Abstract
The synthesis and evaluation of a key series of analogs of duocarmycin SA, bearing a single substituent at the C5’ position of the DNA binding subunit, are described.
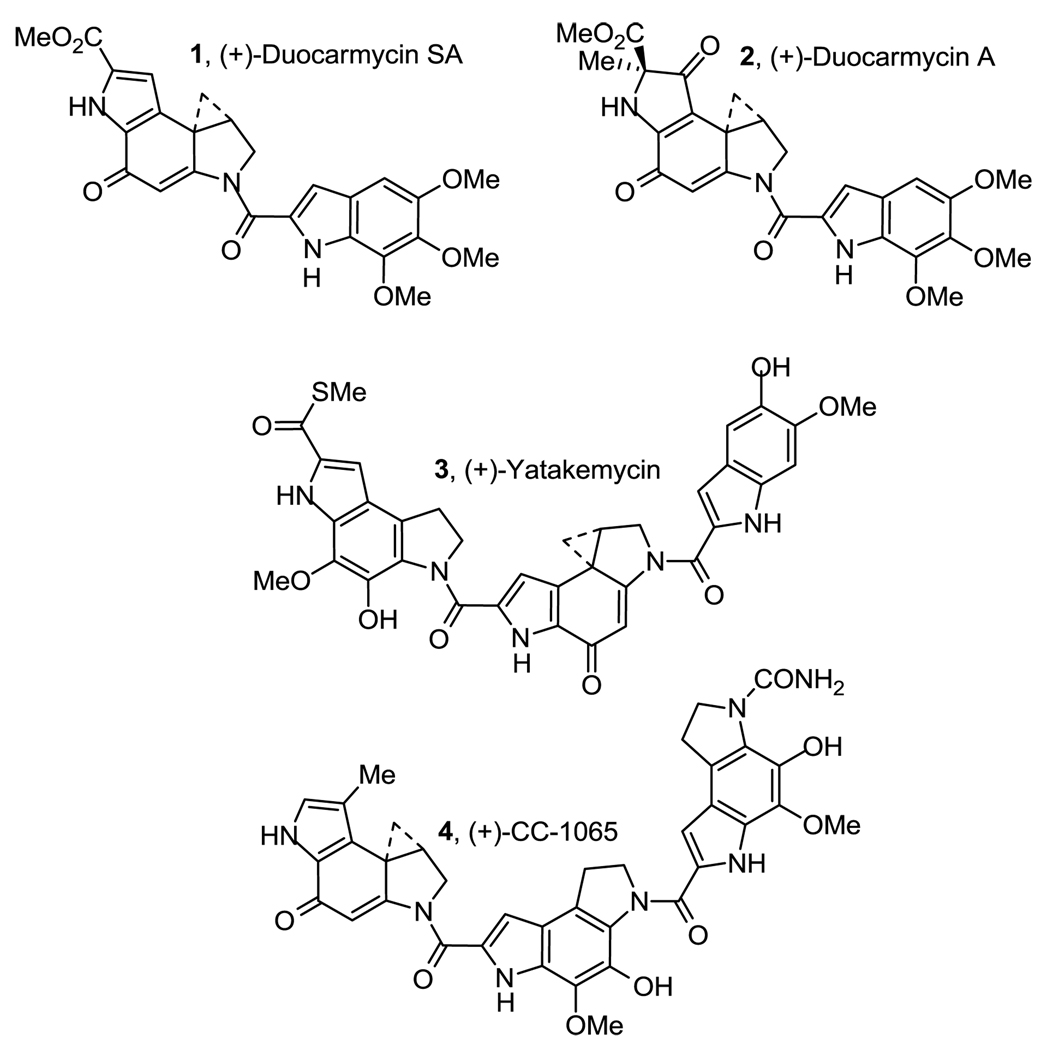
Duocarmycin SA (1, IC50 = 10 pM)1 represents one of the most cytotoxic members of a class of natural products that also include duocarmycin A (2),2 yatakemycin (3),3 and CC-1065 (4, Figure 1).4 Members of this family of natural products derive their biological properties from a characteristic sequence-selective alkylation of duplex DNA,5–9 in which a stereoelectronically controlled nucleophilic addition of adenine occurs at the least substituted carbon of the activated cyclopropane within the deeper, narrower minor groove at AT-rich sites in DNA.
Figure 1.
Natural products.
