Abstract
Background and Purpose
To evaluate the proximity, variance, predictors of dose, and complications to the sigmoid in cervical-cancer brachytherapy using 3D planning.
Materials and Methods
Over 36 months, 50 patients were treated for cervical cancer with either low-dose-rate (LDR) or high-dose-rate (HDR) brachytherapy. The distance from the central tandem to the sigmoid, the D0.1cc and the D2cc to the sigmoid, rectum and bladder doses, and toxicity were analyzed.
Results
The median sigmoid EQD2 D0.1cc and D2cc were 84 Gy and 68.3 Gy for HDR versus 71.1 Gy and 65.9 Gy for LDR (p=0.02 and 0.98, respectively). Twenty percent of the HDR fractions required manipulation of the superior dwell positions to decrease the sigmoid dose. The median distance from the sigmoid to the tandem was 1.7 cm (range [rg], 0.1 – 6.16 cm) for HDR and 2.7 cm (rg, 1.17 – 4.52 cm) for LDR; from the sigmoid to the 100% isodose region the median distances were – 0.1 cm (rg, -1.4 – 2.5 cm) and 0.44 cm (rg. -0.73 – 5.2 cm), respectively. The proximity of the sigmoid to the tandem is significantly related to sigmoid dose (p<0.0001). Within-patient (among-fraction) variation in sigmoid-to-tandem distance during HDR was substantial (coefficient of variation = 40%). No grade 3-4 sigmoid toxicity was seen after a median 31-month follow-up period.
Conclusions
3D imaging in cervical cancer brachytherapy shows the sigmoid in close proximity to the tandem. The sigmoid to tandem distance varies substantially between fractions, indicating the importance of sigmoid dose-volume evaluation with each fraction.
Keywords: cervical cancer, brachytherapy, normal tissue dose
Introduction
Intracavitary brachytherapy (BT), in conjunction with external-beam radiation (EB) and chemotherapy, is the standard of care in the curative management of locally advanced cervical cancer.(1) The rectal and bladder reference dose points of the International Commission on Radiation Units (ICRU) 38 are the standard for reporting normal tissue doses with BT. However, these points are based on plain films and two-dimensional (2D) BT planning(2) and do not relate to the maximum dose delivered or toxicity.(3-10) Stereo x-ray photogrammetry has been used to estimate dose to the sigmoid colon near the low-dose-rate LDR applicator. This method has shown that simple 2D planning cannot determine the maximal dose to the sigmoid, which is possible with ICRU rectal and bladder doses.(11) Therefore, no sigmoid dose point is described in the ICRU recommendations for 2D planning in cervical cancer BT. Previous publications have estimated a 0.6-6.4% sigmoid complication rate for low-dose-rate (LDR) radiation, including the risk of obstructive sigmoiditis and sigmoid perforation.(12-18)
Three-dimensional (3D) imaging allows for the delineation of the organs at risk (OAR), including the bladder, rectum, and sigmoid. In 2005, the GEC-ESTRO published guidelines for magnetic resonance (MR)-based contouring of tumor volumes in cervical-cancer BT.(19, 20) CT-based planning for the OAR has been shown to be similar to MR-based contours if the time interval between the MR and CT scans is relatively short, patient positioning is similar, and the bladder is drained with a Foley catheter in place.(21) GEC-ESTRO II recommended the reporting of the minimum dose to the most irradiated tissue volume (D0.1, D1, and D2cc). This was based on the assumption that the OAR received the full dose of external-beam radiation and that the brachytherapy dose to the OAR should be recorded per fraction in order to calculate the worst-case scenario of cumulative dose received.
Sigmoid dose has been reported in other 3D-based studies of cervical cancer treatment with high-dose-rate (HDR) (22-24) or pulsed-dose-rate (PDR) brachytherapy.(25, 26) However, no study to date has reported within-patient variation between fractions in sigmoid location and determined predictors of sigmoid dose in cervical-cancer BT. The goal of this study is to evaluate these factors and to report sigmoid complications in this population of patients.
