Abstract
Smoking is associated with multiple adverse pregnancy outcomes, including fetal growth restriction. The objective of this study was to determine whether cigarette smoke exposure during pregnancy in a mouse model affects the functional properties of maternal uterine, mesenteric, and renal arteries as a possible mechanism for growth restriction. C57Bl/CJ mice were exposed to whole body sidestream smoke for 4 h/day. Smoke particle exposure was increased from day 4 of gestation until late pregnancy (day 16–19), with mean total suspended particle levels of 63 mg/m3, representative of moderate-to-heavy smoking in humans. Uterine, mesenteric, and renal arteries from late-pregnant and virgin mice were isolated and studied in a pressure-arteriograph system (n = 23). Plasma cotinine was measured by ELISA. Fetal weights were significantly reduced in smoke-exposed compared with control fetuses (0.88 ± 0.1 vs. 1.0 ± 0.08 g, P < 0.02), while litter sizes were not different. Endothelium-mediated relaxation responses to methacholine were significantly impaired in both the uterine and mesenteric vasculature of pregnant mice exposed to cigarette smoke during gestation. This difference was not apparent in isolated renal arteries from pregnant mice exposed to cigarette smoke; however, relaxation was significantly reduced in renal arteries from smoke-exposed virgin mice. In conclusion, we found that passive cigarette smoke exposure is associated with impaired vascular relaxation of uterine and mesenteric arteries in pregnant mice. Functional maternal vascular perturbations during pregnancy, specifically impaired peripheral and uterine vasodilation, may contribute to a mechanism by which smoking results in fetal growth restriction.
Keywords: intrauterine growth restriction, passive smoke, vasorelaxation, arterial relaxation, myogenic reactivity
in 2005, over 20 million (18.1%) American women smoked cigarettes (5). While ∼25% of smoking women quit after becoming pregnant, an estimated 10.7% of all pregnant women continue to smoke during pregnancy. The impact of smoking on reproductive-aged women includes reductions in fertility and increases in early pregnancy loss, as well as complications later in pregnancy, including spontaneous miscarriage, low birth weight, prematurity, intrauterine growth restriction, placental abruption, perinatal death, and postnatal morbidity (1, 44, 68). Premature delivery and low birth weight are common among smoking mothers and remain a primary cause of neonatal death (4). Approximately 65% of infant deaths occur among infants with birth weight <2,500 g (26, 27). Maternal smoking remains the single largest modifiable risk factor for intrauterine growth restriction (4, 12, 33, 34, 39–41, 53, 61, 69).
Pregnancy is characterized by profound changes in the maternal vasculature, particularly the uterine circulation (49). Vascular adaptation to pregnancy includes both dilation and angiogenesis (47). Systemic cardiovascular changes occur early in normal pregnancy, with a marked decline in peripheral vascular resistance and blood pressure, an increase in cardiac output and blood volume, and a decrease in perfusion pressure (14). The marked reduction in systemic vascular resistance occurs at the level of the resistance vessels, with an increase in cross-sectional area and vessel compliance. Vascular responsiveness to pressors such as angiotensin II and norepinephrine is attenuated, and vasodilatory responses are enhanced via an endothelium-dependent mechanism (22, 32, 45). Changes in the renal circulation are initiated even prior to pregnancy in the luteal phase of the menstrual cycle. By 4–6 wk of gestation, renal blood flow and glomerular filtration rate are increased to 20% above baseline (15). Overall, effective renal plasma flow increases by 50–85% during gestation (14). Unlike changes in uterine blood flow that occur relatively late in pregnancy, reduced total peripheral vascular resistance, increased cardiac output, and increased renal blood flow are early changes that anticipate the needs of the growing uterus, placenta, and fetus. The mechanisms underlying the functional and structural changes of the uterine and peripheral arteries during early- and late-pregnancy vascular adaptations are not fully recognized. Striking similarities in the uteroplacental vascular structure, as well as the response of peripheral vascular beds to pregnancy, have been found between humans and other species with hemochorial-type placentation (including rodents) (2, 13, 14, 47, 50). Associations between fetal growth restriction and inadequate plasma volume expansion in early pregnancy, hemodynamic alterations, and lower cardiac output have been postulated as a cause of fetal growth restriction (17, 18, 20, 58, 60, 63). Thus, failure or perturbations of normal vascular adaptations may be a cause of poor pregnancy outcomes such as fetal growth restriction.
Smoking during pregnancy has been associated with reduced vascularization of the placenta, and three-dimensional Doppler ultrasound measures flow at 11–14 wk of gestation without impacting placental volume (57). Smoking has also recently been associated with impaired flow-mediated dilation in pregnant women, similar to previous reports in nonpregnant subjects (54). Increases in fetal and maternal heart rate are seen immediately following smoking (52). While smoking has been demonstrated to increase resistance in the uterine and umbilical arteries, no change in uterine blood flow was detected (3, 7, 30, 31, 48, 52), or if a change was observed, uterine blood flow returned to baseline within minutes of smoke or nicotine exposure (10).
Maternal cigarette smoke exposure could impair the normal vascular adaptations to pregnancy; however, the mechanism that causes fetal growth restriction is not well defined. The primary objective of this study was to determine whether maternal vascular adaptation to pregnancy was affected by smoke exposure in an animal model with cigarette smoke-induced fetal growth restriction. Use of an animal model of inhaled cigarette smoke during pregnancy is crucial to examining mechanisms for smoking-related pregnancy complications as well as testing potential interventional strategies.
