Velopharyngeal incompetence is a complication following cleft palate surgery, with an incidence ranging from 5 to 38 percent following palatoplasty.1 It occurs when the velopharyngeal valving mechanism is incapable of complete closure to isolate the oropharynx from the nasopharynx during speech production. The anatomical components of the velopharynx are the velum (soft palate) and the three pharyngeal walls: posterior, right lateral, and left lateral. Velopharyngeal closure depends on three basic factors: (1) superior and posterior movement of the soft palate produced by the contraction of the levator veli palatini muscle, (2) medial movement of the lateral pharyngeal walls, and (3) bulging forward of the posterior pharyngeal wall, creating a Passavant's ridge to facilitate velopharyngeal closure. Both the levator veli palatini muscle and the soft palate play a major role in the pathogenesis of velopharyngeal incompetence. Dysfunction of the levator veli palatini muscle or shortness of the soft palate may cause velopharyngeal incompetence, yet treatment options are quite different. Therefore, accurate diagnosis is crucial. Procedures that rely on restoration of a functional levator sling require the presence of adequate levator musculature for reconstruction of the levator sling.
When the diagnosis of velopharyngeal incompetence is confirmed, speech therapy is initiated. Should the patient fail to demonstrate sufficient improvement in nasality despite speech therapy, secondary surgical management to restore a competent velopharyngeal valving mechanism may be indicated. If it is possible to do so by restoration of the levator muscular sling, this may obviate the need for surgical alteration of the posterior or lateral pharyngeal walls as occurs during a pharyngeal flap or sphincter pharyngoplasty. Dynamic reconstruction of the levator sling can be accomplished using either a Furlow palatoplasty in patients with submucous cleft palate or postpalatoplasty velopharyngeal incompetence,2 or by palate re-repair in patients with postpalatoplasty velopharyngeal incompetence as advocated by Sommerlad and colleagues.3 However, should the levator mechanism be negligible or replaced with scar following prior surgery, attempting dynamic levator reconstruction would not result in a competent velopharyngeal valving mechanism, and pharyngeal flap or sphincter pharyngoplasty should be considered. Therefore, the ability to visualize different soft-tissue planes of the velopharynx and to visualize the muscular anatomy preoperatively become crucial when planning secondary surgical management of velopharyngeal incompetence.
Current methods for functional visualization of the velopharynx are either invasive (nasendoscopy) or impart ionizing radiation (speech videofluoroscopy), and do not provide anatomical definition of palatal soft-tissue planes. In addition to its invasiveness, nasendoscopy allows only a single viewpoint (from a ventral and cephalad observation point) of the velum. Furthermore, both the passage of the endoscope itself and the local anesthetic agent used during scoping may affect speech. In contrast, although speech videofluoroscopy allows visualization of dynamic interaction (i.e., functional evaluation) of velopharyngeal structures from different angles, interpretation is difficult because of shadows introduced by the overlying structures. Moreover, relative proportion distortion is inevitable in speech videofluoroscopy because three-dimensional structures are converted into two-dimensional images. An ideal test that is noninvasive and does not impart ionizing radiation is not in widespread use for preoperative assessment of the velopharyngeal mechanism in children. Magnetic resonance imaging is noninvasive and poses no ionizing radiation hazard, and allows imaging of the velopharyngeal mechanism during limited speech production. Magnetic resonance imaging also allows anatomical assessment of soft-tissue planes involved in the velopharyngeal valving mechanism, which is not possible with other techniques currently in use.
The major hurdle that prevents the widespread application of magnetic resonance imaging for functional evaluation of the velopharyngeal mechanism is poor image quality. To examine the function of the velopharyngeal mechanism during active phonation using magnetic resonance imaging, patients are instructed to sustain phonation of a chosen vowel or consonant during the image-acquisition process. Because most patients, especially children, are only able to sustain phonation for 15 to 20 seconds comfortably, image-acquisition time is limited. This in turn limits the resolution of the acquired image. Fortunately, magnetic resonance image quality is dependent not only on the length of acquisition time but also on the magnetic field strength and head coil design. By using more powerful magnetic resonance imaging scanners and better head coil design, improvement in magnetic resonance images is possible without increasing image-acquisition time.