Extensive investigations on the natural products in this class as well as their synthetic analogs have defined key and subtle features that contribute to their properties.9,10 Most notable of these are the structural features that contribute to the AT-rich noncovalent binding selectivity dominating the alkylation selectivity,11 those that are responsible for the source of catalysis for the DNA alkylation reaction,12,13 and those that subtly impact the unusual and intrinsic stability of their alkylation subunits.9,13–17
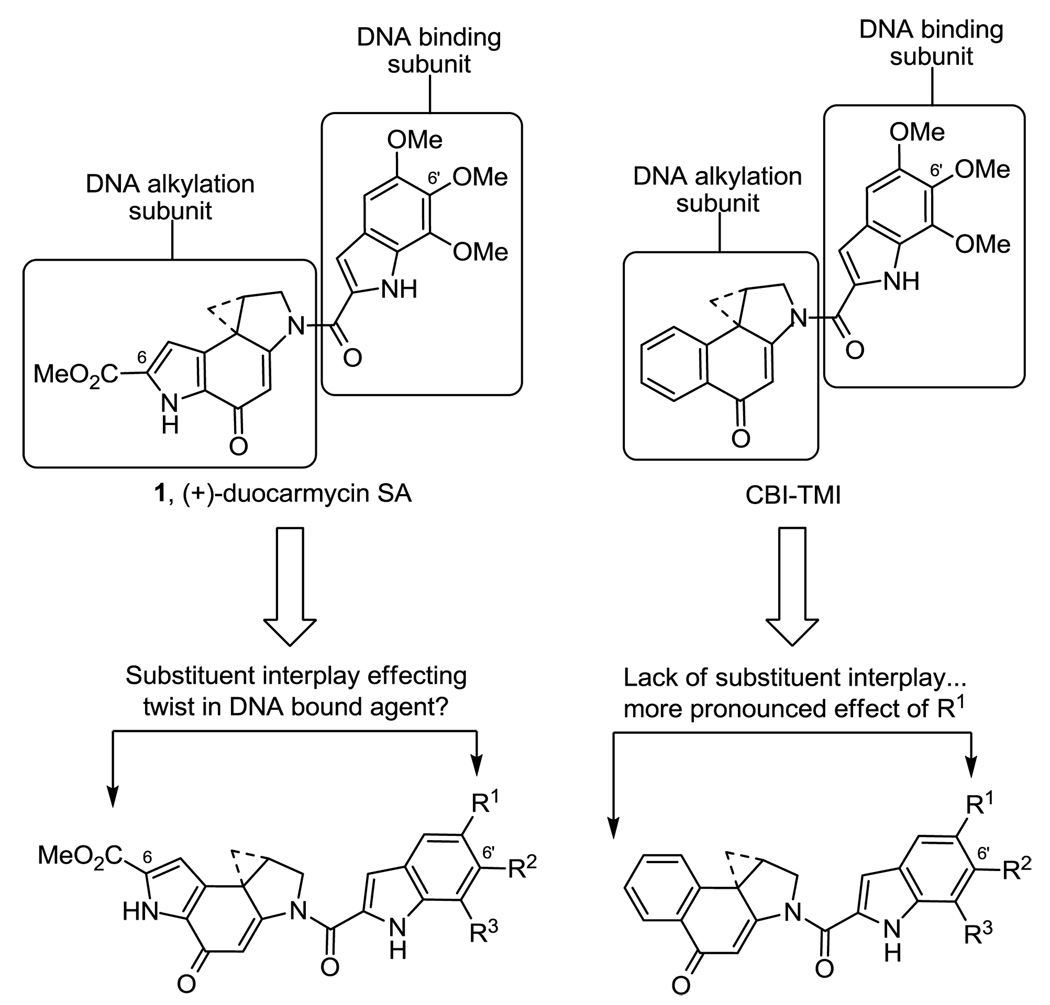
Studies have shown that the role of the attached DNA binding subunit goes beyond that of simply providing DNA binding affinity and selectivity,11 but that it also contributes to and is largely responsible for the DNA alkylation catalysis.12,18 We have suggested that this catalysis is derived from a DNA binding induced conformational change in the compound that serves to disrupt the vinylogous amide stabilization of the alkylation subunit activating it for nucleophilic attack. Responsible for this conformational change and the resulting activation is the rigid extended and planar structures of both the alkylation subunit and the attached DNA binding subunit. The DNA bound molecule adopts a bent versus planar conformation following the overall curvature and helical pitch of the minor groove with the twist accommodated by the region surrounding the linking amide, the only flexible portion of the molecule.12,13,19 Both the C-terminus substituent (C6 methyl ester for 1) on the alkylation subunit and the minor groove imbedded C5’ substituent on the DNA binding subunit contribute to the effective overall rigid length of the compounds. Hence, both can impact the extent of the conformational change or twist in the DNA bound compound, the degree of activation for nucleophilic attack, and the extent of the catalysis of the DNA alkylation reaction. As a result, minor groove bound substituents on both the DNA binding subunit and the alkylation subunit each have been shown to have a pronounced effect on the rate and efficiency of DNA alkylation and the resulting biological potency of the compounds. It has been shown that the substituent at the C5’ position of the indole DNA binding subunit of duocarmycin SA is of predominant importance, and that it can alone provide a fully active agent with little or no contribution derived from the C6’ and C7’ substituents found in duocarmycin A and duocarmycin SA (Figure 2).12 Similarly, the removal of the C6 methyl ester from the alkylation subunit of duocarmycin SA was shown to reduce the rate (12–13 fold) and efficiency (10-fold) of DNA alkylation illustrating an important role for the minor groove imbedded substituent.15v Additionally, and as first systematically explored with CBI derivatives, the nature of the C5’ substituent proved to have pronounced impact that correlated with its presence, size, rigidity and length, but not electronic properties.18 Herein, we systematically explore the importance of the C5’ substituent within duocarmycin SA analogs additionally bearing the minor groove imbedded C6 methyl ester.