Materials and Methods
Patients
We conducted a retrospective review of all biopsy-confirmed cervical-cancer patients treated using 3D planning techniques with curative intent from April, 2004 to December, 2007 at the Brigham and Women's (BWH)/Dana-Farber Cancer Center after approval by the Human Subjects Research Committee. Exclusions included one patient who had a supracervical hysterectomy treated with a short tandem and another patient treated with BT alone for Stage IB1 cervical cancer. Fifty patients were identified who received BT with either LDR (tandem and ovoid (T/O), tandem and interstitial (T/I)) or HDR BT (tandem and ovoid (T/O), tandem and ring (T/R), or tandem and cylinder (T/C)) following EB radiation therapy and chemotherapy.
Brachytherapy Procedure and Treatment Planning
All patients were brought into a dedicated BT suite at BWH. The suite includes a CT scanner with a custom-designed tabletop that allows the patient to be moved through the bore in treatment position without having to shift off the procedure table. Patients were given either spinal or general anesthesia and were examined in lithotomy position to document the extent of cancer. After sterile prepping and draping of the patient, a Foley catheter was inserted into the bladder, and the Foley balloon was inflated with 7-10 cc of hypaque contrast. The uterus was sounded with ultrasound guidance if necessary. Serial dilation of the cervical os was followed by insertion of the intrauterine tandem. Depending on disease extent, a para-cervical ring, ovoids, cylinder, or interstitial needles were placed. Prior to September 2005, hypaque contrast was placed into the Foley balloon but not into the bladder directly. After September 2005, the bladder was emptied and then instilled with 10 cc of contrast material in 40 cc of saline to define the bladder wall; barium was instilled into the sigmoid and rectum with a rectal tube. Radio-opaque markers were placed within the applicators for visualization and treatment planning.
All patients underwent CT simulation (General Electric Medical Systems, Milwaukee, WI) using 2.5 mm slices. The patient remained anesthetized throughout the procedure and simulation, and the images were obtained with the patient in treatment position with the legs down. Patients receiving HDR treatments remained anesthetized during both planning and delivery. The CT images were transferred to the Advantage Sim (GE Medical Systems) Virtual Simulation Software and to Plato (brachytherapy planning v14.2.6 Nucletron Systems, Veenendaal, The Netherlands) for HDR or to Brachyvision (Planning 6.5 build 7.3.10) for LDR contouring and planning. External contours of the sigmoid, rectum, and bladder were made. The window and level settings of the images were manipulated to decrease the scatter artifact from the LDR applicator. One physician (C.H.) contoured the sigmoid from the rectosigmoid junction to the level where the sigmoid crosses anteriorly to the pubic symphysis.(27) Rectal contrast helped define the rectosigmoid junction and distinguish the large bowel from the small bowel. The post-implant dose-volume histograms (DVHs) of the sigmoid, rectum, and bladder were generated for the D0.1cc and D2cc volumes.
The prescription for HDR was 27.5 Gy in 5 fractions to Point A, and for LDR approximately 40 Gy over 1–2 fractions at an average of 0.6Gy/hr (range 0.4–0.8 Gy/hr). Equivalent doses were calculated for HDR treatments using the linear quadratic model normalized to 2 Gy/fraction using an α/β of 3 (EQD2). 3D manual optimization of the HDR plans restricted the per-fraction D2cc dose to the sigmoid and bladder, making the cumulative EB plus total BT EQD2 dose <75 Gy.(22) The LDR applicator was loaded to the tip in all LDR cases. The EQD2 dose to the sigmoid and rectum was limited to 70-75 Gy. The closest single point distance from the tandem to the sigmoid was measured on axial images, as was the closest distance from the 100% isodose to the sigmoid.
Analysis
Linear regression was used to determine predictors of the D2cc sigmoid dose. Univariate analysis included tandem length, distance from tandem to sigmoid, type of applicator (categorical T/O vs. T/R vs. T/I vs. T/C), dose rate (HDR vs. LDR), and bladder and rectal dose. The variation of sigmoid to tandem distance between fractions within individual patients was estimated. The coefficient of variation (CV) is the ratio of a standard deviation to a mean; in this study the CV is the ratio of the standard deviation of within-patient (among-fraction) sigmoid-to-tandem distances and the mean sigmoid-to-tandem distance. A single factor ANOVA test was used to evaluate whether the mean sigmoid distances differed among patients given the estimated within-patient variation. Complications were recorded using the NCI Common Toxicity Criteria.
