Abstract
Placental insufficiency results in intrauterine growth restriction (IUGR) and hypertension in adult male growth-restricted rats. Although renal ANG II and plasma renin activity do not differ between growth-restricted and control rats, blockade of the renin-angiotensin system (RAS) abolishes hypertension in growth-restricted rats, suggesting that the RAS contributes to IUGR-induced hypertension. Moreover, castration abolishes hypertension in growth-restricted rats, indicating an important role for testosterone. Therefore, we hypothesized that enhanced responsiveness to ANG II contributes to hypertension in this model of IUGR and that androgens may play a pivotal role in this enhanced response. Physiological parameters were determined at 16 wk of age in male rats pretreated with enalapril (40 mg·kg−1·day−1) for 1 wk. Baseline blood pressures were similar between growth-restricted (112 ± 3 mmHg) and control (110 ± 2 mmHg) rats; however, an enhanced pressor response to acute ANG II (100 ng·kg−1·min−1 for 30 min) was observed in growth-restricted (160 ± 2 mmHg) vs. control (136 ± 2 mmHg; P < 0.05) rats. Castration abolished the enhanced pressor response to acute ANG II in growth-restricted (130 ± 2 mmHg) rats with no significant effect on blood pressure in controls (130 ± 2 mmHg). Blood pressure was increased to a similar extent above baseline in response to acute phenylephrine (100 μg/min) in control (184 ± 5 mmHg) and growth-restricted (184 ± 8 mmHg) rats, suggesting the enhanced pressor response in growth-restricted rats is ANG II specific. Thus, these results suggest that growth-restricted rats exhibit an enhanced responsiveness to ANG II that is testosterone dependent and indicate that the RAS may serve as an underlying mechanism in mediating hypertension programmed in response to IUGR.
Keywords: sex steroids, blood pressure, developmental programming
adverse influences during early life can lead to an increased risk for cardiovascular and renal disease (4, 21). Using a model of reduced uterine perfusion in the pregnant rat, our laboratory has begun to elucidate the mechanisms linking intrauterine growth restriction (IUGR) and blood pressure (1). We previously reported that male growth-restricted offspring from reduced uterine perfusion dams exhibit a marked increase in arterial pressure in adulthood that is associated with a marked increase in intrarenal renin and angiotensinogen mRNA expression, and a marked increase in renal angiotensin I-converting enzyme (ACE) activity (9). However, despite these increases in intrarenal renin and angiotensinogen mRNA expression and intrarenal ACE activity, expression of intrarenal ANG II and its receptor, AT1R, are similar in hypertensive male growth-restricted rats compared with normotensive age-matched male control rats (9). Moreover, plasma renin activity, plasma renin substrate, and serum ACE activity are not altered in adult male growth-restricted rats relative to control (9, 22) suggesting that inappropriate activation of the systemic renin angiotensin system (RAS) is not observed in hypertensive male growth-restricted offspring. However, blockade of the RAS abolishes IUGR-induced hypertension (22), suggesting that the RAS contributes to the etiology of hypertension in male growth-restricted offspring; yet, the exact mechanism by which the RAS contributes to hypertension in this model of IUGR is not clear.
Castration abolishes hypertension in adult male growth-restricted rats with no significant effect on blood pressure in adult male control rats, suggesting that hypertension in adult male growth-restricted rats is testosterone dependent (22). The mechanism by which testosterone modulates blood pressure in adult male growth-restricted rats is not yet elucidated. Furthermore, whether modulation of the RAS by testosterone plays an important role in mediating hypertension induced by placental insufficiency in male growth-restricted offspring is also not known.
