Abstract
Endothelial nitric oxide synthase (eNOS) is associated with a number of physiological functions involved in the regulation of metabolism; however, the functional role of eNOS is poorly understood. We tested the hypothesis that eNOS is critical to muscle cell signaling and fuel usage during exercise in vivo, using 16-wk-old catheterized (carotid artery and jugular vein) C57BL/6J mice with wild-type (WT), partial (+/−), or no expression (−/−) of eNOS. Quantitative reductions in eNOS expression (∼40%) elicited many of the phenotypic effects observed in enos−/− mice under fasted, sedentary conditions, with expression of oxidative phosphorylation complexes I to V and ATP levels being decreased, and total NOS activity and Ca2+/CaM kinase II Thr286 phosphorylation being increased in skeletal muscle. Despite these alterations, exercise tolerance was markedly impaired in enos−/− mice during an acute 30-min bout of exercise. An eNOS-dependent effect was observed with regard to AMP-activated protein kinase signaling and muscle perfusion. Muscle glucose and long-chain fatty acid uptake, and hepatic and skeletal muscle glycogenolysis during the exercise bout was markedly accelerated in enos−/− mice compared with enos+/− and WT mice. Correspondingly, enos−/− mice exhibited hypoglycemia during exercise. Thus, the ablation of eNOS alters a number of physiological processes that result in impaired exercise capacity in vivo. The finding that a partial reduction in eNOS expression is sufficient to induce many of the changes associated with ablation of eNOS has implications for chronic metabolic diseases, such as obesity and insulin resistance, which are associated with reduced eNOS expression.
Keywords: mitochondria, oxidative phosphorylation, blood flow
endothelial nitric oxide synthase (eNOS) is one of three NOS isozymes, which catalyze the reaction of l-arginine to l-citrulline and nitric oxide (NO) (35). eNOS is expressed in a number of different tissues (28), and on the basis of studies using genetically modified mice, the production of NO via eNOS is thought to play a role in a number of physiological functions ranging from, but not limited to, the regulation of vascular tone (13, 20, 25, 32), cardiovascular function (4), and skeletal muscle mitochondrial function and biogenesis (26, 47). A partial impairment in eNOS expression in skeletal muscle also correlates with the pathogenesis of chronic metabolic disease states such as obesity and insulin resistance (11, 18, 47). However, the degree to which the diverse biochemical and morphological changes associated with the reduction of eNOS translate into functional metabolic effects is poorly understood.
In skeletal muscle, moderate-intensity exercise increases total NOS activity [i.e., the sum of eNOS, neuronal (n) NOS and inducible (i) NOS, as well as AMP-activated protein kinase (AMPK) activity (27, 40)]. It has been suggested that the activation of AMPK is, in part, regulated by endogenous NO in a positive feedback mechanism, such that an increase in NO activates AMPK, which further augments NOS activity and NO production (8, 29, 50). Thus, it could be hypothesized that impaired skeletal muscle NOS activity and NO production would suppress AMPK activity. In line with this positive feedback paradigm, mice expressing a catalytically inactive AMPKα2 subunit in skeletal muscle are unable to increase total NOS activity during moderate-intensity exercise (27). These mice are exercise intolerant (16, 27, 37), as are mice with a full deletion of eNOS (34, 39).
In humans and rodents, the nonspecific NOS inhibitors NG-monomethyl-l-arginine and Nω-nitro-l-arginine methyl ester have been shown to impair skeletal muscle glucose uptake (MGU) and GLUT-4 translocation during exercise in vivo (5, 21, 41), and attenuate MGU in response to contraction in situ (42); however, other rodent studies utilizing contraction in vitro (14, 19, 46) and exercise in vivo (43) show no effect of NOS inhibition on MGU. A caveat to the use of these NOS inhibitors is that they affect all NOS isozymes, and thus, the role of specific isozymes in the physiological, metabolic, and enzymatic response to exercise is unclear. Here, we studied mice with heterozygous (enos+/−) and homozygous (enos−/−) eNOS deletion, and wild-type littermates (WT). This allowed us to study the specific eNOS isozyme, as well as quantifiable eNOS dose-response effects. Physical exercise in vivo was used as a means to unmask phenotypes associated with eNOS action. We tested the hypotheses that 1) exercise capacity, 2) skeletal muscle enzymatic signaling, and 3) the uptake of metabolic fuels by skeletal muscle during exercise in vivo is critically dependent on the extent to which eNOS is expressed.
MATERIALS AND METHODS
Animal maintenance.
All procedures were approved by the Vanderbilt University Animal Care and Use Committee. WT, enos+/−, and enos−/− littermate mice were generated by mating C57BL/6J enos+/− mice (45) purchased from Jackson Laboratories (Bar Harbor, ME). Twenty-one days after birth, littermates were separated by sex and maintained in microisolator cages until they were 16 wk of age. Genotyping was performed using standard PCR techniques on genomic DNA isolated from tail biopsies. Following separation, mice were fed a standard chow diet (5.5% fat by weight; 5001 Laboratory Rodent Diet; Purina, Richmond, IN) and had access to water ad libitum. Studies were conducted on 16-wk-old mice.
Body composition, feeding, and indirect calorimetry.
At 16 wk of age, body composition was determined using a mq10 NMR analyzer (Bruker Optics, The Woodlands, TX). For food intake measurements, mice were placed in individual cages with measured amounts of food and bedding. Food was weighed 48 h later, excluding fecal matter and bedding. Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) was measured using an Oxymax indirect calorimetry system (Columbus Instruments, Columbus, OH), as described previously (2). The respiratory exchange ratio (RER) was calculated as V̇co2/V̇o2, and energy expenditure was calculated as described previously (2).
Metabolic experiments.
Seven days prior to the experiment, surgical procedures were performed as previously described (3). Briefly, mice were anesthetized with pentobarbital sodium (70 mg/kg body wt ip), the carotid artery and jugular vein were catheterized, and the free ends of the catheters were tunneled under the skin to the back of the neck, where they were attached via stainless-steel connectors to lines made of Micro-Renathane. These lines were exteriorized, sealed with stainless-steel plugs, and kept patent with saline containing 200 U/ml of heparin and 5 mg/ml of ampicillin. Mice were housed individually postsurgery, and body weight was recorded daily. Five days following surgery, all mice were familiarized to treadmill exercise by performing a 10-min bout of exercise at 13 m/min (0% incline). Experiments were performed 2 days later. Male and female mice were utilized for all experiments, and no sex-specific effects were observed.