METHODS
C57Bl/6J adult mice (n = 23; Jackson Laboratories, Bar Harbor, ME) were maintained on a 12:12-h light-dark cycle, with food (breeder chow) and water available ad libitum. The C57Bl mouse has been reported to be more susceptible than other mouse strains to cigarette smoke when lung inflammation and oxidative end points were measured, so this strain was selected for our studies (73). Two female and one male mouse were caged together for natural breeding. The morning of a positive vaginal plug was considered to be day 0 of gestation (n = 11). On day 4 of gestation, pregnant dams (n = 6) as well as virgins (n = 6) were exposed to whole body sidestream smoke in an inhalation chamber (model TE-10, Teague Enterprises, Davis, CA) for 4 h, with smoke exposure increased gradually over the first 5 days from 30 mg/m3 total suspended particles (TSP) to 100 mg/m3 TSP (average 67 mg/m3) and maintained at 100 mg/m3 TSP through day 16–17 of pregnancy (late gestation). TSP matter was monitored hourly using a particle impacter. The average TSP reading for the exposure was 63 mg/m3, which is representative of moderate-to-heavy smoking in humans. Mice were weighed throughout pregnancy, and percent weight gain from the initial weight to day 17 of pregnancy was calculated. Additionally, animals were monitored throughout the exposure period for signs of morbidity (weight loss and lack of activity or grooming). No animal exhibited signs of severe stress, and no experiments had to be discontinued. Control animals (n = 6 virgin controls and n = 5 pregnant controls) were placed in an adjacent chamber for the same amount of time as the exposed mice, without smoke exposure, to control for stress related to the noise generated from the smoke inhalation chamber. Smoke exposure was determined in EDTA plasma samples by measurement of cotinine levels by a Direct ELISA kit (Immunalysis, Pomona, CA). The sensitivity of the kit is 1 ng/ml. Plasma samples were obtained 18–19 h after smoke exposure. The Magee-Womens Research Institute Animal Care and Use Committee reviewed and approved this protocol.
Isolated arteriograph experiments.
Mice were killed with an intraperitoneal injection of pentobarbital sodium (Nembutal), and the arteries were immediately removed from virgin or day 17 or 18 pregnant mice (with the exception of 1 animal at gestational day 16) and placed in cold HEPES-buffered physiological saline solution at pH 7.4 (HPSS). HPSS contained (mmol/l) 142 sodium chloride, 4.7 potassium chloride, 1.17 magnesium sulfate, 2.5 calcium chloride, 1.18 potassium phosphate, 10 HEPES, and 5.5 dextrose. Second-order mesenteric arteries, segments of the main uterine artery and renal arteries, were isolated and cleaned of fat and connective tissue. Vessels were transferred to dual-chamber pressurized arteriographs (Living Systems, Burlington, VT) and mounted in parallel on microcannulas suspended inside the chambers. Residual blood was flushed from the lumen with HPSS, and the distal cannula was occluded to prevent flow. The proximal cannula was attached to a solid-state pressure transducer, a pressure servo-controller, and a peristaltic pump. This system enabled the intraluminal pressure to be maintained and controlled. The arteriograph system was placed on an inverted microscope stage equipped with a video camera-and-dimension analyzing system (Living Systems) that recorded lumen diameter and wall thickness. Further description of this system is provided elsewhere (28). The arteriograph chamber filled with HPSS was maintained at 37°C and pH 7.4. A conditioning stretch was performed after 45 min of equilibration: the intraluminal pressure was increased from 60 to 100 mmHg and returned to 60 mmHg (1–2 min total) and then equilibrated for an additional 15 min (28).
Myogenic reactivity assessment.
Myogenic reactivity is a dynamic and complex integrative vascular behavior that can be assessed in isolated arteries with use of a pressurized arteriograph. An approach modified from MacPherson et al. was utilized in these experiments (21, 37). Arteries with intraluminal pressure stabilized at 60 mmHg were subjected to a rapid increase in pressure to 80 mmHg. This manipulation was performed in triplicate, with 4- to 6-min intervals between pressure steps. Arteries studied in this manner have a beginning steady-state diameter at 60 mmHg (D1) followed by a rapid increase in diameter with the pressure step and then an active constriction to a final diameter (D2) at 80 mmHg. The pressure-induced tone is the percent change in diameter from D1 to D2.
Agonist concentration response.
Arteries were first preconstricted with phenylephrine to ∼50% of the baseline diameter at 60 mmHg. Preconstricted arteries were exposed to cumulative concentrations of the endothelium-dependent vasodilator methacholine (5 × 10−9–10−5 mol/l). Cumulative concentration-response curves to methacholine were determined. The arteries were then rinsed with HPSS. Arteries were reconstricted with phenylephrine and exposed to cumulative concentrations of the nitric oxide (NO) donor sodium nitroprusside (10−9–10−4 mol/l) to assess non-endothelium-dependent vasodilatory capacity.
Arterial smooth muscle was inactivated by treatment with a calcium-free HPSS in combination with 1 × 10−4 mol/l papaverine and 1 × 10−4 mol/l EGTA in calcium-free HPSS for ≥10 min; then passive luminal diameter and wall thickness were measured at 0–150 mmHg.
Calculations.
Passive mechanical properties of arteries were assessed using pressure-diameter relationships for distensibility and stress-strain calculations. Distensibility is defined as the relative change in diameter per unit change in pressure in arteries with inactivated smooth muscle. To obtain the relative change in diameter, the diameter measured at each pressure was normalized to an initial diameter at 5 mmHg. The slopes of the linear portion (5–100 mmHg) of the pressure-diameter curves were used to compare distensibilities between groups. The circumferential stress-strain relationship was calculated to further describe the passive mechanical properties of the arteries. This parameter was normalized for wall thickness and characterized the stiffness of the vascular wall. Circumferential stress describes the force exerted on the vascular wall per unit of tissue and is derived from the following equation: stress = (P × D)/2T, where P is transmural pressure in millinewtons per square millimeter (1 mmHg = 0.133 mN/mm2), D is diameter, and T is wall thickness. Circumferential strain represents the response of an artery to the force or intraluminal pressure it experiences. Strain was calculated as follows: (Df − D0)/D0, where D0 is the initial diameter at 5 mmHg and Df is the diameter at the new pressure. Stress was calculated for specified strains and compared between groups.