A role for modulation of the RAS by testosterone is observed in genetic models of experimental hypertension. Testosterone is implicated to play a critical role in mediating hypertension in the spontaneously hypertensive rat (SHR) (13, 19, 25), and modulation of the RAS by testosterone exacerbates hypertension in male SHR (26). Upregulation of renal angiotensinogen expression by testosterone in the SHR may serve as one mechanism by which modulation of the RAS by testosterone contributes to the development of hypertension in the SHR (5, 7). However, testosterone mediated increases in renal angiotensinogen expression are not always associated with increases in expression of the renal ANG II receptors (24). Moreover, expression of the renal AT1R is not altered by castration in the New Zealand genetically hypertensive (NZGH) rat (29), yet the enhanced pressor response to acute ANG II observed in male NZGH rats is abolished by castration, indicating that increased sensitivity to ANG II is testosterone dependent in this genetic model of hypertension (29). Thus, testosterone-dependent hypertension may be mediated via modulation of the RAS; and modulation of the RAS may not always involve upregulation of the intrarenal RAS.
A critical role for the RAS is indicated in models of developmental programming of hypertension (17, 27, 31, 32), and exaggerated vasomotor responses to acute ANG II (33) may serve as a potential mechanism. Thus, the aims of this study were to determine whether an enhanced responsiveness to ANG II is observed in adult male growth-restricted rats and whether the enhanced responsiveness to ANG II in adult male growth-restricted rats is testosterone dependent.
MATERIALS AND METHODS
Animals.
All experimental procedures were in accordance with National Institutes of Health guidelines, and our protocol was approved by the Animal Care and Use Committee at the University of Mississippi Medical Center. Rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle with food and water available ad libitum. Timed pregnant Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). At day 14 of gestation, rats destined for reduced uterine perfusion were clipped as described in Reduced uterine perfusion in the pregnant rat. All dams were allowed to deliver at term with birth weight recorded within 12 h of delivery. At this time, the number of pups in control and reduced uterine perfusion litters were culled to eight pups per dam to ensure equal nutrient access for all offspring. The ratio of male to female pups remained equivalent after culling when possible; however, only male pups were utilized for this study. Animals were weighed twice weekly. Pups were weaned at 3 wk of age. Male offspring from 15 control pregnant and 12 reduced uterine perfusion pregnant litters were randomly assigned into four groups from different dams: 11 intact control and 6 castrated (CTX) control offspring from control pregnant dams and 8 intact growth-restricted and 6 castrated growth-restricted offspring from reduced uterine perfusion pregnant rats for all hemodynamic measurements; the pressor response to ANG I and phenylephrine (PE) were determined in a subset of five intact male control and six intact male growth-restricted offspring. Vessels for analysis of morphology were from a separate set of animals and included six groups: intact untreated control and intact untreated growth-restricted, intact treated control, and intact treated growth-restricted (enalapril; 40 mg·kg−1·day−1 for 1 wk), and untreated castrated control and untreated castrated growth-restricted (n = 4 rats per group).
Reduced uterine perfusion in the pregnant rat.
Reduced utero-placental perfusion, as previously described (1), was utilized for induction of IUGR. Control dams were subjected to a sham reduced uterine perfusion procedure that involved exposure of the uterine horn. Briefly, rats undergoing surgical procedures were anesthetized with 2% isoflurane. At day 14 of gestation, a silver clip (0.203-mm ID) was placed around the lower abdominal aorta above the iliac bifurcation. Since compensation of blood flow occurs through an adaptive increase in ovarian blood flow, a silver clip was slipped around each branch of the ovarian arteries (0.100-mm ID).
Castration in male offspring.
As previously described (22), rats were anesthetized at 10 wk of age with isoflurane, and a small median incision was made at the distal tip of the scrotum. Subcutaneous connective tissue was cleared, and the testes were visualized. The muscular sac of the testes was excised and exposed by gently pulling on the cauda epididymis. The blood vessels were tied, and the vas deferens with the testes removed followed by closure of the muscle and the skin. Sham operation involved exposure of the testes without isolation.
Drug administration.