On the day of the experiment, mice were fasted for 5 h prior to commencing exercise. Approximately 1 h prior to the experiment, Micro-Renathane tubing was connected to the exteriorized catheters, and all mice were placed in the enclosed treadmill to acclimate to the environment. At t = 0 min, a baseline arterial blood sample (∼100 μl) was taken to measure arterial levels of glucose, insulin, nonesterified fatty acids (NEFA), lactate, and hematocrit. Mice then remained sedentary or performed treadmill exercise for a maximum of 30 min at 16 m/min (0% incline). Sedentary mice were allowed to move freely in the stationary treadmill for 30 min. In all mice, a bolus containing 2[14C]deoxyglucose (2[14C]DG; 13 μCi) and [9,10-3H]-(R)-2-bromopalmitate (3H-R-BrP; 26 μCi) was injected into the jugular vein at t = 5 min to provide an index of tissue-specific glucose and long-chain fatty acid (LCFA) uptake and clearance, respectively. At t = 7, 10, 15, and 20 min, ∼50 μl of arterial blood was sampled to determine arterial glucose, NEFA, 2[14C]DG, and 3H-R-BrP. Hematocrit was also measured at t = 20 min. At t = 30 min or exhaustion (if earlier than 30 min), ∼100 μl of arterial blood was taken for the measurement of arterial glucose, insulin, NEFA, lactate, 2[14C]DG, and 3H-R-BrP. Following the final arterial sample, 50 μl of yellow DYE-TRAK Microspheres (15 μm; Triton Technology, San Diego, CA) were injected into the carotid artery to assess percent cardiac output to the gastrocnemius (%QG), as well as to the left and right kidney. Mice were then anesthetized with an arterial infusion of pentobarbital sodium (3 mg). Tissues were rapidly excised and frozen in liquid nitrogen and stored at −70°C. For microsphere analysis, the left gastrocnemius and left and right kidneys were placed into 15-ml polypropylene tubes and stored at 4°C until analyzed.
Plasma and tissue analyses.
Plasma 2[14C]DG radioactivity was assessed following deproteinization, as previously described (3), while plasma 3H-R-BrP radioactivity was determined directly from the plasma (44). Tissue phosphorylated 2[14C]DG (2[14C]DG-P) and 3H-R-BrP was determined using a modified method of Folch et al. (15), which has been previously described (27). Immunoreactive plasma insulin was assayed with a double antibody method (36), and plasma NEFAs were measured spectrophotometrically using an enzymatic colorimetric assay (Wako NEFA C kit; Wako Chemicals, Richmond, VA). Plasma lactate was determined using the enzymatic method of Lowry and Passoneau (30), using lithium L-lactate (Sigma, St. Louis, MO) as the standard.
Glycogen was determined using the method of Chan and Exton (7). ATP was measured using HPLC (1). %QG was determined as previously described (27).
Muscle samples were homogenized in lysis buffer containing (final concentrations) 50 mM Tris·HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, 10 μg/ml trypsin inhibitor, 5 μl/ml protease inhibitor cocktail, 50 mM NaF, and 5 mM Na pyrophosphate, as previously described (27). Protein content in the supernatant was determined using the Bradford method. Protein expression of eNOS, nNOS, iNOS, GLUT-4, hexokinase (HK) II, AMPK α, acetyl-CoA carboxylase (ACC) β, Ca2+/calmodulin kinase (CaMKII), as well as AMPKα Thr172 phosphorylation, ACCβ Ser221 phosphorylation, and CaMKII Thr286 phosphorylation in the gastrocnemius muscle was determined from 100 μg of protein. Proteins were resolved on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidine fluoride membranes. Blots were probed with anti-eNOS rabbit polyclonal (Abcam, Cambridge, MA), anti-nNOS and anti-iNOS mouse monoclonal (BD Biosciences, San Jose, CA), anti-GLUT-4 and anti-HK II rabbit polyclonal (Abcam), anti-AMPKα pan and anti-ACCβ rabbit polyclonal (Cell Signaling, Beverly, MA), as well as anti-AMPKα Thr172, anti-ACCβ Ser79 (equivalent to Ser221 in skeletal muscle), and anti-CaMKII Thr286 rabbit polyclonal (Cell Signaling) antibodies. Oxidative phosphorylation (OXPHOS) complexes I to V of the electron transport chain were determined from 25 μg of protein, and MitoProfile Total OXPHOS Rodent WB Antibody Cocktail, according to manufacturer instructions (MitoSciences, Eugene, OR). Protein expression was normalized to GAPDH (Abcam). OXPHOS complexes were normalized to voltage-dependent anion channel (VDAC) expression, a known mitochondrial marker (12). AMPKα Thr172, ACCβ Ser221, and CaMKII Thr286 phosphorylation was normalized to AMPKα, ACCβ, and CaMKII protein levels, respectively. Antibody binding was detected with either IRDye 800-conjugated anti-rabbit IgG or IRDye 700-conjugated anti-mouse IgG secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA). ACCβ expression was detected using IRDye 800-labeled streptavidin (Rockland Immunochemicals).
AMPKα2 and NOS activities were measured using 200 μg and ∼70 μg of protein, respectively, as previously described (27). Briefly, AMPKα2 was immunoprecipitated using Recomb protein A beads (Pierce, Rockford, IL), and activity was measured for 24 min at 30°C in the presence of 200 μM AMP and 100 μM SAMS peptide (Genway Biotech, San Diego, CA) and was calculated as picomoles of phosphate incorporated into the SAMS peptide per minute per milligram of protein. NOS activity was measured on whole cell lysates, and it is the difference between samples incubated with or without Nω-nitro-l-arginine methyl ester. NOS activity was calculated as the amount of l-[3H]-arginine converted to l-[3H]-citrulline (in disintegrations/min) per minute per milligram of protein.
Calculations.
The tissue-specific clearance of 2[14C]DG and 3H-R-BrP (Kg and Kf, respectively), and the metabolic index for glucose and LCFA (Rg and Rf) were calculated as previously described (23, 27). Kg and Kf are used as concentration-independent indices of muscle glucose and LCFA uptake, respectively.
