Abstract
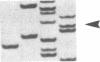
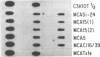
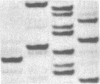
The polymerase chain reaction was used to amplify DNA surrounding the codon 12 region of the c-Ki-ras gene from C3H/10T1/2 cells and from a number of 3-methylcholanthrene (MCA)-transformed derivatives of these cells. Sequence analysis demonstrated that tumorigenic MCAC116/39 cells, known by DNA-mediated transfection to contain an activated c-Ki-ras oncogene, had a G----T transversion in the first position of codon 12 of this gene, resulting in a Gly12----Cys mutation. A combination of polymerase chain-reaction amplification and oligonucleotide hybridization demonstrated that three additional tumorigenic MCA transformants of C3H/10T1/2 cells had an identical mutation in the c-Ki-ras gene. In contrast, this mutation was not present in an MCA-induced C3H/10T 1/2 transformant that was not tumorigenic. The molecular specificity of this MCA-induced mutation resulting in C3H/10T1/2 tumorigenic transformants should provide an excellent system in which to study the roles of transcription, replication, repair, and exogenous factors in the establishment and expression of transformation and tumorigenicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Birren B. W., Taplitz S. J., Herschman H. R. Rat metallothionein-1 structural gene and three pseudogenes, one of which contains 5'-regulatory sequences. Mol Cell Biol. 1986 Jan;6(1):302–314. doi: 10.1128/mcb.6.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bertram J. S., Heidelberger C. Cell cycle dependency of oncogenic transformation induced by N-methyl-N'-nitro-N-nitrosoquanidine in culture. Cancer Res. 1974 Mar;34(3):526–537. [PubMed] [Google Scholar]
- Billings P. C., Shuin T., Lillehaug J., Miura T., Roy-Burman P., Landolph J. R. Enhanced expression and state of the c-myc oncogene in chemically and X-ray-transformed C3H/10T1/2 Cl 8 mouse embryo fibroblasts. Cancer Res. 1987 Jul 15;47(14):3643–3649. [PubMed] [Google Scholar]
- Borek C., Guernsey D. L., Ong A., Edelman I. S. Critical role played by thyroid hormone in induction of neoplastic transformation by chemical carcinogens in tissue culture. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5749–5752. doi: 10.1073/pnas.80.18.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrello M. G., Carbone G., Pierotti M. A., Molla A., Della Porta G. Activated c-K-ras and c-N-ras oncogenes in 3-methylcholanthrene-induced BALB/c fibrosarcomas. Carcinogenesis. 1988 Aug;9(8):1517–1519. doi: 10.1093/carcin/9.8.1517. [DOI] [PubMed] [Google Scholar]
- Chen A. C., Herschman H. R. A continuum of transformed phenotypes in C3H/10T1/2 derivatives. Carcinogenesis. 1988 Sep;9(9):1695–1700. doi: 10.1093/carcin/9.9.1695. [DOI] [PubMed] [Google Scholar]
- DuBridge R. B., Tang P., Hsia H. C., Leong P. M., Miller J. H., Calos M. P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987 Jan;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W., Greenberg D. S., Kaufman D. G., Smith G. J. Cycle-related toxicity and transformation in 10T1/2 cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4813–4817. doi: 10.1073/pnas.77.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernsey D. L., Leuthauser S. W. Correlation of thyroid hormone dose-dependent regulation of K-ras protooncogene expression with oncogene activation by 3-methylcholanthrene: loss of thyroidal regulation in the transformed mouse cell. Cancer Res. 1987 Jun 15;47(12):3052–3056. [PubMed] [Google Scholar]
- Herschman H. R., Brankow D. W. Ultraviolet irradiation transforms C3H10T1/2 cells to a unique, suppressible phenotype. Science. 1986 Dec 12;234(4782):1385–1388. doi: 10.1126/science.3787250. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mondal S., Brankow D. W., Heidelberger C. Two-stage chemical oncogenesis in cultures of C3H/10T1/2 cells. Cancer Res. 1976 Jul;36(7 Pt 1):2254–2260. [PubMed] [Google Scholar]
- Mordan L. J., Bergin L. M., Budnick J. E., Meegan R. R., Bertram J. S. Isolation of methylcholanthrene-"initiated" C3H/10T1/2 cells by inhibiting neoplastic progression with retinyl acetate. Carcinogenesis. 1982;3(3):279–285. doi: 10.1093/carcin/3.3.279. [DOI] [PubMed] [Google Scholar]
- Mordan L. J. Induction by growth factors from platelets of the focus-forming transformed phenotype in carcinogen-treated C3H/10T1/2 fibroblasts. Carcinogenesis. 1988 Jul;9(7):1129–1134. doi: 10.1093/carcin/9.7.1129. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Weinberg R. A. Presence of a Kirsten murine sarcoma virus ras oncogene in cells transformed by 3-methylcholanthrene. Mol Cell Biol. 1983 Dec;3(12):2298–2301. doi: 10.1128/mcb.3.12.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Patterson R. M., Maronpot R. R., Aaronson S. A., Anderson M. W. Activated oncogenes in B6C3F1 mouse liver tumors: implications for risk assessment. Science. 1987 Sep 11;237(4820):1309–1316. doi: 10.1126/science.3629242. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. J., Grisham J. W. Activation of the Ha-ras gene in C3H 10T1/2 cells transformed by exposure to N-methyl-N'-nitro-N-nitrosoguanidine. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1194–1199. doi: 10.1016/s0006-291x(87)80196-1. [DOI] [PubMed] [Google Scholar]
- Stowers S. J., Wiseman R. W., Ward J. M., Miller E. C., Miller J. A., Anderson M. W., Eva A. Detection of activated proto-oncogenes in N-nitrosodiethylamine-induced liver tumors: a comparison between B6C3F1 mice and Fischer 344 rats. Carcinogenesis. 1988 Feb;9(2):271–276. doi: 10.1093/carcin/9.2.271. [DOI] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]