Figure 2.
(+)-Duocarmycin SA and CBI analog series.
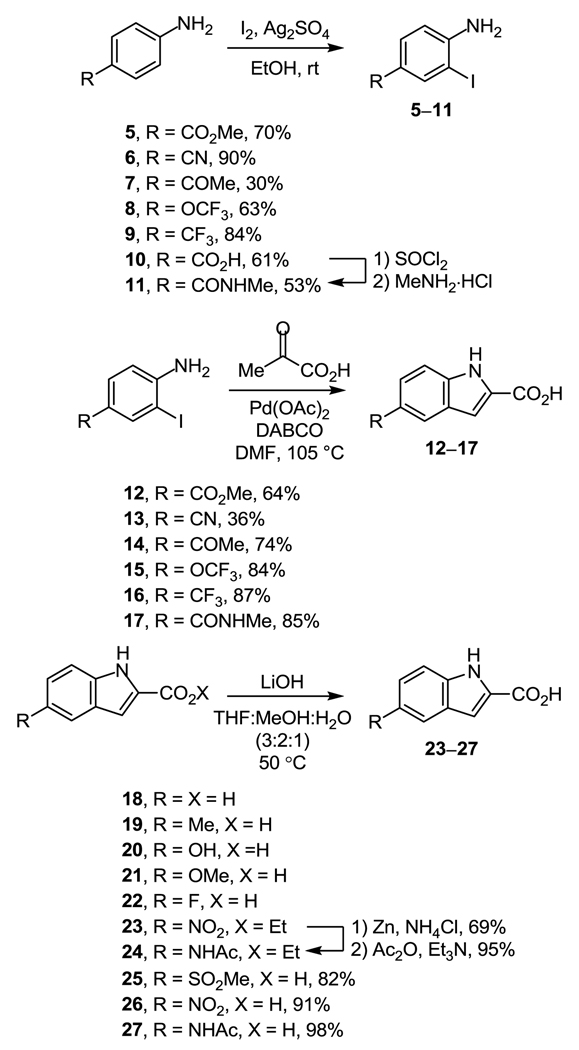
The synthesis of the duocarmycin SA alkylation subunit was carried out by a sequence first developed for the total synthesis of duocarmycin SA,20 which relied on a late-stage chiral resolution to provide optically active material. In most cases, a synthesis of the C5-substituted indole-2-carboxylic acids was required to obtain the appropriately substituted DNA binding subunit. Their synthesis was initially pursued using a Hemetsberger indole synthesis21 followed by saponification of the methyl indole-2-carboxylate to provide the desired indole-2-carboxylic acid. However, the Hemetsberger reaction, enlisting 3-substituted benzaldehydes, often produced a near 1:1 mixture (by 1H NMR) of the C5- and C7-substituted indoles and attempts to separate these regioisomers by chromatography proved challenging. Alternatively, a route employing a palladium-catalyzed indole-forming reaction22 to regioselectively access the desired C5-substituted indole-2-carboxylic acids was explored and proved to be a synthetically attractive route (Scheme 1).
Scheme 1.
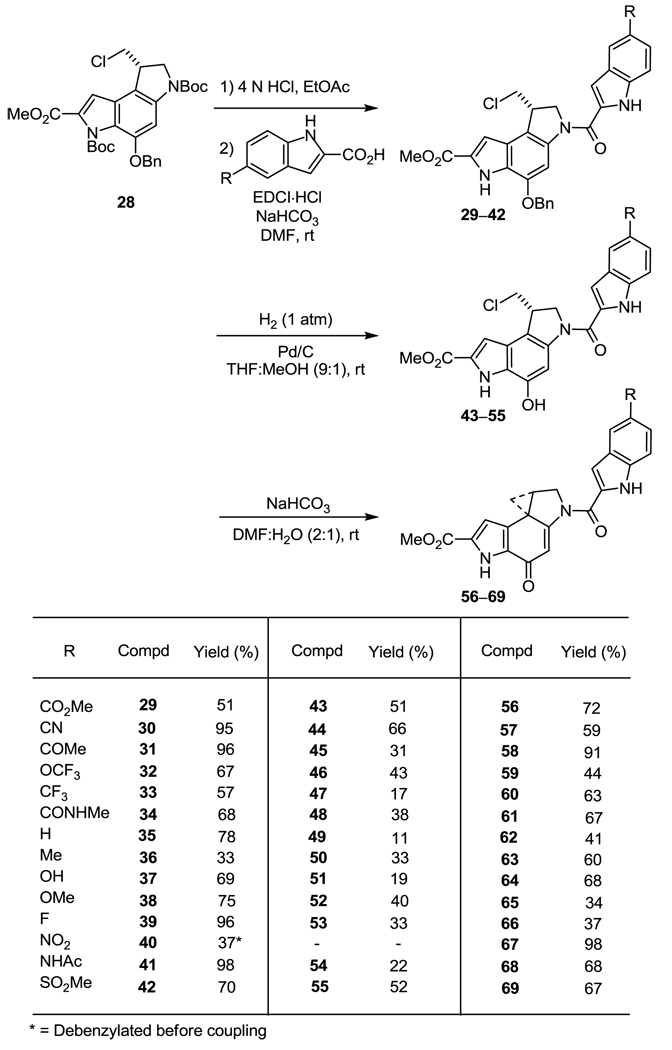
Starting with the commercially available 4-substituted anilines, electrophilic aromatic iodination (1.0 equiv I2, 1.0 equiv Ag2SO4, EtOH, rt, 30–60 min, 30–90%)23 cleanly afforded the 4-substituted-2-iodoanilines (5–11). Treatment of the iodoanilines 5–11 with pyruvic acid (3.0 equiv), DABCO (3.0 equiv), and Pd(OAc)2 (0.05 equiv) at 105 °C for 4 