Previous magnetic resonance imaging studies involving functional evaluation of the velopharyngeal valving mechanism in children used magnetic resonance imaging scanners with a strength up to only 1.5 T.4–7 By using a 3.0-T magnetic resonance imaging scanner with a multichannel head coil, we hypothesize that the quality of the images acquired will be sufficient to allow functional evaluation of the velopharyngeal valving mechanism in children. Moreover, resolution of the images will allow delineation of palatal soft-tissue planes for surgical planning.
PATIENTS AND METHODS
A total of three adults and three children were enrolled in the study. The three adults were used first to test the feasibility of the study protocol. Once the feasibility was confirmed and the protocol optimized, three children were enrolled. One child (7 years old) with velopharyngeal incompetence secondary to submucous cleft palate was scanned before and after Furlow palatoplasty. Two normal children (aged 9 and 11 years) were used as controls. Studies were carried out using a 3.0-T GE Long Bore Signa Excite scanner and a GE 8-channel brain array coil assembly (General Electric, Milwaukee, Wis.) with summations of signals from the eight channels for the best signal-to-noise ratio. The sequence used is known as FSE-XL. Technical parameters were optimized, arriving at the following values: repetition time, 750 msec; echo time, minimum; matrix, 256 × 256; field of view, 18 × 18 cm; slice, 3 mm; number of excitations, 1; echo-train length, 16; and scan time, 14 seconds. A single 3-mm sagittal slice was acquired that was carefully centered at the midline. Partial volume blurring was negligible. The optimum in image quality was obtained when using 16 echoes in this turbo sequence.
Anatomical position of the velopharynx was studied during rest and sustained phonation (14 seconds) of “e” and “n.” An investigator monitored the phonation to verify that the desired sound was sustained for the entire imaging time. An image was also obtained with the subject relaxed and breathing normally through the nose. The short acquisition time made it possible to immediately verify that the image was of high quality and to repeat scans if necessary. All phonations were performed at least twice to ensure consistent and reproducible results. In nearly all cases, the appearance of the two scans performed for the same phonation was identical. If this was not the case, the phonation was repeated as necessary.
The images were analyzed by examination of the resting position of the soft palate to determine its length and shape and the location of the posterior pharyngeal wall. The phonation images were then evaluated for the completeness of velopharyngeal closure by observing the presence or absence of air space between the soft palate and the posterior pharyngeal wall. All images were evaluated independently by three investigators and the results compared.
RESULTS
The following findings are visualized on the magnetic resonance imaging scans (Fig. 1):
Normal subjects demonstrated ample muscle in the midline palate, with relaxation and descent during sounds not requiring nasopharyngeal closure (Fig. 1, first and second columns).
The soft-tissue planes can be readily delineated in all images despite the short acquisition time of 14 seconds.
Preoperative palatal anatomy in the submucous cleft palate patient consisted of a short hard palate consistent with absence of the posterior nasal spine, and an attenuated soft palate in which muscle was devoid in the midline, consistent with the zona pellucidum. There was lack of muscular activity in the posterior velum, resulting in a velopharyngeal gap during phonation (Fig. 1, second row, third column).
After Furlow palatoplasty, the submucous cleft palate patient demonstrated increased muscle bulk in the posterior half of the soft palate. Consistent closure of the velopharyngeal gap was demonstrated (Fig. 1, second row, fourth column), consistent with muscle rearrangement in the velum.
Inspection of the images suggests that an improved multichannel coil targeting the orofacial cavity would be valuable in improving the image quality. The image quality of the velopharyngeal region was adequate but clearly inferior to the image quality at a level only a few centimeters superior to the velum.