Percent cardiac output to the tissue was calculated from fluorescent intensity as previously described (27) and is expressed as the percentage of microspheres in the gastrocnemius muscle relative to the total amount infused. Adequacy of microsphere mixing was assumed if microsphere content in the left and right kidney was within 10%. On average, the difference between %Q to the left and right kidney was 6 ± 3% across genotypes.
Statistical analyses.
Data are presented as means ± SE. Statistical analysis was performed using either a Student's t-test, one-way ANOVA, or two-way repeated-measures ANOVA where appropriate with the statistical software package SigmaStat. If the ANOVA were significant (P < 0.05), specific differences were located using Fisher's least significant difference (LSD) test.
RESULTS
Baseline characteristics.
At 16 wk of age, body weight of WT and enos+/− mice was significantly greater than enos−/− mice (Table 1). enos+/− and enos−/− mice had less muscle mass than WT (Table 1). In absolute terms, fat mass was elevated in enos+/− mice vs. WT (Table 1). Under sedentary conditions, no genotype effects were observed for arterial levels of glucose (11 ± 1 vs. 10 ± 1 vs. 9 ± 1mM for WT, enos+/− and enos−/−, respectively) or NEFA (1.7 ± 0.1 vs. 1.8 ± 0.1 vs. 1.8 ± 0.2mM, respectively). eNOS protein expression was reduced ∼40% in skeletal muscle of enos+/− mice, and was undetectable in enos−/− mice (Fig. 1A). Neuronal nitric oxide synthase mu (nNOSμ)expression did not significantly differ between genotypes in skeletal muscle (Table 2), while iNOS expression was undetectable. GLUT-4 and HKII protein levels were also similar between genotypes (Table 2). Basal ATP levels in skeletal muscle differed between genotypes with WT > enos+/− > enos−/− (55 ± 1 vs. 47 ± 2 vs. 41 ± 1 μmol·100g-1, respectively; P < 0.05).
Table 1.
Body weight, body composition, and indirect calorimetry measurements of 16 wk of chow-fed C57BL/6J mice with wild type, partial, or no expression of endothelial NOS
| WT | enos+/− | enos−/− | |
|---|---|---|---|
| Body weight, g | 25.5 ± 0.8 | 24.8 ± 0.8 | 22.3 ± 0.8† |
| Lean mass, g | 21.4 ± 0.7 | 19.1 ± 0.5* | 17.5 ± 0.6* |
| Lean mass, % | 84.1 ± 1.4 | 77.0 ± 0.7* | 78.2 ± 0.6* |
| Fat mass, g | 1.5 ± 0.2 | 2.3 ± 0.2* | 1.7 ± 0.1 |
| Fat mass, % | 6.2 ± 1.0 | 9.1 ± 0.7** | 7.6 ± 0.5 |
| Light cycle (0600–1800) | |||
| V̇o2, ml·kg−1·min−1 | 52 ± 2 | 52 ± 3 | 53 ± 2 |
| RER | 0.92 ± 0.02 | 0.97 ± 0.01* | 0.95 ± 0.01 |
| CHOox, μmol·kg−1·min−1 | 50.1 ± 4.1 | 62.9 ± 4.5* | 59.0 ± 3.0 |
| Fatox, μmol·kg−1·min−1 | 7.1 ± 1.4 | 2.8 ± 1.1* | 4.2 ± 1.0 |
| Dark cycle (1800–0600) | |||
| V̇o2, ml·kg−1·min−1 | 66 ± 2 | 66 ± 3 | 69 ± 2 |
| RER | 0.99 ± 0.02 | 0.98 ± 0.01 | 0.95 ± 0.01* |
| CHOox, μmol·kg−1·min−1 | 85.4 ± 3.1 | 81.6 ± 5.1 | 76.1 ± 2.3 |
| Fatox, μmol·kg−1·min−1 | 2.1 ± 1.4 | 2.6 ± 0.8 | 6.0 ± 1.0† |
| 24-h food consumption, g | 7.3 ± 0.5 | 6.7 ± 0.6 | 6.6 ± 0.6 |
Data are expressed as means ± SE for n = 6–8 per group.
P < 0.05 vs. WT;
P < 0.05 vs. wild type (WT) and enos+/−;
P = 0.05 vs. WT. eNOS, endothelial nitric oxide synthase; WT, wild type; enos+/−, partial expression of eNOS; enos−/−, no expression of eNOS; RER, respiratory exchange ratio; V̇o2, oxygen consumption; CHOox and Fatox, carbohydrate and fat oxidation, respectively.
Fig. 1.
Baseline characteristics of 16-wk-old chow-fed C57BL/6J mice with wild type (WT), partial (+/−) or no expression (−/−) of endothelial nitric oxide synthase (eNOS). Protein expression of eNOS (A), oxidative phosphorylation (OXPHOS) complexes I to V of the electron transport chain (B and C), NOS activity (D), AMP-activated protein kinase (AMPK) α2 activity (E), and Ca2+/calmodulin protein kinase (CaMK) II Thr286 phosphorylation (F) was determined in gastrocnemius muscle of sedentary mice that had been fasted for 5 h. Data are expressed as means ± SE for n = 5–7 per group. *P < 0.05 vs. WT; †P < 0.05 vs. WT and enos+/−; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; +ve, positive control (rat brain mitochondria).
Table 2.
Protein expression of the skeletal muscle isoform of neuronal nitric oxide synthase, GLUT-4, hexokinase II, AMP-activated protein kinase α, acetyl-CoA carboxylase β, LKB1, and Ca2+/calmodulin protein kinase II in gastrocnemius muscle of 16-wk-old chow-fed C57BL/6J mice with WT, +/−, or −/− of eNOS
| Protein | WT | enos+/− | enos−/− |
|---|---|---|---|
| nNOSμ | 0.16 ± 0.02 | 0.18 ± 0.02 | 0.15 ± 0.02 |
| GLUT-4 | 1.09 ± 0.14 | 1.09 ± 0.21 | 1.10 ± 0.14 |
| HK II | 0.36 ± 0.05 | 0.29 ± 0.05 | 0.39 ± 0.09 |
| AMPKα | 0.41 ± 0.04 | 0.41 ± 0.02 | 0.39 ± 0.03 |
| ACCβ | 0.55 ± 0.07 | 0.45 ± 0.04 | 0.53 ± 0.07 |
| LKB1 | 0.62 ± 0.04 | 0.56 ± 0.03 | 0.63 ± 0.03 |
| CaMKII | 1.25 ± 0.08 | 1.30 ± 0.14 | 1.26 ± 0.15 |
Data are expressed as arbitrary units normalized to glyceraldehyde-3-phosphate dehydrogenase and are means ± SE for n = 6–8 per group. nNOSμ, neuronal nitric oxide synthase mu; AMPK, AMP-activate protein kinase; HK II, hexokinase II; ACCβ, acetyl-CoA carboxylase beta; CaMKII, calmodulin protein kinase II.