h in a thoroughly degassed solution of DMF cleanly provided the desired C5-substituted indole-2-carboxylic acids (12–17, 36–86%) as a single regioisomeric product.22 Most C5-substituted indole-2-carboxylic acids were prepared by this method, with a few purchased from commercially available sources either as the free acid (18–22) or obtained by hydrolysis of the corresponding methyl or ethyl esters (23–27) (10 equiv LiOH, THF:MeOH:H2O 3:2:1, 50 °C, 2 h, 57–91%). Boc deprotection of enantiopure 2820 was achieved by treatment with 4 N HCl/EtOAc (18 h, 25 °C) to afford the crude amine hydrochloride salt. Without purification, subsequent coupling with the C5-substituted indole-2-carboxylic acid (1.4 equiv, 4.0 equiv EDCI, 2.0 equiv NaHCO3, DMF, rt, 30–45 min, 19–98%) provided the benzyl protected coupled products 29–42 (Scheme 2).
Scheme 2.
Hydrogenolysis of the benzyl ether to liberate the free phenol (10% Pd/C, 1 atm H2, THF/MeOH, rt, 2 h, 11–66%) afforded derivatives 43–55. In the case of 40, debenzylation of 28 (10% Pd/C, 25% aqueous HCO2NH4, THF, rt, 2 h, 28%) prior to Boc deprotection and coupling was employed to avoid competitive reduction of the nitro group under the hydrogenolysis conditions (Scheme 2). Finally, spirocyclization was achieved with relative ease (1 equiv NaHCO3, DMF, H2O, rt, 30 min, 33–98%) to provide 56–69 (Scheme 2).
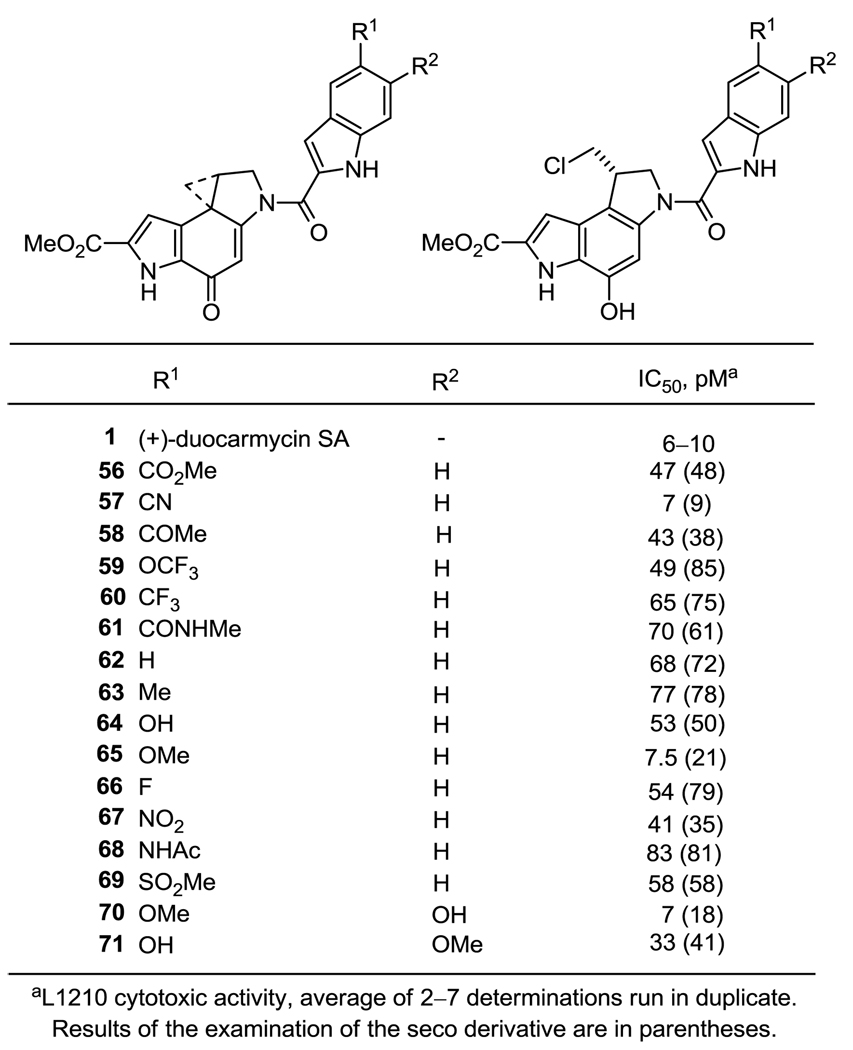
The results of the examination of the C5’-substituted (+)-duocarmycin SA analogs in a L1210 cytotoxic assay are summarized in Figure 3.
