Abstract
The objective of this study was to determine whether the previously observed effects of photoperiod on body weight in Siberian hamsters were due to changes in the daily patterns of locomotor activity, energy expenditure, and/or feeding behavior. Adult males were monitored through a seasonal cycle using an automated comprehensive laboratory animal monitoring system (CLAMS). Exposure to a short-day photoperiod (SD; 8:16-h light-dark cycle) induced a significant decline in body weight, and oxygen consumption (V̇o2), carbon dioxide production (V̇co2), and heat production all decreased reaching a nadir by 16 wk of SD. Clear daily rhythms in locomotor activity, V̇o2, and V̇co2 were observed at the start of the study, but these all progressively diminished after prolonged exposure to SD. Rhythms in feeding behavior were also detected initially, reflecting an increase in meal frequency but not duration during the dark phase. This rhythm was lost by 8 wk of SD exposure such that food intake was relatively constant across dark and light phases. After 18 wk in SD, hamsters were transferred to a long-day photoperiod (LD; 16:8-h light-dark cycle), which induced significant weight gain. This was associated with an increase in energy intake within 2 wk, while V̇o2, V̇co2, and heat production all increased back to basal levels. Rhythmicity was reestablished within 4 wk of reexposure to long days. These results demonstrate that photoperiod impacts on body weight via complex changes in locomotor activity, energy expenditure, and feeding behavior, with a striking loss of daily rhythms during SD exposure.
Keywords: daily rhythms, seasonality, Siberian hamster, locomotor activity, energy expenditure, feeding behavior
innate rhythmicity is a fundamental element of an organism's biology, allowing it to anticipate predictable changes in the external environment. Such endogenous control is exemplified by annual rhythmicity in the Siberian hamster, which ensures the optimal behavioral and physiological strategies are engaged to survive winter (13). This species reduces food intake in anticipation of winter, and loses a significant degree of body weight, which largely reflects catabolism of intra-abdominal fat reserves (3). The Siberian hamster also displays torpor in winter, a lowering of body temperature and metabolic rate for several hours at a time, decreasing the caloric needs of the animal when at its nadir of body weight cycle (16, 21). Despite research on metabolic physiology in this species spanning four decades, it remains controversial as to whether the primary cause for body weight loss is reduced energy intake, increased energy expenditure, or decreased assimilation efficiency. Some studies have recorded decreased food intake prior to changes in energy metabolism when hamsters are exposed to short photoperiods (19), whereas other researchers have been unable to temporally separate food intake, energy intake/assimilation, and body weight (33). An initial objective of the current study was to use a contemporary apparatus to simultaneously measure parameters of energy intake and expenditure to determine whether they could be temporally separated during a photoperiodically regulated cycle of weight loss and gain.
Circadian oscillators not only regulate metabolic and behavioral processes across the day-night cycle in hamsters and most other species, but also play a key role in photoperiodic time measurement, such that the annual rhythms in energy metabolism and reproduction are synchronized by the changes in ambient day length (22). Studies of daily and circadian rhythmicity in rodents have largely relied upon recording of wheel-running behavior (1, 24, 26). This voluntary form of activity is very amenable to continuous long-term recordings, but it is not necessarily a direct reflection of the overall pattern of locomotor activity that would occur in a home cage environment, as it clearly has some self-rewarding properties. Daily changes in the other parameters that contribute to the net changes in body weight, namely feeding behavior and metabolic rate, have generally been overlooked as they have been more difficult to quantify over extended periods. However, technological advances in open loop indirect calorimetry systems now provide the opportunity to study these parameters across multiple day-night cycles (18). Moreover, some studies have found that provision of a running wheel in a home cage confounds the development of the physiological state induced by short days in seasonal rodents (7, 30), although this is not universally the case (32). A second objective of the current study was, therefore, to monitor profiles of locomotor activity in the absence of a running wheel by using disturbance of infrared beams. Since little is known about the daily organization of other aspects of energy expenditure over the seasonal cycle, other than observations of torpor bouts using radiotelemetry, this study aimed to characterize temporal profiles of energy expenditure during a seasonal cycle of body weight loss and gain. Finally, although it has been demonstrated that Siberian hamsters show a short-day induced decline in overall food intake (12), the actual changes in the pattern of food intake over a 24-h period have not been identified. Therefore, this study also assessed the temporal profiles of feeding behavior.
MATERIALS AND METHODS
Animals.