A partial reduction of eNOS was sufficient to impair OXPHOS complexes I through V in skeletal muscle (Fig. 1, B and C). A further reduction in complex II and V expression was seen in enos−/− mice when compared with enos+/− mice. The changes in OXPHOS complex expression between genotypes was not due to changes in mitochondrial content, as assessed via expression of mitochondrial VDAC (2.4 ± 0.3 vs. 2.2 ± 0.2 vs. 2.0 ± 0.3 arbitrary units for WT, enos+/− and enos−/−, respectively).
We next assessed whether changes in eNOS protein expression altered enzymatic signaling under basal conditions. Partial and full deletion of eNOS resulted in a paradoxical increase in total NOS activity in skeletal muscle (Fig. 1D). Basal AMPKα2 activity was similar between genotypes (Fig. 1E), as was total AMPKα protein expression (Table 2) and total AMPKα Thr172 phosphorylation. Expression of the upstream AMPK kinase LKB1 was similar between genotypes, as was ACCβ, a downstream target of AMPK (Table 2). Basal ACCβ Ser221 phosphorylation was similar between genotypes. Basal CaMKII Thr286 phosphorylation was elevated by 34 ± 15 and 46 ± 7% in skeletal muscle of enos+/− and enos−/− mice, respectively, when compared with WT mice (Fig. 1F). This increase was not due to changes in CaMKII protein expression (Table 2).
Reductions in eNOS alter whole body substrate oxidation in the light and dark cycle.
In all genotypes, V̇o2 (Table 1) and V̇co2 (data not shown) were significantly elevated in the dark cycle. In WT mice, RER increased from the light to dark cycle, whereas no change was observed in enos+/− and enos−/− mice (Table 1). Furthermore, in the light cycle, WT RER was reduced compared with enos+/− mice (P < 0.02) and tended to be lower when compared with enos−/− mice (P = 0.1). During the dark cycle, RER was reduced in enos−/− vs. WT mice (P < 0.05) and tended to be lower in enos−/− vs. enos+/− mice (P = 0.1). Energy expenditure was similar between WT, enos+/− and enos−/− mice during the light (15.4 ± 0.7 vs. 15.7 ± 0.8 vs. 15.8 ± 0.6 kcal·kg−1·h−1, respectively) and dark cycle (19.9 ± 0.7 vs. 19.8 ± 0.9 vs. 20.6 ± 0.6 kcal·kg−1·h−1).
Because of the differences in RER within cycles, whole body substrate utilization also differed. In the light cycle, carbohydrate (CHO) oxidation was elevated in enos+/− compared with WT mice; however, CHO oxidation was similar between enos−/− mice and WT mice (P = 0.15). Fat oxidation during the light cycle was reduced in enos+/− mice compared with WT (P < 0.02) and tended to be lower in enos−/− vs. WT mice (P = 0.09). In the dark cycle, CHO oxidation was similar between genotypes; however, fat oxidation was elevated in enos−/− mice compared with WT and enos+/− mice.
A reduction in eNOS reduces exercise-stimulated increases in AMPK signaling in skeletal muscle in vivo.
Exercise significantly increased AMPKα Thr172 phosphorylation in skeletal muscle of WT and enos+/− mice, whereas this increase was abolished in enos−/− mice (Fig. 2A). Regression analysis revealed correlations between eNOS protein expression and the exercise-induced increase in AMPKα Thr172 phosphorylation (r = 0.63, P = 0.02; Fig. 2B). Changes in ACCβ Ser221 phosphorylation (Fig. 2C) were similar to that observed for AMPKα Thr172 phosphorylation, and a significant correlation between eNOS protein expression and the exercise-induced increase in ACCβ Ser221 phosphorylation was also observed (r = 0.84 P < 0.001; Fig. 2D). Exercise elicited a robust increase in AMPKα2 activity in WT and enos+/− mice, whereas no increase was observed for enos−/− mice (Fig. 2E).
Fig. 2.
AMP-activated protein kinase (AMPK) α Thr172 phosphorylation (A), linear regression analysis for the exercise-induced increase in AMPKα Thr172 phosphorylation (B), acetyl-CoA carboxylase (ACC) β Ser221 phosphorylation (C), linear regression analysis for the exercise-induced increase in ACCβ Ser221 phosphorylation (D), AMPKα2 activity (E), nitric oxide synthase (NOS) activity (F), and Ca2+/calmodulin protein kinase (CaMK) II Thr286 phosphorylation (G) in gastrocnemius muscle of 16-wk-old chow fed C57BL/6J mice with wild-type (WT), partial (+/−), or no expression (−/−) of eNOS. Mice either remained sedentary (Sed) or performed a maximum of 30 min of treadmill exercise (Ex) following a 5-h fast. Data are expressed as means ± SE for n = 5–7 per group. ‡ P < 0.05 vs. corresponding rest value; *P < 0.05 vs. WT; †P < 0.05 vs. WT and enos+/−.
In WT mice, exercise tended to increase total NOS activity (65 ± 33%; P = 0.09 vs. sedentary, Fig. 2F); no changes were observed for enos+/− or enos−/− mice. CaMKII Thr286 phosphorylation increased 54 ± 12% in WT mice during exercise, whereas no increase was observed in enos+/− or enos−/− mice (Fig. 2G). However, because of the elevated basal activity of these enzymes in enos+/− and enos−/− mice, absolute levels of total NOS activity and CaMKII Thr286 phosphorylation were similar between genotypes during exercise.
A reduction in eNOS expression alters running time, arterial metabolites, and arterial hormones during steady state exercise in vivo.
