is available on ERP effects associated with an anger-out style (Patrick & Verona, 2007), and none has specifically examined ERPs associated with an anger-in style. ERP studies of aggression have primarily used oddball tasks consisting of either auditory or visual stimuli and have focused on the parietally distributed P300 component, typically in patient or inmate populations (e.g., Barratt et al., 1997; Bernat et al., 2007; Harmon-Jones et al., 1997; Stanford et al., 2003), although college and community populations have also been examined (e.g., Gerstle et al., 1998; Mathias & Stanford, 1999; Surguy & Bond, 2006). P300, a positive deflection typically occurring 300 to 600 ms post-stimulus-onset, is thought to reflect stimulus evaluation, attention allocation, and context updating (e.g., Coles et al., 2000; Donchin & Coles, 1988). Results of these P300 studies suggest a link between impulsive aggression and reductions in parietal P300 amplitude (Barratt et al., 1997; Harmon-Jones et al., 1997).
Most ERP studies of aggression have employed non-emotional words or sounds as oddball stimuli, so it is unclear whether the association between reduced P300 amplitude and aggression generalizes to or differs from anger- or aggression-related stimuli. The single ERP study incorporating negatively valenced stimuli found that male community members high in aggression displayed reduced frontal P3a in response to non-target aggressive words in a visual oddball task, but these P3a reductions were in the absence of parietal P300 decrements in response to target-neutral food-related words (Surguy & Bond, 2006).
Whereas P300 is thought to reflect evaluation of stimulus significance, N100 (a negative deflection occurring around or shortly after 100 ms) and P200 (a positive deflection occurring about 200 ms) are components with frontocentral scalp distributions thought to reflect attention to stimuli during relatively early, perceptual stages of processing (e.g., Hillyard et al., 1998; Junghöfer et al., 2001; Nätäänen et al., 1982). In contrast to earlier components like the N100 and P200, the visual N200 is a negative-going ERP component occurring 200–300 ms post-stimulus, with a right-lateralized frontocentral scalp distribution localized to a right prefrontal source (Strik et al., 1998), thought to reflect response inhibition and/or conflict monitoring (e.g., van Veen & Carter, 2002). N400 indexes elaborative stimulus processing in that it is modulated by semantic meaning. Larger N400 amplitude is associated with improbable words, whereas smaller N400 amplitude is associated with facilitated processing (e.g., for words of higher lexical frequency or words primed within a particular sentence context; Kutas & Hillyard, 1980; van Petten & Kutas, 1990). Although N400 is reduced for emotional stimuli that are primed (Schirmer et al., 2002, 2005), it is also attenuated for emotional stimuli compared to neutral stimuli in the absence of explicit priming (Kanske & Kotz, 2007). If amplitudes of earlier components such as N100 or P200 are reduced in size, conclusions about aggression and its relationship to attention cannot be limited to later, “top-down” processes involving attentional control such as N200, P300, or N400. ERP studies of aggression have not typically analyzed components other than P300, although one study using neutral stimuli found no relationship between aggression and N100, P200, or N200 amplitude (Barratt et al., 1997).
The present study examined whether approach and withdrawal anger expression styles were differentially associated with attentional bias to negative words in an emotion-word Stroop task above and beyond measures of negative affect that are highly comorbid with anger expression such as depression, anxiety, and trait anger (e.g., Deffenbacher et al., 1996). Neural mechanisms involved in attention to emotional stimuli were measured using N100, P200, N200, P300, and N400 amplitude scores. The specificity of any such effects to negative stimuli was evaluated by inclusion of positive and neutral stimuli.
Differential predictions were made regarding ERP amplitude to negative stimuli in anger styles, despite lack of guidance from the literature. It was hypothesized that higher anger-in scores would predict larger N200, P300, and N400 amplitude in response to negative words, as more resources may be needed for high anger-in individuals to suppress outward angry responses. In contrast, it was predicted that anger-out would be linked to reduced N200, P300, and N400 amplitudes to negative stimuli based on prior P300 research utilizing non-emotional stimuli with aggressive individuals. It was predicted that the two anger styles would diverge only when executive control was needed (reflected in N200, P300, and N400 amplitude) to override attention to negative valence in order to select the correct color response.
It was likely that differences in brain activation as a function of anger style would occur without behavioral differences in RT or error rates (e.g., longer RT and more errors for negative stimuli than for positive or neutral stimuli), since in nonclinical samples, including samples indexing traits such as anxiety, RT impairment from emotional content is attenuated in the emotion-word Stroop task (e.g., Franken et al., 2009; Thomas et al., 2007). Thus, in the present study the focus was on ERP indices of attentional bias to emotional stimuli.
Method
Participants
Participants were 102 paid undergraduates (54 female, 81% Caucasian, mean age = 19.02, SD = 1.74) recruited via group questionnaire sessions in which measures of anger, anxiety, and depression were administered. Participants completed the Anger Expression-In, Anger Expression-Out, and Trait Anger scales from the State-Trait Anger Expression Inventory 2 (STAXI-2; Spielberger, 1999). STAXI-2 Anger Expression-In and Anger Expression-Out are 8-item scales on which participants rate how they generally react or behave when angry or furious (1 = almost never, 2 = sometimes, 3 = often, 4 = almost always). Examples of Anger Expression-In items are “I boil inside but don’t show it” and “I withdraw from people.” Examples of Anger-Expression-Out items are “I strike out at whatever infuriates me” and “I do things like slam doors.” STAXI-2 Trait Anger is a 10-item scale that measures individual differences in the predisposition to express anger and react angrily to situations involving frustration or negative evaluation. In addition to the Trait Anger scale, participants were administered other measures of negative affect to assess depression and types of anxiety that may co-occur with anger styles: the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990) to assess anxious apprehension, or worry, and the Anxious Arousal (AA) and Anhedonic Depression (AD) scales of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995) to assess anxious arousal, or somatic anxiety, and anhedonic depression. A subscale of 8 items from the MASQ-AD was used that identifies depressed mood and loss of interest distinct from other items reflecting low positive affect (Nitschke et al., 2001). Means and standard deviations for STAXI-2, PSWQ, MASQ-AA, and 8-item MASQ-AD scales are provided in Table 1, and correlations between scales are presented in Table 2.1 Results indicate that, although higher anger-in and anger-out scores were both associated with higher trait anger scores, anger-in (but not anger-out) was positively linked to depression and types of anxiety, with anger-in possessing a significantly higher correlation with anxious apprehension than anger-out (p < .01). All participants provided informed consent, were right-handed with average Edinburgh Handedness (Oldfield, 1971) laterality quotient M = 78.96 (SD = 17.52), native speakers of English with self-reported normal color vision who were free of traumatic brain injury and other medical conditions known to affect central nervous system function (e.g., epilepsy). This study was approved by the university IRB before participants were recruited, and therefore this research has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Table 1.