All animal procedures were approved by the University of Nottingham Local Ethical Review Committee and were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 (project license PPL 40/3065). Eight male Siberian hamsters (Phodopus sungorus) of ∼3 mo of age, obtained from an in-house bred colony (11), were initially maintained on long photoperiod (16:8-h light-dark cycle, hereafter 16L:8D)—lights off at 1100, lights on at 1900—in a temperature-controlled (21 ± 1°C) holding room. Our entire colony of hamsters are on a reversed photoperiod with darkness during the human day to facilitate behavioral observations during the nocturnal phase. The hamsters were fed ad libitum on a 9% protein-extruded laboratory chow (Teklad 2019; Harlan, Loughborough, UK). The same diet was coarsely ground into a rough powder during the calorimetry studies. At week 0, the light cycle in the holding room was switched to short days (8:16-h light-dark cycle, hereafter 8L:16D), with lights off at 1100 and lights on at 0300. At week 18, the lighting was returned to the long-photoperiod regime (16L:8D). Body weight and pelage were measured weekly. Pelage color was evaluated on a nominal scale ranging from 4 (dark summer fur) to 1 (white winter fur) (10).
Comprehensive lab animal monitoring system.
Multiple energy expenditure and feeding behavior parameters were measured using a comprehensive lab animal monitoring system (CLAMS) (Linton Instrumentation, Linton, UK, and Columbus Instruments, Columbus, OH), a modified open-circuit calorimeter, configured for Siberian hamsters. This consisted of eight mouse chambers, where the hamsters were individually housed, with dropper-style water bottles and food hoppers in the center of each chamber. Metabolic parameters measured included oxygen consumption (V̇o2) and CO2 production (V̇co2), such that respiratory exchange ratio (RER = V̇co2/V̇o2), and heat production could be calculated. Heat production (kcal/h) was calculated from V̇o2 (l/h) and RER, using heat = V̇o2 × [3.815 + (1.232 × RER)]. Locomotor activity was defined as successive linear infrared beam breaks recorded in 9-min bins.
Feeding behavior parameters measured included timing and duration of feeding bouts, food intake within a bout (meal size), and total food intake per unit time, which was used to calculate energy intake. Locomotor activity was also measured, using two sets of infrared beams lining each cage to monitor linear and vertical movement. The system was operated with an air intake of 0.6 l/min per chamber and an extracted outflow of 0.4 l/min. All measurements were taken at an ambient temperature of 21–22°C. Lighting in both the long-term holding room and the room containing the CLAMS was provided by fluorescent strip lighting, which provided about 350–400 lux at the level of the upper row of CLAMS chambers and 250–300 lux for the lower level of chambers.
Experimental design.
All of the eight hamsters were initially placed in the CLAMS shortly before lights off during long-day photoperiod (LD), designated as week 0. The parameters mentioned above were measured for 68–72 h, and the animals were then removed and put back into their home cages in the holding room on short-day photoperiod (SD). The eight animals were again placed into the CLAMS for 72 h on weeks 1, 2, 4, 8, 12, 16, and 18 under the SD lighting regime (Fig. 1), and again at weeks 1, 2, 4, and 8 after returning to LD (Fig. 1). Raw data were collected from the CLAMS using OxyMax software (ver. 4.2, Columbus Instruments). As we were unsure as to whether the hamsters would require a period of time for habituation to the apparatus, the first 16 h of data were discarded, and only the data collected over the next 48 h were used for analysis. Average values over the 48-h period were calculated for locomotor activity (breaks/9 min), V̇o2 (ml/h), V̇co2 (ml/h), RER, heat production (kcal/h), and energy intake (kcal/h) for each hamster at each CLAMS session. Subsequent analyses revealed that food intake and metabolic parameters for the initial 16-h “habituation” period did not differ significantly from values in the corresponding 16-h period on the second day in the CLAMS (data not shown); thus, the “habituation” period was not necessary.
Fig. 1.
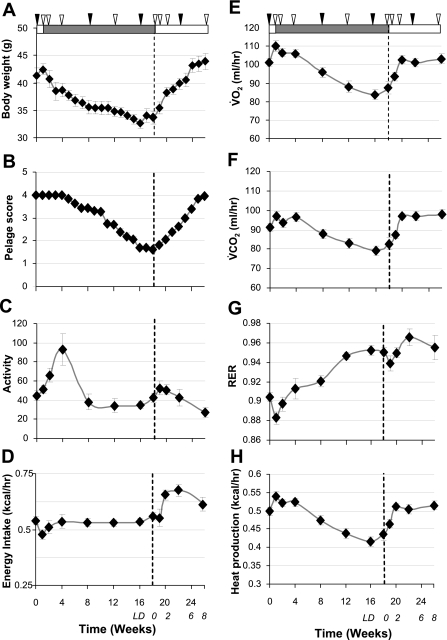
Body weight (A) and pelage scores (B) of male Siberian hamsters exposed to 18 wk of short photoperiod (shaded bar: 8 h light:16 h dark) followed by 8-wk exposure to long photoperiod (open bar: 16 h light:8 h dark). Solid and open arrowheads indicate times of CLAMS analyses. C–H: mean values for each parameter calculated over 48 h: locomotor activity (C), caloric intake (D), V̇o2 (E), V̇co2 (F), respiratory exchange ratio (G), and heat production (H). Solid arrows indicate times of the analyses of daily rhythms presented in subsequent figures. Values are expressed as group means ± SE; n = 8.
Data analyses.