The ACE inhibitor, enalapril (40 mg·kg−1·day−1) (Sigma Aldrich, St. Louis, MO) was administered in the drinking water from 15 to 16 wk of age at a dose previously shown to block the endogenous production of ANG II in the rat (2). Water consumption was monitored daily for the duration of the treatment period. At 16 wk of age, ANG II (100 ng·kg−1·min−1 in 0.9% saline solution; Sigma Aldrich) was administered acutely during the measurement of renal and systemic hemodynamics. In a subset of animals the dose response to acute ANG I (0.025, 0.25, 2.5, and 25 mg/kg body wt in 0.9% saline solution) was used to ensure complete blockade of endogenous RAS. In addition, a dose response to PE (1, 10, and 100 μg·kg−1·min−1 in 0.9% saline solution, Sigma Aldrich) was administered acutely to determine whether the enhanced pressor response to acute ANG II in growth-restricted rats was specific to ANG II. For these studies, doses were delivered at 50 μl/min, and blood pressure values were allowed to return to baseline between each dose.
Measurement of systemic and renal hemodynamics.
As previously described (1), during isoflurane anesthesia, rats were surgically instrumented with flexible catheters (PE-50 tubing) in the right jugular vein for infusion of ANG I, ANG II, and PE, and in the right carotid artery for measurement of arterial pressure and collection of blood; the bladder was instrumented with a flexible catheter (PE-90 tubing) for collection of urine. All catheters were tunneled to the nape of the neck and exteriorized. Renal function and arterial pressure measurements were performed in the conscious state after a 2-day recovery phase. Mean arterial pressure (MAP) was monitored in conscious, chronically instrumented rats via connection of the arterial catheter to a pressure transducer and a data acquisition kit (DATAQ Instruments, Akron, OH) with a computer for continuous recording. Glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) were calculated from radioactivity of I125-labeled iothalamate and concentration of PAH, respectively, in plasma and urine. Renal vascular resistance (RVR) and filtration fraction (FF) were calculated as RVR = (MAP/ERPF) × (1 − hematocrit) and FF = (GFR/ERPF), respectively. Data were collected during an initial 20-min clearance and then for an additional 20-min clearance during the acute infusion of ANG II, and ANG I or PE for comparison between adult male control and adult male growth-restricted rats.
Tissue morphology.
Thoracic aortas were collected from male control and male growth-restricted offspring randomly assigned from different dams into six groups: intact untreated control, intact untreated growth-restricted, intact treated control, intact treated growth-restricted (enalapril; 40 mg·kg−1·day−1 for 1 wk), untreated castrated control, and untreated castrated growth-restricted. After collection, vessels were placed in 10% phosphate buffered formalin, embedded in paraffin, sectioned (4-μm thickness), then stained with hematoxylin and eosin (Suripath Medical Industries, Leica, IL). Aortic medial wall thickness was determined by software analysis (NIS Elements, ver. 3; Nikon, Tokyo, Japan). Measurement of aortic wall thickness was obtained from four sections from each rat by an examiner blinded to sample identity.
Plasma testosterone levels.
Blood samples were collected following decapitation at the end of the study to determine serum testosterone using a commercially available radioimmunoassay kit (Coat-A-Count Total Testosterone Assay kit; Diagnostics Products, Los Angeles, CA).
Statistics.
Data are presented as mean values ± SE. Systemic and renal hemodynamic responses to ANG II, ANG I, and PE, comparison of body and kidney weights, and vessel morphology in groups studied were compared using one-way repeated-measures ANOVA (Prism 5.0, GraphPad, San Diego, CA). Post hoc testing was performed using Student-Newman-Keuls multiple-comparisons test where appropriate. Differences were reported as significant when P < 0.05.
RESULTS
Birth weight, body weight, kidney weight, and water consumption.
Birth weight was significantly reduced in male growth-restricted rats compared with male control rats (6.3 ± 0.2 vs. 7.1 ± 0.2 g; P < 0.05, IUGR vs. control, respectively). However, by 16 wk of age, body weight did not differ upon comparison of male growth-restricted to male control; castration had no significant effect on body weight (Table 1). Kidney weight at 16 wk of age did not differ upon comparison of male growth-restricted rats to male control (1.6 ± 0.3 vs. 1.3 ± 0.3 g; IUGR vs. control, respectively), intact or castrated, and the kidney-to-body weight ratio did not differ upon comparison of male growth-restricted to male control rats at 16 wk of age, intact or castrated (Table 1). Water consumption was monitored daily during the administration of the ACE inhibitor, enalapril, and the average daily volume did not differ between male growth-restricted vs. male control rats, intact, or castrated (Table 1).
