Abstract
In this study, we investigated the effects of bladder outlet obstruction (BOO) on the expression and function of large conductance (BK) and small conductance (SK) Ca2+-activated K+ channels in detrusor smooth muscle. The bladder from adult female Sprague-Dawley rats with 6-wk BOO were used. The mRNA expression of the BK channel α-subunit, β1-, β2-, and β4-subunits and SK1, SK2, and SK3 channels were investigated using real-time RT-PCR. All subunits except for the BK-β2, SK2, and SK3 channels were predominantly expressed in the detrusor smooth muscle rather than in the mucosa. The mRNA expression of the BK channel α-subunit was not significantly changed in obstructed bladders. However, the expression of the BK channel β1-subunit and the SK3 channel was remarkably increased in obstructed bladders. On the other hand, the expression of the BK channel β4-subunit was decreased as the severity of BOO-induced bladder overactivity progressed. In detrusor smooth muscle strips from obstructed bladders, blockade of BK channels by iberiotoxin (IbTx) or charybdotoxin (CTx) and blockade of SK channels by apamin increased the amplitude of spontaneous contractions. These blockers also increased the contractility and affinity of these strips for carbachol during cumulative applications. The facilitatory effects elicited by these K+ channel blockers were larger in the strips from obstructed bladders compared with control bladders. These results suggest that long-term exposure to BOO for 6 wk enhances the function of both BK and SK types of Ca2+-activated K+ channels in the detrusor smooth muscle to induce an inhibition of bladder contractility, which might be a compensatory mechanism to reduce BOO-induced bladder overactivity.
Keywords: bladder outlet obstruction, Ca2+-activated K+ channels, spontaneous contraction, muscarinic stimulation
bladder outlet obstruction (BOO) causes various structural and functional changes in the lower urinary tract and induces not only obstructive but also detrusor overactivity, as well as irritative symptoms, such as urgency, urge incontinence, urinary frequency, and nocturia. This situation has been simulated using animal models with partial urethral obstruction, and numerous mechanisms including myogenic and neurogenic changes, have been proposed as a source of detrusor overactivity. In the case of BOO, there is evidence that detrusor overactivity is closely related to increased excitability of detrusor smooth muscle (1, 53).
One of the important functions of K+ channels is to stabilize the membrane potential and reduce the excitability of nerves and muscle cells (38). The relationship between detrusor overactivity and K+ channels has been reported (18, 31, 33, 45, 46), and several kinds of modulators of these K+ channels have been developed in an attempt to manage detrusor overactivity (10, 27, 34). Ca2+-activated K+ channels are one of the major groups of six/seven transmembrane potassium-selective channels. These channels are divided into several groups according to the genetic, electrical, and structural properties of each channel (50). Large-conductance Ca2+-activated K+ (BK) channels, which are activated by membrane potential depolarization and by increases in cytosolic Ca2+ concentration (50), mainly mediate repolarization phases of detrusor smooth muscle cells (13, 15, 46). BK channel knockout mice show reduced bladder capacity, increased amplitude of spontaneous contractions, and increased responses to electrical and carbachol-induced stimulation (31, 46). The BK channels consist of pore-forming α-subunits associated with accessory β-subunits, which contribute to the functional diversity of the channels (5, 11, 12, 29, 30, 33, 51). Four different β-subunits with tissue-specific distribution have been cloned (5, 21, 30, 49). The smooth muscle-specific β1-subunit was first identified in bovine tracheal and aortic smooth muscle (21). The detrusor smooth muscle from β1-subunit knockout mice shows altered phasic contractions (33). A recent study has also demonstrated the presence of β4-subunits in the rat detrusor smooth muscle both at mRNA and protein levels (7), although β4-subunits were thought to be expressed mainly in neurons (11, 30, 51). Small-conductance Ca2+-activated K+ (SK) channels, which are Ca2+ sensitive, but not voltage sensitive, are also important determinants of detrusor smooth muscle excitability and contractility. They are divided into three subtypes (SK1, SK2, and SK3). Genetic modification of these channels in mice has shown critical roles of these channels in the control of detrusor smooth muscle activity (18, 45).
In this study, we examined how BOO affects the expression of BK and SK channels and investigated the impact of altered expressions of these channels on the contractile properties of detrusor smooth muscle in rats.
MATERIALS AND METHODS
Animals.
A total of 63 adult female Sprague-Dawley rats, weighing 200–250 g, were used. All experimental procedures were in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Partial urethral obstruction.
Partial urethral obstruction was produced as previously described (8, 20). The bladder and proximal urethra were exposed via a lower abdominal midline incision under isoflurane anesthesia. A 4–0 silk ligature was placed around the proximal urethra, and an adjacent 1.1-mm diameter steel rod tied tightly, after which the rod was removed. The abdominal wound was closed, and ampicillin (100 mg/kg sc) and buprenorphine (0.05 mg/kg sc) were given to all animals to control postoperative infection and pain. In preliminary experiments, there were no significant differences in the mRNA expression of each channel between a nonoperated (intact) group and a sham-operated group. Therefore, nonoperated rats were used as a control group in this study.
Cystometry.
Six weeks after surgery, the bladder was exposed through a lower midline incision under isoflurane anesthesia. A PE-50 catheter (Clay Adams, Parsippany, NJ) was inserted into the bladder through the bladder dome, and the abdomen was closed with sutures. Local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5% (AstraZeneca, Wilmington, DE) was applied to the wound to suppress pain. The rats were then placed in restraining cages and allowed to recover from the anesthesia for 1.5 h before cystometry. The intravesical catheter was connected via a three-way stopcock to a pressure transducer (World Precision Instruments, Sarasota, FL) and a syringe pump for recording intravesical bladder pressure and infusing saline into the bladder, respectively. Intravesical pressure changes were recorded by the PowerLab system (AD Instruments, Castle Hill, NSE, Australia), and data were analyzed using data acquisition software (AD Instruments). Saline at room temperature was infused into the bladder at 2.4 ml/h in control rats. In BOO rats, because of the large bladder capacity, the infusion rate of saline was adjusted to 3–15 ml/h to obtain an intercontraction interval of 10–20 min. Saline was infused until rhythmic bladder contractions became stable. Parameters listed in Table 1 were determined from cystometrograms as previously described (20). Nonvoiding contractions (NVCs) were defined as bladder contractions without voiding prior to micturition, the amplitudes of which were more than 4 cmH2O.