Questionnaire Statistics (N = 102)
| Scale | Mean | SD | N below 50th Percentile |
N 50th–80th Percentile |
N above 80th Percentile |
|---|---|---|---|---|---|
| STAXI-2 Trait Anger | 18.28 | 5.14 | 54 | 27 | 21 |
| STAXI-2 Anger Expression-In | 16.69 | 4.79 | 51 | 30 | 21 |
| STAXI-2 Anger Expression-Out | 14.58 | 3.51 | 57 | 14 | 31 |
| Penn State Worry Questionnaire | 44.60 | 16.91 | 50 | 33 | 19 |
| MASQ Anxious Arousal | 24.59 | 6.66 | 53 | 26 | 23 |
| MASQ 8-item Anhedonic Depression |
15.71 | 4.93 | 56 | 24 | 22 |
Note: STAXI-2 = State Trait Anger Expression Inventory-2. MASQ = Mood and Anxiety Symptom Questionnaire.
Table 2.
Questionnaire Correlations (N = 102)
| STAXI-2 Anger Expression- Out |
STAXI-2 Anger Expression- In |
PSWQ† | MASQ-AA | 8-item MASQ-AD |
|
|---|---|---|---|---|---|
| STAXI-2 Trait Anger |
.56** | .44** | .38** | .27** | .20* |
| STAXI-2 Anger Expression-Out |
.25* | −.02 | .10 | .12 | |
| STAXI-2 Anger Expression-In |
.50** | .25* | .32** | ||
| PSWQ | .25* | .16 | |||
| MASQ-AA | .47** |
Note: Significant at .05 level.
Significant at .01 level.
The relationship between PSWQ and anger-in was stronger than that between PSWQ and anger-out (p < .01). Tests are two-tailed. Three participants with STAXI-2 Anger Expression-Out scores greater than three standard deviations from the mean are not included in correlations involving that scale (N = 99). STAXI-2 = State Trait Anger Expression Inventory-2. MASQ = Mood and Anxiety Symptom Questionnaire. PSWQ = Penn State Worry Questionnaire. AA = Anxious Arousal. AD = Anhedonic Depression.
Stimuli and Experimental Design
Participants2 completed a color-word Stroop task and an emotion-word Stroop task. Both tasks were administered during an EEG session and again during an fMRI session. The order of presentation of the two Stroop tasks within a session was counterbalanced across participants, as was the order of the EEG and fMRI sessions, with a diagnostic interview session in-between (49 participants were assigned EEG sessions first, whereas the remaining 53 were assigned fMRI sessions first). Data from the emotion-word Stroop task completed during the EEG session are reported in the present study.3
Word presentation and response recording were controlled by STIM software (James Long Company, Caroga Lake, NY). Several pilot studies for this project as well as published work show that a blocked design is more effective in eliciting emotion-word Stroop interference than is an intermixed design (e.g., Compton et al., 2003; Dalgleish, 1995). The emotion-word Stroop task consisted of blocks of positive or negative emotion words alternating with blocks of neutral words. Participants received 256 trials in 16 blocks (4 positive, 8 neutral, 4 negative) of 16 trials. A trial began with the presentation of a word for 1500 ms, followed by a fixation cross for 275 to 725 ms (onset to onset ITI 2000 +/−225 ms). Each trial consisted of one word presented in 1 of 4 ink colors (red, yellow, green, blue) on a black background, with each color occurring equally often with each word type (positive, neutral, negative). In the EEG and the fMRI sessions, each participant was randomly assigned 1 of 8 possible orders designed specifically to control stimulus order effects. In 4 of the 8 presentation orders, the first and third blocks were neutral words, with positive and negative blocks second or fourth, with valence order counterbalanced across participants. The remaining 4 presentation orders complemented these, with the first and third blocks being either positive or negative emotion words and the neutral words second and fourth. These 8 orders of presentation were designed to ensure that the neutral and emotional words preceded each other equally often in order to avoid order effects. Stimulus familiarity was controlled by presenting each word just once per session. Within a block, each color appeared 4 times, and trials were pseudo-randomized such that no more than 2 trials featuring the same color appeared in a row. After every fourth block, there was a brief rest period. In addition to the 16 word blocks, there were 4 fixation blocks, one at the beginning, one at the end, and two in the middle of the experiment: instead of a word, a brighter fixation cross was presented for 1500 ms, followed by the fixation cross that followed word stimuli.
The 256 word stimuli were selected from the Affective Norms for English Words set (ANEW: Bradley & Lang, 1999). Sixty-four positive (e.g., birthday, ecstasy, laughter), 64 negative (e.g., suicide, war, victim), and two sets of 64 neutral (e.g., hydrant, moment, carpet) words were carefully selected on the basis of established norms for valence, arousal, and frequency of usage in the English language (Bradley & Lang, 1999; Toglia & Battig, 1978). Specifically, positive and negative words were chosen to be particularly high in arousal. Words ranged from three to eight letters in length. Words were presented in capital letters using Tahoma 72-point font at a distance of 1.35 m from the participant's eyes, for a vertical span of 1.5 degrees and a horizontal span between 2.5 and 9.3 degrees. Participants were instructed to press one of four buttons (red, yellow, green, blue, mapped to the first two fingers of each hand) to indicate the color of each word as quickly as possible. Instructions were read verbatim by experimenters to assure that participants understood task requirements. The participant performed 32 practice trials before the actual task began. No participants failed to understand the task instructions or the mapping between colors and buttons after completing practice trials.
Electrophysiological Recording and Data Reduction
Participants were seated in a comfortable chair in a quiet room that was adjacent to a room where the experimenter controlled stimulus presentation and EEG data collection. The participant room was connected to the experimenter room by intercom. EEG was recorded with a custom-designed Falk Minow 64-channel cap with Ag/AgCl electrodes spaced equidistantly, extending inferiorly to the F9/F10 ring of the 10-10 System. The left mastoid served as the online reference for all EEG and EOG sites. Electrodes placed above and below each eye and near the outer canthus of each eye recorded vertical and horizontal EOG for off-line eye-blink artifact correction of EEG. Electrode impedances were maintained below 20 kΩ, in line with the high input impedance of the amplifiers. Half-power amplifier bandpass was .1 to 100 Hz, and data were digitized at 250 Hz. Electrode positions were recorded using a Zebris ELPOS digitizer (Zebris Medizintechnik, Tübingen, Germany).
Via Brain Electrical Source Analysis (BESA 5.1.8) software, muscle, movement, blink, and other artifacts were removed (Berg & Scherg, 1991, 1994). The electrode configuration was then transformed to BESA’s standard 81-channel montage using spherical spline interpolation (Perrin et al., 1989), reflecting the 10-10 system and facilitating comparison with other studies. An average reference was computed for each time point as the mean voltage over the 81 standard virtual scalp electrodes. Data were exported from BESA and each channel baseline-adjusted by subtracting the average amplitude for the 200 ms before stimulus onset in custom Matlab software. Waveform averages were smoothed using a 101-weight, .1–8 Hz digital filter (Cook & Miller, 1992; Edgar et al., 2005; Nitschke et al., 1998).