Table 1.
Renal hemodynamic parameters and body and kidney weights under chronic ACE inhibition (enalapril for 7 days at 40 mg/kg/day) in intact and CTX male control and IUGR rats
| Intact Control | Intact IUGR | CTX Control | CTX IUGR | |
|---|---|---|---|---|
| Baseline | ||||
| GFR, ml/min | 5.8 ± 0.5 | 3.9 ± 0.4 | 5.0 ± 0.6 | 4.5 ± 0.5 |
| GFR, ml·min−1·g kidney−1 | 2.0 ± 0.1 | 1.3 ± 0.1* | 2.1 ± 0.2 | 1.7 ± 0.2 |
| eRPF, ml/min | 9.4 ± 0.5 | 9.6 ± 0.5 | 10.1 ± 0.6 | 10.0 ± 0.7 |
| RVR, mmHg·ml−1·min−1 | 6.6 ± 0.3 | 6.7 ± 0.4 | 6.9 ± 0.5 | 6.8 ± 0.4 |
| Filtration fraction | 0.3 ± 0.04 | 0.4 ± 0.05 | 0.3 ± 0.05 | 0.4 ± 0.09 |
| ANG II infusion | ||||
| GFR, ml/min | 3.7 ± 0.3 | 0.5 ± 0.1* | 2.8 ± 0.1 | 3.4 ± 0.3† |
| GFR, ml·min−1·g kidney−1 | 1.3 ± 0.1 | 0.1 ± 0.1* | 1.2 ± 0.1 | 1.3 ± 0.1† |
| eRPF, ml/min | 5.8 ± 0.3 | 2.9 ± 0.4* | 5.5 ± 0.6 | 6.4 ± 0.5† |
| RVR, mmHg·ml−1·min−1 | 15.2 ± 0.3 | 35.8 ± 5.2* | 16.8 ± 1.3 | 15.5 ± 0.6† |
| Filtration fraction | 0.2 ± 0.03 | 0.2 ± 0.06 | 0.2 ± 0.02 | 0.5 ± 0.04† |
| Body weight, g | 399 ± 9 | 388 ± 12 | 375 ± 9 | 375 ± 8 |
| Kidney/body weight ratio | 0.70 ± 0.04 | 0.80 ± 0.03 | 0.60 ± 0.04 | 0.70 ± 0.03 |
| Water consumption, ml/day | 60.8 ± 0.7 | 62.0 ± 1.0 | 60.8 ± 0.6 | 59.6 ± 0.6 |
Data are presented as means ± SE. IUGR, intrauterine growth restriction; CTX, castrated; GFR, glomerular filtration rate; ERPF, effective renal plasma flow; RVR, renal vascular resistance.
P < 0.05 vs. Intact Control;
P < 0.05 vs. Intact IUGR.
Pressor responses to acute ANG II.
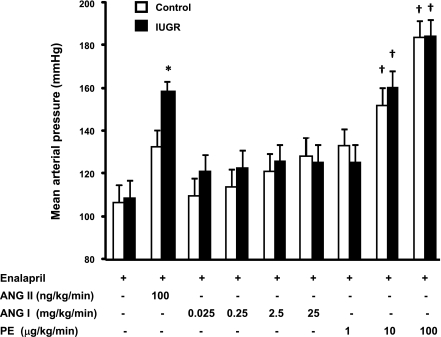
Following chronic treatment with the ACE inhibitor, enalapril, blood pressure was comparable in intact male control and intact male growth-restricted rats (Fig. 1). Both intact male control and intact male growth-restricted rats exhibited a pressor response to an acute infusion of ANG II; however, the increase in blood pressure in response to acute ANG II was significantly greater in the intact male growth-restricted rats (increase of 48 mmHg) compared with the increase in blood pressure in response to an acute infusion of ANG II in intact male control rats (increase of 28 mmHg) (P < 0.05) (Fig. 1). Acute infusion of PE increased blood pressure in control and growth-restricted rats relative to baseline values in a dose-dependent manner; however, the increase in blood pressure in male growth-restricted rats was not enhanced relative to the increase in blood pressure in male control rats, suggesting that the enhanced pressor response observed in male growth-restricted rats is specific for ANG II (Fig. 2). Acute infusion of ANG I did not lead to a significant increase in blood pressure in either control or growth-restricted rats, indicating that blockade of the endogenous RAS was complete (Fig. 2).