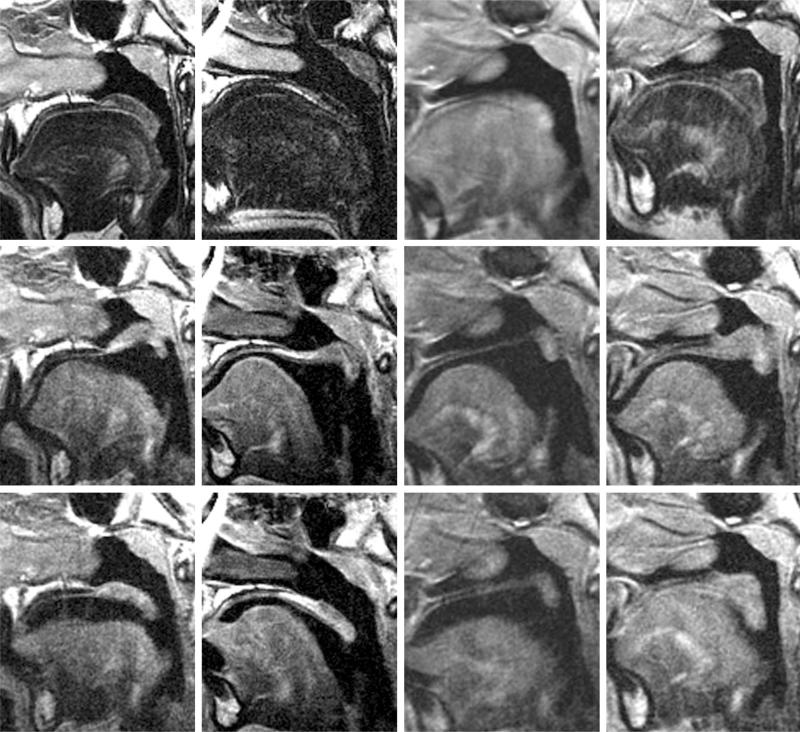
Fig. 1.
Velopharyngeal mechanism visualized on magnetic resonance imaging. (Above) Velum at rest during nasal breathing. (First column) Control child 1; (second column) control child 2; (third column) submucous cleft palate child preoperatively; (fourth column) submucous cleft palate child postoperatively. The velum rests passively on the tongue during nasal breathing, maintaining airflow through the nasopharynx. (Center) Velum during phonation of “e” sound, demonstrating closure of the nasopharynx. (First column) Control child 1; (second column) control child 2; (third column) submucous cleft palate child preoperatively; (fourth column) submucous cleft palate child postoperatively. Note the absence of muscle in the velum of the submucous cleft palate patient preoperatively, with a persistent velopharyngeal gap on phonation (third column) compared with considerable muscle bulk in this location postoperatively with closure of the velopharyngeal gap (fourth column). (Below) Velum during phonation of “n” sound, with airflow through both the nasal and oral cavities. (First column) Control child 1; (second column) control child 2; (third column) submucous cleft palate child preoperatively; (fourth column) submucous cleft palate child postoperatively. The velum rests in an intermediate position, with airflow through both the nasopharynx and the oropharynx.
Figure 1 demonstrates the magnetic resonance images of the velopharyngeal mechanism obtained during nasal breathing with the velum at rest (first row), with velopharyngeal closure during phonation of “e” (second row), and with the velopharyngeal mechanism in midposition during phonation of “n” (third row). The first and second columns demonstrate these activities in normal children, the third column demonstrates the patient with submucous cleft palate preoperatively, and the fourth column demonstrates the patient with submucous cleft palate postoperatively.
In the patient with submucous cleft palate, preoperative rating on the velopharyngeal function assessment scale (range, 0 to 13; where 0 = no nasality and 13 = maximum nasality) was 6 because of consistent audible nasal air emission, with notable articulation errors, nasal turbulence, and mild to moderate hypernasality. Postoperative velopharyngeal assessment demonstrated a score of 2, with only occasional audible nasal air emission and hypernasality. This correlated with the change in muscular anatomy visualized in the posterior half of the soft palate by magnetic resonance imaging. Absence of muscle in the velum of the submucous cleft palate patient was observed preoperatively, with a persistent velopharyngeal gap on phonation (Fig. 1, second row, third column) compared with considerable muscle bulk in this location postoperatively with closure of the velopharyngeal gap on phonation (Fig. 1, second row, fourth column).