During acute treadmill exercise, WT and enos+/− ran for 30.0 ± 0.0 and 29.6 ± 0.4 min, respectively, whereas enos−/− mice ran for 21.3 ± 1.8 min (P < 0.001 vs. WT and enos+/−). An eNOS-dependent effect on arterial glucose was observed during exercise (Fig. 3A). enos+/− mice exhibited lower glucose levels during exercise than WT mice, while enos−/− mice exhibited even lower glucose levels than enos+/− mice and became hypoglycemic at the end of exercise. A main effect for genotype (P < 0.05) and time (P < 0.05) was observed for arterial NEFA (Fig. 3B) and insulin (Fig. 3C). Arterial lactate increased during exercise in all genotypes (Fig. 3D); however, the increase in enos−/− mice (11.3 ± 1.4-fold) was significantly greater than in both enos+/− (3.9 ± 0.6-fold) and WT mice (2.9 ± 0.4 fold). Thus, exercise in vivo elicited an eNOS-dependent phenotype characterized by reductions in exercise capacity and arterial glucose, altered arterial NEFA and insulin, and increased arterial lactate levels.
Fig. 3.
Arterial blood glucose (A), plasma nonesterified fatty acids (NEFAs; B), plasma insulin (C), and plasma lactate (D) at rest and during exercise in 16-wk-old chow fed C57BL/6J mice with wild-type (WT), partial (+/−), or no expression (−/−) of eNOS. Following a 5-h fast, chronically catheterized mice performed a maximum of 30 min of running on a motorized treadmill, and arterial blood was sampled at times shown. Data are expressed as means ± SE for n = 7 (WT), n = 15 (enos+/−), and n = 10 (enos−/−). ‡P < 0.05 vs. corresponding rest value: *P < 0.001 vs. WT; †P < 0.001 vs. WT and enos+/−; fMain effect for genotype, P < 0.05; §Main effect for time, P < 0.05.
Percent cardiac output to skeletal muscle during exercise in vivo is highly dependent on eNOS.
In the sedentary state, %QG did not differ between genotypes (Fig. 4A). In response to exercise, %QG was proportional to eNOS expression, increasing ∼6-, 3-, and 2-fold in WT, enos+/−, and enos−/− mice, respectively (Fig. 4A). Regression analysis revealed a correlation between eNOS expression and the increase in %QG in response to exercise (r = 0.73, P < 0.01; Fig. 4B). On the basis of the equation of the line, 20 ± 5% of the increase in %QG during exercise in WT mice was eNOS independent.
Fig. 4.
Exercise-induced increase in percent cardiac output to gastrocnemius muscle (%QG) in 16-wk-old chow fed C57BL/6J mice with wild-type (WT), partial (+/−), or no expression (−/−) of eNOS. Following a 5-h fast, mice either remained sedentary or performed a maximum of 30 min of running on a motorized treadmill. At the end of exercise, 50 μl of yellow DYE-TRAK Microspheres were injected into the carotid artery to determine %QG (see materials and methods). A: %QG in sedentary mice and exercised mice. B: exercise-induced increase in %QG. eNOS protein expression in gastrocnemius muscle is also shown. Data are expressed as means ± SE; n = 5 per group. *P < 0.01 vs. WT; ‡P < 0.05 vs. corresponding rest value.
Ablation of eNOS markedly accelerates indices of muscle substrate uptake in vivo.
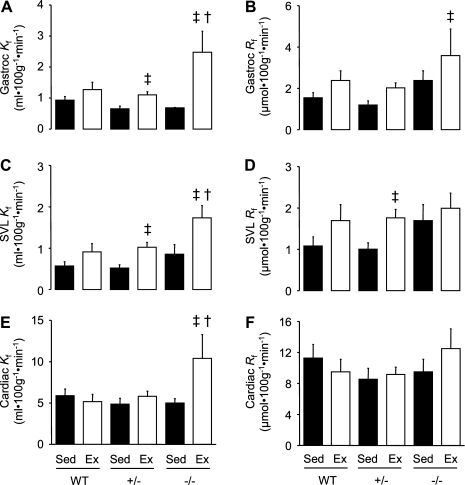
Compared with WT, sedentary Kg (Fig. 5A) and Rg (Fig. 5B) in gastrocnemius muscle was elevated in enos+/− mice. Exercise significantly increased gastrocnemius Kg and Rg in WT and enos−/− mice; however, this increase was considerably higher (∼5-fold) in enos−/− mice (Fig. 5, A and B). To determine whether this phenomenon occurred in other tissues, we assessed Kg and Rg in superficial vastus lateralis (SVL) (Fig. 5, C and D) and cardiac muscle (Fig. 5, E and F). In these tissues, Kg and Rg during exercise were also markedly elevated in enos−/− mice.
Fig. 5.
Tissue-specific indexes of glucose clearance (Kg) and glucose uptake (Rg) in gastrocnemius (Gastroc; A, B), superficial vastus lateralis (SVL; C, D), and cardiac muscle (E, F) at rest and during exercise in 16-wk-old chow fed C57BL/6J mice with wild-type (WT), partial (+/−) or no expression (−/−) of eNOS. Mice were chronically catheterized and allowed to recover for 7 days. Following a 5-h fast, mice either remained sedentary (Sed) or performed a maximum of 30 min of treadmill exercise (Ex). At 5 min, mice were given a bolus injection containing 13 μCi of 2[14C]deoxyglucose. Kg and Rg were calculated as described previously (see materials and methods). In Sed, data are expressed as means ± SE for n = 6 (WT), n = 7 (enos+/−) and n = 4 (enos−/−). For Ex, n = 6, n = 12, and n = 7, respectively. †P < 0.05 vs. WT and enos+/−; ‡P < 0.05 vs. corresponding sedentary value; *P < 0.05 vs. WT.
No genotype differences in gastrocnemius Kf (Fig. 6A) or Rf (Fig. 6B) were observed in the sedentary state. Kf increased during exercise in enos+/− and enos−/− mice, whereas Rf only significantly increased in enos−/− mice. As with Kg, gastrocnemius Kf increased to the greatest extent in enos−/− mice during exercise. Similar findings were observed in SVL muscle (Fig. 6, C and D). In cardiac muscle of enos+/− and enos−/− mice, basal Kf (Fig. 6E) and Rf (Fig. 6F) were similar between genotypes. Exercise increased Kf in enos−/− mice, whilst Rf was not altered in any genotype.
Fig. 6.