Statistical analysis.
Data are expressed as means ± SE. Student's t-test was used for all pairwise comparisons of parametric data. All data were first analyzed by one- or two-factor ANOVA. If significant main effects or interactions were observed, then individual group means were compared, with the level of significance for each test adjusted by Bonferroni's method to account for multiple comparisons or by orthogonal contrasts. Two-way repeated-measures ANOVA was used to compare changes in diameter at different doses between groups. P < 0.05 represented statistical significance.
RESULTS
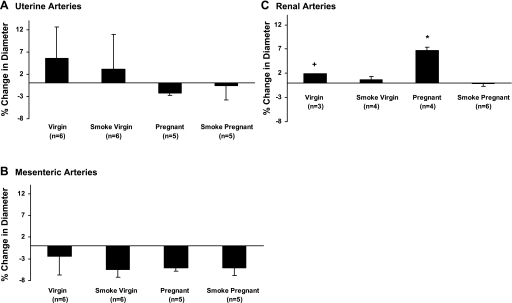
Pregnancy-associated maternal vascular remodeling as assessed by isolated arterial diameter at 60 mmHg was seen only in the uterine vasculature, where diameters increased in both control and smoke-exposed arteries from pregnant mice (Table 1). Renal arteries isolated from smoke-exposed virgin mice were significantly smaller than those isolated from virgin controls (Table 1). Maternal weight gain during pregnancy was not significantly different between smoke-exposed mothers and controls (44% vs. 37% increase in weight during pregnancy, P = 0.158; Table 2). Fetal weights were significantly reduced in smoke-exposed compared with control fetuses (0.88 ± 0.1 vs. 1.0 ± 0.08 g, P = 0.02), while litter sizes were not different (Table 2). Fetoplacental ratios were significantly reduced in smoke-exposed mothers due to the >10% reduction in fetal weights (Table 2). The levels of the nicotine metabolite cotinine in smoking mice were significantly elevated at 18–19 h after exposure to 4 h of cigarette smoke compared with control mice [51.9 ± 9.2 vs. 4.2 ± 0.8 (virgin) and 33.2 ± 21 vs. 2.8 ± 0.4 ng/ml (pregnant)]. Cotinine levels >50 ng/ml are typically associated with active smokers (61).
Table 1.
Passive (smooth muscle-inactivated) isolated mouse arterial diameters at 60 mmHg
| Diameter at 60 mmHg, μm |
||||
|---|---|---|---|---|
| Treatment Group | n | Uterine Arteries | Mesenteric Arteries | Renal Arteries |
| Virgin | ||||
| Control | 6 | 166 ± 22 | 225 ± 9 | 196 ± 10† |
| Smoke-exposed | 6 | 199 ± 12 | 231 ± 15 | 130 ± 19 |
| Pregnant | ||||
| Control | 5 | 320 ± 17*† | 246 ± 8 | 183 ± 20 |
| Smoke-exposed | 6 | 328 ± 26*† | 232 ± 10 | 156 ± 9 |
Values are means ± SE.
P < 0.05 vs. control virgin;
P < 0.05 vs. smoke virgin (by 2-way ANOVA within a vascular bed).
Table 2.
Pregnancy data
| n | Litter Size | Pup Wt, g | Placental Wt, g | Fetoplacental Ratio | %Maternal Wt Gain During Pregnancy | Gestational Age, days | |
|---|---|---|---|---|---|---|---|
| Control pregnant | 5 | 6.0 ± 1.3 | 1.0 ± 0.1* | 0.1 ± 0.01 | 8.9 ± 0.7* | 37.1 ± 4.0 | 17.6 ± 0.2 (17–18) |
| Smoke pregnant | 6 | 6.8 ± 0.6 | 0.9 ± 0.1 | 0.1 ± 0.01 | 7.1 ± 1.1 | 43.8 ± 2.2 | 17.5 ± 0.3 (16–18) |
Values are means ± SE, with range in parentheses.
P < 0.05 (by Student's t-test).
Relaxation responses.
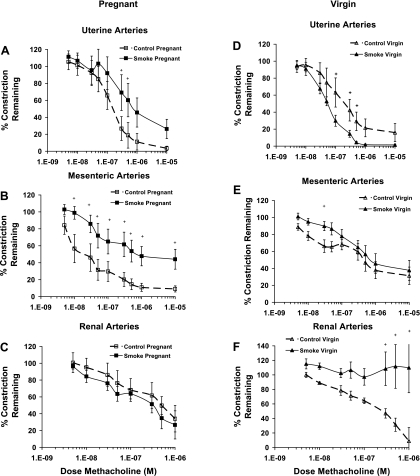
Isolated arteries were preconstricted with phenylephrine to ∼50% of their initial diameter. There was no significant difference in phenylephrine responses between the groups. Maximal endothelium-mediated relaxation responses to methacholine were significantly impaired in both the uterine (3.4 ± 3% constriction remaining in control pregnant vs. 26.3 ± 11% in smoke-exposed pregnant mice) and mesenteric (9.1 ± 4% constriction remaining in control pregnant vs. 44.1 ± 12% in smoke-exposed pregnant mice) vasculature of pregnant mice exposed to cigarette smoke during gestation (Fig. 1, A and B, Table 3). No difference in endothelium-mediated relaxation was seen in renal arteries isolated from pregnant mice exposed to cigarette smoke (Fig. 1C); however, relaxation was significantly reduced in renal arteries from virgin mice exposed to smoke (109.9 ± 35% constriction remaining in smoke-exposed virgin vs. 8.9 ± 19% in control virgin mice; Fig. 1F, Table 3). In contrast, relaxation responses to methacholine were unchanged in mesenteric arteries from virgin mice (Fig. 1E), and uterine arteries from smoke-exposed virgin mice were more sensitive than those from controls (Fig. 1D). The EC50 for methacholine was not significantly different in any of the vascular beds.