DISCUSSION
Magnetic resonance imaging allows visualization of the velopharyngeal sphincter in the sagittal, coronal, and axial planes without the need for patient repositioning. The full range of motion of the soft palate and its interaction with the posterior pharyngeal wall can be evaluated easily using midsagittal cuts, whereas lateral pharyngeal wall motion can be assessed by means of coronal cuts. Changes in the aperture/closure of the velopharyngeal port can be seen using axial cuts.4 However, conventional magnetic resonance imaging is limited to the study of static structures because of lengthy time required to obtain images with sufficient anatomical detail. Moving structures produce blurry images. This mismatch between the time necessary to acquire sufficiently detailed images and the rapidity of movement of the vocal tract during speech has forced investigators to acquire images either at rest or during sustained phonation/utterances.4,6–10
In cleft palate surgery, treatment priorities for most surgeons consist of (1) intelligible speech, (2) integrity of the repair without fistula formation, (3) normal growth and development of the palate and facial structures, and (4) development of normal dental occlusion. Approximately 20 to 30 percent of patients who undergo palatoplasty have velopharyngeal incompetence with an incompetent velopharyngeal valving mechanism regardless of the technique used for the initial palatoplasty.1 A substantial portion of these patients may require secondary surgical management for the correction of velopharyngeal incompetence.9 With magnetic resonance imaging, it is possible to visualize the anatomy of the palate and the mechanism for velopharyngeal closure and to examine the performance of the soft palate following palatoplasty.
This is the first study in which magnetic resonance imaging has been used to visualize the changes in the velopharyngeal valving mechanism with active phonation before and after dynamic levator muscle reconstruction. Kuehn et al.11 used magnetic resonance imaging to study the course of the levator veli palatini muscles in patients with occult submucous cleft palate before and after Furlow palatoplasty. However, the patients were sedated during the study, without attempted phonation, making it a static anatomical study rather than a functional study where dynamic changes in the velopharyngeal valving mechanism can be evaluated. These authors subsequently looked at the anatomy of the levator muscles before and after primary palatoplasty in infants, but the patients were too young to cooperate with active phonation and were anesthetized during the study.12 Özgür et al.4 used magnetic resonance imaging to visualize the dynamic change in the anatomy of the velopharynx before and after pharyngeal flap surgery. However, no attempt was made to reconstruct the levator musculature as is done in a Furlow palatoplasty, and therefore the authors did not attempt to use the preoperative magnetic resonance data to assess the potential for levator muscle reconstruction. In addition, these authors visualized the velopharynx by means of the midsagittal view, which does not demonstrate the lateral pharyngeal ports that are the dynamic components of a pharyngeal flap. Therefore, useful information regarding preoperative assessment or dynamic postoperative outcome could not be obtained from previous techniques.
Cephalometric measurements may be a useful adjunct and may provide another application of magnetic resonance imaging with which to follow the anatomy of the velum over time following palatoplasty. The soft-palate length (distance between the posterior nasal spine and the tip of the uvula), pharyngeal depth (distance between the posterior nasal spine and the posterior pharyngeal wall), and the ratio between the pharyngeal depth and the soft-palate length (the need ratio) were reported to be crucial factors influencing the ability of the velum to stretch. Subtelny13 and Wu et al.14 found that a need ratio greater than 70 percent indicates an unfavorable relationship between soft palate length and pharyngeal depth, placing these patients at risk of velopharyngeal incompetence. Near the age of 10 years, involution of adenoid tissue combined with rapid pharyngeal enlargement results in maximization of the need ratio. This corresponds to a period when velopharyngeal incompetence worsens. As the soft palate continues to lengthen throughout life, whereas other dimensions (such as the pharyngeal depth) remain relatively unchanged after age 15, the need ratio decreases.9 Postpalatoplasty patients who are found to have deterioration in velopharyngeal assessment scores over time could be reassessed using magnetic resonance imaging, which would provide an opportunity to evaluate alteration in levator muscle position relative to standard cephalometric measures.