The effects of photoperiod on body weight and pelage scores were analyzed by repeated-measures ANOVA (Prism ver. 5.0; GraphPad Software, San Diego, CA). Similarly, the effects of photoperiod on 48-h average values for locomotor activity (breaks/9 min), V̇o2 (ml/h), V̇co2 (ml/h), RER, heat production (kcal/h), and energy intake (kcal/4 h) were analyzed by repeated-measures one-way ANOVA (Genstat ver. 12 statistical software), including body weight as a covariate, as suggested by Arch et al. (2). Cosinor analysis was used to determine whether significant 24-h periodicity was present for V̇o2, V̇co2, RER, and locomotor activity. Nonlinear regression analysis was used to predict a single cosinor curve for each parameter at the key time points (weeks 0, SD8, SD16, and LD4) according to the equation: y = M+ACos(2πt/24) = BSin(2πt/24) (Eq. 1), where y is the units of the parameter in question, t is zeitgeber time, and A, B, and M were predicted by nonlinear regression. Daily patterns were accepted when the corrected regression coefficient of the fitted cosinor curve was significantly different from zero, and the number of animals displaying significant rhythms on each occasion is shown in Table 1. Where significant daily periodicity was suggested, the acrophase (ϕ) was predicted using Eq. 2, with A and B predicted from Eq. 1: ϕ = ArcTan(−B/A) (Table 1).
Table 1.
Summary of statistical analysis of all parameters measured in the comprehensive laboratory animal monitoring system
| Week 0 LD | Week 8 SD | Week 16 SD | Week 4 LD | ||
|---|---|---|---|---|---|
| Activity (successive beam breaks) | n* | 8 | 1 | 3 | 8 |
| A† | 108 ± 14 | 31 ± 7 | 33 ± 9 | 81 ± 18 | |
| Oxygen consumption | n* | 8 | 0 | 3 | 7 |
| A† | 328 ± 51 | 105 ± 22 | 179 ± 36 | 293 ± 45 | |
| Carbon dioxide output | n* | 8 | 1 | 4 | 7 |
| A† | 250 ± 37 | 91 ± 18 | 165 ± 24 | 245 ± 38 | |
| Respiratory quotient | n* | 8 | 5 | 2 | 8 |
| A† | 0.04 ± 0.003 | 0.03 ± 0.006 | 0.02 ± 0.004 | 0.06 ± 0.004 | |
| Meal frequency‡ | F = 10.8, P < 0.0001 | ns | ns | F = 4.92, P < 0.0001 | |
| Meal duration‡ | F = 8.54, P < 0.0001 | F = 2.50, P < 0.001 | F = 2.37, P < 0.05 | F = 10.6, P < 0.0001 | |
| Total food intake‡ | ns | F = 4.92, P < 0.0001 | F = 2.12, P < 0.05 | F = 3.53, P < 0.001 |
Number of animals out of a total of 8 for which a single cosine nonlinear regression significantly fits the data for the indicated locomotor activity or gas exchange variable.
Values are expressed as means ± SE amplitude of the best-fit cosinor curve for each hamster (n = 8).
Repeated-measures ANOVA to determine the effect of time on each parameter of ingestive behavior; n = 8 hamsters on each sampling occasion.
For feeding behaviors (meal frequency, meal duration, total food intake), the 48 h of data were split into 4-h bins, and each CLAMS session analyzed separately using repeated-measures one-way ANOVA. A bout of feeding (meal) was defined as an intake of greater than 0.02 g. Meal frequency was defined as the number of meals in each 4-h bin. Meal duration in each 4-h interval was represented as the mean length of time the animal spent at the hopper. Total food intake was the total amount of food removed from the automated hopper within the 4-h bin. Data are expressed as the means ± SE; statistical significance was accepted at P < 0.05, and statistical analyses were performed using GraphPad Prism or Genstat statistical software (as indicated).
RESULTS
Effects of photoperiod on body weight, pelage, and energy balance.
Body weight changed significantly during exposure to SD and subsequent return to LD (F = 23.8; P < 0.001, Fig. 1A). Dunnett's post hoc tests revealed that body weight was significantly reduced by week 3 in SD, and reached a nadir at week 16, at which point, it had decreased by ∼20%. Following transfer to LD after 18-wk exposure to SD, body weight rapidly increased and was significantly elevated after 2 wk in LD (week 20, Fig. 1A). All of the hamsters molted their summer coat and grew a winter pelage (F = 113.4; P < 0.001, Fig. 1B). A significant decrease in the pelage score was first detected after 6 wk in SD, and the greatest degree of molt was observed after 16 wk in SD (Fig. 1B). Following the return to LD, the hamsters progressively molted their winter coat and regrew a summer pelage, such that the summer coat was almost completely restored 8 wk after returning to LD (week 26, Fig. 1B).