Individual correct trials were averaged for each emotion condition of interest (positive, neutral, and negative). Since N100, P200, N200, and N400 ERP components have predominantly frontocentral distributions, peak latency and amplitude of these components were scored at frontal (Fz, F1, F2, F3, F4), frontocentral (FCz, FC1, FC2, FC3, FC4), and central (Cz, C1, C2, C3, C4) sites. P300 was scored at centroparietal (CPz, CP1, CP2, CP3, CP4), parietal (Pz, P1, P2, P3, P4), and parietooccipital (POz, PO3, PO4, PO7, PO8) sites, reflecting its more posterior distribution. Electrode sites and scoring windows for N100 (80–140 ms), P200 (160–260 ms), N200 (240–360 ms), P300 (450–580 ms), and N400 (448–580 ms) were chosen by examining grand-average waveforms and individual participant data and consulting recent Stroop ERP literature measuring these components (e.g., Curtin & Fairchild, 2003; Sass et al., in press; Thomas et al., 2007). The amplitude score was created by averaging data surrounding the scored peaks, in order to obtain a more reliable measure of ERP amplitude. For N100, P200, and N200, 12 ms were averaged before and after peak latency (7 points spanning 24 ms). Since P300 and N400 are much longer in duration, 48 ms were averaged before and after peak latency (25 points spanning 96 ms). Two electrodes from each hemisphere were selected and their amplitude scores averaged together for each region in order to examine hemisphere differences in amplitude (for N100, P200, N200, and N400: F1 and F3 = left frontal, F2 and F4 = right frontal, FC1 and FC3 = left frontocentral, FC2 and FC4 = right frontocentral, C1 and C3 = left central, C2 and C4 = right central; for P300: CP1 and CP3 = left centroparietal, CP2 and CP4 = right centroparietal, P1 and P3 = left parietal, P2 and P4 = right parietal, PO3 and PO7 = left parietooccipital, PO4 and PO8 = right parietooccipital).
Electrophysiological Data Analysis
Replication of emotion-word Stroop effects
In order to compare present ERP results of the present study to prior ERP research using the emotion-word Stroop task, univariate ANOVAs were computed separately for each ERP component, with condition (positive, neutral, negative) and midline electrode (for N100, P200, N200, and N400: Fz, FCz, and Cz; for P300, CPz, Pz, and POz) as within-subject variables and session counterbalancing order (EEG-first, MRI-first) as the between-subject variable. P-values reflect the Huynh-Feldt correction for sphericity where appropriate.
Anger style
Since prior work indicated that anger is associated with an attentional bias toward negative stimuli, examining ERP amplitude differences as a function of anger style for negative words was of primary interest. Thus, anger style score (STAXI-2 Anger Expression-In for anger-in, or STAXI-2 Anger Expression-Out for anger-out) was first correlated with two ERP amplitude difference scores for two comparisons: 1) negative minus neutral, and 2) negative minus positive. Next, for correlations in which either anger-style scores contributed significant variance to ERP amplitude (p < .05), the anger-style score of interest (either STAXI-2 anger-in or anger-out) was entered as a predictor in regression analyses after variance associated with other types of negative affect (PSWQ for anxious apprehension, MASQ-AA for anxious arousal, 8-item MASQ-AD subscale for anhedonic depression, and STAXI-2 Trait Anger) was removed. In other words, four measures of negative affect were entered together in the first step of a hierarchical linear regression to predict ERP amplitude, and then anger style was entered in the second step. All questionnaire scores were z-scored (pooling across subjects within questionnaire) before they were entered as regression predictors. Results from regressions involving anger style were considered (and reported) only if anger-in or anger-out predicted at least marginally unique variance (p < .10) when entered after other measures of negative affect. When anger-in and/or anger-out contributed significant variance to ERP amplitude, the difference between anger-in and anger-out effects was compared by calculating the 95% confidence interval for each beta (Cohen et al., 2003) and examining whether these confidence intervals overlapped.4
Behavioral Data Analysis
Replication of emotion-word Stroop effects
In order to compare RT and error-rate results of the present study to prior behavioral research using an emotion-word Stroop task, univariate ANOVAs were computed separately for RT and error rate, with condition (positive, neutral, negative) as the within-subject variable and session counterbalancing order (EEG-first, MRI-first) as the between-subject variable. P-values reflect the Huynh-Feldt correction for sphericity where appropriate.
Anger style
Identical regressions involving anger styles were executed with the dependent variable being RT or error rate instead of ERP amplitude.
Results
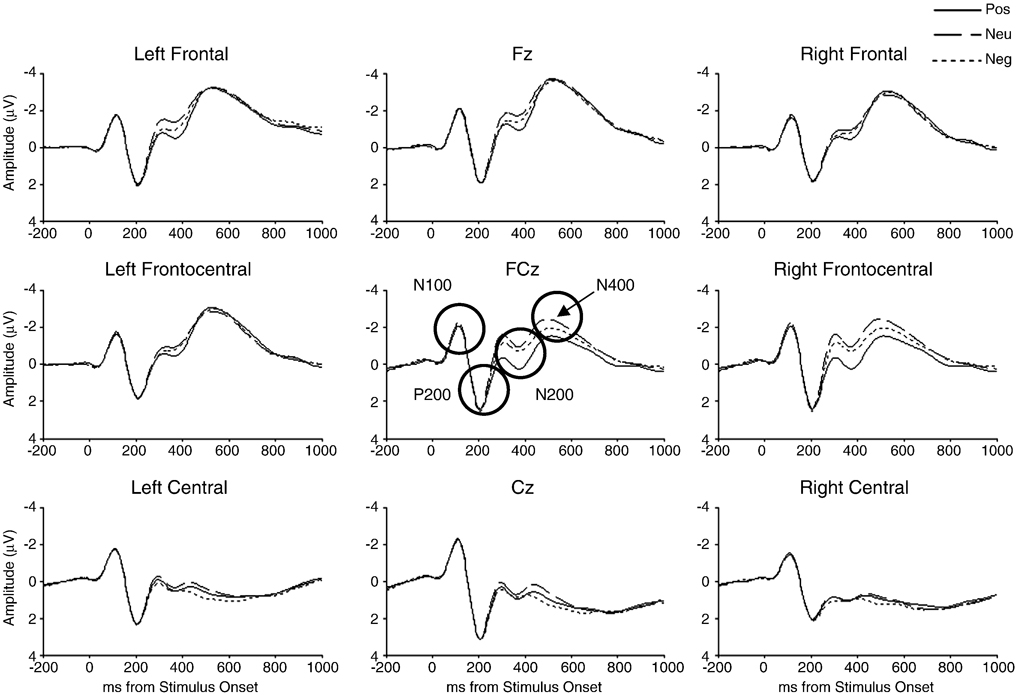
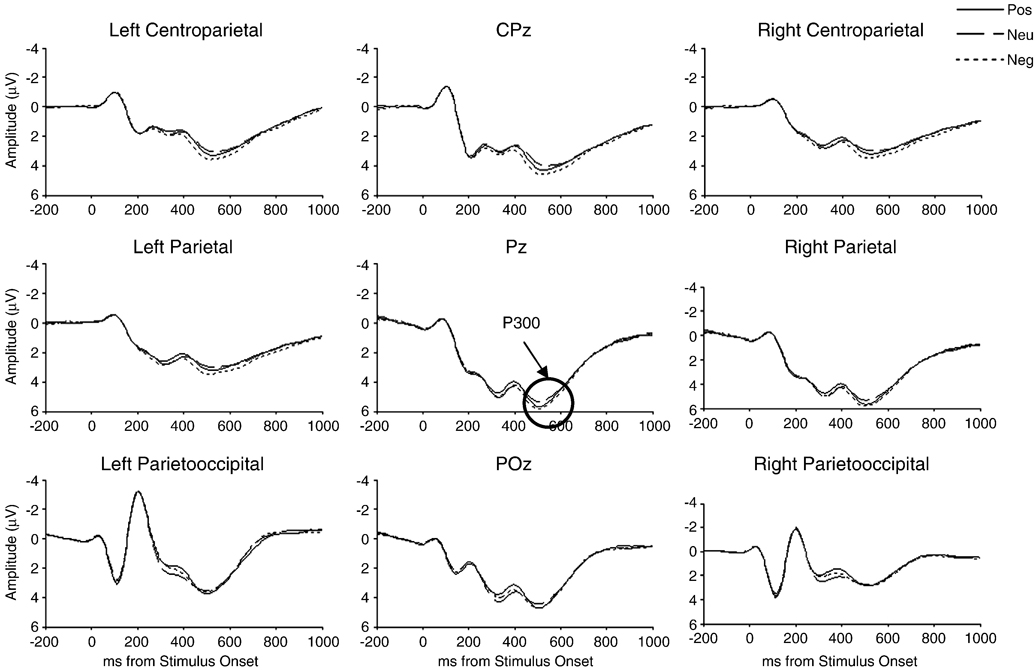
Grand-average ERP waveforms are shown in Figure 1 and Figure 2. Participants who had ERP amplitude scores greater than 3 SD from the mean for at least one electrode site (two for N100, one for P200, four for N200 and P300, and five for N400) were removed from analyses for that particular component.