Figure 3.
In vitro cytotoxic activity, L1210
From these data all analogs, except 65 (R = OMe) and 57 (R = CN), exhibit approximately equal potency (IC50 = 40–80 pM), all being ≤ 10-fold less potent than (+)-duocarmycin SA, (1, IC50 = 6–10 pM). Additionally and according to expectations, the spiro and seco analogs containing the same C5’-substituent on the DNA binding subunit exhibited equal potency and were essentially indistinguishable. This indicates that the seco analogs, regardless of the C5’-substituent, are able to cyclize in situ to the corresponding cyclopropane, the active DNA alkylating agent. Contrary to initial expectations, all C5’-substituted analogues with the exception of 65 and 57, were found to exhibit an approximately 10-fold decrease in cytotoxic potency as compared to 1 and to approach the potency of 62 lacking a C5’ substituent altogether. This is in contrast to observations18 made for the series of C5’-substituted analogs containing the CBI alkylation subunit, where the presence of a simple C5’ substituent, regardless of that substituent’s electronic nature, typically produced analogs that substantially increased the activity (as much as 100-fold) and some that matched or even exceeded the potency of 1 (1000-fold increase). This suggests that within the duocarmycin SA structure that already incorporates a more extended alkylation subunit and its minor groove imbedded C6 methyl ester, the addition of a simple indole C5’ substituent typically does little to further enhance its properties. The exceptions to this generalization are 65 and 57, incorporating a C5’ methoxy group or nitrile, respectively. The former incorporates a substituent (OMe) found in the natural products themselves, and it is significantly more potent than even its most closely related derivative (OH, OCF3). The latter incorporates an electron-withdrawing versus electron-donating substituent, and one that is sterically small, but extended linearly. Because other electron-withdrawing substituents proved noticeably less potent, it does not appear that it is electronic properties of these two substituents that are responsible for their enhanced activity. Additional potential contributing features including hybridization (sp2 vs sp3) or hydrogen bond donor or acceptor properties do not appear to account for the unique properties of 65 and 57. Although it is easy to rationalize the observations that a C5’ substituent does not have such a significant impact with duocarmycin SA (10-fold difference between 1 and 62), at present it is difficult to rationalize the origin of the observations with the two unique substituents (OMe, CN) that significantly impact the activity.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA041986) and the Skaggs Institute for Chemical Biology. WMR and DBK were Skaggs Fellows.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data. Full experimental details are provided. The supplementary data associated with this article can be found in the online version at doi:
References and notes
- 1.Ichimura M, Ogawa T, Takahashi K, Kobayashi E, Kawamoto I, Yasuzawa T, Takahashi I, Nakano H. J. Antibiot. 1990;43:1037. doi: 10.7164/antibiotics.43.1037. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi I, Takahashi K, Ichimura M, Morimoto M, Asano K, Kawamoto I, Tomita F, Nakano H. J. Antibiot. 1988;41:1915. doi: 10.7164/antibiotics.41.1915. [DOI] [PubMed] [Google Scholar]
- 3. Igarashi Y, Futamata K, Fujita T, Sekine A, Senda H, Naoki H, Furumai T. J. Antibiot. 2003;56:107. doi: 10.7164/antibiotics.56.107. Structure revision: Tichenor MS, Kastrinsky DB, Boger DL. J. Am. Chem. Soc. 2004;126:8396. doi: 10.1021/ja0472735.