Table 1.
Effects of BOO on characteristics of the bladder and cystometrical parameters
| Control | m-BOO | s-BOO | |
|---|---|---|---|
| n | 7 | 6 | 7 |
| BW, mg | 79.4 (3.62) | 281.5 (31.5)* | 798.4 (139.2)† |
| MVP, cmH2O | 24.9 (1.28) | 112.2 (9.45)† | 76.3 (9.29)†‡ |
| TP, cmH2O | 6.05 (0.65) | 6.54 (1.13) | 18.96 (4.08)*‡ |
| BP, cmH2O | 1.31 (0.72) | 2.79 (0.38) | 11.05 (2.98)*‡ |
| BC, ml | 0.83 (0.09) | 1.79 (0.31) | 9.26 (2.53)*‡ |
| VE, % | 89.0 (5.39) | 70.1 (6.79) | 50.1 (7.45)* |
m-BOO, mild bladder outlet obstruction; s-BOO, severe bladder outlet obstruction; BW, bladder weight; MVP, maximal voiding pressure; TP, threshold pressure; BP, baseline pressure; BC, bladder capacity; VE, voiding efficacy. Values are expressed as means ± SE.
P < 0.01,
P < 0.001 vs. control group.
P < 0.05 versus m-BOO group.
RNA extraction and cDNA synthesis.
After cystometric evaluation, rats were killed by carbon dioxide inhalation. Immediately after death, the whole bladder above the ureteral orifices was dissected out and weighed. Some bladders (n = 7 in the control group; n = 13 in the BOO group) were cut into small pieces containing all layers of the bladder wall, whereas others (n = 5 in each group) were placed in Krebs-Henseleit solution aerated with 95% oxygen and 5% carbon dioxide followed by separation of the bladder into the mucosa and muscle layers under a dissecting microscope, which were then cut into small pieces. These specimens were frozen in liquid nitrogen immediately after preparation and stored at −80°C until further processing. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), according to manufacturer's instructions. All samples were treated with DNase (Promega, Madison, WI) to prevent the contamination of genomic DNA followed by cleaning up with an RNeasy mini kit (Qiagen, Valencia, CA) or TRIzol reagent. RNA was quantified by a spectrophotometer (Biochrom, Cambridge, UK). Five micrograms total RNA from whole bladder tissues and 1 μg from separated bladder tissues were reverse-transcribed using Thermoscript with oligo (dT) primers (Invitrogen) following manufacturer's instructions.
Real-time RT-PCR.
Real-time PCR was performed in 96-well reaction plates using the MX3000P (Stratagene, La Jolla, CA). The reaction mixture contained 1 μl diluted cDNA, 12.5 μl 2 × SYBR Green PCR Master Mix (Qiagen) and 0.3 μM primer pair in total volume of 25 μl. Primers, details of which are listed in Table 2, were designed using Primer3 (Whitehead Institute for Biomedical Research, Cambridge, MA) or PrimerQuest free software (Integrated DNA Technologies, Coralville, IA), except where referenced otherwise. These primers were tested in silico for specificity against sequences for Rattus norvegicus using BLAST software (National Center for Biotechnology Information, Bethesda, MD) and synthesized by Integrated DNA Technologies. The cycle conditions comprised 15-min polymerase activation and 40 cycles with denaturation at 94°C for 15 s, annealing at 55°C or 58°C for 30 s and extension at 72°C for 30 s followed by dissociation from 58°C to 95°C. Five-fold dilution series of cDNA were used to establish standard curves.
Table 2.
Characteristics of primers
| Gene Namea (abb.) | Gene Symbol | Primer Sequenceb | Accession Numberc | Amplicon Sizesd, bp | Annealing Tme, °C |
|---|---|---|---|---|---|
| Potassium large-conductance calcium-activated channel, subfamily M, α member 1 (BK-α) | Kcnma1 | F:AGGCTCTGTTCAAACGGCAT | NM_031828 | 250 | 58 |
| R:TTGAGCAGATGGGCCTTGTT | |||||
| Potassium large-conductance calcium-activated channel, subfamily M, β member 1 (BK-β1) | Kcnmb1 | F:ACCCATGCCTTTGGGTCAAT | NM_019273 | 236 | 58 |
| R:ATAGAGGCGCTGGTACACAA | |||||
| Potassium large-conductance calcium-activated channel, subfamily M, β member 2 (BK-β2) | Kcnmb2 | F:TGCCCTGCTGAATGTGTCAA | NM_176861 | 231 | 58 |
| R:ACACTCACAAGGGACATGGA | |||||
| Potassium large-conductance calcium-activated channel, subfamily M, β member 4 (BK-β4) | Kcnmb4 | F:TCAACATCAAAGACCAGAGGAC | NM_023960 | 107 | 58 |
| R:TGAGGACACCCACCACAAAC | |||||
| Potassium intermediate/small-conductance calcium-activated channel, subfamily N, member 1 (SK1) | Kcnn1 | F:AGCTCCGGACTGTGAAGATT | NM_019313 | 213 | 55 |
| R:TGGCTTGGGCTATGAGACTT | |||||
| Potassium intermediate/small-conductance calcium-activated channel, subfamily N, member 2 (SK2) | Kcnn2 | F:ACGCTAGTGGATCTGGCAAA | NM_019314 | 240 | 58 |
| R:ACGCTCAGCATTGTAGGTGA | |||||
| Potassium intermediate/small-conductance calcium-activated channel, subfamily N, member 3 (SK3) | Kcnn3 | F:CAGGAAACACCAGAGGAAGT | NM_019315 | 228 | 58 |
| R:AGGGAATTGAAGCTGGCTGT | |||||
| Ribosomal protein L13a (Rpl13a) | Rpl13a | F:ACAAGAAAAAGCGGATGGTGf | NM_173340 | 172 | 58 |
| R:TTCCGGTAATGGATCTTTGCf | |||||
| Hypoxanthine phosphoribosyltransferase 1 (HPRT) | Hprt1 | F:TTGTTGGATATGCCCTTGACTg | NM_012583.2 | 105 | 58 |
| R:CCGCTGTCTTTTAGGCTTTGg |
Abbreviation used in this article (abb.).