Figure 1.
Average-reference grand-average ERPs for frontal, frontocentral, and central regions elicited during the emotion-word Stroop task (N = 102).
Figure 2.
Average-reference grand-average ERPs for centroparietal, parietal, and parietooccipital regions elicited during the emotion-word Stroop task (N = 102).
Replication of Emotion-Word Stroop Effects
Behavioral Data
A valence by session-order effect emerged for RT (F(1, 100) = 5.75, p = .018, partial η2 = .05), indicating that the EEG-first group took longer to respond to positive (M = 667.18, SE = 12.86) and negative words (M = 675.63, SE = 13.35) than neutral words (M = 659.23, SE = 12.86), whereas no differences emerged for the MRI-first group (positive: M = 637.37, SE = 12.36, neutral: M = 640.87, SE = 12.36, negative: M = 638.77, SE = 12.84). In addition, a valence effect emerged for error rate (F(1, 100) = 19.69, p < .001, partial η2 = .17), indicating that the neutral condition was associated with a higher number of errors (M = 4.51, SE = .41) than positive and negative conditions (M = 2.95, SE = .21, and M = 3.32, SE = .27, respectively).
N100
A session-order by electrode interaction emerged (F(2, 196) = 4.02, p = .042, partial η2 = .04), indicating that N100 at Fz was larger for EEG-first (M = −2.40, SE = .14) than MRI-first (M = −1.95, SE = .13) participants.
P200
A main effect of channel emerged (F(2, 198) = 47.56, p < .001, partial η2 = .32), demonstrating that P200 was larger at Cz (M = 3.27, SE = .21) followed by FCz (M = 2.56, SE = .20) and Fz (M = 2.03, SE = .18) (all channels differed from each other at p < .001).
N200
Main effects of condition (F(2, 192) = 7.92, p = .001, partial η2 = .08) and channel (F(2, 192) = 51.58, p < .001, partial η2 = .35) were qualified by a condition by channel interaction (F(4, 384) = 3.52, p = .025, partial η2 = .04) indicating that N200 was larger for the neutral condition than 1) the positive condition at Fz (p < .001) and FCz (p = .001), and 2) the negative condition at Fz (p = .007), FCz (p = .003), and Cz (p = .022). In addition, for the positive condition, N200 was larger at Fz and FCz than Cz (both p < .001), whereas, for the neutral and negative conditions, N200 was larger at Fz than FCz (p < .05), and Fz and FCz were larger than Cz (both p < .001). Finally, a session-order by condition interaction emerged (F(2, 192) = 3.75, p = .025, partial η2 = .04), demonstrating that the neutral condition elicited larger N200 than the negative condition for the EEG-first group (p = .004), whereas the neutral and negative conditions were associated with larger N200 than the positive condition for the MRI-first group (p < .001 and p = .021, respectively).
P300
Main effects of condition (F(2, 192) = 5.15, p = .007, partial η2 = .05) and channel (F(2, 192) = 15.65, p < .001, partial η2 = .14) were qualified by a condition by channel interaction, indicating that P300 was larger for the negative condition than 1) the neutral condition at CPz (p < .001) and Pz (p = .002) and 2) the positive condition at CPz (p = .024). In addition, P300 was larger at Pz than CPz or POz for positive, neutral, and negative conditions (all p < .001).
N400
A main effect of condition emerged (F(2, 190) = 3.88, p = .022, partial η2 = .04), demonstrating that N400 was larger for neutral than negative stimuli (p = .007). In addition, a main effect of channel (F(2, 190) = 157.82, p < .001, partial η2 = .62) indicated that N400 was largest at Fz, followed by FCz, then Cz (all p < .001).
Anger Style
Three participants endorsing STAXI-2 Anger Expression-Out scores greater than 3 SD from the mean were excluded from all analyses involving anger-out as a predictor.
Behavioral Data
Neither anger-in nor anger-out contributed unique variance to RT or error rate.
N100
Although higher anger-out scores predicted larger N100 amplitude for the negative minus neutral comparison at FCz, this effect became marginal once other indices of negative affect (anxious apprehension, anxious arousal, anhedonic depression, and trait anger) were included in the model (Table 3 illustrates the zero-order relationship between anger-out and N100 amplitude for FCz, and Table 4 demonstrates variance accounted for by anger-out when measures of negative affect are included in the model). Anger-in did not contribute variance to N100 amplitude.
Table 3.