Tissue-specific indexes of long-chain fatty acid (LCFA) clearance (Kf) and LCFA uptake (Rf) in gastrocnemius (Gastroc; A, B), superficial vastus lateralis (SVL; C, D), and cardiac muscle (E, F) at rest and during exercise in 16-wk-old chow fed C57BL/6J mice with wild-type (WT), partial (+/−) or no expression (−/−) of eNOS. Mice were chronically catheterized and allowed to recover for 7 days. Following a 5-h fast, mice either remained sedentary (Sed) or performed a maximum of 30 min of treadmill exercise (Ex). At 5 min, mice were given a bolus injection containing 26 μCi of [9,10-3H]-(R)-2-bromopalmitate. Kf and Rf were calculated as described previously (see materials and methods). In Sed, data are expressed as means ± SE for n = 7 (WT), n = 9 (enos+/−) and n = 4 (enos−/−). For Ex, n = 7, n = 12, and n = 7, respectively. †P < 0.05 vs. WT and enos+/−; ‡P < 0.05 vs. corresponding sedentary value.
Ablation of eNOS accelerates net muscle and liver glycogenolysis in vivo.
Gastrocnemius glycogen levels were similar between WT, enos+/− and enos−/− mice under sedentary conditions, and exercise caused a similar decrement in glycogen (Table 3). Liver glycogen was also similar at rest and decreased to a similar extent in response to exercise in WT, enos+/−, and enos−/− mice (Table 3). However, the average running time for enos−/− mice was ∼30% less than for WT and enos+/− mice. Thus, the rate of glycogenolysis was ∼3-fold greater in enos−/− mice in the gastrocnemius and liver (Table 3).
Table 3.
Glycogen levels and glycogenolysis in liver and gastrocnemius of 16-wk-old chow-fed C57BL/6J mice with WT, +/−, or −/− of eNOS
| WT | enos+/− | enos−/− | |
|---|---|---|---|
| Gastrocnemius glycogen, mmol/100 g | |||
| Sedentary | 2.1 ± 0.4 | 2.3 ± 0.2 | 2.2 ± 0.5 |
| Exercise | 1.0 ± 0.3* | 1.1 ± 0.4* | 0.6 ± 0.2*† |
| Glycogenolysis, μmol·100 g−1·min−1 | 39 ± 9 | 47 ± 8 | 100 ± 15† |
| Hepatic glycogen, mmol/100 g | |||
| Sedentary | 6.1 ± 2.5 | 6.6 ± 2.4 | 8.2 ± 2.4 |
| Exercise | 1.4 ± 0.5* | 1.1 ± 0.38 | 0.4 ± 0.1*† |
| Glycogenolysis, μmol·100 g−1·min−1 | 157 ± 15 | 193 ± 19 | 468 ± 60† |
Data are expressed as means ± SE for n = 5–8 per group. Gastrocnemius and liver glycogen levels were assessed from mice that remained sedentary or performed a maximum of 30-min treadmill exercise, and they are measured as described in materials and methods.
P < 0.05 vs. corresponding sedentary value;
P < 0.05 vs. WT and enos+/−.
DISCUSSION
Here, we examined the role of eNOS in the regulation of enzymatic signaling and fuel metabolism in skeletal muscle in vivo. Novel findings were that a partial reduction in eNOS was sufficient to induce many of the phenotypic effects observed in enos−/− mice under fasting, sedentary conditions. Likewise, physical exercise was able to resolve physiological deficits, which were dependent upon eNOS expression, and precipitate others that were unique to enos−/− mice. Collectively, our findings show that eNOS plays a multifaceted role in the interaction of physiological responses both at rest and during exercise in vivo. These findings emphasize the exquisite sensitivity of skeletal muscle to deficits in eNOS expression.
The finding that a partial deletion of eNOS is sufficient to induce a phenotypic effect under basal conditions that is similar to that of enos−/− mice has important implications for metabolic disease states. Obese individuals have a partial reduction in eNOS protein expression in skeletal muscle (18); high-fat fed enos+/− mice exhibit exaggerated insulin resistance during an insulin clamp (11); genetic and environmental induction of obesity attenuates eNOS protein expression and lowers ATP levels in skeletal muscle of rodents (47). Findings from the present and aforementioned studies suggest that a reduction in eNOS expression does not play a causative role with regard to obesity per se. Indeed, enos+/− and enos−/− mice do not weigh more than WT mice; however, enos+/− mice do have slightly elevated fat mass, while fat oxidation is reduced in the light cycle. It is likely that obesity impairs eNOS protein expression, potentially via elevated levels of tumor necrosis factor α (47), and this downregulation of eNOS exerts subsequent effects on ATP levels and OXPHOS complexes in skeletal muscle, as seen in the present study.
Here, we show that partial and full deletion of eNOS decreases OXPHOS complexes in skeletal muscle without altering the mitochondrial marker VDAC. It has previously been shown that enos−/− mice have impaired skeletal muscle mitochondrial β-oxidation, although this occurred in parallel with reduced mitochondrial content (26). Presently, the role of eNOS with regard to mitochondrial content and biogenesis is unclear, with some (26, 38), but not all studies (34, 48), showing a regulatory role for eNOS in these processes. Furthermore, differences have been observed in mice generated from the same group (45), with Wadley et al. (48) finding no differences in mitochondrial content of enos−/− and WT mice, and Le Gouill et al. (26) showing decreased mitochondrial content in enos−/− mice. Similar to Wadley et al. (48), we found no differences in mitochondrial content between genotypes. Likewise, whereas Le Gouill et al. (26) find reduced energy expenditure and V̇o2 in enos−/− mice compared with WT, here, we report no difference between WT, enos+/−, and enos−/− mice. It is possible that age differences account for these contrasting observations (49). Indeed, basal V̇o2 is ∼20% lower in 12-mo-old enos−/− mice compared with WT mice (39).
An unexpected finding from the present study was that basal NOS activity was elevated in enos+/− and enos−/− mice when compared with WT mice. As shown in the present study and by others (34), iNOS expression and activity are not present in enos+/− and enos−/− mice. As such, the elevated rates of basal NOS activity in these mice likely reflect an increase in nNOSμ activity. This is in line with previous observations showing that nNOSμ activity predominates over eNOS activity under basal conditions (40). Presently, the role of eNOS in the regulation of NOS activity is unclear, as other studies in enos−/− mice have observed no change (24) or a partial reduction (26) in NOS activity. However, our finding of a similar elevation in NOS activity in enos+/− mice corroborates our findings in enos−/− mice. It is possible that the elevated level of NOS activity in enos+/− and enos−/− mice relates to alterations in Ca2+ or CaM, which both serve as cofactors for the activation of eNOS and nNOS (6, 9). Indeed, CaMKII Thr286 phosphorylation was also elevated in enos+/− and enos−/− mice, and it has been well established that phosphorylation of CaMKII at the Thr286 residue occurs following CaM binding.