Sequences of forward (F) and reverse (R) primers.
Accession number of GenBank (http://www.ncbi.nlm.nih.gov/).
Amplicon length in base pairs (bp).
Annealing temperature (Tm).
Sequences referenced to Bonefeld et al. (2).
Sequences referenced to van Wijngaarden et al. (47).
Tissue preparations for isolated detrusor smooth muscle studies.
The bladders from separate groups of control and BOO rats were removed immediately after death with carbon dioxide inhalation and placed in the aerated Krebs-Henseleit solution. One × four millimeter detrusor muscle strips, in which the mucosa was removed, were cut out longitudinally from the bladder body above the ureteral orifices under a dissecting microscope. Each strip was mounted in an 8-ml organ bath containing aerated 37°C Krebs-Henseleit solution with one end fixed to a stationary rod and the other to a transducer. Tension generated was measured, recorded, and analyzed with the same equipment as cystometry. The initial load was set to 10 mN. After a 60–90 min equilibration period accompanied by periodical changes of the solution in the organ bath, test drugs were applied. All procedures were conducted in the presence of 1 μM tetrodotoxin to reduce the influence of nerve activity. At the end of the experiment, wet weight was recorded for each strip.
Evaluation of spontaneous contractions in bladder strips.
To stabilize spontaneous contractions, the concentration of K+ in the Krebs-Henseleit solution was increased to 20 mM by equimolar substitution of NaCl with KCl as previously described (10, 43). After the equilibration period, 10, 30, and 100 nM iberiotoxin (IbTx) and charybdotoxin (CTx), which are blockers of BK channels, and 0.1, 1, 10, and 100 nM apamin, which is a blocker of SK channels, were applied cumulatively at 20-min intervals. Amplitudes and frequency of spontaneous contractions during the last 5 min of each application period were measured.
Evaluation of carbachol-induced contractions.
After the equilibration period, carbachol was applied in increasing cumulative concentrations of 10−8, 3 × 10−8, 10−7, 3 × 10−7, 10−6, 3 × 10−6, 10−5, 3 × 10−5, 10−4 and 3 × 10−4 M. When contraction force reached the maximum at one concentration, the next concentration was added. After conducting the first concentration-response study, carbachol was washed out by changing the Krebs-Henseleit solution of the organ bath 5 times at 10-min intervals. Following the washout period, 100 nM of each K+ channel blocker was added to the bath, and after a 20-min incubation time, the second carbachol concentration-response study was conducted.
Drugs.
Krebs-Henseleit solution contained: 143 mM Na+, 5.9 mM K+, 2.5 mM Ca2+, 1.2 mM Mg2+, 127.7 mM Cl−, 1.2 mM SO42−, 1.2 mM PO43−, 25 mM HCO3− and 11 mM glucose. Tetrodotoxin and carbachol were purchased from Sigma-Aldrich (St. Louis, MO). IbTx, CTx, and apamin were purchased from Alomone (Jerusalem, Israel). All drugs were reconstituted in distilled water as concentrated stock solutions and diluted with Krebs-Henseleit solution immediately before use.
Data analysis.
To evaluate the expression level of K+ channel mRNA, we used ribosomal protein L13a (Rpl13a) and hypoxanthine guanine phosphoribosyl-transferase-1 mRNA as internal controls, which were not significantly different in control and BOO groups, according to an algorithm proposed previously (48). This was necessary because some studies have reported increased expression of GAPDH and β-actin in the hypertrophied bladder (3, 28). Also in our preliminary study, the mRNA levels of GAPDH were markedly increased in mucosa-intact obstructed bladders and in mucosa-removed smooth muscle layers of obstructed bladders (n = 7 in both groups, P < 0.001; n = 5 in both groups, P < 0.01, respectively). The expression of β-actin was also significantly increased in mucosa-removed smooth muscles of obstructed bladders (n = 5 in both groups, P < 0.01) (data not shown).
In the mucosa-removed muscle strip study, responses to carbachol are expressed as a percentage of the maximum force (Emax) induced by the first application of carbachol. ΔEmax was obtained by subtracting the Emax value in the first carbachol application from that in the second application. Cumulative concentration-response curves were analyzed by nonlinear regression in each individual experiment using GraphPad Prism 4.03 (GraphPad Software, San Diego, CA). EC50 values were calculated for concentration-response curves before and after a test drug application, and are presented as −logEC50. Δ−logEC50 was then obtained by subtracting the −logEC50 value of the first carbachol application from that of the second carbachol application.
All data are expressed as means ± SE. Student's t-test or one-way ANOVA followed by Bonferroni's multiple-comparison test was used to determine significance among two groups or more than two groups, respectively. For all statistical tests, P < 0.05 was considered significant. In the isolated muscle strip study, n represents the number of strips and N represents the number of animals.
RESULT
Cystometry.