Fig. 1.
Mean arterial pressure (MAP) at baseline and following an acute infusion of ANG II (100 ng·kg−1·min−1) in intact and castrated conscious, chronically instrumented animals pretreated with the angiotensin I-converting enzyme (ACE) inhibitor, enalapril. *P < 0.05 vs. baseline counterpart. †P < 0.05 vs. Intact Control ANG II. ‡P < 0.05 vs. Intact intrauterine growth restricted (IUGR) + ANG II. All data are expressed as means ± SE.
Fig. 2.
The pressor response to an acute infusion of ANG I, ANG II, or phenylephrine (PE) in conscious, chronically instrumented control and IUGR offspring pretreated with the ACE inhibitor, enalapril. *P < 0.05 vs. ANG II Control. †P < 0.05 vs. baseline counterpart. All data are expressed as means ± SE.
Renal hemodynamic responses to acute ANG II.
RVR, GFR, and ERPF did not significantly differ upon comparison of intact male growth-restricted rats to intact male control rats following chronic treatment with the ACE inhibitor, enalapril (Table 1). However, in response to an acute infusion of ANG II, RVR was increased to a greater extent in intact male growth-restricted rats compared with intact male control rats (P < 0.05, IUGR vs. control), whereas GFR and ERPF were decreased to a greater extent in intact male growth-restricted rats compared with intact male control rats (P < 0.05, IUGR vs. control) (Table 1). Under baseline conditions, GFR normalized to kidney weight decreased in male growth-restricted rats relative to male control rats (P < 0.05, IUGR vs. control). In response to acute ANG II, the decrease in GFR normalized to kidney weight was also potentiated in male growth-restricted rats compared with male control (P < 0.05, IUGR vs. control) (Table 1). FF did not differ significantly upon comparison of intact male growth-restricted with intact male control rats under baseline conditions or in response to acute ANG II (Table 1).
Response to castration and acute ANG II.
Castration had no effect on the pressor response to acute ANG II in control rats (Fig. 1). However, castration abolished the enhanced pressor response to acute ANG II in male growth-restricted rats (P < 0.05 CTX IUGR vs. intact IUGR), normalizing it relative to the pressor response to acute ANG II observed in control rats (Fig. 1). Castration also abolished the enhanced increase in RVR in male growth-restricted rats observed in response to acute ANG II (P < 0.05 CTX IUGR vs. intact IUGR) with no significant effect on RVR in control rats (Table 1). The enhanced decrease in GFR induced in response to acute ANG II was also abolished by castration in male growth-restricted rats (P < 0.05, CTX IUGR vs. intact IUGR); a similar observation was noted when GFR was adjusted per kidney weight (Table 1). Castration also abolished the enhanced decrease in ERPF in male growth-restricted rats (P < 0.05, CTX IUGR vs. intact IUGR). FF was not altered by castration (Table 1).
Vessel morphology.
Aortic medial wall thickness was not altered in intact untreated male growth-restricted offspring relative to untreated intact male control offspring at 16 wk of age (Fig. 3). Aortic medial wall thickness at 16 wk of age was not altered by castration, castrated control, and castrated growth-restricted animals vs. intact control or intact growth-restricted animals (Fig. 3). In addition, treatment with enalapril for 1 wk, as utilized in the experimental protocol for acute ANG II, did not alter aortic medial wall thickness (Fig. 3).
Fig. 3.
Aortic medial wall thickness in untreated intact control and untreated intact IUGR offspring, in castrated (CTX) control and castrated growth-restricted offspring, and in intact enalapril-treated control and intact enalapril treated growth-restricted offspring. All data are expressed as means ± SE.
Serum testosterone levels.