Results
A total of 170 HDR fractions were delivered to 34 patients with tandem and ovoid (17 patients), tandem and ring (5 patients), tandem and cylinder (2 patients), or a combination (10 patients). Twenty-four LDR fractions were delivered to 16 patients with tandem and ovoid (5 patients), tandem and interstitial (8 patients), or both (3 patients). Table 1 lists patient and tumor characteristics. Proportionally more Stage III and IVA patients received LDR radiation than HDR. There was no difference in the median tandem length inserted: 6 cm for both HDR (range [rg], 4–7 cm) and LDR (rg, 5–7 cm).
Table 1.
Patient and tumor characteristics.
| HDR | LDR | ||
|---|---|---|---|
| Patient | n = 34 | n = 16 | |
| Median Age in years (range) | 56 (28–84) | 50 (19–79) | |
| Tumor | n (%) | n (%) | |
| Stage (%) | I | 6 (18) | 1 (6) |
| IIA | 9 (26) | 0 | |
| IIB | 14 (41) | 6 (38) | |
| III | 5 (15) | 6 (38) | |
| IVA | 0 | 3 (19) | |
| Pathology (%) | Squamous | 23 (68) | 11 (69) |
| Adenosquamous | 2 (6) | 1 (6) | |
| Adenocarcinoma | 6 (18) | 2 (13) | |
| Other | 3 (9) | 2 (13) |
The median EB dose for both HDR and LDR patients was 45 Gy (rg, 40–50.4 Gy). The median cumulative BT dose was 27.5 Gy (rg, 25-27.5Gy) in 4–5 fractions prescribed to Point A for the HDR patients, and 42.5 Gy (rg, 36–50 Gy) over 1–2 fractions prescribed at 0.4–0.8 Gy/hr for the LDR patients. The median activity at the tip of the LDR tandem was 13.5 mgRaeq (rg, 6.2–14.3 mgRaeq).
The median distance between the sigmoid and the tandem was 1.7 cm (rg, 0.1–6.16 cm) for HDR plans and 2.7 cm (rg, 1.17–4.52 cm) for LDR. One or more dwell positions at the tip of the tandem were turned off in 20% (30/146) of HDR fractions as part of the dose optimization process. The median distance from the sigmoid to the 100% isodose line in the HDR plans was -0.1 cm (rg, -1.4–2.5 cm), with the sigmoid falling within the high-dose region; for LDR the corresponding median distance was 0.44 cm (rg, -0.73–5.2 cm). Among HDR fractions, the coefficient of variation (CV) of the within-patient (among-fraction) sigmoid-to-tandem distance was 40%. The variation among patients as evaluated by ANOVA was significant, given the estimated within-patient variation (p<0.01).
Only the proximity of the sigmoid to the tandem was significantly related to sigmoid dose (p<0.01). Tandem length, type of applicator (categorical T/O vs. T/R vs. T/I vs. T/C), dose rate (HDR vs LDR), and bladder and rectal dose were not significantly related to sigmoid dose using linear regression. Therefore, multivariate analysis was not performed.
Table 2 lists the D0.1cc and D2cc total doses EQD2 (EB+BT) delivered to the OAR. The cumulative LDR EB + BT dose was significantly higher for the bladder and rectal D2cc (p=0.02, p=<0.01). Figure 1 depicts the ratio of prescribed sigmoid dose (D0.1cc and D2cc) to point A prescription dose for HDR and LDR patients. The BT dose to the sigmoid, rectum, and bladder and the ratio of delivered BT dose to the OAR as compared with the prescribed BT dose to point A are reported in Table 2.
Table 2.
Sigmoid, bladder and rectum EQD2 with an α/β = 3.