The changes in body weight were associated with significant changes in various components of energy expenditure, including locomotor activity (F = 5.82; P < 0.001, Fig. 1C), V̇o2 (F = 5.38; P < 0.001, Fig. 1E), V̇co2 (F=4.80; P < 0.001, Fig. 1F), RER (F = 13.93; P < 0.001, Fig. 1G), and heat production (F = 4.93; P < 0.001, Fig. 1H). Post hoc tests revealed that locomotor activity (Fig. 1C) was significantly increased (P < 0.05) at week 4 but had returned to basal levels by week 8 and did not differ significantly thereafter. V̇o2 (Fig. 1E) and heat production (Fig. 1H) were significantly increased (P < 0.05) at week 1, but significantly decreased (P < 0.05) by weeks 12–18 of SD compared with week 0. There was no initial increase (P > 0.1) in V̇co2 (Fig. 1F), but there was a significant decrease at weeks 12–18 compared with week 0 (P < 0.05). Two weeks after the switch back to LD (week 20), V̇o2 (Fig. 1E), V̇co2 (Fig. 1F), and heat production (Fig. 1H) had all returned to week 0 levels and remained constant thereafter. In contrast, RER (Fig. 1G) gradually increased throughout the study period, being significantly increased from week 12 onward. This indicates a gradual increase in the use of glucose as an energy substrate, possibly as a consequence of the gradual loss of adipose tissue.
Interestingly, energy intake (Fig. 1D) did not change significantly relative to week 0 during the SD period but was increased 2 wk after switching back to LD (week 20) and may, therefore, be the driver for body weight gain after switching from SD back to LD. Importantly, all of these effects were significant when including body weight as a covariate in the statistical analyses, indicating effects of photoperiod independent of (or causing) the changes in body weight. Energy balance was calculated by subtracting the average heat production (kcal/h, Fig. 1H) from the average energy intake (kcal/h, Fig. 1D) and indicated that the hamsters were in negative energy balance in weeks 1 and 2 of SD, despite the intake in the CLAMS (ground diet) probably being higher than in the home cage (pelleted diet)—see discussion.
Effects of photoperiod on daily rhythms.
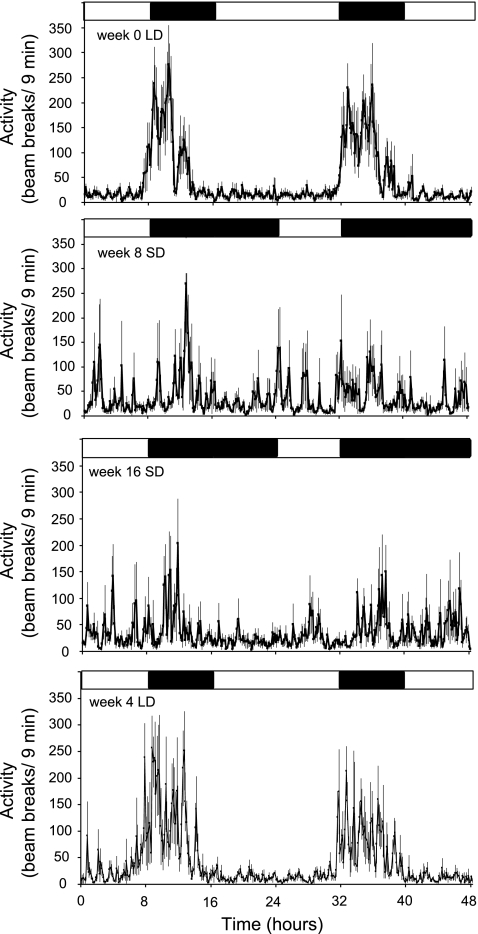
The hamsters displayed clear daily rhythms in locomotor activity in LD with a striking increase in activity in anticipation of the dark phase, which gradually diminished through the later part of the 8-h dark phase (week 0, Fig. 2). Significant high-amplitude rhythms were present in all eight individuals (Table 1). This clear nocturnal increase in activity persisted for the first few weeks of SD exposure, locomotor activity increasing at the start of the dark phase but gradually extending through a greater part of the 16-h dark phase (Supplemental Fig. 1). However, by week 8 in SD, the robust rhythm had diminished, with locomotor activity spread throughout both the light and dark phases (Fig. 2), with only one individual hamster showing a significant rhythm (Table 1). This lack of rhythmicity in locomotor activity persisted for the remainder of the SD exposure (Fig. 2; see Supplemental Fig. 1 in the online version of this article). A significant cosinor rhythm could only be fitted to 3 of the 8 hamsters after a 16-wk exposure (Table 1), and the amplitude of this rhythm remained very low (Table 1). Rhythmicity was gradually restored following reexposure to LD (Supplemental Fig. 1), and by week 4 in LD, all individuals had significant high-amplitude rhythms (Table 1) with the majority of activity again confined to the dark phase (Fig. 2).
Fig. 2.