Serum testosterone measured at 16 wk of age was significantly increased in intact male growth-restricted rats compared with intact male control rats; testosterone levels were significantly reduced by castration in male control and male growth-restricted rats compared with their intact counterparts (Fig. 4).
Fig. 4.
Serum testosterone levels in intact and castrated control and IUGR offspring. *P < 0.05 vs. Intact Control. †P < 0.05 vs. Intact counterpart. All data are expressed as means ± SE.
DISCUSSION
This study tested the hypothesis that male growth-restricted rats exhibit an enhanced pressor response to acute ANG II and that testosterone contributes to this enhanced response. The main findings indicate that not only is the pressor response to acute ANG II enhanced in male growth-restricted offspring from reduced uterine perfusion dams, but the enhanced pressor response to ANG II in male growth-restricted rats is abolished by castration. Previously, we demonstrated that blockade of the RAS by chronic ACE inhibition abolishes hypertension in male growth-restricted rats, decreasing blood pressure to levels comparable to chronically treated control rats (22). However, the mechanism by which the RAS contributes to male IUGR-induced hypertension is not clear. Intrarenal ANG II levels and density of its type 1 receptor are not increased in adult male growth-restricted rats relative to adult male control rats (9, 22); however, intrarenal ACE activity is significantly elevated in adult male growth-restricted rats (9). Whether regional differences in ANG II and expression of its receptors are present in male growth-restricted rats has not been confirmed. Accordingly, this study was conducted in rats in which the endogenous RAS was blocked by chronic ACE inhibition. Importantly, this study indicated that an enhanced response to acute ANG II was observed in male growth-restricted rats that demonstrated a baseline blood pressure comparable to control rats under conditions of RAS blockade. Therefore, this study conducted under conditions of chronic RAS blockade suggests that the enhanced pressor response to acute ANG II observed in male growth-restricted rats is not due to differences in endogenous RAS but may involve an increased responsiveness to ANG II that is programmed in response to placental insufficiency and subsequent IUGR. Importantly, the pressor response to increasing doses of another vasoconstrictor, phenylephrine, was similar in control and growth-restricted rats, suggesting that the enhanced pressor response to acute ANG II in growth-restricted rats is specific to ANG II.
The kidneys play a critical role in the long-term control of arterial pressure via regulation of sodium and fluid homeostasis (10). The RAS, an important regulator of arterial pressure and body fluid volume, mediates its effects via alterations in tubular reabsorption and/or glomerular filtration (8, 11). We previously reported that under basal conditions, or in the absence of RAS blockade, GFR and GFR normalized to kidney weight do not differ in adult male growth-restricted rats relative to adult male control rats (1). However, in response to acute ANG II GFR and GFR normalized to kidney weight were significantly decreased in male growth-restricted rats relative to the decrease in observed in response to acute ANG II in control rats, suggesting male growth-restricted rats exhibit an enhanced renal vascular sensitivity to ANG II. RVR is also not altered in adult male growth-restricted rats relative to adult male control rats under basal conditions (1). However, in this study, RVR was markedly increased in both male control and male growth-restricted rats in response to acute ANG II. However, the increase in RVR observed in male growth-restricted rats was potentiated relative to the increase in RVR observed in male control rats. Thus, these findings indicate that an enhanced responsiveness of the renal vasculature to ANG II may contribute to IUGR hypertension programmed in response to fetal insult.
Enhanced renal vascular responses to ANG II are observed in the SHR (16), and hypertension in the male SHR is androgen dependent (26). Previously, we reported that hypertension in male growth-restricted rats is abolished by castration, suggesting a critical role for testosterone in IUGR hypertension (22). The current study demonstrates that the enhanced pressor response to acute ANG II in male growth-restricted rats was abolished by castration. This finding suggests that the enhanced responsiveness to ANG II in male growth-restricted rats is testosterone dependent and importantly, indicates that involvement of the RAS in IUGR-induced hypertension may necessitate modulation by testosterone.