Currently, the pharyngeal flap and the sphincter pharyngoplasty are the most commonly used procedures for secondary surgical management of velopharyngeal incompetence following palatoplasty.15 Augmentation of the posterior pharyngeal wall, Furlow palatoplasty, and cleft palate re-repair are other surgical alternatives. However, only the latter two techniques attempt to restore velopharyngeal competence by restoring a dynamic levator mechanism. A superiorly based pharyngeal flap narrows the area of the velopharyngeal port; a sphincter pharyngoplasty seeks to create a sphincter at the entry to the nasopharynx; and augmentation of the posterior pharyngeal wall decreases the velopharyngeal gap.16 No attempt for dynamic levator reconstruction is made with these three techniques. In contrast, Furlow palatoplasty repositions the levator muscle posteriorly and medially and reconstructs the levator sling while simultaneously lengthening the soft palate. Palatal re-repair seeks to free the levator musculature from surrounding scar tissue and to reposition the levator sling in a more favorable position for creation of a competent velopharyngeal valve.3 It is imperative that adequate levator muscle be present if Furlow palatoplasty or palatal re-repair are to be considered, and we therefore feel that magnetic resonance imaging would provide the most useful information if one of these techniques is anticipated. Such use of magnetic resonance images of the velopharynx would make it an important part of the preoperative assessment of these patients and would help to identify those patients who may not be candidates for dynamic levator reconstruction. Were such protocols to come into widespread clinical use, it would require that the test be performed when the patient is old enough to cooperate with phonation in the magnetic resonance imaging scanner (presumably age 5 years or older). To date, use of magnetic resonance imaging scans to assess velopharyngeal function has been largely descriptive. The present study attempts to establish a protocol that may serve to guide treatment by first establishing the pattern of phonation of a standard set of sounds in normal populations and then evaluating preoperative and postoperative outcomes in a patient with velopharyngeal incompetence secondary to submucous cleft palate.
Magnetic resonance imaging offers a noninvasive method for anatomical and functional evaluation of the velopharynx. As no exposure to ionizing radiation is involved, the examination can be used safely and repeatedly during treatment and rehabilitation of pediatric patients. Technological development will make magnetic resonance imaging a more useful investigation tool for velopharyngeal function; a magnetic resonance imaging scanner with higher magnetic power and a better designed multichannel head coil will enable investigators to capture images in a shorter acquisition time without compromising resolution. Magnetic resonance imaging has great potential for evaluation of the palate before and after surgery. We feel that preoperative visualization of the levator musculature as demonstrated by magnetic resonance imaging can be invaluable for the surgeon when deciding which surgical approach will be used for secondary management of velopharyngeal incompetence. Moreover, magnetic resonance imaging can easily be standardized by protocols in normal and cleft patients to yield reproducible results that allow comparison over time. Such an application of magnetic resonance imaging in the future will make this a useful test in guiding speech therapy and subsequent surgical planning in the growing child, in whom change in velopharyngeal function over time is commonly seen.
CONCLUSIONS
Magnetic resonance imaging holds the potential for refining the management of patients with velopharyngeal incompetence secondary to cleft palate. The velopharyngeal muscular anatomy can be reliably visualized in normal subjects using magnetic resonance imaging, and this technique can better define the changes in velopharyngeal function following surgical correction of velopharyngeal incompetence in cleft patients. Younger patients would not be able to undergo the same dynamic assessment of the velopharynx without sedation, and we therefore advocate use of this test in patients age 5 years or older so they can cooperate with a protocol for dynamic assessment of the velopharynx. Cleft patients eligible for such a protocol would consist of patients with postpalatoplasty velopharyngeal incompetence and those with velopharyngeal incompetence secondary to unrepaired submucous cleft palate. The present report is the first to demonstrate dynamic changes in velopharyngeal muscular anatomy using magnetic resonance imaging following surgical management for velopharyngeal incompetence. We believe this technique can be applied to identify and localize the anatomical defect in patients with cleft-related velopharyngeal incompetence and serve as an aid in presurgical planning for dynamic velopharyngeal reconstruction. Future developments that will make this technique more effective include magnetic resonance imaging scanners with higher magnetic power and a better multichannel head coil that will decrease image acquisition time and improve image quality. In addition, the development of open magnetic resonance imaging machines will make this technology applicable even to patients with claustrophobia. We believe that these technologies will make magnetic resonance imaging more practical for diagnostic use in the pediatric population and help to bring it into widespread use in the future.