Six weeks after urethral ligation, bladder weight was significantly increased in BOO rats (79.4 ± 3.62 mg in control rats vs. 559.8 ± 104.6 mg in BOO rats). However, the degree of hypertrophy and an occurrence of NVCs were variable among animals. Therefore, to investigate the influence of severity of altered properties induced by BOO on K+ channel function, BOO rats were divided into mildly obstructed (m-BOO) and severely obstructed (s-BOO) groups, based on the absence and presence of NVCs greater than 4 cmH2O during cystometry, respectively. Bladder weight and bladder capacity in the s-BOO group were more than 10 times larger than those in the control group (Table 1). Significantly increased maximum voiding pressure was noted in both BOO groups although the value in the s-BOO group was lower compared with the m-BOO group. Voiding efficacy was also significantly lower in the s-BOO group than in the m-BOO group. These results indicate that s-BOO bladders exhibit more pronounced detrusor overactivity (i.e., NVCs) along with decompensated contractile properties during voiding compared with m-BOO rats.
mRNA expressions.
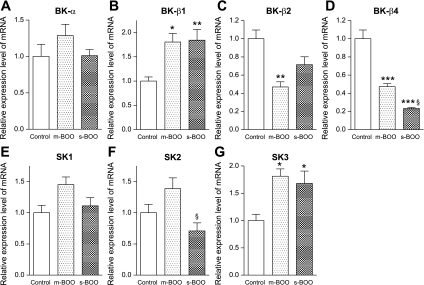
In our preliminary experiments, mRNA expression of β1-, β2-, and β4-subunits, but not β3-subunits, of the BK channel was detected in the bladder tissue as previously described (35). Therefore, we investigated the BK channel α-subunit, β1-, β2-, and β4-subunits and all 3 types of SK channels in this study. To evaluate the influence of BOO on the expression of each channel transcript, we compared the mRNA expression in control, m-BOO and s-BOO groups. There were no significant differences of the BK channel α-subunit transcripts expression among groups, although the expression in the m-BOO group tended to be higher compared with other groups (Fig. 1A). Similar patterns were seen in the expression of the SK1 and SK2 channel transcripts (Fig. 1, E and F). However, BOO markedly increased the expression of the BK channel β1-subunit and the SK3 channel transcripts regardless of the severity of BOO (Fig. 1, B and G). On the other hand, the expression of the β4-subunit transcripts was significantly decreased in parallel with the severity of BOO (Fig. 1D).
Fig. 1.
Relative mRNA expression levels of large-conductance (BK) channel α-subunit (BK-α) (A), BK channel β1-subunit (BK-β1) (B), BK channel β2-subunit (BK-β2) (C), BK channel β4-subunit (BK-β4) (D), small-conductance (SK)1 channel (SK1) (E), SK2 channel (SK2) (F), and SK3 channel (SK3) (G) in mucosa-intact whole bladders from control (n = 7), mild bladder outlet obstruction (m-BOO) (n = 6), and severe bladder outlet obstruction (s-BOO) (n = 7) groups. Each value is expressed as a percentage of the expression level of the control group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group. §P < 0.05 vs. m-BOO group.
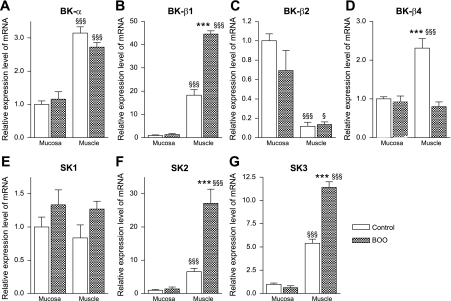
To examine mRNA expression in different layers of the bladder, RT-PCR was performed on the mucosa and smooth muscle layers of bladder tissues from control and BOO rats, which showed increased bladder weight (659.2 ± 99.0 mg) and NVCs during cystometry similar to s-BOO rats. Muscle and mucosa exhibited marked differences in expression of the different genes; BK-α, SK2 and SK3 transcripts, as well as the β1 and β4 subunit transcripts, were more prominently expressed in muscle. Among these channels and subunit transcripts in the muscle layer, changes in expression similar to the whole bladder experiment were observed after BOO except the SK2 channel, which was significantly increased compared with controls (Fig. 2).
Fig. 2.
Relative mRNA expression levels of BK-α (A), BK-β1 (B), BK-β2 (C), BK-β4 (D), SK1 (E), SK2 (F), and SK3 (G) in mucosa and detrusor layers separated from bladder tissues in control and BOO groups (n = 5 in each). Each value is expressed as a percentage of the expression level in the mucosa of control group. ***P < 0.001 vs. control group. §P < 0.05, §§§P < 0.001 vs. mucosa of the same group.
Spontaneous activity in muscle strips.
To investigate whether functional changes are elicited by the altered expression of BK channel subunits and SK channel transcripts following BOO, we examined responses of detrusor smooth muscle strips, in which the mucosa was removed, to BK channel blocking agents, IbTx and CTx, and a SK channel blocker, apamin.