Locomotor activity in adult male hamsters studied at week 0 (top), week 8 in SD (second panel), week 16 in SD (third panel) and 4 wk after exposure to LD (bottom). Lighting regimes are represented by bars above each graph (solid bars denote dark phase, and open bars denote light phase). Values are expressed as the group means ± SE number of successive infrared beam breaks per 9-min bin; n = 8.
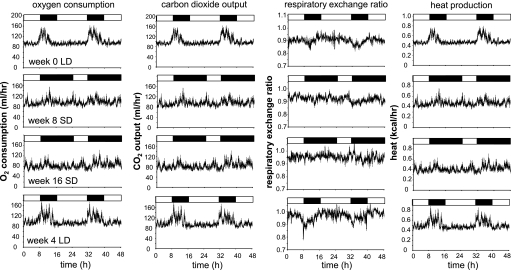
There were also clear daily rhythms in V̇o2, V̇co2, RER, and heat production in LD (Fig. 3 and Table 1; see Supplemental Figs. 2 and 3 in the online version of this article). As with locomotor activity, in week 0, V̇o2/heat production began to increase in anticipation of the dark phase and peaked in the early night. The rhythm of V̇co2 followed a very similar pattern, but it was slightly phase delayed; consequently, RER decreased at the end of the light phase and early dark phase, and then it increased as the dark phase progressed (Supplemental Fig. 3). These clear rhythms in gas exchange were displayed by all of the hamsters in the initial LD-sampling period but gradually dissipated in SD. Significant rhythmicity in V̇o2 persisted for 4 wk in SD (Supplemental Fig. 2), but it was lost in all hamsters by week 8 in SD (Fig. 3; Table 1) and all subsequent sampling occasions in SD (Fig. 3, Supplemental Fig. 2). Rhythmicity in V̇co2 was also lost in most hamsters by 8- and 16-wk exposure to SD (Fig. 3), but it was restored when the hamsters were returned to LD (Fig. 3; Table 1). Although some significant rhythmicity in RER values persisted in the majority of hamsters after 8 wk of exposure to SD (Table 1), the amplitude of the rhythms decreased such that by week 16 in SD, very little rhythmicity was evident (Fig. 3; Supplemental Fig. 3). Exposure to LD resulted in the restoration of clear rhythms in RER values (Table 1) with the same temporal pattern as during the initial period of exposure to LD (Fig. 3).
Fig. 3.
Oxygen consumption (left), carbon dioxide production, respiratory exchange ratio, and heat production (right) in adult male hamsters at week 0 (top), week 8 in SD (second row), week 16 in SD (third row) and 4 wk after return to LD (bottom). Lighting regimes are represented by bars above each graph (solid bars denote dark phase, and open bars denotes light phase). Values are expressed for each 9-min bin as group means ± SE; n = 8.
Effects of SD on feeding behavior.
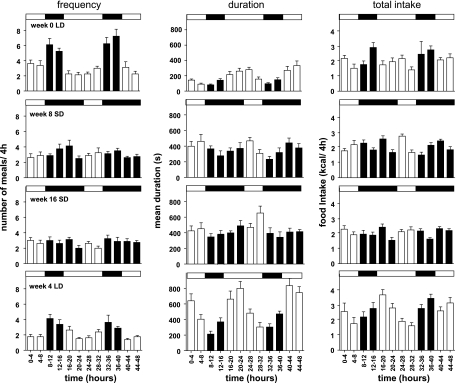
Significant daily rhythms in feeding behavior were observed during the initial sampling occasion in LD. Meal frequency was higher in the dark phase than the light phase (F = 10.8, P < 0.0001, Fig. 4), and this rhythm continued until week 4 in SD with meal frequency being higher in the early part of the dark phase (see Supplemental Fig. 4 in the online version of this article). In contrast, meal duration in LD was higher in the light phase but lower in the dark phase (F = 8.54, P < 0.0001, Fig. 4). Meal duration is directly correlated with the amount of food ingested (see Supplemental Fig. 5 in the online version of this article). As a consequence, overall food intake did not vary significantly across the light-dark cycle (Table 1). The rhythmicity in meal frequency was lost by week 8 in SD (Fig. 4, Table 1). No rhythmicity in meal frequency was observed for the remainder of the SD exposure (Fig. 4, Supplemental Fig. 4), but the rhythm reemerged under LD and was significant by week 4 in LD (Fig. 4, Table 1). Significant rhythmicity in meal duration did persist throughout the whole study (Fig. 4 and Table 1), with the peak in duration corresponding to the early part of the light phase, although the amplitude of the rhythm was greater in LD than in SD (Fig. 4). The combination of changes in meal frequency and meal duration did result in significant temporal variation in the overall food intake in SD, albeit at a rather low amplitude (Fig. 4). Reexposure to LD established a more pronounced rhythm in total food intake, which was lowest in the light phase but gradually increased during the dark phase to peak at the end of the 8-h dark phase/start of the 16-h light phase (Fig. 4).
Fig. 4.