| HDR | LDR | |||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Sigmoid | ||||
| D0.1cca | 40.8 | 10.5–76.3 | 27.7 | 9–52 |
| D0.1ccb | 0.87 | 0.09–5.29 | 0.65 | 0.14–1.3 |
| D0.1ccc | 84.0 | 53.7–119.5 | 71.1 | 52.2–95.2 |
| D2cca | 25.3 | 7.8–36.5 | 22 | 7.6–42.9 |
| D2ccb | 0.53 | 0.06–2.6 | 0.53 | 0.11–0.99 |
| D2ccc | 68.3 | 51–79.7 | 65.9 | 50.8–86.1 |
| Bladder | ||||
| D0.1cca | 53.3 | 18.1–98.8 | 53.8 | 17.5–81.5 |
| D0.1ccb | 1.12 | 0.11–4.03 | 1.18 | 0.33–2.04 |
| D0.1ccc | 96.5 | 61.3–142 | 97 | 60.7–124 |
| D2cca | 32.3 | 11.6–52.3 | 40.3 | 14.8–57.6 |
| D2ccb | 0.7 | 0.08–1.54 | 0.85 | 0.28–1.44 |
| D2ccc | 75.7 | 54.8–95.5 | 83.5 | 58.0–100.8 |
| Rectum | ||||
| D0.1cca | 35.3 | 11–62.2 | 36 | 12–123.2 |
| D0.1ccb | 0.7 | 0.16–1.91 | 0.8 | 0.3–3.1 |
| D0.1ccc | 78.5 | 54.2–105.4 | 81.8 | 55.2–166.4 |
| D2cca | 20.6 | 7.2–40.8 | 27.5 | 10.1–83 |
| D2ccb | 0.42 | 0.02–1.53 | 0.6 | 0.25–2.1 |
| D2ccc | 63.8 | 50.4–84 | 72.5 | 53.3–126.2 |
Brachytherapy dose alone.
Ratio of delivered OAR brachytherapy dose to prescribed target dose.
External beam and brachytherapy dose.
Figure 1.
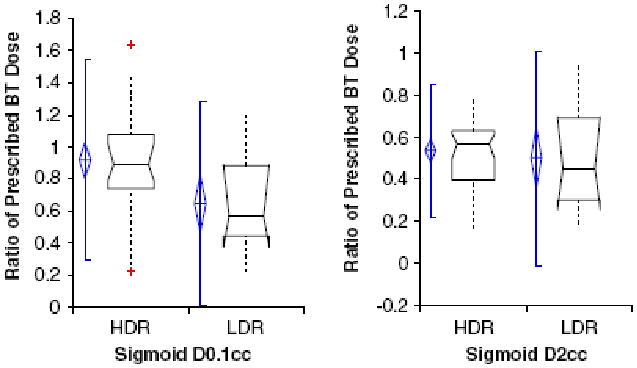
The ratio of sigmoid DVH dose to prescribed point A dose as DO.1cc or D2cc for HDR and LDR brachytherapy. The diamond shape reflects the 95% Confidence Interval of the mean. The bar represents two standard deviations above and below the mean. The box plot width related to sample size, and the horizontal line through the box represents the median value. The dashed lines above and below the box plot are data points.
The ratio of sigmoid D0.1 cc to prescribed point A dose in T/O patients showed no significant difference between HDR and LDR, although the significance was limited by the small number of subjects (median D0.1cc and D2cc for HDR, 0.78 and 0.51; median D0.1cc and D2cc for LDR, 0.69 and 0.57; for comparison of HDR versus LDR, D0.1cc p=0.52 and D2cc p=0.65).
With a median follow-up of 31.2 months (rg, 10.8 – 50.4 months), there were no grade 3–4 sigmoid toxicities in either the HDR or LDR group. Two patients in the HDR group and one in the LDR group required rehydration for diarrhea, which had not resolved from the EB portion of treatment. In the LDR group, one patient with extensive Stage IVA disease at diagnosis developed a recto-vaginal fistula requiring surgical correction.
Discussion
Using 3D imaging to analyze the dose delivered, this study classifies the sigmoid as an OAR. We found that the distance of the sigmoid relative to the tandem was significantly related to sigmoid dose. This study indicates that the relation of the sigmoid colon to the tandem changes between fractions, with a possible variance in the same patient of approximately 40%. It is important to note that this variance is determined by the proximity of the highest-dose region to the sigmoid, which may be an important factor for future toxicity. CT planning may minimize sigmoid toxicity by allowing optimization of HDR dose. The distance of the sigmoid from the tandem differed significantly between patients, indicating that a standard sigmoid point defined as a certain distance from the tandem would not be valid. The within-patient variance seen in this study indicates the necessity of sigmoid dose calculation with re-planning of each fraction for every patient to accurately calculate a worst-case scenario cumulative sigmoid dose. Whether the exact same geographic region of high dose was treated from fraction to fraction cannot be determined with modern imaging. A recent abstract reporting observer-based sigmoid topography(28) concluded that in 68% of their cases sigmoid movement was easily identified by different observers, which confirms variation in position.