Fig. 1.
Endothelium-mediated relaxation responses to methacholine in arteries isolated from pregnant (A–C) and virgin (D–F) mice. Relaxation responses were impaired in uterine (A) and mesenteric (B) arteries from smoke-exposed pregnant mice but not in uterine (D) and mesenteric (E) arteries from virgin mice. In renal arteries (C and F), relaxation was significantly reduced only in smoke-exposed virgin mice (F). +P ≤ 0.05 (by 2-way repeated-measures ANOVA).
Table 3.
Summary arterial data
| Uterine Artery |
Mesenteric Artery |
Renal Artery |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virgin |
Pregnant |
Virgin |
Pregnant |
Virgin |
Pregnant |
|||||||
| Control | Smoke-exposed | Control | Smoke-exposed | Control | Smoke-exposed | Control | Smoke-exposed | Control | Smoke-exposed | Control | Smoke-exposed | |
| Endothelial relaxation, %constriction remaining | 15.9 ± 10.7 | 1.4 ± 0.9 | 3.4 ± 2.5 | 26.3 ± 11.3 | 31.3 ± 10.2* | 37.7 ± 11.7* | 9.1 ± 3.6 | 44.1 ± 11.7* | 9.0 ± 11.5 | 100 ± 34.5† | 34.1 ± 15.8 | 26.7 ± 16.7 |
| EC50 methacholine, μM | 0.19 ± 0.06 | 0.06 ± 0.009 | 0.29 ± 0.18 | 0.61 ± 0.20 | 0.18 ± 0.08 | 0.31 ± 0.14 | 0.05 ± 0.02 | 0.19 ± 0.07 | 0.16 ± 0.05 | 0.24 ± 0.14 | 0.31 ± 0.16 | 0.12 ± 0.05 |
| NO relaxation, %constriction remaining | 24.8 ± 7.9 | 24.1 ± 8.8 | 19.8 ± 10.0 | 20.7 ± 5.9 | 8.8 ± 8.8 | 12.1 ± 6.2 | 7.6 ± 5.4 | 0.4 ± 0.4 | NA | NA | NA | NA |
| EC50 sodium nitroprusside, μM | 0.49 ± 0.17 | 1.6 ± 1.1‡ | 0.28 ± 1.8 | 0.08 ± 0.06 | 0.51 ± 0.49* | 1.8 ± 1.8‡ | 0.00057 ± 0.00019 | 0.002 ± 0.001 | NA | NA | NA | NA |
| Myogenicity, %change in diameter | 5.5 ± 7.1 | 3.2 ± 7.7 | −2.2 ± 0.6 | −0.6 ± 3.2 | −2.4 ± 4.3 | −5.4 ± 1.9 | −5.0 ± 0.8 | −5.1 ± 1.7 | 1.9 ± 0.1 | 0.7 ± 0.7† | 6.7 ± 0.7 | −0.1 ± 0.6* |
| Stress, 106 dyn/cm2 at strain = 1 | 2.7 ± 0.9 | 1.5 ± 0.3*‡ | 4.9 ± 0.8†‡ | 3.3 ± 0.5 | 2.4 ± 0.2 | 2.6 ± 0.2 | 2.3 ± 0.5 | 2.0 ± 0.3 | NA | NA | NA | NA |
Values are means ± SE. Endothelial relaxation is expressed as %phenylephrine constriction remaining at maximum dose of methacholine. Nitric oxide (NO) relaxation is %phenylephrine constriction remaining at maximum dose of sodium nitroprusside. Myogenic tone is %change in diameter of the artery in response to a step in intraluminal pressure from 60 to 80 mmHg; positive values indicate increase in diameter and negative values indicate constriction. Arterial stress at strain = 1 is calculated stress when diameter of the artery has doubled in response to increasing intraluminal pressure. NA, not assessed.
P < 0.05 vs. control pregnant;
P < 0.05 vs. virgin control;
P < 0.05 vs. smoke-exposed pregnant (by 2-way ANOVA comparing within a vascular bed).
Smoke exposure did not change endothelium-independent relaxation responses to the NO donor sodium nitroprusside in either vessel bed (mesenteric and uterine). There was a significant increase in sensitivity to NO in arteries from pregnant compared with virgin mice, in both control and smoke-exposed mesenteric arteries and among smoke-exposed uterine arteries (Table 3).
Pressure stimulated arterial tone.