The mechanism by which testosterone mediates the enhanced sensitivity to acute ANG II in male growth-restricted rats is not clear. ANG II activation in hypertension may involve an ANG II postreceptor phenomenon (30). Thus, in the absence of an increase in renal AT1R expression in male growth-restricted rats, the enhanced response to acute ANG II may indicate that functional differences in response to acute ANG II are not mediated via differences in renal AT1R expression but may involve testosterone-mediated alterations in signaling cascades downstream of the renal ANG II receptor. Expression of the renal AT1R is not elevated in the NZGH rat (29). In this genetic model of hypertension, testosterone potentiated renal vascular responses to ANG II are partially mediated through upregulation of the Rho kinase signaling pathway (28). Thus, testosterone-dependent hypertension may be mediated via modulation of the RAS, although modulation of the RAS may not always involve upregulation of the intrarenal RAS. Whether testosterone-modulated control of Rho kinase or other downstream signaling pathways contributes to the enhanced responsiveness to acute ANG II in male growth-restricted rats is unknown. However, the enhanced increase in RVR in male growth-restricted rats relative to control rats in response to acute ANG II, the decrease in GFR in male growth-restricted rats in response to acute ANG II, and the attenuation of renal sensitivity to acute ANG II by castration in male growth-restricted rats suggests that testosterone potentiates sensitivity to ANG II via renal mediated mechanisms in this model of IUGR and will be the focus of future investigation.
Testosterone may also potentiate sensitivity to ANG II via centrally mediated mechanisms. Androgen receptors colocalize in areas of the central nervous system critical to the long-term regulation of blood pressure (12). Moreover, central AT1R expression is markedly increased in areas of the brain critical to cardiovascular regulation in offspring programmed by low protein during fetal life (23). Hypertension in low-protein offspring is abolished by central blockade of the AT1R (23), indicating a critical role for central ANG II in this model of developmental programming. Moreover, vascular responses to ANG II are potentiated in low-protein offspring (33). Thus, testosterone-mediated activation of the central RAS may be one mechanism by which an enhanced responsiveness to acute ANG II is programmed in response to gestational influences. Central actions of ANG II can also modulate renal sympathetic nerve activity (6). We previously reported that bilateral renal denervation abolishes hypertension in male growth-restricted rats, suggesting that increased renal sympathetic nerve activity (RSNA) contributes to IUGR-programmed hypertension (3). Whether an increase in RSNA is secondary to the generation of ANG II and whether central cardiovascular effects of ANG II contribute to the etiology of hypertension in this model of IUGR is unknown and requires further investigation. However, activation of the central RAS by testosterone, leading to an increase in renal sympathetic nerve activity, may be another mechanism that contributes to hypertension in male growth-restricted rats.
The enhanced response to ANG II observed in this study may also be an effect of preexisting vascular hypertrophy. Hypertension is associated with remodeling of the resistance vessels, resulting in amplified responses to vasoconstrictors (20). Treatment with chronic ACE inhibition may (18) or may not (14) reverse vascular hypertrophy, suggesting that preexisting vascular hypertrophy could continue following chronic ACE inhibition in this study. Additionally, testosterone is reported to promote cardiac hypertrophy and vascular remodeling (15), suggesting that testosterone-mediated alterations in vascular structure could potentiate vascular hypertrophy in male growth-restricted offspring and contribute to the enhanced pressor response to acute ANG II. Structurally mediated increases in the wall:lumen ratio of resistance vessels can lead to an increase in peripheral resistance (20). However, we previously reported that RVR is not increased in adult male growth-restricted offspring (1). Moreover, in this study, we report that aortic medial wall thickness does not differ upon comparison of intact growth-restricted rats relative to intact control rats, whether untreated or treated with chronic ACE inhibition. In addition, castration at 10 wk of age did not alter vessel morphology. Thus, these data indicate that the enhanced response to acute ANG II is not due to preexisting vascular hypertrophy amplifying the response. Given that an enhanced pressor response to acute PE is not observed in growth-restricted rats relative to control and that castration abolishes the ANG II-specific response, these findings suggest that growth-restricted rats exhibit an enhanced responsiveness to ANG II that is ANG II specific and testosterone dependent; therefore, indicating that the RAS may serve as an underlying mechanism in mediating hypertension programmed in response to IUGR.