In the present study, we used exercise in vivo as a means to unmask phenotypes associated with impaired eNOS expression. The use of mice with a wide range of eNOS expression allowed for a comprehensive assessment of eNOS in the regulation of physiological responses to exercise. It is intriguing that despite similar decreases in OXPHOS complexes and lower ATP levels, and similar levels of total NOS activity and CaMKII Thr286 phosphorylation between enos+/− and enos−/− mice, the enos−/− mice were relatively exercise intolerant. This finding suggests that alterations in OXHPOS capacity within skeletal muscle, or the activity of NOS and CaMKII, do not affect exercise capacity within the 30-min exercise period utilized. Impaired substrate delivery arising from reduced muscle perfusion likely accounted for the enos−/− phenotype. Indeed, substrate delivery was also closely tied to eNOS expression during exercise (i.e., %QG in WT > enos+/− > enos−/− mice). It should be noted that heart rate is reduced by ∼5–10% under basal conditions in enos−/− mice (45). Although heart rate during exercise in these mice is unknown, a reduced heart rate could contribute to the impaired blood flow to contracting muscle seen in these mice. Moreover, the inability of enos−/− mice to significantly increase %QG during exercise will result in impaired oxygen delivery, which would lead to hypoxia. In skeletal muscle, hypoxia is a potent stimulator of glucose uptake, as well as phosphocreatine and glycogen breakdown (17, 22, 51). Also, although exercise increases plasma lactate, hepatic glucose production, and muscle glycogenolysis, these processes are further augmented during exercise performed under hypoxic conditions (22, 51). The fact that muscle metabolic flux, glycogenolysis, and plasma lactate levels were markedly enhanced in enos−/− mice during exercise suggests that hypoxia, arising from impairments in %QG, may have elicited an additive effect on substrate uptake and metabolism. We have previously observed a similar finding in AMPKα2 kinase-dead mice, whereby skeletal muscle complex I and IV activities, muscle perfusion during exercise in vivo, and exercise tolerance were impaired when compared with WT littermates (27).
The finding that the exercise-induced increase in AMPK signaling, and AMPKα2 activity, was ablated in skeletal muscle of enos−/− mice is remarkable. AMPKα2 is the predominant α subunit activated in skeletal muscle during moderate to high-intensity exercise (10, 27). Previous studies have suggested that the activation of AMPK is regulated via endogenous NO in a positive-feedback mechanism (29, 50). Our findings argue against absolute rates of NOS activity per se, and thus absolute NO production, as a regulator of AMPKα2 activity in skeletal muscle, for AMPKα2 activity and AMPK signaling was sequentially reduced in an eNOS-dependent manner during exercise despite similar rates of NOS activity in all genotypes. Thus, while exercise in rodents increases eNOS and nNOSμ activity in skeletal muscle (40), our findings provide evidence that it is eNOS-derived NO, and thus specifically eNOS activity in skeletal muscle, which interacts with AMPK under these conditions.
To date, contrasting effects have been observed regarding the role of NOS in the direct regulation of substrate uptake during contraction in vivo (5, 21, 43) and in isolated or perfused hindlimb muscle (14, 19, 33, 41–43). This study is the first to test the role of eNOS, and further the dose-response effect of eNOS, in these processes in vivo. Despite similar total NOS activity in all genotypes in response to exercise, MGU increased to the greatest extent in enos−/− mice, indicating that the absolute level of NOS activity does not regulate MGU during exercise in vivo. It has been suggested that the relative increase in NOS activity during contraction, as opposed to absolute rates, may more accurately reflect the role of NOS in contraction-mediated MGU (33); however, again our findings argue against this as WT mice had the greatest relative increase in NOS activity from rest to exercise, yet MGU was substantially lower than that for enos−/− mice who had no relative increase in NOS activity.
An interesting observation from the present study was that enos+/− mice responded differently in a number of parameters to WT and/or enos−/− mice. Indeed, upon initial inspection, these mice do not differ from WT mice in terms of total body weight; however, this is due to an increase in fat mass, which compensates for a reduction in lean mass. This increase in fat mass may be, at least, partly due to reduced fat oxidation in the light cycle. Despite a ∼30% reduction in expression of OXPHOS complexes when compared with WT mice, enos+/− mice are able to adequately perform 30 min of treadmill exercise, suggesting that mitochondrial dysfunction in the basal state does not transfer to gross alterations during exercise, at least under the conditions used in the present study. Furthermore, enos+/− mice do not increase MGU during exercise in vivo, an effect that was observed across all tissues examined. This was at least partially due to elevated basal MGU in these mice, meaning that absolute levels of MGU in response to exercise were similar to WT.
Despite a ∼3-fold increase in hepatic glycogen breakdown in enos−/− mice, marked hypoglycemia occurred, further emphasizing the magnitude of the increase in glucose disposal with exercise in these mice. The elevated indices of MGU in enos−/− mice were not due to increased total GLUT-4 or HKII protein in these mice, suggesting that other factors are involved. Likewise, Kf and Rf were also increased to the greatest extent in gastrocnemius of enos−/− mice during exercise, although this finding is in line with previous observations showing that enos−/− mice have elevated levels of intramyocellular lipid (26). Therefore, our findings suggest that neither expression of eNOS nor an increase in total NOS activity is essential for increases in MGU during stressful exercise. This is also in agreement with previous findings in AMPKα2 kinase-dead mice, as the extraction of glucose by skeletal muscle (27) and GLUT-4 translocation (31) is normal during exercise despite the absence of an increase in total NOS activity (27). Nevertheless, it cannot be discounted that at least part of the reason for differences in MGU relates to exercise intensity. Had the WT and enos+/− mice also been run to exhaustion, it is possible that MGU would have increased to rates observed in enos−/− mice.