STAXI-2 Anger-Out Entered in the First Step Predicting ERP Amplitude
| Dependent Variable | R2 | β | p |
|---|---|---|---|
| N100 Amplitude, Negative Minus Neutral | |||
| FCz | .05 | −.21 | .036 |
| N200 Amplitude, Negative Minus Positive | |||
| FCz | .13 | −.36 | < .001 |
| Left Frontocentral | .08 | −.28 | .006 |
| Left Central | .04 | −.21 | .041 |
| Right Frontocentral | .07 | −.27 | .008 |
| Right Central | .05 | −.22 | .034 |
| P300 Amplitude, Negative Minus Neutral | |||
| CPz | .05 | .21 | .039 |
| Pz | .08 | .27 | .007 |
| Right Centroparietal | .05 | .22 | .032 |
| Right Parietal | .06 | .25 | .015 |
| P300 Amplitude, Negative Minus Positive | |||
| Pz | .04 | .21 | .043 |
| N400 Amplitude, Negative Minus Neutral | |||
| Fz | .06 | −.25 | .017 |
| Left Frontal | .06 | −.25 | .015 |
| Right Frontal | .09 | −.29 | .004 |
Note. STAXI-2 = State Trait Anger Expression Inventory 2.
Table 4.
STAXI-2 Anger-Out Added in the Second Step Predicting ERP Amplitude
| Full Model | NA added first | Anger-Out | ||||
|---|---|---|---|---|---|---|
| Dependent Variable | R2 | p | R2 | p | ΔR2 | p |
| N100 Amplitude, Negative Minus Neutral | ||||||
| FCz | .05 | .484 | .02 | .849 | .03 | .081 |
| N200 Amplitude, Negative Minus Positive | ||||||
| FCz | .18 | .002 | .13 | .014 | .06 | .014 |
| Left Frontocentral | .13 | .032 | .08 | .089 | .04 | .042 |
| Left Central | .14 | .016 | .11 | .025 | .03 | .095 |
| Right Frontocentral | .09 | .108 | .05 | .324 | .05 | .038 |
| Right Central | .08 | .170 | .05 | .307 | .03 | .088 |
| P300 Amplitude, Negative Minus Neutral | ||||||
| CPz | .10 | .087 | .05 | .313 | .05 | .029 |
| Pz | .12 | .051 | .07 | .151 | .04 | .039 |
| Right Centroparietal |
.08 | .205 | .01 | .934 | .07 | .012 |
| Right Parietal | .09 | .114 | .06 | .273 | .04 | .055 |
| P300 Amplitude, Negative Minus Positive | ||||||
| Pz | .14 | .022 | .09 | .080 | .05 | .030 |
| N400 Amplitude, Negative Minus Neutral | ||||||
| Fz | .08 | .189 | .03 | .580 | .05 | .034 |
| Left Frontal | .07 | .231 | .02 | .741 | .05 | .029 |
| Right Frontal | .13 | .030 | .07 | .164 | .06 | .016 |
Note: DV = Dependent Variable. NA = STAXI-2 Trait Anger, Penn State Worry Questionnaire, MASQ-Anxious Arousal, and 8-item MASQ-Anhedonic Depression entered together as a negative affect set. STAXI-2 = State-Trait Anger Expression Inventory 2. MASQ = Mood and Anxiety Symptom Questionnaire.
P200
Neither anger-in nor anger-out contributed variance to P200 amplitude.
N200
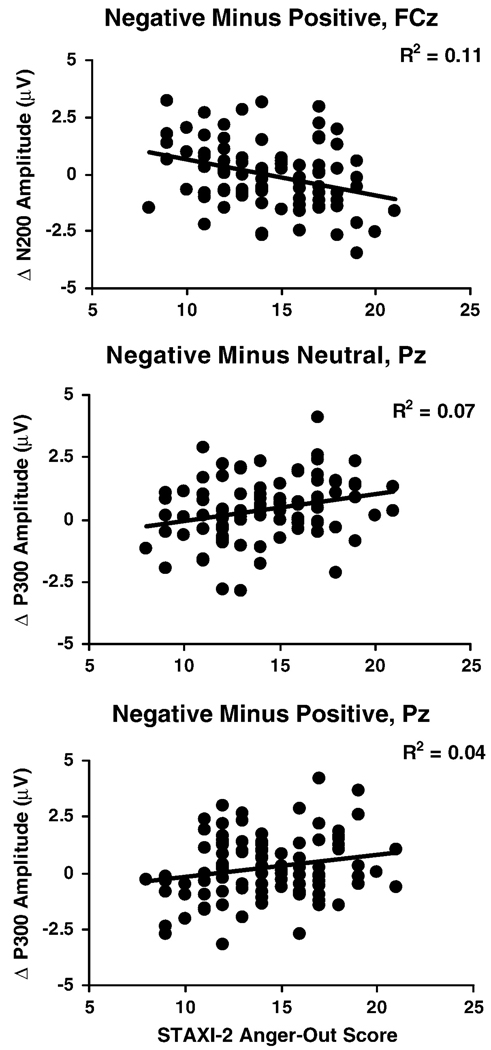
Two participants displayed one negative minus neutral score greater than 3 SD (one for the left frontal region and the other for the left frontocentral region) and were excluded from regression analyses for that particular region. Higher anger-out scores predicted larger N200 amplitude for the negative minus positive comparison (see Figure 3 at FCz, and zero-order relationships between anger-out and N200 amplitude in Table 3). Overall, these effects remained significant after measures of negative affect were included in the model (see Table 4).
Figure 3.
Scatterplots of STAXI-2 Anger Expression-Out scores predicting N200 difference score at FCz for the negative minus positive comparison (upper panel), P300 at Pz for the negative minus neutral comparison (middle panel), and P300 at Pz for the negative minus positive comparison (lower panel).
P300
Table 3 and Table 4 illustrate that higher anger-out scores predicted larger P300 amplitude for the negative minus neutral comparison at central and right posterior sites and a larger negative minus positive effect on P300 at Pz (see Figure 3 for examples of P300 effects at Pz).
N400
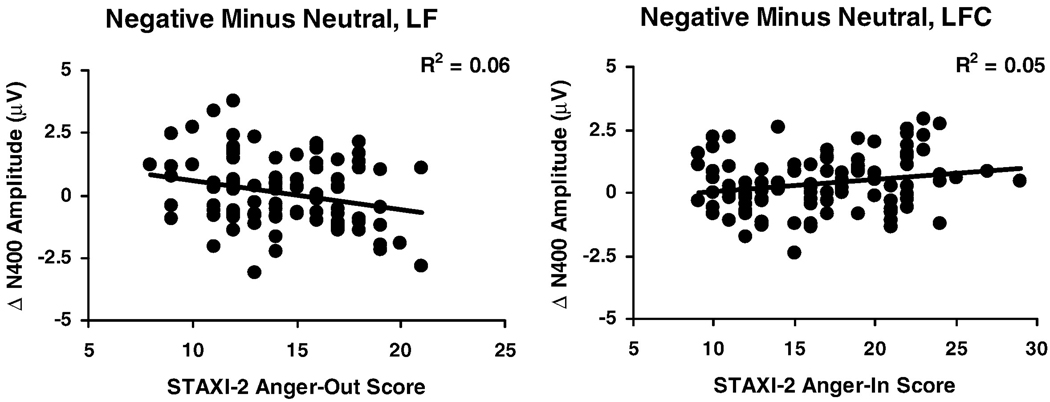
Higher anger-out scores predicted larger N400 amplitude at frontal channels for the negative minus neutral comparison (see Table 3). These effects remained significant when measures of negative affect were included (see Table 4). In contrast, higher anger-in scores predicted smaller N400 amplitude for the negative minus neutral comparison (left frontocentral: R2 = .05, β = .22, p = .034; right central: R2 = .06, β = .24, p = .019), but these effects became marginal after measures of negative affect were added to the model (left frontocentral: entire model R2 = .06 / p = .292, negative affect added first R2 = .04 / p = .505, anger-in added second R2 = .03 / p = .096; right central: entire model R2 = .14 / p = .019, negative affect added first R2 = .11 / p = .031, anger-in added second R2 = .03 / p = .090). Figure 4 illustrates differential patterns of N400 amplitude associated with anger-in versus anger-out recorded at frontal sites of the left hemisphere.