Footnotes
Disclosure: The authors declare that they had no financial interests or commercial associations during the course of this study.
REFERENCES
- 1.Witt PD, D'Antonio LL. Velopharyngeal insufficiency and secondary palatal management: A new look at an old problem. Clin. Plast. Surg. 1993;20:707. [PubMed] [Google Scholar]
- 2.Chen PK, Wu JT, Chen YR, Noordhoff MS. Correction of secondary velopharyngeal insufficiency in cleft palate patients with the Furlow palatoplasty. Plast. Reconstr. Surg. 1994;94:933. [PubMed] [Google Scholar]
- 3.Sommerlad BC, Henley M, Birch M, Harland K, Moiemen N, Boorman JG. Cleft palate re-repair: A clinical and radiographic study of 32 consecutive cases. Br. J. Plast. Surg. 1994;47:406. doi: 10.1016/0007-1226(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 4.Özgür F, Tunçbilek G, Cila A. Evaluation of velopharyngeal insufficiency with magnetic resonance imaging and nasoendoscopy. Ann. Plast. Surg. 2000;44:8. doi: 10.1097/00000637-200044010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Akgüner M, Karaça C, Barutca A, Özaksoy D, Yurt A, Vayvada H. Evaluation of velopharyngeal pathophysiology and velopharyngeal insufficiency with magnetic resonance imaging. Eur. J. Plast. Surg. 1998;21:118. [Google Scholar]
- 6.McGowan J, Hatabu H, Yousem D, Randall P, Kressel H. Evaluation of soft palate function with MRI: Application to the cleft palate patient. J. Comput. Assist. Tomogr. 1992;16:877. doi: 10.1097/00004728-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Vadodaria S, Goodacre T, Anslow P. Does MRI contribute to the investigation of palatal function? Br. J. Plast. Surg. 2000;53:191. doi: 10.1054/bjps.1999.3308. [DOI] [PubMed] [Google Scholar]
- 8.Story B, Titze I, Hoffman E. Vocal tract area functions for an adult female speaker based on volumetric imaging. J. Acoust. Soc. Am. 1998;104:471. doi: 10.1121/1.423298. [DOI] [PubMed] [Google Scholar]
- 9.Akgüner M. Velopharyngeal anthropometric analysis with MRI in normal subjects. Ann. Plast. Surg. 1999;43:142. [PubMed] [Google Scholar]
- 10.Fitch W, Giedd J. Morphology and development of the human vocal tract: A study using magnetic resonance imaging. J. Acoust. Soc. Am. 1999;106:1511. doi: 10.1121/1.427148. [DOI] [PubMed] [Google Scholar]
- 11.Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC, Wachtel JM. Magnetic resonance imaging in the evaluation of occult submucous cleft palate. Cleft Palate Craniofac. J. 2001;38:421. doi: 10.1597/1545-1569_2001_038_0421_mriite_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 12.Kuehn DP, Ettema SL, Goldwasser MS, Bark-meier JC. Magnetic resonance imaging of the levator veli palatini muscle before and after primary palatoplasty. Cleft Palate Craniofac. J. 2004;41:584. doi: 10.1597/03-060.1. [DOI] [PubMed] [Google Scholar]
- 13.Subtelny JD. A cephalometric study of the soft palate. Plast. Reconstr. Surg. 1957;19:49. doi: 10.1097/00006534-195701000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Huang CS, Noordhoff MS. Nasopharyngoscopic evaluation and cephalometric analysis of velopharynx in normal and cleft palate patients. Ann. Plast. Surg. 1996;36:117. doi: 10.1097/00000637-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Witt PD, D'Antonio LL, Zimmerman GJ, Marsh JL. Sphincter pharyngoplasty: A preoperative and postoperative analysis of perceptual speech characteristics and endoscopic studies of velopharyngeal function. Plast. Reconstr. Surg. 1994;93:1154. doi: 10.1097/00006534-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Jackson IT. Sphincter pharyngoplasty. Clin. Plast. Surg. 1985;12:711. [PubMed] [Google Scholar]