- 4.Martin DG, Biles C, Gerpheide SA, Hanka LJ, Krueger WC, McGovren JP, Mizsak SA, Neil GL, Stewart JC, Visser J. J. Antibiot. 1981;34:1119. doi: 10.7164/antibiotics.34.1119. [DOI] [PubMed] [Google Scholar]
- 5.Boger DL, Johnson DS, Yun W. J. Am. Chem. Soc. 1994;116:1635. [Google Scholar]
- 6.(a) Parrish JP, Kastrinsky DB, Wolkenberg SE, Igarishi Y, Boger DL. J. Am. Chem. Soc. 2003;125:10971. doi: 10.1021/ja035984h. [DOI] [PubMed] [Google Scholar]; (b) Trzupek JD, Gottesfeld JM, Boger DL. Nature Chem. Biol. 2006;2:79. doi: 10.1038/nchembio761. [DOI] [PubMed] [Google Scholar]; (c) Tichenor MS, Trzupek JD, Kastrinsky DB, Shiga F, Hwang I, Boger DL. J. Am. Chem. Soc. 2006;128:15683. doi: 10.1021/ja064228j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tichenor MS, MacMillan KS, Trzupek JD, Rayl TJ, Hwang I, Boger DL. J. Am. Chem. Soc. 2007;129:10858. doi: 10.1021/ja072777z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Hurley LH, Lee C-S, McGovren JP, Warpehoski MA, Mitchell MA, Kelly RC, Aristoff PA. Biochemistry. 1988;27:3886. doi: 10.1021/bi00410a054. [DOI] [PubMed] [Google Scholar]; (b) Hurley LH, Warpehoski MA, Lee C-S, McGovren JP, Scahill TA, Kelly RC, Mitchell MA, Wicnienski NA, Gebhard I, Johnson PD, Bradford VS. J. Am. Chem. Soc. 1990;112:4633. [Google Scholar]; (c) Boger DL, Johnson DS, Yun W, Tarby CM. Bioorg. Med. Chem. 1994;2:115. doi: 10.1016/s0968-0896(00)82007-6. [DOI] [PubMed] [Google Scholar]; (d) Boger DL, Coleman RS, Invergo BJ, Sakya SM, Ishizaki T, Munk SA, Zarrinmayeh H, Kitos PA, Thompson SC. J. Am. Chem. Soc. 1990;112:4623. [Google Scholar]; (e) Boger DL, Munk SA, Zarrinmayeh H, Ishizaki T, Haught J, Bina M. Tetrahedron. 1991;47:2661. [PubMed] [Google Scholar]
- 8.(a) Boger DL, Ishizaki T, Zarrinmayeh H, Munk SA, Kitos PA, Suntornwat O. J. Am. Chem. Soc. 1990;112:8961. [Google Scholar]; (b) Boger DL, Ishizaki T, Zarrinmayeh H. J. Am. Chem. Soc. 1991;113:6645. [Google Scholar]; (c) Boger DL, Yun W, Terashima S, Fukuda Y, Nakatani K, Kitos PA, Jin Q. Bioorg. Med. Chem. Lett. 1992;2:759. [Google Scholar]; (d) Boger DL, Yun W. J. Am. Chem. Soc. 1993;115:9872. [Google Scholar]; (e) Boger DL, Wysocki RJ, Ishisaki T. J. Am. Chem. Soc. 1990;112:5230. [Google Scholar]; (f) Boger DL, Ishizaki T, Zarrinmayeh H, Kitos PA, Suntornwat O. J. Org. Chem. 1990;55:4499. [Google Scholar]; (g) Boger DL, McKie JA, Nishi T, Ogiku T. J. Am. Chem. Soc. 1997;119:311. [Google Scholar]
- 9.Reviews: Boger DL, Johnson DS. Angew. Chem. Int. Ed. Engl. 1996;35:1438. Boger DL. Acc. Chem. Res. 1995;28:20. Boger DL, Johnson DS. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3642. doi: 10.1073/pnas.92.9.3642. Boger DL, Garbaccio RM. Acc. Chem. Res. 1999;32:1043. Tichenor MS, Boger DL. Natural Prod. Rep. 2008;25:220. doi: 10.1039/b705665f. MacMillan KS, Boger DL. J. Med. Chem. 2009;52:5771. doi: 10.1021/jm9006214.
- 10.Warpehoski MA, Gebhard I, Kelly RC, Krueger WC, Li L, McGovern JP, Praire MD, Wienienski N, Wierenga W. J. Med. Chem. 1988;31:590. doi: 10.1021/jm00398a017. [DOI] [PubMed] [Google Scholar]
- 11.(a) Boger DL, Coleman RS, Invergo BJ, Zarrinmayeh H, Kitos PA, Thompson SC, Leong T, McLaughlin LW. Chem.-Biol. Interact. 1990;73:29. doi: 10.1016/0009-2797(90)90107-x. [DOI] [PubMed] [Google Scholar]; (b) Boger DL, Zarrinmayeh H, Munk SA, Kitos PA, Suntornwat O. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1431. doi: 10.1073/pnas.88.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Boger DL, Munk SA, Zarrinmayeh H. J. Am. Chem. Soc. 1991;113:3980. [Google Scholar]; (d) Boger DL, Johnson DS. J. Am. Chem. Soc. 1995;117:1443. [Google Scholar]; (e) Boger DL, Zhou J, Cai H. Bioorg. Med. Chem. 1996;4:859. doi: 10.1016/0968-0896(96)00073-9. [DOI] [PubMed] [Google Scholar]
- 12.(a) Boger DL, Bollinger B, Hertzog DL, Johnson DS, Cai H, Mesini P, Garbaccio RM, Jin Q, Kitos PA. J. Am. Chem. Soc. 1997;119:4987. [Google Scholar]; (b) Boger DL, Hertzog DL, Bollinger B, Johnson DS, Cai H, Goldberg J, Turnbull P. J. Am. Chem. Soc. 1997;119:4977. [Google Scholar]; (c) Boger DL, Bollinger B, Johnson DS. Bioorg. Med. Chem. Lett. 1996;6:2207. [Google Scholar]
- 13.(a) Boger DL, Garbaccio RM. Bioorg. Med. Chem. 1997;5:263. doi: 10.1016/s0968-0896(96)00238-6. [DOI] [PubMed] [Google Scholar]; (b) Ambroise Y, Boger DL. Bioorg. Med. Chem. Lett. 2002;12:303. doi: 10.1016/s0960-894x(01)00740-5. [DOI] [PubMed] [Google Scholar]; (c) Boger DL, Santillan A, Jr, Searcey M, Jin Q. J. Am. Chem. Soc. 1998;120:11554. [Google Scholar]; (d) Boger DL, Garbaccio RM. J. Org. Chem. 1999;64:5666. doi: 10.1021/jo990762g. [DOI] [PubMed] [Google Scholar]; (e) Boger DL, Santillian A, Jr, Searcey M, Jin Q. J. Org. Chem. 1999;64:5241. doi: 10.1021/jo990452y. [DOI] [PubMed] [Google Scholar]
- 14.Reviews: Wolkenberg SE, Boger DL. Chem. Rev. 2002;102:2477. doi: 10.1021/cr010046q. Tse WC, Boger DL. Chem. Biol. 2004;11:1607. doi: 10.1016/j.chembiol.2003.08.012. Tse WC, Boger DL. Acc. Chem. Res. 2004;37:61. doi: 10.1021/ar030113y.