Though sigmoid dose has been reported in the most recent 3D reports, no report to date has assessed proximity to the applicator or other factors that may influence sigmoid dose. In a study by Kim et al. (7), the sigmoid D3% was 65% and the small bowel D3% was 56% of the prescribed dose. This is consistent with our data, which reflect that after optimization, the sigmoid D2cc received 60% of the prescribed HDR BT dose. Interestingly, we found the cumulative doses to the rectum and bladder but not the sigmoid were higher in LDR treatments. In LDR patients treated with tandem and interstitial implants, we hypothesized that needles adjacent to the prescription point (Point A) may have decreased the dose to the sigmoid. In sub-analysis of T/O fractions in HDR versus LDR, no difference in sigmoid dose was seen, though small sample size was a limitation. In a retrospective analysis by Ferrigno et al. of cervical-cancer patients treated with either LDR or HDR BT with 5-year follow-up, LDR patients had twice the probability of rectal complications than HDR patients (16% versus 8%; p=0.03).(29) This speaks to the potential of HDR BT to spare normal tissue when treatment plans are optimized. The multiple fractions used with HDR prescriptions allow for variance in the position of the rectum and bladder and the potential for optimal packing with each fraction.
To date, it is not known whether the D0.1cc or the D2cc will be more valuable in predicting normal tissue complications. Koom et al. (30) noted that the D0.1cc, D1cc, D2cc, and D5cc predicted radiation-induced telangiectasia in the sigmoid as evaluated by sigmoidoscopy. The probability of telangiectasia increased when the EQD2 D0.1cc was >85 Gy and the D2cc was >70 Gy. In this analysis, the sigmoid D2cc in HDR and LDR BT were similar when using a central tandem and restricting the cumulative sigmoid dose in HDR to EQD2 75 Gy. With a median follow-up of 31 months, there have been no sigmoid complications, but further follow-up is required. This study cannot evaluate whether the brachytherapy dose is delivered to the same region of the sigmoid from one fraction to the next; therefore, we assumed a worst-case scenario for reporting: that the same region of the sigmoid always receives the highest dose. Cumulating the D2cc dose is therefore a limitation of this study, but the cumulative dose represents a potential maximum dose to a single region of the sigmoid. Other limitations of this study include use of only axial images to determine the distance from the tandem to the sigmoid and the small number of patients treated with low-dose-rate radiation, which restricts our ability to make comparisons between specific applicator types, or between HDR and LDR.
The sigmoid is a mobile organ within the pelvis; toxicity in relation to dose cannot be assessed through plain X-ray images. The use of 3D-based dosimetry may permit more organ sparing and give a better estimate of the dose to the OAR, ultimately decreasing toxicity.
Conclusions
3D imaging in cervical cancer brachytherapy shows the sigmoid in close proximity to the tandem. The maximum sigmoid dose is significantly related to its proximity to the tandem, which varies significantly between fractions, indicating the importance of sigmoid dose-volume evaluation with each fraction. Long-term follow up is necessary to define the dose-volume relationship of the sigmoid.
Acknowledgments
Thank you to Eric Macklin, PhD for reviewing and commenting on the statistical sections of the manuscript.
Footnotes
Presented at the American Society for Therapeutic Radiology and Oncology Annual Meeting, Philadelphia, PA, November 7, 2006.
Conflict of interest notification: None.