In conclusion, through the use of WT, enos+/− and enos−/− littermate mice, we have shown for the first time an eNOS-dependent phenotype under basal conditions, characterized by reductions in OXPHOS complex expression, impaired ATP levels, and increased total NOS activity and CaMKII Thr286 phosphorylation in skeletal muscle. Thus, a partial reduction in eNOS protein expression is sufficient to induce many of the effects observed in enos−/− mice, demonstrating that even a quantitative reduction in eNOS protein expression can result in metabolic dysregulation in vivo. A 30-min bout of acute exercise in vivo also elicits a number of physiological processes that are dependent upon eNOS expression, with the exercise-induced increase in AMPKα Thr172 phosphorylation, ACCβ Ser221 phosphorylation, and %QG all being reduced in parallel with reduced eNOS expression. Ablation of eNOS also results in impaired exercise capacity, hypoglycemia, and increased plasma lactate levels. The alterations associated with partial impairment of eNOS have important implications for chronic metabolic disease states such as obesity and insulin resistance, conditions that are characterized by reduced eNOS protein expression in skeletal muscle.
GRANTS
This work was supported by National Institutes of Health Grants U24 DK-59637 and R01 DK-54902 (DHW). Robert S. Lee-Young was supported by a Mentor-Based Fellowship from the American Diabetes Association.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Pat Donahue of the Vanderbilt Hormone Assay and Analytical Services Core for performing the HPLC analysis.
Present address for J. E. Ayala: Diabetes and Obesity Research Center, Burnham Institute for Medical Research, 6400 Sanger Road, Orlando, FL 32827.
REFERENCES
- 1.Ally A, Park G. Rapid determination of creatine, phosphocreatine, purine bases and nucleotides (ATP, ADP, AMP, GTP, GDP) in heart biopsies by gradient ion-pair reversed-phase liquid chromatography. J Chromatogr 575: 19–27, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56: 1025–1033, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48: 1815–1821, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87: 682–685, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan TM, Exton JH. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem 71: 96–105, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JYJ. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 104: 496–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52: 2205–2212, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Cook S, Hugli O, Egli M, Menard B, Thalmann S, Sartori C, Perrin C, Nicod P, Thorens B, Vollenweider P, Scherrer U, Burcelin R. Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. Diabetes 53: 2067–2072, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Csukly K, Ascah A, Matas J, Gardiner PF, Fontaine E, Burelle Y. Muscle denervation promotes opening of the permeability transition pore and increases the expression of cyclophilin D. J Physiol 574: 319–327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104: 342–345, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Etgen GJ, Jr, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes 46: 1915–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 16.Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S, Hirshman MF, Goodyear LJ. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle. Insight from analysis of a transgenic mouse model. Diabetes Res Clin Pract 77Suppl 1: S92–S98, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49: 527–531, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hickner RC, Kemeny G, Stallings HW, Manning SM, McIver KL. Relationship between body composition and skeletal muscle eNOS. Int J Obes (Lond) 30: 308–312, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 50: 241–247, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes 51: 2572–2580, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kjaer M, Hanel B, Worm L, Perko G, Lewis SF, Sahlin K, Galbo H, Secher NH. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am J Physiol Regul Integr Comp Physiol 277: R76–R85, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol Endocrinol Metab 248: E353–E362, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Lacza Z, Snipes JA, Zhang J, Horváth EM, Figueroa JP, Szabó C, Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med 35: 1217–1228, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Le Gouill E, Jimenez M, Binnert C, Jayet PY, Thalmann S, Nicod P, Scherrer U, Vollenweider P. Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes 56: 2690–2696, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem 284: 23925–23934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Wallerath T, Forstermann U. Physiological mechanisms regulating the expression of endothelial-type NO synthase. Nitric Oxide 7: 132–147, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Lira VA, Soltow QA, Long JHD, Betters JL, Sellman JE, Criswell DS. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab 293: E1062–E1068, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis New York: Academic, 1972 [Google Scholar]
- 31.Maarbjerg SJ, Jorgensen SB, Rose AJ, Jeppesen J, Jensen TE, Treebak JT, Birk JB, Schjerling P, Wojtaszewski JF, Richter EA. Genetic impairment of α2-AMPK signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am J Physiol Endocrinol Metab 297: E924–E934, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation 98: 369–374, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab 298: E577–E585, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Momken I, Lechene P, Ventura-Clapier R, Veksler V. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am J Physiol Heart Circ Physiol 287: H914–H920, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Morgan CR, Lazarow A. Immunoassay of pancreatic and plasma insulin following alloxan injection of rats. Diabetes 14: 669–671, 1965 [DOI] [PubMed] [Google Scholar]
- 37.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101: 16507–16512, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojaimi C, Li W, Kinugawa S, Post H, Csiszar A, Pacher P, Kaley G, Hintze TH. Transcriptional basis for exercise limitation in male eNOS-knockout mice with age: heart failure and the fetal phenotype. Am J Physiol Heart Circ Physiol 289: H1399–H1407, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol Endocrinol Metab 277: E390–E394, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol Endocrinol Metab 273: E220–E225, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Ross RM, Wadley GD, Clark MG, Rattigan S, McConell GK. Local nitric oxide synthase inhibition reduces skeletal muscle glucose uptake but not capillary blood flow during in situ muscle contraction in rats. Diabetes 56: 2885–2892, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Rottman JN, Bracy D, Malabanan C, Yue Z, Clanton J, Wasserman DH. Contrasting effects of exercise and NOS inhibition on tissue-specific fatty acid and glucose uptake in mice. Am J Physiol Endocrinol Metab 283: E116–E123, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Shearer J, Coenen KR, Pencek RR, Swift LL, Wasserman DH, Rottman JN. Long-chain fatty acid uptake in vivo: Comparison of [(125)I]-BMIPP and [(3)H]-bromopalmitate. Lipids 43: 703–711, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens TJ, Canny BJ, Snow RJ, McConell GK. 5′-aminoimidazole-4-carboxyamide-ribonucleoside-activated glucose transport is not prevented by nitric oxide synthase inhibition in rat isolated skeletal muscle. Clin Exp Pharmacol Physiol 31: 419–423, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 116: 2791–2798, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadley GD, Choate J, McConell GK. NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol 585: 253–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wadley GD, Choate J, McConell Reply from G. D. Wadley, G. K. Choate, and J. McConell. J Physiol 586: 915–916, 2008 [Google Scholar]
- 50.Zhang J, Xie Z, Dong Y, Wang S, Liu C, Zou MH. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem 283: 27452–27461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Zinker BA, Wilson RD, Wasserman DH. Interaction of decreased arterial Po2 and exercise on carbohydrate metabolism in the dog. Am J Physiol Endocrinol Metab 269: E409–E417, 1995 [DOI] [PubMed] [Google Scholar]