- 15.(a) Boger DL, Coleman RS. J. Am. Chem. Soc. 1988;110:1321 and 4796. [Google Scholar]; (b) Boger DL, Ishizaki T, Wysocki RJ, Jr, Munk SA, Kitos PA, Suntornwat O. J. Am. Chem. Soc. 1989;111:6461. [Google Scholar]; (c) Boger DL, Wysocki RJ., Jr J. Org. Chem. 1989;54:1238. [Google Scholar]; (d) Boger DL, Ishizaki T. Tetrahedron Lett. 1990;31:793. [Google Scholar]; (e) Boger DL, Wysocki RJ, Jr, Ishizaki T. J. Am. Chem. Soc. 1990;112:5230. [Google Scholar]; (f) Boger DL, Ishizaki T, Kitos PA, Suntornwat O. J. Org. Chem. 1990;55:5823. [Google Scholar]; (g) Boger DL, Munk SA, Ishizaki T. J. Am. Chem. Soc. 1991;113:2779. [Google Scholar]; (h) Boger DL, Munk SA. J. Am. Chem. Soc. 1992;114:5487. [Google Scholar]; (i) Boger DL, Yun W. J. Am. Chem. Soc. 1994;116:5523. [Google Scholar]; (j) Boger DL, Mesini P, Tarby CM. J. Am. Chem. Soc. 1994;116:6461. [Google Scholar]; (k) Boger DL, Mesini P. J. Am. Chem. Soc. 1994;116:11335. [Google Scholar]; (l) Mohamadi F, Spees MM, Staten GS, Marder P, Kipka JK, Johnson DA, Boger DL, Zarrinmayeh H. J. Med. Chem. 1994;37:232. doi: 10.1021/jm00028a005. [DOI] [PubMed] [Google Scholar]; (m) Boger DL, Yun W. J. Am. Chem. Soc. 1994;116:7996. [Google Scholar]; (n) Boger DL, McKie JA, Cai H, Cacciari B, Baraldi PG. J. Org. Chem. 1996;61:1710. doi: 10.1021/jo952033g. [DOI] [PubMed] [Google Scholar]; (o) Boger DL, Han N, Tarby CM, Boyce CW, Cai H, Jin Q, Kitos PA. J. Org. Chem. 1996;61:4894. [Google Scholar]; (p) Boger DL, Jenkins T. J. Am. Chem. Soc. 1996;118:8860. [Google Scholar]; (q) Boger DL, Turnbull P. J. Org. Chem. 1997;62:5849. [Google Scholar]; (r) Boger DL, Garbaccio RM, Jin Q. J. Org. Chem. 1997;62:8875. [Google Scholar]; (s) Boger DL, Turnbull P. J. Org. Chem. 1998;63:8004. [Google Scholar]; (t) Boger DL, Garbaccio RM. J. Org. Chem. 1999;64:8350. doi: 10.1021/jo991301y. [DOI] [PubMed] [Google Scholar]; (u) Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Boyce CW, Boger DL. Bioorg. Med. Chem. Lett. 1999;9:3087. doi: 10.1016/s0960-894x(99)00533-8. [DOI] [PubMed] [Google Scholar]; (v) Boger DL, Wolkenberg SE, Boyce CW. J. Am. Chem. Soc. 2000;122:6325. [Google Scholar]; (w) Boger DL, Santillian A, Jr, Searcey M, Brunette SR, Wolkenberg SE, Hedrick MP, Jin Q. J. Org. Chem. 2000;65:4101. doi: 10.1021/jo000297j. [DOI] [PubMed] [Google Scholar]; (x) Boger DL, Boyce CW. J. Org. Chem. 2000;65:4088. doi: 10.1021/jo000177b. [DOI] [PubMed] [Google Scholar]; (y) Ellis DA, Wolkenberg SE, Boger DL. J. Am. Chem. Soc. 2001;123:9299. doi: 10.1021/ja010769r. [DOI] [PubMed] [Google Scholar]; (z) Boger DL, Hughes TV, Hedrick MP. J. Org. Chem. 2001;66:2207. doi: 10.1021/jo001772g. [DOI] [PubMed] [Google Scholar]; (aa) Boger DL, Brunette SR, Garbaccio RM. J. Org. Chem. 2001;66:5163. doi: 10.1021/jo010309g. [DOI] [PubMed] [Google Scholar]; (bb) Parrish JP, Kastrinsky DB, Boger DL. Org. Lett. 2003;5:2577. doi: 10.1021/ol035000t. [DOI] [PubMed] [Google Scholar]; Parrish JP, Kastrinsky DB, Hwang I, Boger DL. J. Org. Chem. 2003;68:8984. doi: 10.1021/jo035119f. [DOI] [PubMed] [Google Scholar]; (cc) MacMillan KS, Boger DL. J. Am. Chem. Soc. 2008;130:16521. doi: 10.1021/ja806593w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (dd) MacMillan KS, Nguyen T, Hwang I, Boger DL. J. Am. Chem. Soc. 2009;131:1187. doi: 10.1021/ja808108q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (ee) Gauss CM, Hamasaki A, Parrish JP, MacMillan KS, Rayl TJ, Hwang I, Boger DL. Tetrahedron. 