Feeding behavior in adult male hamsters studied at week 0 (top), week 8 in SD (second row), week 16 in SD (third row) and 4 wk after exposure to LD (bottom). Lighting regimes are represented by bars above each graph (solid bars denote dark phase, and open bars denote light phase). Meal frequency (left), mean meal duration (middle), and total food intake (right) were calculated for 4-h bins over 48 h. Values are expressed for each bin as group means ± SE; n = 8.
DISCUSSION
The Siberian hamster has been extensively used as an animal model to understand the photoperiodic regulation and central control of seasonal rhythms of energy metabolism and body weight. As noted in the introduction, it is not clear whether the initial body weight loss in short days reflects reduced energy intake (19) or increased energy expenditure (33). Through an analysis of the mean values for these parameters calculated for each of the 48-h periods when the hamsters were placed in the CLAMS, we did not detect an immediate significant decrease in energy intake in short days, but we did detect an initial but transient increase in V̇o2 and heat production, as well as an increase in locomotor activity early in SD. This would support the view that an increase in energy expenditure underlies the initial phase of weight loss. Indeed, the data indicate that the hamsters were in negative energy balance at weeks 1 and 2 in SD. In contrast, after prolonged exposure to SD, there were significant decreases in V̇o2, heat production, and V̇co2, even though body weight was included as a covariate in the analysis. This could, in part, reflect decreased locomotor activity, since the nocturnal increase in activity was substantially decreased after 8-wk exposure to SD (discussed later). As body weight progressively decreases in SD, largely reflecting decreases in abdominal fat depots, there may also be decreases in resting energy expenditure of specific tissues. As white adipose tissue only constitutes a small proportion of resting energy expenditure in mammals (6), it seems likely that resting energy expenditure in skeletal muscle, liver, and brain, which collectively account for over 60% of energy expenditure at rest (6), must also be decreased during prolonged exposure to SD.
Our interpretation of the current data set is that changes in energy expenditure by muscle related to locomotor activity are likely to be a major determinant of energy balance and thus body weight. The hamsters showed increased nocturnal activity when initially transferred to SD, then a striking loss of activity rhythms and a decrease in overall activity by 8 wk of SD exposure when body weight had decreased. This loss of day-night rhythmicity is not without precedence in this species, as studies investigating the central mechanisms underlying energy balance have observed a surprising lack of rhythmicity across the light-dark cycle, at least under laboratory housing conditions. For example, patterns of circulating leptin in hamsters in both long and short photoperiods show little variation (17), and likewise, patterns of expression of the major genes involved in homeostatic control of energy balance are fairly constant over 24 h in both LD and SD (14). The capability of the CLAMS has now allowed us to characterize daily rhythmicity of several variables related to energy balance. The principal findings are that 1) robust rhythms of locomotor activity, V̇o2, and V̇co2 do occur in LD, but they are lost during prolonged exposure to SD, and 2) while particular aspects of feeding behavior (meal frequency and duration) do vary over the course of the light-dark cycle, the hamsters do not display a clear nocturnal increase in overall food intake.
Monitoring locomotor activity by the disruption of infrared beams has an advantage over using running wheels because provision of these can result in a change in the temporal distribution of activity (31), and in some (30) but not all (32), studies, it has been shown to compromise the seasonal energetic responses of the hamster. In the current study, the removal of hamsters from their home cages into the CLAMS did not affect the timing of the SD-induced weight loss, although the degree of weight loss (24%) was slightly less than that observed in some previous studies in our colony (29). This attenuated weight loss could relate to greater than expected food intake in the CLAMS during SD exposure as the ground lab chow is more palatable to the hamsters. In a separate study, we provided ground lab chow ad libitum in the home cage and found that SD-induced weight loss was significantly attenuated: hamsters fed ground chow lost 9% of body weight after 10 wk of exposure to SD compared with a loss of 17% body weight in hamsters fed pelleted chow (M. Murphy and A. Warner, unpublished data). The fact that the hamsters continued to lose body weight between week 4 and 16 in SD in the current study, even though they appeared to be in neutral or positive energy balance in the CLAMS, would indicate that they were in negative energy balance in the home cage and eating less of the pelleted chow. There was no significant effect of SD photoperiod on the hamsters' total food intake while in the CLAMS sessions, whereas our previous studies and those of several other researchers have detected SD-induced decreases in food intake in hamsters monitored in their home cages (12, 33). In contrast, when hamsters were transferred to LD after 18 wk exposure to SD, a significant increase in food intake was detected. Thus, the CLAMS is capable of detecting changes in overall food intake, but the requirement for the diet to be ground for use in the CLAMS affects its palatability and, therefore, increases intake compared with home cage (pelleted diet) measurements.
Although we noted clear rhythms in the frequency of visits to the food hopper in hamsters in LD and in the initial period of exposure to SD, the duration of the visits and the amounts of food taken were generally inversely related to the frequency of visits. Thus, the hamsters ate significant amounts during the light phase and showed far lower amplitude rhythmicity in total food intake than in overall locomotor activity or in gas exchange. In general, the hamsters appeared to be far less nocturnal in their feeding behavior than mice and rats studied in comparable metabolic cage systems (5, 18, 20).