Figure 4.
Scatterplots of STAXI-2 Anger Expression-Out scores (left panel) and STAXI-2 Anger Expression-In scores (right panel) predicting N400 for the negative minus neutral comparison. LF = left frontal region. LFC = left frontocentral region.
Discussion
With anger-out and anger-in expression styles believed to be associated with opposing motivational directions (approach, withdrawal), the present study investigated whether they were associated with distinct patterns of behavior and brain activation during a selective attention task that involves an emotional challenge. Findings illustrated differences in attentional bias to negative stimuli as a function of anger style that was not due to other types of negative affect. Results have implications for the hypotheses of the present study as well as for conceptualizing differences in anger style.
First, it was predicted that anger-in and anger-out would not differ in attention to negative stimuli until later stimulus processing stages, and this was generally confirmed. Higher anger-out predicted larger N100 to negative stimuli, interpretable as heightened perceptual processing of negative words. However, this effect was present only at one electrode site and was reduced to marginal significance once measures of negative affect were included in the model. In addition, neither anger-in nor anger-out predicted P200 amplitude to negative words. Overall, these findings suggest that biases toward negative stimuli were confined to during later elaborative stimulus processing involving attentional control for both anger styles.
Second, it was hypothesized that an anger-in style would be associated with larger N200, P300, and N400 responses to negative stimuli, due to heightened resources needed to override or inhibit the impact of negative words. This hypothesis was not supported. Anger-in was unrelated to N200 and P300 amplitude and predicted smaller N400 amplitude for negative than neutral words, a relationship that became marginally significant when indices of negative affect were included in the model. It could be the case that individuals with an anger-in style require fewer resources to process negative words, because negative content is already primed (the STAXI-2 Anger Expression-In scale includes items of a ruminative quality such as “I tend to harbor grudges that I don’t tell anyone about” and “I boil inside, but I don’t show it”). Since no ERP literature exists on the anger-in style, hypotheses for the present study were preliminary, and additional research is needed to explore how anger suppression taxes attentional resources in situations that elicit anger.
Contrary to the third hypothesis, anger-out style was associated with preferential bias for negative stimuli as indexed by N200, P300, and N400 amplitude. These findings suggest that individuals with high levels of aggression need to exert attentional effort to override their focus on negative (likely anger-related) information. Distinct interpretations of N200, P300, and N400 effects in the literature suggest several avenues for further research.
Since research on response inhibition using Go-Nogo and Stop-Signal tasks has indicated that N200 is larger for a withheld response than for a non-withheld response, and flanker-task studies have found N200 larger for high-conflict than low-conflict stimuli, present N200 amplitude results suggest that negative stimuli are a source of conflict and require extra resources to suppress. Stimulus features in the emotion-word Stroop task occasion no direct conflict (e.g., responding to the color of the word does not directly compete with processing the meaning of the word; Algom et al., 2004; but see Dalgleish, 2005, and Mohanty et al., 2007), so additional research is needed to pursue this hypothesis.
Higher anger-out scores were associated with increased P300 amplitude, results that are inconsistent with research demonstrating reduced P300 in aggressive individuals (e.g., Barratt et al., 1997; Bernat et al., 2007; Harmon-Jones et al., 1997; Stanford et al., 2003). However, most of those studies examined inmate or inpatient populations, used non-emotional stimuli, and/or did not partial out variance related to psychopathology. The latter is often associated with P300 effects. The present large sample and diverse measures allowed these to be unconfounded. It is also possible that aggression in an undergraduate sample is qualitatively or quantitatively different from aggression linked with violent offending and psychopathology requiring hospitalization and that aggression can motivate approach behavior (as indexed by increased attentional resources) to override distraction by negative information in higher-functioning individuals. It would be informative to examine anger-out styles in non-incarcerated individuals who have high levels of trait anger to see whether frequent anger experience (or higher severity) reduces P300 amplitude in response to negatively valenced, or more specifically anger-relevant, stimuli.
The fact that anger-out was linked to enhanced N400 to negative stimuli suggests a specific cognitive deficit in such individuals. Perhaps negative information is not primed or readily available for access as may be the case for an anger-in style, thus requiring more resources to process.
Replication of Emotion-Word Stroop ERP Results
Midline ERP results were generally in line with relevant literature. Consistent with traditional work on attentional load (e.g., Hillyard et al., 1973) and studies using the emotion-word Stroop task (e.g., Thomas et al., 2007), early ERP components (N100 and P200 at midline electrode sites) in the present paradigm with low perceptual load showed no sensitivity to stimulus valence. With respect to elaborative stimulus processing in the entire sample, N200 amplitude was larger for neutral words than for positive or negative words, results somewhat consistent with Perez-Edgar and Fox (2003), who found smaller N200 amplitude for negative but not positive words. Thomas et al. (2007) found no N200 difference between neutral and threat words, but their N200 window was much wider than that used in the present study (260–500 ms versus 240–360 ms), potentially encompassing multiple components, and their sample was much smaller (22 versus 102 participants), perhaps limiting the power to detect such effects. The present study also found that P300 enhancement for negative than neutral stimuli, indicating enhanced salience of threat, replicates research examining attentional processing of emotional stimuli (e.g., Franken et al., 2009; Thomas et al., 2007). Furthermore, N400 amplitude was larger for neutral than negative words, consistent with work indicating facilitated processing of emotional words (e.g., Kanske & Kotz, 2007; Schirmer et al., 2002).
It is worth noting that what has been called an N450 component peaking at approximately 400–500 ms with a frontocentral distribution has been reported in color-word Stroop studies, with greater negativity in incongruent than in neutral and/or congruent trials (e.g., Curtin & Fairchild, 2003; Liotti et al., 2000; Rebai et al., 1997; West & Alain, 1999). This component is presumably associated with conflict detection or selection of competing responses, with N450 amplitude perhaps reflecting the amount of cognitive resources devoted to cognitive control. Given the pattern of effects in the present study, it seems likely that emotional words were easier to process than neutral words, consistent with other studies involving emotional word stimuli (Kanske & Kotz, 2007). Future research could readily address the issue of whether negativity occurring around 400–500 ms is associated with greater control of attention or facilitated processing of emotion by manipulating task demands that require different levels of attentional control in the context of emotional words.