2009;65:6591. doi: 10.1016/j.tet.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]; (ff) Boyle KE, MacMillan KS, Ellis DA, Lajiness JP, Robertson WM, Boger DL. Bioorg. Med. Chem. Lett. 2010;20:1854. doi: 10.1016/j.bmcl.2010.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Parrish JP, Hughes TV, Hwang I, Boger DL. J. Am. Chem. Soc. 2004;126:80. doi: 10.1021/ja038162t. [DOI] [PubMed] [Google Scholar]; (b) Parrish JP, Trzupek JD, Hughes TV, Hwang I, Boger DL. Bioorg. Med. Chem. 2004;12:5845. doi: 10.1016/j.bmc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 17.(a) MacMillan KS, Lajiness JP, Cara CL, Romagnoli R, Robertson WM, Hwang I, Baraldi PG, Boger DL. Bioorg. Med. Chem. Lett. 2009;19:6962. doi: 10.1016/j.bmcl.2009.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tichenor MS, MacMillan KS, Stover JS, Wolkenberg SE, Pavani MG, Zanella L, Zaid AN, Spalluto G, Rayl TJ, Hwang I, Baraldi PG, Boger DL. J. Am. Chem. Soc. 2007;129:14092. doi: 10.1021/ja073989z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Parrish JP, Kastrinsky DB, Stauffer F, Hedrick MP, Hwang I, Boger DL. Bioorg. Med. Chem. 2003;11:3815. doi: 10.1016/s0968-0896(03)00194-9. [DOI] [PubMed] [Google Scholar]; (b) Boger DL, Stauffer F, Hedrick MP. Bioorg. Med. Chem. Lett. 2001;11:2021. doi: 10.1016/s0960-894x(01)00372-9. [DOI] [PubMed] [Google Scholar]; (c) Boger DL, Schmitt H, Fink BF, Hedrick MP. J. Org. Chem. 2001;66:6654. doi: 10.1021/jo010454u. [DOI] [PubMed] [Google Scholar]
- (a) Schnell JR, Ketchem RR, Boger DL, Chazin WJ. J. Am. Chem. Soc. 1999;121:5645. [Google Scholar]; (b) Eis PS, Smith JA, Rydzewski JM, Case DA, Boger DL, Chazin WJ. J. Mol. Biol. 1997;272:237. doi: 10.1006/jmbi.1997.1223. [DOI] [PubMed] [Google Scholar]; (c) Smith JA, Bifulco G, Case DA, Boger DL, Gomez-Paloma L, Chazin WJ. J. Mol. Biol. 2000;300:1195. doi: 10.1006/jmbi.2000.3887. [DOI] [PubMed] [Google Scholar]; (d) Bassarello C, Cimino P, Bifulco G, Boger DL, Smith JA, Chazin WJ, Paloma LG. ChemBioChem. 2003;4:1188. doi: 10.1002/cbic.200300642. [DOI] [PubMed] [Google Scholar]
- 20. Boger DL, Machiya K. J. Am. Chem. Soc. 1992;114:10056. Boger DL, Machiya K, Hertzog DL, Kitos PA, Holmes D. J. Am. Chem. Soc. 1993;115:9025. (c) Review: Boger DL, Boyce CW, Garbaccio RM, Goldberg JA. Chem. Rev. 1997;97:787. doi: 10.1021/cr960095g.
- 21.(a) Hemetsberger H, Knittel D, Weidmann H. Montash. Chem. 1969;100:1599. [Google Scholar]; (b) Henn L, Hickey DMB, Moody CJ, Rees CW. J. Chem. Soc., Perkin Trans. 1. 1984:2189. [Google Scholar]; (c) Hickey DMB, MacKenzie AR, Moody CJ, Rees CW. J. Chem. Soc., Perkin Trans. 1. 1987:921. [Google Scholar]
- 22.Chen C-Y, Lieberman DR, Larsen RD, Verhoeven TR, Reider PJ. J. Org. Chem. 1997;62:2676. doi: 10.1021/jo970278i. [DOI] [PubMed] [Google Scholar]
- 23.Sy W-W. Syn. Commun. 1992;22:3215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.