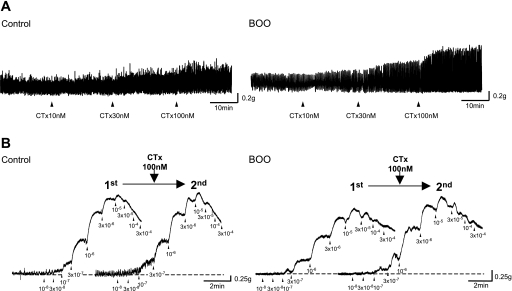
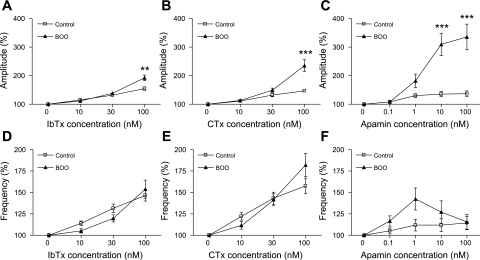
One series of experiments investigated the influence of blockers on spontaneous bladder contractions. Before application of K+ channel blockers, the amplitude of spontaneous contractions was significantly higher in the control group compared with the BOO group when values were corrected with wet weight (wet wt) of muscle strips (0.20 ± 0.012 vs. 0.12 ± 0.011 mN/mg wet wt, P < 0.001). The frequency of spontaneous contractions was also greater in the control group than in the BOO group. (0.154 ± 0.006, n = 35 vs. 0.040 ± 0.002 Hz, n = 40, P < 0.001). All blocking agents increased the amplitude and frequency of spontaneous contractions. Figure 3A shows representative responses to a range of CTx concentrations in control and BOO groups. CTx application at 100 nM elicited larger changes in amplitude of spontaneous contractions in the BOO group compared with the control group (235.3 ± 20.8% vs. 146.9 ± 4.3% of predrug values, P < 0.001, Figs. 3A and 4B). IbTx application at 100 nM also showed significantly larger responses in the BOO group compared with the control group (191.2 ± 9.6% vs. 155.0 ± 6.0% of predrug values, P < 0.01, Fig. 4A). Apamin also increased the amplitude of spontaneous activity, and this increase was greater in the BOO group compared with the control group at concentrations of 10 and 100 nM (309.5 ± 39.2% vs. 135.5 ± 9.0% at 10 nM, P < 0.001, 335.7 ± 44.8% vs. 136.8 ± 10.0% at 100 nM, P < 0.001, Fig. 4C). Meanwhile, although the frequency of spontaneous contractions also tended to be increased by all blockers, the magnitude of this effect was not significantly different between control and BOO rats.
Fig. 3.
Representative force tracings produced by detrusor smooth muscle strips. A: effect of cumulative applications of charybdotoxin (CTx; 10, 30, 100 nM) on spontaneous contractions of smooth muscle strips from control and BOO groups. B: effect of 100 nM CTx on contractility in response to cumulative applications of carbachol (10−8, 3 × 10−8, 10−7, 3 × 10−7, 10−6, 3 × 10−6, 10−5, 3 × 10−5, 10−4, and 3 × 10−4 M). Cumulative application of carbachol before a treatment with 100 nM CTx (1st) and after a treatment with 100 nM CTx (2nd).
Fig. 4.
Alterations of amplitudes (A, B, C) and frequency (D, E, F) of spontaneous contractions of detrusor smooth muscle strips elicited by iberiotoxin (IbTx) (A and D: n = 12, N = 4 in control group; n = 14, N = 6 in BOO group, where n represents the number of strips and N represents the number of animals), CTx (B and E: n = 14, N = 6 in control group; n = 15, N = 6 in BOO group), and apamin (C and F: n = 10, N = 5 in control group; n = 11, N = 5 in BOO group). Values are presented as a percentage of the value before an application of each blocker. **P < 0.01, ***P < 0.001 vs. control group.
Carbachol evoked contractions.
The influence of K+ blocking agents on the contractions evoked by carbachol, a cholinergic agonist, were also examined in control and BOO strips (Fig. 3B and Fig. 5) To quantify the effect of multiple applications of carbachol on muscle strips' contractility, we investigated carbachol-evoked contractions of time-matched controls (TMC) in the absence of blocking agent.
Fig. 5.
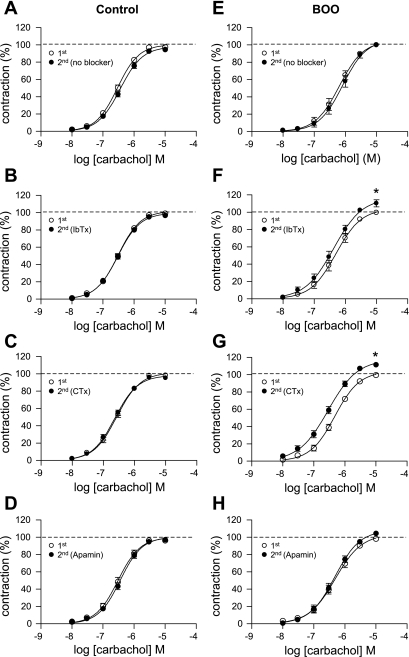
Effect of K+ channel blockers on concentration-response curves for carbachol in detrusor smooth muscle strips from the control (A–D) and BOO (E–H) groups. Curves in each panel were obtained from cumulative applications of carbachol in time-matched controls (TMC) without blocking agents (A and E: n = 8, N = 8 in control group; n = 7, N = 4 in BOO group) and in preapplication and postapplication phases of 100 nM IbTx (B and F: n = 11, N = 6 in control group; n = 9, N = 5 in BOO group), 100 nM CTx (C and G: n = 11, N = 6 in control group; n = 11, N = 6 in BOO group) and 100 nM apamin (D and H: n = 10, N = 5 in control group; n = 10, N = 5 in BOO group). 1st denotes cumulative applications of carbachol before a treatment with each blocker or first carbachol applications in TMC. 2nd denotes cumulative applications of carbachol after a treatment with each blocker or second carbachol application in TMC. *P < 0.05 vs. TMC (ΔEmax).
Before the blocker application, maximum force (Emax) values corrected for wet weight of strips were larger in the control group than in the BOO group (16.4 ± 0.71 mN/mg wet wt, n = 40 vs. 11.6 ± 0.83 mN/mg wet wt, n = 37, P < 0.001). The EC50 values in the control group are smaller than in the BOO group (309.0 ± 20.2 nM vs. 526.0 ± 46.28 nM, P < 0.001). These results indicate decreased contractility and affinity to carbachol of the BOO smooth muscle.