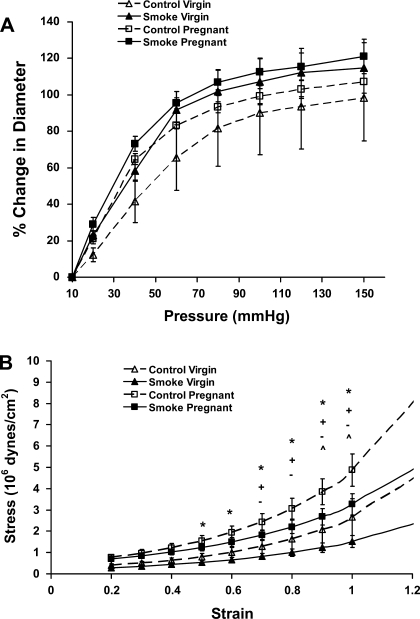
Myogenic reactivity was assessed in isolated arterial segments using repeated steps in pressure from 60 to 80 mmHg. The percent change in diameter of uterine and mesenteric arteries was not significantly different between the groups (Fig. 2, A and B). The percent change in diameter of renal arteries isolated from control pregnant mice was significantly greater (i.e., less pressure-induced reactivity) than that of arteries isolated from virgin mice, indicative of less myogenic tone, while the diameter of renal arteries from smoke-exposed pregnant mice was not changed from that of arteries from either control or smoke-exposed virgin mice (Fig. 2C).
Fig. 2.
Myogenic reactivity assessed in isolated arterial segments using repeated steps in pressure from 60 to 80 mmHg. Myogenic reactivity of uterine (A) or mesenteric (B) arteries was not significantly different between control and smoke-exposed mice. Myogenic reactivity was significantly less in renal arteries from control pregnant mice (C) than in renal arteries from either smoke-exposed pregnant or virgin mice. Myogenic reactivity in renal arteries was significantly less (P < 0.05) in control pregnant mice than in control virgin (+) and smoke-exposed pregnant (*) mice (by 1-way ANOVA).
Passive mechanical properties of isolated arteries.
The distensibility of isolated uterine arteries, demonstrated by percent change in diameter plotted against change in pressure, is shown in Fig. 3A. There was no significant difference in distensibility of isolated uterine or mesenteric arteries from any of the study groups as determined by an analysis of the pressure vs. percent change in diameter over the linear portion of the pressure-diameter curve (10–60 mmHg). There was a significant difference in wall thickness only at low pressures in uterine arteries from smoke-exposed pregnant mice compared with virgin controls (33 ± 3 vs. 22 ± 2 μm at 20 mmHg, P < 0.05); comparison of control pregnant mice and virgin controls showed a similar trend (33 ± 5 vs. 26 ± 2 μm at 20 mmHg), but the difference was not significant.
Fig. 3.
Distensibility curves of isolated uterine arteries (A) and stress-strain characteristics (B) of isolated uterine arteries from control virgin (CV), control pregnant (CP), smoke-exposed virgin (SV), and smoke-exposed pregnant (SP) mice. *P ≤ 0.040, CP vs. SV; +P ≤ 0.018, CP vs. CV; −P ≤ 0.030, SP vs. SV; ^P ≤ 0.011, CP vs. SP (by 2-way repeated-measures ANOVA).
There was no significant difference in stress-strain characteristics of uterine arteries from smoking and control virgin mice (Fig. 3B, Table 3). There was a greater stress associated with a given strain in uterine arteries from pregnant mice than in arteries from virgin mice, with the greatest pregnancy-associated change in the control mice (Fig. 3B). Thus, while the modulus of elasticity (or stiffness) was higher in uterine vessels from control pregnant mice than control virgin mice, stiffness was decreased in vessels from smoke-exposed pregnant mice compared with control pregnant mice.
DISCUSSION
This study used a pregnant mouse model of passive cigarette smoke exposure at levels causing reduced fetal weight to determine if this exposure would adversely affect the maternal vascular adaptation to pregnancy. Smoke exposure beginning on day 4 of pregnancy did not affect the litter size or maternal weight gain during pregnancy. Cigarette smoke exposure during days 4–17 of pregnancy in mice caused a reduction in the fetal-to-placental ratio due to a low fetal weight relative to placental weight. Disproportionate fetal-to-placental growth has previously been reported in humans (31, 70, 72) and in mice (19). A >10% reduction in fetal weight is consistent with previously published data in rats and mice based on the dose and time frame of exposure (19, 23, 36). In mice, mainstream or sidestream (passive) exposure to cigarette smoke prior to day 6 of gestation has been reported to decrease fetal weight and crown-rump length in C57Bl mice (19). The reduction in fetal weight independent of placental weight reduction has been postulated to result from an abnormal placenta with impaired function and/or fetal/placental exposure to toxicants present in smoke (9).
Arterial function and structure measures after smoke exposure.
Three maternal vascular beds were examined in the current study. Endothelium-mediated relaxation was impaired in mouse mesenteric and uterine vessels exposed to cigarette smoke during pregnancy and in the renal bed of virgin mice. Impaired maternal vascular function of the endothelium has also recently been demonstrated through calculations of flow in the brachial artery of smoking women at 28–32 wk of gestation in response to reactive hyperemia (54). Our data indicate that specific maternal arterial beds may be more susceptible to cigarette smoke exposure during pregnancy, and work is ongoing to begin to elucidate the mechanisms of the endothelial dysfunction.
Arterial diameter was increased only within the uterine vascular bed compared with arteries from the same vascular bed isolated from virgin mice. The dramatic expansion of arterial diameter within the uterus encompasses the main uterine artery as well as the arteries supplying the implantation site. Gokina et al. (24) showed that the most profound changes occur in the arteries directly supplying the implantation site in the uterus of rodents, while vasodilation is less profound in ancillary arteries and veins. A 40–50% increase in main uterine artery diameter is expected during a mouse pregnancy (65, 66). In the mouse, arterial fetoplacental vasculature (measured as arterial surface area and volume) is unchanged between days 13.5 and 15.5 of gestation but more than doubles from day 15.5 to 18.5 (56). Changes in the fetoplacental structure and fetal growth restriction have been demonstrated in response to subcutaneous injection of polycyclic aromatic hydrocarbon acting through the aryl hydrocarbon receptor (16).