References
- 1.Eifel PJ. Chemoradiotherapy in the treatment of cervical cancer. Seminars in Radiation Oncology. 2006 Jul;16(3):177–85. doi: 10.1016/j.semradonc.2006.02.007. Review. [DOI] [PubMed] [Google Scholar]
- 2.International Commission on Radiation Units and Measurements. Dose and volume specification for reporting intracavitary therapy in gynecology. Vol. 38. Bethesda: International Commission on Radiation Units and Measurements; 1985. [Google Scholar]
- 3.Pelloski CE, Palmer M, Chronowski GM, Jhingran A, Horton J, Eifel PJ. Comparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary radiotherapy for cervical cancer. International Journal of Radiation Oncology, Biology, Physics. 2005 May 1;62(1):131–7. doi: 10.1016/j.ijrobp.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 4.Katz A, Eifel PJ. Quantification of intracavitary brachytherapy parameters and correlation with outcome in patients with carcinoma of the cervix. International Journal of Radiation Oncology, Biology, Physics. 2000 Dec 1;48(5):1417–25. doi: 10.1016/s0360-3016(00)01364-x. see comment. [DOI] [PubMed] [Google Scholar]
- 5.Fellner C, Potter R, Knocke TH, Wambersie A. Comparison of radiography- and computed tomography-based treatment planning in cervix cancer in brachytherapy with specific attention to some quality assurance aspects. Radiotherapy & Oncology. 2001 Jan;58(1):53–62. doi: 10.1016/s0167-8140(00)00282-6. [DOI] [PubMed] [Google Scholar]
- 6.Kapp KS, Stuecklschweiger GF, Kapp DS, Hackl AG. Dosimetry of intracavitary placements for uterine and cervical carcinoma: results of orthogonal film, TLD, and CT-assisted techniques. Radiotherapy & Oncology. 1992 Jul;24(3):137–46. doi: 10.1016/0167-8140(92)90372-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim RY, Pareek P. Radiography-based treatment planning compared with computed tomography (CT)-based treatment planning for intracavitary brachytherapy in cancer of the cervix: analysis of dose-volume histograms. Brachytherapy. 2003;2(4):200–6. doi: 10.1016/j.brachy.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Ling CC, Schell MC, Working KR, Jentzsch K, Harisiadis L, Carabell S, et al. CT-assisted assessment of bladder and rectum dose in gynecological implants. International Journal of Radiation Oncology, Biology, Physics. 1987 Oct;13(10):1577–82. doi: 10.1016/0360-3016(87)90327-0. [DOI] [PubMed] [Google Scholar]
- 9.Schoeppel SL, LaVigne ML, Martel MK, McShan DL, Fraass BA, Roberts JA. Three-dimensional treatment planning of intracavitary gynecologic implants: analysis of ten cases and implications for dose specification. International Journal of Radiation Oncology, Biology, Physics. 1994 Jan 1;28(1):277–83. doi: 10.1016/0360-3016(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 10.Shin KH, Kim TH, Cho JK, Kim JY, Park SY, Kim DY, et al. CT-guided intracavitary radiotherapy for cervical cancer: Comparison of conventional point A plan with clinical target volume-based three-dimensional plan using dose-volume parameters. International Journal of Radiation Oncology, Biology, Physics. 2006 Jan 1;64(1):197–204. doi: 10.1016/j.ijrobp.2005.06.015. Evaluation Studies. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers T. Stereo X Ray Photogrammetry Applied For Prevention of Sigmoid-Colon Damage Caused By Radiation From Intrauterine Sources. International Journal of Radiation Oncology, Biology, Physics. 1982 June;8(6):1011–7. doi: 10.1016/0360-3016(82)90170-5. [DOI] [PubMed] [Google Scholar]
- 12.Deore SM, Shrivastava SK, Viswanathan PS, Dinshaw KA, Deore SM, Shrivastava SK, et al. The severity of late rectal and recto-sigmoid complications related to fraction size in irradiation treatment of carcinoma cervix stage III B. Strahlentherapie und Onkologie. 1991 Nov;167(11):638–42. Clinical Trial Randomized Controlled Trial. [PubMed] [Google Scholar]
- 13.Eifel PJ, Levenback C, Wharton JT, Oswald MJ. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. International Journal of Radiation Oncology, Biology, Physics. 1995 Jul 30;32(5):1289–300. doi: 10.1016/0360-3016(95)00118-I. see comment. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen D, Bentzen SM, Overgaard J. Early and late radiotherapeutic morbidity in 442 consecutive patients with locally advanced carcinoma of the uterine cervix. International Journal of Radiation Oncology, Biology, Physics. 1994 Jul 30;29(5):941–52. doi: 10.1016/0360-3016(94)90387-5. see comment. [DOI] [PubMed] [Google Scholar]