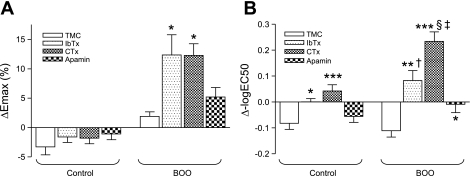
Emax values at the second carbachol application were reduced by 3.29 ± 1.36% without blocker application in the control group (Figs. 5A and 6A). IbTx, CTx, and apamin treatments tended to prevent this spontaneous decline in Emax, but differences in ΔEmax with and without blocker treatments were not statistically significant. On the other hand, significant increases in ΔEmax values were seen in the strips from the BOO group after IbTx and CTx treatment (12.36 ± 3.42%, P < 0.05 and 12.26 ± 2.01%, P < 0.05, respectively) (Figs. 5, F and G and 6A), compared with the changes (1.89 ± 0.79%) in the TMC of BOO rats (Figs. 5E and 6A). The apamin treatment showed a larger but insignificant increase of ΔEmax (5.22 ± 1.61%, Figs. 5H and 6A) compared with the TMC.
Fig. 6.
Comparisons of the effects of K+ channel blockers on the carbachol-induced contractile responses in detrusor smooth muscle strip from control and BOO rats. A: percentage changes of Emax between first and second applications of carbachol (ΔEmax). B: differences of −logEC50 between first and second applications of carbachol (Δ−logEC50). Blocking agents were applied before the second application of carbachol. TMC, time-matched controls. *P < 0.05, **P < 0.01, ***P < 0.001 vs. TMC. §P < 0.05 vs. IbTx application (BOO). †P < 0.05, ‡P < 0.001 vs. control group.
The potassium channel blockers also influenced the EC50 values. The −logEC50 values in the TMC of control and BOO groups were decreased to an equal degree (i.e., decreased affinity) at second applications of carbachol without blockers, while the −logEC50 tended to increase after blocker application (i.e., increased affinity). In both groups, the Δ-logEC50 obtained after IbTx or CTx treatment was larger than that seen in the TMC (Fig. 6B). Furthermore, the CTx treatment showed a greater increase in the Δ−log EC50 than in the IbTx treatment in the BOO group. However, changes in the Δ−log EC50 after the IbTx or CTx treatment were larger in the BOO group than in the control group. On the other hand, there is no significant difference in the Δ−logEC50 obtained after apamin treatment between control and BOO groups, although the apamin treatment showed a significant increase compared with the TMC in the BOO model.
DISCUSSION
The present study describes the impact of BOO on function and expression of Ca2+-activated K+ channels. BOO for 6 wk greatly influenced the mRNA expression of SK2 and SK3 channels, as well as β1 and β4 modulatory subunits of the BK channel. Functional studies using detrusor smooth muscle strips also revealed enhanced effects of BK and SK channel blockers in the detrusor smooth muscle of the obstructed bladder, suggesting an up-regulated inhibitory function of BK and SK channels after BOO.
In this study, we found that mRNA expression of BK modulatory β1 and β4 subunits were increased and decreased, respectively, in the detrusor, while there was no change in the BK α-subunit expression 6 wk after BOO. The BK channel β1-subunit, which is mainly expressed in the smooth muscle dramatically increases Ca2+ and voltage sensitivity of the BK α-subunit, resulting in facilitation of the BK channel function (5, 29, 33). On the other hand, the β4-subunit, which is known to be expressed mainly in neurons, but was proven to be present also in bladder smooth muscle (7), decreases the voltage sensitivity and slows activation kinetics, thereby being called a “down regulator” of the BK channel (5, 51). Therefore, it is assumed that increased and decreased mRNA expression of the BK β1- and β4-subunits, respectively, contributes to the facilitated inhibitory function of BK channels in the detrusor smooth muscle of BOO rats, even though the BK channel α-subunit expression was not changed.
We also detected changes in expression of SK2 and SK3 channel transcripts in the BOO bladder. However, there was discordance in the expression level of the SK2 channel transcripts between mucosa-attached and mucosa-removed tissues (Figs. 1F and 2F). Because the mucosal expression of SK2 channel mRNA was minimal (Fig. 2F), the discrepancy is likely to be related to the detrusor muscle expression. One possible explanation for this discrepancy might be the difference in severity of bladder hypertrophy induced by BOO because the bladder weight of BOO bladders used for mucosa-removed preparations was lower (660 vs. 790 mg) than the weight of s-BOO bladders used for whole bladder experiments although both groups of BOO rats showed NVCs during cystometry. Thus, it is possible that the expression of SK2 channel transcripts in the detrusor is sensitive to the bladder condition after BOO, although mRNA and protein expression of SK2 channels might not correlate with each other after BOO. Nevertheless, the functional study using a SK channel blocker, apamin, showed overall SK channel activity is enhanced in the detrusor smooth muscle of obstructed bladders.
In this study, we did not evaluate protein expression of BK and SK channel and subunits due to the lack of high-quality antibodies against Ca2+-activated K+ channels, especially β-subunits. Therefore, we instead performed muscle strip experiments to examine whether changes in BK and SK channel transcripts are correlated with functional changes in muscle activity. Smooth muscle strips are known to develop spontaneous contractions. These contractions are initiated by action potentials and associated Ca2+ transients through L-type Ca2+ channels (13, 14). This Ca2+ entry through L-type Ca2+ channels can activate both BK and SK channels (17), which regulates the repolarization phase and after hyperpolarization phase of the action potential, respectively (15, 17, 46). Thus BK and SK channel blockers can increase the amplitude of spontaneous bladder contractions (17, 44). In the present study, this effect of IbTx, CTx, and apamin was enhanced in BOO detrusor muscle strips, indicating that both BK and SK channel activity is enhanced following BOO and suppresses spontaneous contractions induced by Ca2+ influx through the L-type Ca2+ channel.