Component of cigarette smoke with potential to impact vascular function.
The main constituents of environmental tobacco smoke are very similar to those of inhaled cigarette smoke, including tar, nicotine, carbon monoxide, cadmium, and aromatic hydrocarbons. Nicotine alone has previously been shown to reduce uterine blood flow in pregnant sheep and rats (8, 11, 42, 43), mediated through release of epinephrine. However, some reports have shown no significant fetal growth restriction with nicotine or high levels of epinephrine (8, 29, 64). A limitation of these studies was the route of nicotine dosing (via oral gavages, implantable osmotic minipump, subcutaneous intravenous injections, or parenteral bolus); these routes require high nicotine doses to achieve relevant plasma concentrations for heavy smokers that also cause maternal cardiovascular effects in rodents. Nicotine does not reproduce all the effects seen with cigarette smoke, leading us and others to believe that nicotine alone cannot cause the acute endothelial toxicity related to passive smoking (6) and that other constituents of cigarette smoke during pregnancy likely play a role in fetal growth restriction (29). It is important to note that the vascular effects reported here were observed 18–19 h after the last smoke exposure. Nicotine has a short half-life of 5–8 min in C57Bl mice, so the effects are not likely to be the direct effect of nicotine in the maternal circulation (51, 62).
The inhalation chamber permits a route of delivery and dosing levels of cigarette smoke relevant in humans. It has been shown in vitro that lipid-soluble smoke particles reduce endothelium-dependent relaxation, resulting in endothelial dysfunction in rat mesenteric vessels and human middle cerebral arteries (74). DMSO-soluble particles extracted from smoke are toxic to arterial endothelial and smooth muscle cells in culture. These particles are transported by lipoproteins to the arterial wall, directly impairing function of cells via modification of lipoprotein properties (55, 74).
This is the first study that investigates the myogenic response of vessels from vascular beds of pregnant mice exposed to cigarette smoke. Our study demonstrates that, like rats, there is a significant reduction in pressure-change-induced vascular tone in renal vessels from pregnant compared with virgin mice, and smoking during pregnancy eliminates the pregnancy-associated loss of tone (21). Reduced myogenic tone has also been observed in the small mesenteric and main uterine arteries of pregnant mice (67) and in the mesenteric and renal arteries of pregnant rats (21, 35) and can be attributed to the increased endothelium-derived NO during pregnancy. The pattern of affected vascular beds in the current study may be influenced by the time frame of pregnancy-initiated changes relative to smoke exposure. Early adaptations, including renal changes and the initiation of uterine artery remodeling, would have occurred prior to smoke exposure on day 4 of gestation.
Mechanical properties of vessel walls.
Murine uterine arteries remodel during pregnancy by increasing wall mass early in pregnancy and then increasing intraluminal diameter with no change in densities of elastin and collagen in the extracellular matrix (66). Previous studies showed increased distensibility of uterine arteries of pregnant sheep and guinea pigs and uteroplacental arteries of rats during pregnancy (25, 38, 46), but arteries from pregnant sheep were stiffer than those from virgin sheep (25), while stiffness did not change in the guinea pig (38). This is the first study to show the effect of smoking on the stress-strain relationship of uterine vessels in pregnant mice. There is a significant decrease in the modulus of elasticity (or stiffness) of uterine vessels when pregnant mice are exposed to cigarette smoke; i.e., the vessels become less stiff. In this case, smoking affects the natural adaptations to pregnancy by not enabling the vessels to attain the desired level of stiffness required to accommodate the large increase in blood flow. We found no significant changes in stress-strain relationships of mesenteric and renal vessels of smoking and nonsmoking pregnant mice.
The pathogenesis of the relationship between smoking and growth restriction remains unclear. Potential mechanisms for poor fetal growth include direct effects of nicotine on uterine and umbilical vasculature resulting in vasoconstriction of the uteroplacental circulation (71), increased carboxyhemoglobin in the maternal circulation leading to chronic oxygenation insufficiency of the fetus (59), and chronic exposure of the fetoplacental unit to the thousands of secondary components of tobacco smoke or their metabolites. Smoke exposure results in smaller fetuses in pregnant mice, with significant impairment of the maternal uterine and mesenteric relaxation capacity and renal vascular myogenic tone.
Perspectives and Significance
While smoking is well recognized as a modifiable risk factor associated with numerous pregnancy complications, ∼10% of women will continue to smoke during pregnancy. The uteroplacental vasculature is a likely target of cigarette smoke in both humans and rodent models. The present study and the work of Quinton et al. (54) extend this information to include other maternal vascular beds in mice and humans. Our work also demonstrates that sensitivity to smoke exposure during pregnancy is dependent on the vascular bed examined. The data obtained using a smoke inhalation exposure model in mice and detailed studies of the uteroplacental vascular changes of pregnancy (2, 47, 56) indicate that this is an ideal model system for mechanistic studies as well as for elucidation of both critical time points and components of smoke.
GRANTS
This work was funded by National Institutes of Health Grants P50 ES-12359 and HD-03067.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
Present address of J. A. DeLoia: School of Nursing & Health Studies, Georgetown University, 3700 Reservoir Rd., NW, Washington, DC 20057.