Perspectives and Significance
To conclude, findings from this study suggest that modulation of the RAS by testosterone contributes to hypertension in male growth-restricted rats. The exact mechanism(s) responsible for testosterone modulation of the RAS in this model of IUGR hypertension is not clear but may involve alterations in post-ANG II receptor signaling cascades or contributions from centrally mediated mechanisms. Further studies are needed to clarify the complex pathways that mediate hypertension and ANG II sensitivity in adult male growth-restricted offspring. Importantly, experimental studies investigating the role of sex hormones in mediating hypertension programmed in response to fetal insult may provide insight into the critical mechanisms linking sex hormones and factors key to the long-term control of blood pressure.
GRANTS
Dr. Alexander is supported by the National Institutes of Health Grants HL074927 and HL51971.
DISCLOSURES
The authors have no relationships that could be perceived as real or apparent conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank the Department of Physiology Histology core; specifically, Dr. Christine Maric and Mrs. Stephanie Peters for their excellent assistance with histological evaluation of vessel morphology.
REFERENCES
- 1.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension 38: 742–745, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low birth weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The origins of the developmental origins theory. J Intern Med 261: 412–417, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992 [DOI] [PubMed] [Google Scholar]
- 6.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 175–197, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest 83: 1941–1945, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granger JP, Schnackenberg CG. Renal mechanisms of angiotensin II-induced hypertension. Semin Nephrol 20: 417–425, 2000 [PubMed] [Google Scholar]
- 9.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin-angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol Suppl 12: S258–S265, 1999 [PubMed] [Google Scholar]
- 11.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl 55: S35–S41, 1996 [PubMed] [Google Scholar]
- 12.Heritage AS, Stumpf WE, Sar M, Grant LD. (3-H)-dihydrotestosterone in catecholamine neurons of rat brain stem: combined localization by autoradiography and formaldehyde-induced fluorescence. J Comp Neurol 200: 289–330, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Jenkins C, Salisbury R, Ely D. Castration lowers and testosterone restores blood pressure in several rat strains on high sodium diets. Clin Exp Hypertens 16: 611–625, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Kett MM, Alcorn D, Bertram JF, Anderson WP. Enalapril does not prevent renal arterial hypertrophy in spontaneously hypertensive rats. Hypertension 25: 335–342, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Kienitz T, Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press Res 31: 71–79, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kost CK, Jr, Herzer WA, Li P, Jackson EK. Vascular reactivity to angiotensin II is selectively enhanced in the kidneys of spontaneously hypertensive rats. J Pharmacol Exp Ther 269: 82–88, 1994 [PubMed] [Google Scholar]
- 17.Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans 27: 88–93, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lee RMKW, Berecek KH, Tsoporis J, McKenzie R, Triggle CR. Prevention of hypertension and vascular changes by captopril treatment. Hypertension 17: 141–150, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Martin DS, Biltoft S, Redetzke R, Vogel E. Castration reduces blood pressure and autonomic venous tone in male spontaneously hypertensive rats. J Hypertens 23: 2229–2236, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci 17: 105–109, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ojeda NB, Grigore D, Alexander BT. Developmental programming of hypertension: Insight from animal models of nutritional manipulation. Hypertension 52: 44–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R776, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res 55: 1042–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, Harris RC, Capdevila J, Quigley R. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 287: F452–F459, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 44: 796–799, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in the development of hypertension in SHR: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 104: 607–614, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res 72: 456–463, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Song J, Kost CK, Jr, Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R1608–R1615, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000 [PubMed] [Google Scholar]
- 31.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol 287: F262–F267, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension 50: 579–584, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Yzydorczyk C, Gobeil F, Jr, Cambonie G, Lahaie I, Lê NL, Samarani S, Ahmad A, Lavoie JC, Oligny LL, Pladys P, Hardy P, Nuyt AM. Exaggerated vasomotor response to ANG II in rats with fetal programming of hypertension associated with exposure to a low-protein diet during gestation. Am J Physiol Regul Integr Comp Physiol 291: R1060–R1068, 2006 [DOI] [PubMed] [Google Scholar]