Relationship Between Anger Styles and Indices of Negative Affect
Types of negative affect that have previously been associated with anger styles such as trait anger, anxiety, and depression (e.g., Deffenbacher et al., 1996; Spielberger, 1999) were included in the present study to examine whether variance shared between these constructs accounted for relationships between anger styles and ERP amplitude. Correlations between anger styles and negative affect indicated that anger-in and anger-out were positively correlated with trait anger, replicating previous work (e.g., Spielberger et al., 1999). In addition, the present study replicated recent findings demonstrating that anger-in, not anger-out, is associated with specific types of anxiety and depression, namely anxious apprehension and anhedonic depression (Stewart et al., 2008), results that in conjunction with the present ERP findings support the conceptual distinction between anger-in and anger-out.
Limitations of the Present Study
The paid undergraduate sample in the present study was carefully selected on the basis of depression and anxiety and may not be representative of the greater population. Research employing community samples (either unselected samples, or individuals selected to be high or low on measures of anger-in and anger-out) would assist in addressing electrophysiological differences between anger styles. In addition, examining attentional bias in anger-in and anger-out styles would benefit from a task that elicits angry emotion, which might be more applicable to real-world situations wherein anger regulation (aggression or anger suppression) is warranted.
Summary
The present study demonstrated that individuals with an anger-out style display an attentional bias toward negative stimuli, particularly during later elaborative processing, which can be overcome with the recruitment of additional resources. These results imply that, within a therapy context, relatively high-functioning individuals with an anger-out style might be successfully directed away from anger-inducing stimuli that might otherwise consume their attention and lead to overt aggressive behavior. Subsequently, these clients may be able to pause and examine the benefits and drawbacks of overtly expressing their anger in an aggressive way and then focus on alternative strategies to control aggressive impulses. It would be of interest for future research to examine whether psychophysiological measures such as N200, P300, and N400 amplitude predict therapy outcome in aggressive individuals with and without additional psychopathology.
Acknowledgments
This study was submitted in partial fulfillment of dissertation requirements at the University of Illinois at Urbana-Champaign for Jennifer L. Stewart. This research was supported by the National Institute of Drug Abuse (R21 DA14111), the National Institute of Mental Health (P50 MH079485, R01 MH61358, T32 MH19554), and the University of Illinois Beckman Institute, Department of Psychology, and Intercampus Research Initiative in Biotechnology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All participants in this sample were recruited for a larger study on the basis of high (above the 80th percentile) and low (below the 50th percentile) scores on PSWQ, MASQ-AA, and 8-item MASQ-AD scales gathered during group testing sessions (PSWQ: 72 low-scoring subjects, 30 high; MASQ-AA: 74 low, 28 high; 8-item MASQ-AD: 72 low, 30 high). The STAXI-2, PSWQ, and MASQ questionnaires were readministered to participants during an individual laboratory tour, and these scores were used in the present study. Subjects selected with these criteria represent most of the range of the scales (all but about 1 SD), so this is not a traditional extreme-groups strategy. Furthermore, some regression to the mean occurred from the time of the mass testing session to the lab tour session; Table 1 indicates that 24–32% of the sample moved into the 50th to the 80th percentile range on the PSWQ and MASQ scales, demonstrating a relatively normal distribution of scores on these measures.
The present study includes ERP data from participants reported on in three published studies: 1) 42 anxious and control participants in a fMRI study of the emotion-word Stroop task (Engels et al., 2007), 2) 14 control participants in a fMRI study of color-word and emotion-word Stroop tasks (Mohanty et al., 2007), and 3) 38 anxious and control participants from an ERP study of the emotion-word Stroop task (Sass et al., in press). The present study uses a much larger sample to examine how anger styles moderate ERP amplitude above and beyond depression and anxiety. The three former studies did not address anger. Of the 102 participants in the present study, 39 were not included in any of these studies, and 64 were not included in the ERP studies.
During the diagnostic interview session, each participant was administered the Structured Clinical Interview for DSM-IV (SCID, First, Spitzer, Gibbon, & Williams, 1997) by a PhD student in clinical psychology who had completed a year-long SCID practicum. Interviewers were supervised via group case-conference-review sessions with a senior clinical psychologist (GAM) who has supervised over 2600 SCIDs. Of the 102 participants, 32 met criteria for lifetime Axis I diagnoses (10 of whom met criteria for more than one diagnosis): major depressive disorder (MDD) = 14 (1 of whom met criteria for current major depressive episode); dysthymia = 1; depressive disorder not otherwise specified = 2; social phobia = 2; specific phobia = 4; generalized anxiety disorder = 8; obsessive compulsive disorder = 1; posttraumatic stress disorder = 1; anxiety disorder not otherwise specified = 1; anorexia = 2; alcohol abuse = 5; alcohol dependence = 4; substance abuse = 1. In several exploratory analyses (for emotion-word Stroop replication of the literature and for anger style) presence/absence of a diagnosis did not alter the findings reported here.
Since some models of emotion have postulated hemisphere differences in frontal EEG asymmetry as a function of approach and withdrawal motivation, hemisphere differences were tested, but no left/right differences were found to be associated with anger-in or anger-out. Also, analyses were computed for ERP latency parallel to those for ERP amplitude. No effects of interest were hypothesized or found. That neither anger-in nor anger-out predicted unique variance in ERP component latency scores indicates that anger styles are not associated with processing speed deficits during stimulus updating. The analyses reported here thus focus on amplitude, with implications for resource allocation.