REFERENCES
- 1.Adams EK, Melvin CL. Costs of maternal conditions attributable to smoking during pregnancy. Am J Prev Med 15: 212–219, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 250: 358–373, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque CA, Smith KR, Johnson C, Chao R, Harding R. Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Hum Dev 80: 31–42, 2004 [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetrics and Gynecologists. Smoking and Women's Health Washington, DC: Am. Coll. Obstet. Gynecol., 1997. (Educational Bulletin 240) [Google Scholar]
- 5.Anonymous. Tobacco use among adults—US 2005. Morbidity Mortality Weekly Rep 42: 1145, 2006 [PubMed] [Google Scholar]
- 6.Argacha JF, Fontaine D, Adamopoulos D, Ajose A, van de Borne P, Fontaine J, Berkenboom G. Acute effect of sidestream cigarette smoke extract on vascular endothelial function. J Cardiovasc Pharmacol 52: 262–267, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Ates U, Ata B, Armagan F, Has R, Sidal B. Acute effects of maternal smoking on fetal hemodynamics. Int J Gynaecol Obstet 87: 14–18, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum SC, Kien N, Martucci RW, Gelzleichter TR, Witschi H, Hendrickx AG, Last JA. Nicotine- or epinephrine-induced uteroplacental vasoconstriction and fetal growth in the rat. Toxicology 94: 69–80, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Boyd PA, Keeling JW. Raised maternal serum α-fetoprotein in the absence of fetal abnormality—placental findings. A quantitative morphometric study. Prenat Diagn 6: 369–373, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Bruner JP, Forouzan I. Smoking and buccally administered nicotine. Acute effect on uterine and umbilical artery Doppler flow velocity waveforms. J Reprod Med 36: 435–440, 1991 [PubMed] [Google Scholar]
- 11.Clark KE, Irion GL. Fetal hemodynamic response to maternal intravenous nicotine administration. Am J Obstet Gynecol 167: 1624–1631, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Cnattingius S, Haglund B, Kramer MS. Differences in late fetal death rates in association with determinants of small for gestational age fetuses: population based cohort study. BMJ 316: 1483–1487, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad KP, Gandley RE, Ogawa T, Nakanishi S, Danielson LA. Endothelin mediates renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. Am J Physiol Renal Physiol 276: F767–F776, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Conrad KP, Lindheimer MD. Renal and cardiovascular alterations. In: Chesley's Hypertensive Disorders in Pregnancy (2 ed.), edited by Lindheimer MD, Roberts JM, Cunningham FG. Norwark, CT: Appleton & Lange, 1999, chapt. 8, p. 263–326 [Google Scholar]
- 15.Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol 88: 10–17, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Detmar J, Rennie MY, Whiteley KJ, Qu D, Taniuchi Y, Shang X, Casper RF, Adamson SL, Sled JG, Jurisicova A. Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am J Physiol Endocrinol Metab 295: E519–E530, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Schouten HJ, Peeters LL. Maternal volume homeostasis in early pregnancy in relation to fetal growth restriction. Obstet Gynecol 85: 361–367, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Duvekot JJ, Cheriex EC, Pieters FA, Peeters LL. Severely impaired fetal growth is preceded by maternal hemodynamic maladaptation in very early pregnancy. Acta Obstet Gynecol Scand 74: 693–697, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Esposito ER, Horn KH, Greene RM, Pisano MM. An animal model of cigarette smoke-induced in utero growth retardation. Toxicology 246: 193–202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallery ED, Hunyor SN, Gyory AZ. Plasma volume contraction: a significant factor in both pregnancy-associated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Q J Med 48: 593–602, 1979 [PubMed] [Google Scholar]
- 21.Gandley RE, Conrad KP, McLaughlin MK. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am J Physiol Regul Integr Comp Physiol 280: R1–R7, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaworski CL, Carmines EL, Faqi AS, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicol Sci 79: 157–169, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol 189: 1489–1493, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Griendling KK, Fuller EO, Cox RH. Pregnancy-induced changes in sheep uterine and carotid arteries. Am J Physiol Heart Circ Physiol 248: H658–H665, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Guyer B, Strobino DM, Ventura SJ, MacDorman M, Martin JA. Annual summary of vital statistics—1995. Pediatrics 98: 1007–1019, 1996 [PubMed] [Google Scholar]
- 27.Guyer B, Strobino DM, Ventura SJ, Singh GK. Annual summary of vital statistics—1994. Pediatrics 96: 1029–1039, 1995 [PubMed] [Google Scholar]
- 28.Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann Biomed Eng 12: 463–479, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Hussein J, Farkas S, MacKinnon Y, Ariano RE, Sitar DS, Hasan SU. Nicotine dose-concentration relationship and pregnancy outcomes in rat: biologic plausibility and implications for future research. Toxicol Appl Pharmacol 218: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Jouppila P, Kirkinen P, Eik-Nes S. Acute effect of maternal smoking on the human fetal blood flow. Br J Obstet Gynaecol 90: 7–10, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Kalinka J, Hanke W, Sobala W. Impact of prenatal tobacco smoke exposure, as measured by midgestation serum cotinine levels, on fetal biometry and umbilical flow velocity waveforms. Am J Perinatol 22: 41–47, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Knock GA, Poston L. Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am J Obstet Gynecol 175: 1668–1674, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Kramer MS. Socioeconomic determinants of intrauterine growth retardation. Eur J Clin Nutr 52 Suppl 1: S29–S32, 1998 [PubMed] [Google Scholar]
- 34.Kramer MS, Seguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol 14: 194–210, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Learmont JG, Cockell AP, Knock GA, Poston L. Myogenic and flow-mediated responses in isolated mesenteric small arteries from pregnant and nonpregnant rats. Am J Obstet Gynecol 174: 1631–1636, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Leichter J. Decreased birth weight and attainment of post-natal catch-up growth in offspring of rats exposed to cigarette smoke during gestation. Growth Dev Aging 59: 63–66, 1995 [PubMed] [Google Scholar]