The present study also showed that BOO enhanced the modulatory influence of BK and SK channel on pharmacomechanical coupling in the detrusor smooth muscle as evidenced by increased effects of BK and SK channel blockers on muscle contraction induced by the cholinergic receptor agonist, carbachol. Although the mammalian bladder expresses M2 and M3 muscarinic receptors among five subtypes (M1-M5), physiological bladder contractions largely depend on the M3 receptor subtype activation, which induces an increase of the intracellular Ca2+ concentration essential for initiating the smooth muscle contraction, as well as Ca2+ sensitization due to activation of Rho kinase pathways (4, 32). The proposed cellular mechanisms for muscarinic M3 receptor-mediated increase in intracellular Ca2+ concentrations involve not only Ca2+ influx via L-type Ca2+ channels but also Ca2+ release from intracellular stores, which elicits local increase of Ca2+ concentrations in the cytosol (36, 39, 40, 52).
The increased intracellular Ca2+ concentration following muscarinic receptor activation also activates Ca2+-activated K+ channels. In this study, BK channel blockers increased the Emax and Δ−log EC50 in response to carbachol stimulation as reported previously (16), and the upregulated inhibitory function of BK channels after BOO is indicated by the larger changes of Emax and Δ−log EC50 by BK blockers in obstructed bladders vs. normal bladders. The current study also revealed that the contractile response to carbachol was reduced in the BOO group, as shown by decreased Emax and increased EC50 values compared with the control group. Because BK blockers partially restored Emax and EC50 of carbachol responses in BOO bladders, it seems that enhanced BK channel activity contributes at least, in part, to the reduction in muscarinic receptor-mediated contractility after BOO. However, the effect of apamin on muscarinic receptor-mediated contractions was much smaller compared with the effect of BK channel blockers. This smaller contribution of SK channels to carbachol-induced detrusor contraction might be explained by the previous findings that Ca2+ sparks derived from intracellular Ca2+ stores have smaller effects on SK channel activation compared with BK channels (17).
In contrast to the results of our study, a previous study using 6-wk BOO rats with NVCs reported decreased expression of the BK channel α-subunit and SK channel transcripts in obstructed bladders by using semiquantitative RT-PCR techniques with β-actin as an internal control (24). Although the reason for this discrepancy is unclear, experimental models of BOO in rats are known to yield a range of morphological and functional changes, even if the same surgical procedures are used (41). In our study, we also found a discrepancy in the expression of SK2 channel transcripts in different groups of BOO rats used for whole bladder or detrusor preparations. Therefore, different conditions of experimental models could be one of the reasons of this discrepancy. Another reason that should be considered is the difference of internal controls used to evaluate and standardize the results of the PCR investigation. As the real-time PCR has proven valuable for accurate expression profiling of selected genes, stability of commonly used housekeeping genes adopted from literature as reference genes has been argued (2, 19, 47, 48). In the case of the hypertrophied bladder, several studies reported increased expression of commonly used housekeeping genes such as β-actin and GAPDH at mRNA or protein levels (3, 28). Therefore, in this study, we carefully assessed the variability of several candidates of housekeeping genes and used two selected genes, which showed no significant differences of mRNA expression between control and BOO groups, as internal controls according to an algorithm proposed previously (48).
In animal models of BOO, NVCs are often seen in cystometry, and these observations are regarded as evidence of bladder instability, which is thought to contribute to irritative symptoms (26). Therefore, it is reasonable to speculate that the excitability of detrusor smooth muscle is closely related to bladder instability. However, in the present study, the amplitude and frequency of spontaneous contractions of muscle strips from obstructed rats were smaller than those from control rats. At the cellular level, an increase in excitability of detrusor smooth muscle cell membranes from the obstructed bladder has also been difficult to demonstrate consistently. In terms of spontaneous activity of detrusor muscle, several studies using microelectrode recording in intact bladder tissues reported reduced or unchanged smooth muscle cell excitability in the obstructed bladder (22, 42). On the other hand, increased spontaneous electrical activity was detected using patch-clamp recording techniques in isolated smooth muscle cells from the obstructed bladder (23). Thus, there is the possibility that bladder instability induced by BOO might be related to other factors than increased detrusor smooth muscle excitability. In this regard, recent studies have demonstrated enhanced cell-to-cell interactions of smooth muscle as evidenced by increased expression of gap-junction proteins such as connexin 45 or the increased number of interstitial cells in obstructed bladders (8, 22, 53). Therefore, the enhanced activity of BK and SK channels shown in this study could be a compensatory mechanism to suppress bladder instability induced by BOO. Similar compensatory reactions have been reported in vascular smooth muscle of spontaneous hypertensive rats (6, 9, 25, 37).
These findings support our conclusion that Ca2+-activated K+ channels act in a direction to suppress the excitability of bladder smooth muscle cells in response to either spontaneous or muscarinic stimulation in the BOO condition. Moreover, BOO rats with NVCs (s-BOO group) had reduced voiding efficiency and micturition pressure compared with those without (m-BOO group) in this study. Thus, although inhibition of spontaneous activity might contribute to suppression of BOO-induced bladder instability during the storage phase, a reduction due to BK channel upregulation in muscarinic receptor-mediated contractility, which is directly related to voiding bladder contractions, could be involved in the emergence of voiding dysfunction including decreased voiding efficiency and increased residual volume in BOO.
Perspectives and Significance
Long-term BOO for 6 wk induces changes in the subtype expression of BK and SK channel transcripts of Ca2+-activated K+ channels, which contribute to the enhanced function of these channels in the detrusor smooth muscle. The BOO-induced changes in Ca2+-activated K+ channel activity might be a compensatory mechanism to reduce bladder overactivity following BOO.