- 15.Perez CA, Breaux S, Bedwinek JM, Madoc-Jones H, Camel HM, Purdy JA, et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix. II. Analysis of complications. Cancer. 1984 Jul 15;54(2):235–46. doi: 10.1002/1097-0142(19840715)54:2<235::aid-cncr2820540210>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez PT, Levenback C, Burke TW, Eifel P, Wolf JK, Gershenson DM. Sigmoid perforation following radiation therapy in patients with cervical cancer. Gynecologic Oncology. 2001 Jul;82(1):150–5. doi: 10.1006/gyno.2001.6213. [DOI] [PubMed] [Google Scholar]
- 17.Syed AM, Puthawala AA, Abdelaziz NN, el-Naggar M, Disaia P, Berman M, et al. Long-term results of low-dose-rate interstitial-intracavitary brachytherapy in the treatment of carcinoma of the cervix. International Journal of Radiation Oncology, Biology, Physics. 2002 Sep 1;54(1):67–78. doi: 10.1016/s0360-3016(02)02900-0. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher G, Chassagne D. Possible Causes of Rectosigmoiditis and Sigmoiditis Following Radiotherapy for Cervix. J Radiol Electrol Med Nucl. 1968 Aug - Sept;49(8):639–41. [PubMed] [Google Scholar]
- 19.Haie-Meder C, Potter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiotherapy & Oncology. 2005 Mar;74(3):235–45. doi: 10.1016/j.radonc.2004.12.015. Research Support, Non-U.S. Gov't Review. [DOI] [PubMed] [Google Scholar]
- 20.Potter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiotherapy & Oncology. 2006 Jan;78(1):67–77. doi: 10.1016/j.radonc.2005.11.014. Practice Guideline Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Potter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. International Journal of Radiation Oncology, Biology, Physics. 2007 doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Kirisits C, Potter R, Lang S, Dimopoulos J, Wachter-Gerstner N. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. International Journal of Radiation Oncology, Biology, Physics. 2005 Jul 1;62(3):901–11. doi: 10.1016/j.ijrobp.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Kim RY, Shen S, Duan J. Image-based three-dimensional treatment planning of intracavitary brachytherapy for cancer of the cervix: dose-volume histograms of the bladder, rectum, sigmoid colon, and small bowel. Brachytherapy. 2007 Jul-Sep;6(3):187–94. doi: 10.1016/j.brachy.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Al-Booz H, Ffrrcsi F, Boiangiu I, Frcr M, Appleby H, French C, et al. Sigmoid Colon is an Unexpected Organ at Risk in Brachytherapy for Cervix Cancer. J Egypt Natl Canc Inst. 2006 June;18(2):156–60. [PubMed] [Google Scholar]
- 25.Lindegaard JC, Tanderup K, Nielsen SK, Haack S, Gelineck J. MRI-guided 3D optimization significantly improves DVH parameters of pulsed-dose-rate brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2008 Jul 1;71(3):756–64. doi: 10.1016/j.ijrobp.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 26.De Brabandere M, Mousa AG, Nulens A, Swinnen A, Van Limbergen E. Potential of dose optimisation in MRI-based PDR brachytherapy of cervix carcinoma. Radiother Oncol. 2008 Aug;88(2):217–26. doi: 10.1016/j.radonc.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan AN, Cormack R, Holloway CL, Tanaka C, O'Farrell D, Devlin PM, et al. Magnetic resonance-guided interstitial therapy for vaginal recurrence of endometrial cancer. International Journal of Radiation Oncology, Biology, Physics. 2006 Sep 1;66(1):91–9. doi: 10.1016/j.ijrobp.2006.04.037. Evaluation Studies Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 28.Sturdza A, Berger D, Lang S, Dimopoulos J, Thomas G, Georg P, et al. Uncertainties in assessing sigmoid dose volume parameters in MRI-guided fractionated HDR Brachytherapy. Brachytherapy. 2008;7(2):s109. [Google Scholar]
- 29.Ferrigno R, Nishimoto IN, Novaes PE, Pellizzon AC, Maia MA, Fogarolli RC, et al. Comparison of low and high dose rate brachytherapy in the treatment of uterine cervix cancer. Retrospective analysis of two sequential series. International Journal of Radiation Oncology, Biology, Physics. 2005 Jul 15;62(4):1108–16. doi: 10.1016/j.ijrobp.2004.12.016. Comparative Study. [DOI] [PubMed] [Google Scholar]
- 30.Koom W, Sohn DK, Kim JY, Kim JW, Shin KH. Computed Tomography-Based High-Dose_Rate Intracavitary Brachytherapy for Uterine Cervical Cancer: Preliminary Demonstration of Correlation Between Dose-Volume Parameters and Rectal Mucosal Changes Observed By Flexible Sigmoidoscopy. International Journal of Radiation Oncology, Biology, Physics. 2007 doi: 10.1016/j.ijrobp.2007.02.009. [DOI] [PubMed] [Google Scholar]