- 37.MacPherson RD, McLeod LJ, Rasiah RL. Myogenic response of isolated pressurized rabbit ear artery is independent of endothelium. Am J Physiol Heart Circ Physiol 260: H779–H784, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Mateev SN, Mouser R, Young DA, Mecham RP, Moore LG. Chronic hypoxia augments uterine artery distensibility and alters the circumferential wall stress-strain relationship during pregnancy. J Appl Physiol 100: 1842–1850, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, Johns KA. Factors associated with preterm birth in Cardiff, Wales. I. Univariable and multivariable analysis. Am J Obstet Gynecol 173: 590–596, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, Johns KA. Factors associated with preterm birth in Cardiff, Wales. II. Indicated and spontaneous preterm birth. Am J Obstet Gynecol 173: 597–602, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, Johns KA. Factors associated with term low birthweight in Cardiff, Wales. Paediatr Perinat Epidemiol 11: 287–297, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Mitchell JA, Hammer RE, Goldman H. Concomitant reduction in uterine blood flow and intrauterine oxygen tension in the rat following nicotine administration. Adv Exp Med Biol 159: 231–241, 1983 [DOI] [PubMed] [Google Scholar]
- 43.Monheit AG, VanVunakis H, Key TC, Resnik R. Maternal and fetal cardiovascular effects of nicotine infusion in pregnant sheep. Am J Obstet Gynecol 145: 290–296, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, Kline J. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med 340: 333–339, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Nisell H, Hjemdahl P, Linde B. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol 5: 479–493, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Osol G, Cipolla M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am J Obstet Gynecol 168: 268–274, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ovari L, Aranyosi J, Balla G. Acute effect of cigarette smoking on placental circulation—a study by carbon-monoxide measurement and Doppler assessment. Acta Physiol Hung 96: 243–250, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000–1006, 1992 [PubMed] [Google Scholar]
- 50.Pascoal IF, Lindheimer MD, Nalbantian-Brandt C, Umans JG. Preeclampsia selectively impairs endothelium-dependent relaxation and leads to oscillatory activity in small omental arteries. J Clin Invest 101: 464–470, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos 12: 725–731, 1984 [PubMed] [Google Scholar]
- 52.Pijpers L, Wladimiroff JW, McGhie JS, Bom N. Acute effect of maternal smoking on the maternal and fetal cardiovascular system. Early Hum Dev 10: 95–105, 1984 [DOI] [PubMed] [Google Scholar]
- 53.Pollack H, Lantz PM, Frohna JG. Maternal smoking and adverse birth outcomes among singletons and twins. Am J Public Health 90: 395–400, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quinton AE, Cook CM, Peek MJ. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG 115: 780–784, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol 5: 276–292, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Rennie MY, Whiteley KJ, Kulandavelu S, Adamson SL, Sled JG. 3D visualisation and quantification by microcomputed tomography of late gestational changes in the arterial and venous feto-plaental vasculature of the mouse. Placenta 28: 833–840, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Rizzo G, Capponi A, Pietrolucci ME, Arduini D. Effects of maternal cigarette smoking on placental volume and vascularization measured by 3-dimensional power Doppler ultrasonography at 11 + 0 to 13 + 6 wk of gestation. Am J Obstet Gynecol 200: 415e411–e415, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Rosso P, Donoso E, Braun S, Espinoza R, Fernandez C, Salas SP. Maternal hemodynamic adjustments in idiopathic fetal growth retardation. Gynecol Obstet Invest 35: 162–165, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Salafia C, Shiverick K. Cigarette smoking and pregnancy. II. Vascular effects. Placenta 20: 273–279, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Salas SP, Rosso P, Espinoza R, Robert JA, Valdes G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol 81: 1029–1033, 1993 [PubMed] [Google Scholar]
- 61.Savitz DA, Dole N, Terry JW, Jr, Zhou H, Thorp JM., Jr Smoking and pregnancy outcome among African-American and white women in central North Carolina. Epidemiology 12: 636–642, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Siu EC, Tyndale RF. Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Mol Pharmacol 71: 826–834, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Soffronoff EC, Kaufmann BM, Connaughton JF. Intravascular volume determinations and fetal outcome in hypertensive diseases of pregnancy. Am J Obstet Gynecol 127: 4–9, 1977 [DOI] [PubMed] [Google Scholar]
- 64.Trend SG, Bruce NW. Resistance of the rat embryo to elevated maternal epinephrine concentrations. Am J Obstet Gynecol 160: 498–501, 1989 [DOI] [PubMed] [Google Scholar]
- 65.van der Heijden OW, Essers YP, Fazzi G, Peeters LL, De Mey JG, van Eys GJ. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 72: 1161–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 66.van der Heijden OW, Essers YP, Simkens LH, Teunissen QG, Peeters LL, De Mey JG, van Eys GJ. Aging blunts remodeling of the uterine artery during murine pregnancy. J Soc Gynecol Investig 11: 304–310, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol 283: H2226–H2233, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Wald NJ, Hackshaw AK. Cigarette smoking: an epidemiological overview. Br Med Bull 52: 3–11, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Tager IB, Van Vunakis H, Speizer FE, Hanrahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol 26: 978–988, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Williams LA, Evans SF, Newnham JP. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ 314: 1864–1868, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Windham GC, Eaton A, Hopkins B. Evidence for an association between environmental tobacco smoke exposure and birthweight: a meta-analysis and new data. Paediatr Perinat Epidemiol 13: 35–57, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology 11: 427–433, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Zhang JY, Cao YX, Xu CB, Edvinsson L. Lipid-soluble smoke particles damage endothelial cells and reduce endothelium-dependent dilatation in rat and man. BMC Cardiovasc Disord 6: 3, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]