GRANTS
This work was supported by grants from the National Institutes of Health (DK057267 and DK068557).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 62: 3–10, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bonefeld BE, Elfving B, Wegener G. Reference genes for normalization: a study of rat brain tissue. Synapse 62: 302–309, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Braverman AS, Ruggieri MR., Sr Muscarinic receptor transcript and protein density in hypertrophied and atrophied rat urinary bladder. Neurourol Urodyn 25: 55–61, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman AS, Tibb AS, Ruggieri MR., Sr M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther 316: 869–874, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chang T, Wu L, Wang R. Altered expression of BK channel beta1 subunit in vascular tissues from spontaneously hypertensive rats. Am J Hypertens 19: 678–685, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory β4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christ GJ, Day NS, Day M, Zhao W, Persson K, Pandita RK, Andersson KE. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol 284: R1241–R1248, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cox RH, Lozinskaya I, Dietz NJ. Differences in K+ current components in mesenteric artery myocytes from WKY and SHR. Am J Hypertens 14: 897–907, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68: 442–448, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ghatta S, Nimmagadda D, Xu X, O'Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 110: 103–116, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hanner M, Schmalhofer WA, Munujos P, Knaus HG, Kaczorowski GJ, Garcia ML. The beta subunit of the high-conductance calcium-activated potassium channel contributes to the high-affinity receptor for charybdotoxin. Proc Natl Acad Sci USA 94: 2853–2858, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol 530: 273–286, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6: 279–284, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kaiho Y, Nishiguchi J, Kwon DD, Chancellor MB, Arai Y, Snyder PB, Yoshimura N. The effects of a type 4 phosphodiesterase inhibitor and the muscarinic cholinergic antagonist tolterodine tartrate on detrusor overactivity in female rats with bladder outlet obstruction. BJU Int 101: 615–620, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem 269: 17274–17278, 1994 [PubMed] [Google Scholar]
- 22.Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H, Kohri K. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn 27: 330–340, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Li L, Jiang C, Hao P, Li W, Fan L, Zhou Z, Song B. Changes in T-type calcium channel and its subtypes in overactive detrusor of the rats with partial bladder outflow obstruction. Neurourol Urodyn 26: 870–878, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Li L, Jiang C, Song B, Yan J, Pan J. Altered expression of calcium-activated K and Cl channels in detrusor overactivity of rats with partial bladder outlet obstruction. BJU Int 101: 1588–1594, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension 30: 1403–1409, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Malmgren A, Sjogren C, Uvelius B, Mattiasson A, Andersson KE, Andersson PO. Cystometrical evaluation of bladder instability in rats with infravesical outflow obstruction. J Urol 137: 1291–1294, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 369: 481–489, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Mannikarottu AS, Disanto ME, Zderic SA, Wein AJ, Chacko S. Altered expression of thin filament-associated proteins in hypertrophied urinary bladder smooth muscle. Neurourol Urodyn 25: 78–88, 2006 [DOI] [PubMed] [Google Scholar]
- 29.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14: 645–650, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Michel MC, Barendrecht MM. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol Ther 117: 297–312, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinna C, Sanvito P, Bolego C, Cignarella A, Puglisi L. Effect of the ATP-sensitive potassium channel opener ZM226600 on cystometric parameters in rats with ligature-intact, partial urethral obstruction. Eur J Pharmacol 516: 71–77, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, Klaerke DA. Differential expression of BK channel isoforms and beta-subunits in rat neuro-vascular tissues. Biochim Biophys Acta 1788: 380–389, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Rivera L, Brading AF. The role of Ca2+ influx and intracellular Ca2+ release in the muscarinic-mediated contraction of mammalian urinary bladder smooth muscle. BJU Int 98: 868–875, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Rusch NJ, De Lucena RG, Wooldridge TA, England SK, Cowley AW., Jr A Ca2+-dependent K+ current is enhanced in arterial membranes of hypertensive rats. Hypertension 19: 301–307, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Schneider T, Fetscher C, Krege S, Michel MC. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Ther 309: 1148–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Schneider T, Hein P, Michel MC. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I Phospholipases and Ca2+ sources. J Pharmacol Exp Ther 308: 47–53, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Schroder A, Colli E, Maggi M, Andersson KE. Effects of a vitamin D(3) analogue in a rat model of bladder outlet obstruction. BJU Int 98: 637–642, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Seki N, Karim OM, Mostwin JL. Changes in electrical properties of guinea pig smooth muscle membrane by experimental bladder outflow obstruction. Am J Physiol Renal Fluid Electrolyte Physiol 262: F885–F891, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Shieh CC, Turner SC, Zhang XF, Milicic I, Parihar A, Jinkerson T, Wilkins J, Buckner SA, Gopalakrishnan M. A-272651, a nonpeptidic blocker of large-conductance Ca2+-activated K+ channels, modulates bladder smooth muscle contractility and neuronal action potentials. Br J Pharmacol 151: 798–806, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suarez-Kurtz G, Garcia ML, Kaczorowski GJ. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther 259: 439–443, 1991 [PubMed] [Google Scholar]
- 45.Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ES, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol 294: R1737–R1743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005 [DOI] [PubMed] [Google Scholar]
- 47.van Wijngaarden P, Brereton HM, Coster DJ, Williams KA. Stability of housekeeping gene expression in the rat retina during exposure to cyclic hyperoxia. Mol Vis 13: 1508–1515, 2007 [PubMed] [Google Scholar]
- 48.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes (Online). Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci USA 96: 4137–4142, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, Ge P, Wang C, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, DiStefano PS, Curtis R. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J Neurosci 20: 3563–3570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuest M, Hiller N, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Contribution of Ca2+ influx to carbachol-induced detrusor contraction is different in human urinary bladder compared to pig and mouse. Eur J Pharmacol 565: 180–189, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, Tyagi P. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol 377: 437–448, 2008 [DOI] [PubMed] [Google Scholar]