References
- Algom D, Chajut E, Shlomo L. A rational look at the emotional Stroop phenomenon: A generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133:323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- Averill JR. Studies on anger and aggression: Implications for theories of emotion. In: Parrott WG, editor. Emotions in social psychology: Essential readings. Philadelphia: Psychology Press/Taylor & Francis; 2001. pp. 337–352. [Google Scholar]
- Barratt ES, Stanford MS, Kent TA, Felthous A. Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biological Psychiatry. 1997;41:1045–1061. doi: 10.1016/s0006-3223(96)00175-8. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. Use of prior knowledge in brain electromagnetic source analysis. Brain Topography. 1991;4:143–150. doi: 10.1007/BF01132771. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography & Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. On the formulation and regulation of anger and aggression: A cognitive-neoassociationistic analysis. American Psychologist. 1990;45:494–503. doi: 10.1037//0003-066x.45.4.494. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Hall JR, Steffen BV, Patrick CJ. Violent offending predicts P300 amplitude. Int. J. Psychophysiol. 2007;66:161–167. doi: 10.1016/j.ijpsycho.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ NIMH Center for the Study of Emotion and Attention. Affective norms for English words (ANEW) Gainsville, FL: University of Florida; 1998. [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Eckhardt CI, Schagat KD. Attention allocation and habituation to anger-related stimuli during a visual search task. Aggressive Behavior. 1998;24:399–409. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd edn. Mahwah, NJ: Lawrence Erlbaum; 2003. [Google Scholar]
- Coles MGH, Gratton G, Fabiani M. Event related brain potentials. In: Cacioppo J, Tassinary L, editors. Principles of psychophysiology: Physical, social, and inferential elements. NY: Cambridge University Press; 2000. pp. 413–455. [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Miller GA, Scalf P, et al. Paying attention to emotion: A fMRI investigation of cognitive and emotional Stroop tasks. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:81–93. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Cook EW, Miller GA. Digital filtering: Background and tutorial for psychophysiologists. Psychophysiology. 1992;29:350–367. doi: 10.1111/j.1469-8986.1992.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Performance on the emotional Stroop task in groups of anxious, expert, and control subjects: A comparison of computer and card presentation formats. Cognition & Emotion. 1995;9:341–362. [Google Scholar]
- Deffenbacher JL, Oetting ER, Lynch RS, Morris CD. The expression of anger and its consequences. Behaviour Research & Therapy. 1996;34:575–590. doi: 10.1016/0005-7967(96)00018-6. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- Eckhardt IE, Cohen DJ. Attention to anger-relevant and irrelevant stimuli following naturalistic insult. Personality and Individual Differences. 1997;23:619–629. [Google Scholar]
- Edgar JC, Stewart JL, Miller GA. Digital filtering in EEG/ERP research. In: Handy TC, editor. Event-related potentials: A handbook. Cambridge, MA: MIT Press; 2005. pp. 85–113. [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorder—clinical version, administration booklet. New York, NY: Biometrics Research Department; 1997. [Google Scholar]
- Franken IHA, Gootjes L, van Strien JW. Automatic processing of emotional words during an emotional Stroop task. NeuroReport. 2009;20:776–781. doi: 10.1097/WNR.0b013e32832b02fe. [DOI] [PubMed] [Google Scholar]
- Gerstle JE, Mathias CW, Stanford MS. Auditory P300 and self-reported impulsive aggression. Progress in Neuropsychopharmacology & Biological Psychiatry. 1998;22:575–583. doi: 10.1016/s0278-5846(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Barratt ES, Wigg C. Impulsiveness, aggression, reading, and the P300 of the event-related potential. Personality and Individual Differences. 1997;22:439–445. [Google Scholar]
- Hazebrook JF, Howells K, Day A. Cognitive appraisals associated with high trait anger. Personality and Individual Differences. 2001;30:31–45. [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Teder-Salejarvi WA, Munte TF. Temporal dynamics of early perceptual processing. Current Opinion in Neurobiology. 1998;8:202–210. doi: 10.1016/s0959-4388(98)80141-4. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: A new look at early emotion discrimination. Psychophysiology. 2001;38:175–178. [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Research. 2007;1148:138–148. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kirsch SJ, Olczak PV, Mounts JRW. Violent video games induce an affect processing bias. Media Psychology. 2005;7:239–250. [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38:701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Mathias CW, Stanford MS. P300 under standard and surprise conditions in selfreported impulsive aggression. Progress in Neuropsychopharmacology & Biological Psychiatry. 1999;23:1037–1051. doi: 10.1016/s0278-5846(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research & Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ringo Ho M-H, Banich MT, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Nätäänen R, Simpson M, Loveless NE. Stimulus deviance and evoked potentials. Biological Psychology. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig J, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. [Google Scholar]
- Nitschke JB, Miller GA, Cook EW. Digital filtering in EEG/ERP analysis: Some technical and empirical comparisons. Behavior Research Methods, Instruments & Computers. 1998;30:54–67. [Google Scholar]
- Oldfield OC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Verona E. The psychophysiology of aggression: Autonomic, electrocortical, and neuroimaging findings. In: Flannery D, Vazsonyi A, Waldman I, editors. The Cambridge handbook of violent behavior and aggression. NY: Cambridge University Press; 2007. pp. 111–150. [Google Scholar]
- Perez-Edgar K, Fox NA. Individual differences in children’s performance during an emotional Stroop task: A behavioral and electrophysiological study. Brain and Cognition. 2003;52:33–51. doi: 10.1016/s0278-2626(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Rebai M, Bernard C, Lannou J. The Stroop’s test evokes a negative brain potential, the N400. International Journal of Neuroscience. 1997;91:85–94. doi: 10.3109/00207459708986367. [DOI] [PubMed] [Google Scholar]
- Sass SM, Heller W, Stewart JL, Silton RL, Edgar JC, Fisher JE, et al. Time course of attentional bias in anxiety: Emotion and gender specificity. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00926.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA, Friederici AD. Sex differentiates the role of emotional prosody during word processing. Cognitive Brain Research. 2002;14:228–233. doi: 10.1016/s0926-6410(02)00108-8. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA, Friederici AD. On the role of attention for the processing of emotions in speech: Sex differences revisited. Cognitive Brain Research. 2005;24:442–452. doi: 10.1016/j.cogbrainres.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Smith P, Waterman M. Processing bias for aggression words in forensic and non-forensic samples. Cognition and Emotion. 2003;17:681–701. [Google Scholar]
- Smith P, Waterman M. Role of experience in processing bias for aggressive words in forensic and non-forensic populations. Aggressive Behavior. 2004;30:105–122. [Google Scholar]
- Spielberger CD. State-Trait Anger-Expression Inventory: Professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Spielberger CD. STAXI-2: State-Trait Anger Expression Inventory professional manual. Odessa, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- Stanford MS, Houston RJ, Villemarette-Pittman NR, Greve KW. Premeditated aggression: Clinical assessment and cognitive psychophysiology. Personality and Individual Differences. 2003;34:773–781. [Google Scholar]
- Stewart JL, Levin-Silton R, Sass SM, Heller W, Miller GA. Anger style, psychopathology, and regional brain activity. Emotion. 2008;8:701–713. doi: 10.1037/a0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: Evidence for phasic frontal lobe activation. Electroencephalography and Clinical Neurophysiology. 1998;108:406–413. doi: 10.1016/s0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Surguy SM, Bond AJ. P300 to emotionally relevant stimuli as an indicator of aggression levels. Aggressive Behavior. 2006;32:253–260. [Google Scholar]
- Thomas SJ, Johnstone SJ, Gonsalvez CJ. Event-related potentials during an emotional Stroop task. Int. J. Psychophysiol. 2007;63:221–231. doi: 10.1016/j.ijpsycho.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Toglia MP, Battig WF. Handbook of semantic word norms. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- van Honk J, Tuiten A, van den Hout M, Putman P, de Haan E, Stam H. Selective attention to unmasked and masked threatening words: Relationships to trait anger and anxiety. Personality and Individual Differences. 2001;30:711–720. [Google Scholar]
- van Petten C, Kutas M. Interactions between sentence context and word frequency in event-related brain potentials. Memory and Cognition. 1990;18:380–393. doi: 10.3758/bf03197127. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action monitoring processes in anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Watson D. Locating anger in the hierarchical structure of affect: Comment on Carver and Harmon-Jones. Psychological Bulletin. 2009;135:205–208. doi: 10.1037/a0014413. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Lystad C. Interpretation biases in angry and anxious individuals. Behaviour Research and Therapy. 2005;43:1045–1054. doi: 10.1016/j.brat.2004.02.009. [DOI] [PubMed] [Google Scholar]