The CLAMS revealed clear rhythms in V̇o2 and V̇co2 in LD. These rhythms were lost by 8 wk of exposure to SD, but we never observed major decreases in these parameters that would indicate the occurrence of torpor bouts (15). We have observed torpor in the CLAMS in transgenic mice bearing null mutations of the prokineticin 2 receptor (18), and, using radiotelemetry, we have routinely observed torpor in hamsters in their home cages after ∼12 wk of exposure to SD maintained in the same ambient temperature as the room housing the CLAMS (F. J. P. Ebling, unpublished observations). The failure to observe torpor may reflect a low level of stress induced by the sparse environment in the metabolic chambers, as our experience is that any type of mildly stressful or arousing stimulus, such as an experimenter entering a holding room, will disrupt the occurrence of torpor (F. J. P. Ebling, unpublished observations).
We initially expected to observe a SD-induced decrease in average daily RER values, as the metabolism of the hamsters became more dependent upon lipid mobilization in SD. In fact, average RER values remained above 0.85 in all observation periods (Fig. 1G). In LD, RER levels showed a clear pattern of daily rhythmicity (Supplemental Fig. 3), with the drop in RER values starting toward the end of the 16-h light phase when meal frequency and meal duration were at their lowest, and thus overall food intake was decreased. The drop in RER continued into the dark phase, even though an increase in food consumption had already been initiated, probably because the food needs to be digested and absorbed before it is metabolized, thereby resulting in a lag period before the RER increases again to reflect the high carbohydrate diet. Importantly, the rhythmicity in RER values was initially accentuated in SD, particularly at weeks 1–4, with RER values falling to 0.8 at the end of the light phase after 1 wk in SD (Supplemental Fig. 3), indicating increased fat oxidation. However, this accentuated rhythmicity was subsequently lost in SD (Supplemental Fig. 3), probably reflecting the loss in daily rhythmicity in the temporal patterns of food intake. Under simulated natural conditions Siberian hamsters hoard food externally (8), but we never observed this in the CLAMS, though we could not assess whether there may have been photoperiod-dependent changes in food storage in cheek pouches, which contributed to the loss of rhythmicity in RER.
Perspectives and Significance
This study revealed clear daily rhythms of locomotor activity and metabolic rate inferred from V̇o2, heat production, and V̇co2 in hamsters in long photoperiods, which were lost upon prolonged exposure to short photoperiods. Short photoperiod phenotype maximizes evolutionary fitness by imposing metabolic and behavioral profiles that optimize individual survival in harsh environmental conditions. Although we recognize that the current experimental approach did not reproduce all of the features of a natural winter environment, for example, reduced ambient temperature and limited food supply, the short photoperiod alone was sufficient to induce weight loss associated with a striking loss of overt circadian locomotor activity patterns. We consider that this loss of daily rhythmicity may be an adaptive advantage of the winter phenotype, allowing the animal to resist starvation by releasing them from temporal control of behavior and feeding patterns. Behavioral arrhythmicity is likely to increase the risk of predation (9), and consequently, this strategy is only effective when the risk of starvation outweighs the risk of predation, such as during the winter months when food is restricted. In concurrence with this hypothesis, circadian locomotor rhythms were similarly fragmented with increased daytime activity in calorie-restricted mice (27). Interestingly, seasonal affective disorder, which is thought to represent a remnant of seasonal physiology in humans, was associated with reduced amplitudes of circadian locomotor activity, and lower sleep efficiency in short photoperiods (34). The loss of circadian locomotor rhythmicity under short photoperiod identified in this study is an interesting example of seasonal modulation of circadian physiology, and the mechanisms controlling such interaction warrant further investigation. It will be particularly important to determine whether there is an attenuation of intracellular clock gene rhythmicity, as recently reported in the hibernating European hamster in short photoperiods (28).
GRANTS
This work was supported by a Biotechnology and Biological Sciences Research Council (UK) Priority Targeted studentship and Project Grant BB/E020437/1.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1.Aguilar-Roblero R, Vega-Gonzalez A. Splitting of locomotor circadian rhythmicity in hamsters is facilitated by pinealectomy. Brain Res 605: 229–236, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Arch JRS, Hislop D, Wang SJY, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes 30: 1322–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Hamilton JM, Wade GN, Goldman BD. Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 257: R1533–R1540, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Wade GN. Body weight, food intake and energy regulation in exercising and melatonin-treated Siberian hamsters. Physiol Behav 35: 805–808, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol 294: R344–R351, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bender DA. Introduction to Nutrition and Metabolism, 4th ed.Boca Raton, FL: CRC Press, 2008 [Google Scholar]
- 7.Borer KT, Campbell CS, Tabor J, Jorgenson K, Kandarian S, Gordon L. Exercise reverses photoperiodic anestrus in golden hamsters. Biol Reprod 29: 38–47, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool 289: 162–171, 2001 [PubMed] [Google Scholar]
- 9.Decoursey PJ, Krulas JR, Mele G, Holley DC. Circadian performance of suprachiasmatic nuclei (SCN)-lesioned antelope ground squirrels in a desert enclosure. Physiol Behav 62: 1099–1108, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus, 1. The role of the gonads and pituitary. J Exp Zool 230: 89–96, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Ebling FJP. Photoperiodic differences during development in the dwarf hamsters Phodopus sungorus and Phodopus campbelli. Gen Comp Endocrinol 95: 475–482, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Ebling FJP, Arthurs OJ, Turney BW, Cronin AS. Seasonal neuroendocrine rhythms in the male Siberian hamster persist following monosodium glutamate-induced lesions of the arcuate nucleus in the neonatal period. J Neuroendocrinol 10: 701–712, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ebling FJP, Barrett P. The regulation of seasonal changes in food intake and body weight. J Neuroendocrinol 20: 827–833, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Ellis C, Moar KA, Logie TJ, Ross AW, Morgan PJ, Mercer JG. Diurnal profiles of hypothalamic energy balance gene expression with photoperiod manipulation in the Siberian hamster, Phodopus sungorus. Am J Physiol Regul Integr Comp Physiol 294: R1148–R1153, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Heldmaier G, Klingenspor M, Werneyer M, Lampi BJ, Brooks SP, Storey KB. Metabolic adjustments during daily torpor in the Djungarian hamster. Am J Physiol Endocrinol Metab 276: E896–E906, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Heldmaier G, Steinlechner S. Seasonal pattern and energetics of short daily torpor in the Djungarian hamster. Oecologia (Berl) 48: 265–270, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Horton TH, Buxton OM, Losee-Olson S, Turek FW. Twenty-four-hour profiles of serum leptin in Siberian and golden hamsters: photoperiodic and diurnal variations. Horm Behav 37: 388–398, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Jethwa PH, I'Anson H, Warner A, Prosser H, Hastings MH, Maywood ES, Ebling FJP. Loss of prokineticin receptor 2 signaling predisposes mice to torpor. Am J Physiol Regul Integr Comp Physiol 294: R1968–R1979, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knopper LD, Boily P. The energy budget of captive Siberian hamsters, Phodopus sungorus, exposed to photoperiod changes: mass loss is caused by a voluntary decrease in food intake. Physiol Biochem Zool 73: 517–522, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kohsaka A, Laposky A, Ramsey K, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Mertens A, Stiedl O, Steinlechner S, Meyer M. Cardiac dynamics during daily torpor in the Djungarian hamster (Phodopus sungorus). Am J Physiol Regul Integr Comp Physiol 294: R639–R650, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Morgan PJ, Hazlerigg DG. Photoperiodic signalling beyond the melatonin receptor. J Neuroendocrinol 20: 820–826, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Puchalski W, Lynch GR. Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J Comp Physiol [A] 159: 7–11, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Puchalski W, Lynch GR. Daily melatonin injections affects the expression of circadian rhythmicity in Djungarian hamsters kept under a long-day photoperiod. Neuroendocrinology 48: 280–286, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Puchalski W, Lynch GR. Circadian characteristics of Djungarian hamsters: effects of photoperiodic pretreatment and artificial selection. Am J Physiol Regul Integr Comp Physiol 261: R670–R676, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Refinetti R. Absence of daily and photoperiodic conservation of energy expenditure in three rodent species. J Comp Physiol [A] 177: 309–318, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Resuehr D, Olcese J. Caloric restriction and melatonin substitution: effects on murine circadian parameters. Brain Res 1048: 146–152, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pevet M, Simonneaux V, Saboureau M, Pevet P. The circadian clock stops ticking during deep hibernation in the European hamster. Proc Natl Acad Sci USA 104: 13816–13820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson AJ, Rousseau K, Loudon ASI, Ebling FJP. Cocaine and amphetamine-related transcript (CART) mRNA regulation in the hypothalamus in lean and obese rodents. J Neuroendocrinol 14: 697–709, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Scherbarth F, Petri I, Steinlechner S. Effects of wheel running on photoperiodic responses of Djungarian hamsters (Phodopus sungorus). J Comp Physiol [B] 178: 607–615, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Scherbarth F, Steinlechner S. The annual activity pattern of Djungarian hamsters (Phodopus sungorus) is affected by wheel-running activity. Chronobiol Int 25: 905–922, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Teubner BJ, Bartness TJ. Body mass loss during adaptation to short winter-like days increases food foraging, but not food hoarding. Physiol Behav 97: 135–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight, and body composition in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 246: R26–R30, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Winkler D, Pjrek E, Praschak-Rieder N, Willeit M, Pezawas L, Konstantinidis A, Stastny J, Kasper S. Actigraphy in patients with seasonal affective disorder and healthy control subjects treated with light therapy. Biol Psychiatry 58: 331–336, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.