Abstract
Recent discoveries reveal complex interactions between skeletal muscle and the immune system that regulate muscle regeneration. In this review, we evaluate evidence that indicates that the response of myeloid cells to muscle injury promotes muscle regeneration and growth. Acute perturbations of muscle activate a sequence of interactions between muscle and inflammatory cells. The initial inflammatory response is a characteristic Th1 inflammatory response, first dominated by neutrophils and subsequently by CD68+ M1 macrophages. M1 macrophages can propagate the Th1 response by releasing proinflammatory cytokines and cause further tissue damage through the release of nitric oxide. Myeloid cells in the early Th1 response stimulate the proliferative phase of myogenesis through mechanisms mediated by TNF-α and IL-6; experimental prolongation of their presence is associated with delayed transition to the early differentiation stage of myogenesis. Subsequent invasion by CD163+/CD206+ M2 macrophages attenuates M1 populations through the release of anti-inflammatory cytokines, including IL-10. M2 macrophages play a major role in promoting growth and regeneration; their absence greatly slows muscle growth following injury or modified use and inhibits muscle differentiation and regeneration. Chronic muscle injury leads to profiles of macrophage invasion and function that differ from acute injuries. For example, mdx muscular dystrophy yields invasion of muscle by M1 macrophages, but their early invasion is accompanied by a subpopulation of M2a macrophages. M2a macrophages are IL-4 receptor+/CD206+ cells that reduce cytotoxicity of M1 macrophages. Subsequent invasion of dystrophic muscle by M2c macrophages is associated with progression of the regenerative phase in pathophysiology. Together, these findings show that transitions in macrophage phenotype are an essential component of muscle regeneration in vivo following acute or chronic muscle damage.
Keywords: skeletal muscle, macrophage, neutrophil, muscular dystrophy, chemokines
skeletal muscle has a remarkable capacity for repair, even following severe damage. Experimental findings show that crushing muscle, killing muscle by the injection of toxins, mechanical destruction caused by large, lengthening stretches, or killing muscle fibers by freezing can be followed by the successful regeneration of muscle that leads to recovery of normal structure and function. The regenerative capacity of skeletal muscle relies largely on the presence of a population of mononucleated, myogenic cells, called satellite cells, that retain their ability to proliferate and then differentiate to either fuse with existing fibers or with other myogenic cells to generate new fibers. Thus, perturbations to the processes that normally regulate the activation, proliferation, or differentiation of satellite cells following injury could impair the regenerative capacity of muscle.
Muscle regeneration consists of three major stages, all of which are regulated in part by the activities of a family of muscle-specific transcription factors in the basic helix-loop-helix family (bHLH) (for reviews, see Refs. 52 and 59). These bHLH proteins include MyoD, myogenin, Myf4, and Myf5, each of which can heterodimerize with enhancer proteins, which thereby enables their binding to muscle-specific genes to activate their transcription. The first stage, called the proliferative stage, involves the activation of quiescent satellite cells, leading to their proliferation and expression of MyoD and Myf5 (17, 18, 27, 33, 134). Although these bHLH proteins are present during the proliferative phase, they are held in an inactive state until the satellite cells exit the cell cycle and enter the early differentiation stage. At that time, myogenin and Myf4 expression is initiated, along with transcription factors in the myocyte enhancer binding factor-2 (MEF2) family (17, 18, 27, 33, 72, 124). These factors and activated MyoD and Myf5 then drive the expression of muscle-specific genes that are necessary for muscle cell fusion and transition to the third stage, terminal differentiation. During terminal differentiation, muscle-specific genes are expressed at high levels as the multinucleated muscle cell differentiates into a mature fiber. Progression through these stages of satellite cell activation and differentiation is essential for muscle regeneration following major damage or disease in muscle. In general, the program of satellite cell activation and differentiation recapitulates embryonic development of muscle, with similar patterns of gene expression occurring during the differentiation of embryonic myoblasts and in satellite cells during muscle regeneration.
Although the regulatory mechanisms that affect skeletal muscle regeneration parallel those that occur during muscle development, the microenvironments in which myogenesis occurs varies dramatically between embryonic myogenesis and muscle regeneration. While immune cells are relatively scarce in developing skeletal muscle (1), they can be present in regenerative muscle at concentrations that exceed 100,000 inflammatory cells/mm3 of muscle tissue (129). These immune cells are primarily myeloid cells that are activated, secretory cells capable of releasing numerous soluble molecules, especially cytokines, that can affect the viability and transcriptional activities of regenerative muscle cells. Despite the large number of myeloid cells that are present in regenerating muscle and their capacity to release complex mixtures of proteins that affect the transcriptional activity of target cells, the mechanisms through which they may modulate regeneration have only recently begun to be understood.
The goal of this review is to present and assess recent findings that have led to our rapidly growing understanding of the regulatory interactions between myeloid cells and regenerative skeletal muscle (Fig. 1). Recent discoveries have shown that the response of the immune system to muscle injury and disease is a complexly regulated process, in which multiple myeloid cell populations participate and in which they regulate one another's function, as well as the regeneration of muscle. Furthermore, both injured and healthy muscle cells in the damaged tissue participate in regulating the inflammatory response, and thereby indirectly influence their own regenerative process. We also contrast inflammatory processes that occur in muscle that is subject to acute perturbations that cause injury (acute injuries) with inflammatory processes that occur in diseased muscle, in which repetitive damage occurs over a prolonged period (chronic injuries).
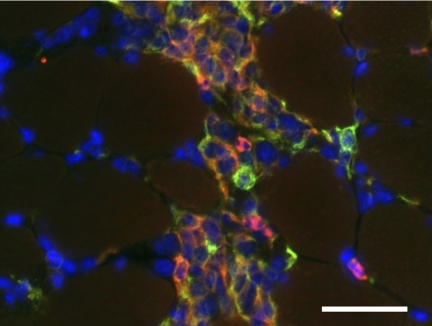
Fig. 1.
Inflammatory lesion in skeletal muscle shows codistribution of M1 and M2 macrophages with activated satellite cells. Anti-F4/80 (red fluorophore) binds all macrophages. Anti-CD206 (green fluorophore) binds M2 macrophages and satellite cells. Hoechts labeling (blue) shows nuclei. CD206−/F4/80+ cells (red) are M1 macrophages. CD206+/F4/80+ cells (orange) are M2 macrophages. CD206+/F4/80− cells (green) are satellite cells. Muscle section was obtained from 4-wk-old mdx quadriceps muscle. Scale bar = 50 μm.
Changes in Myeloid Cell Phenotype in Muscle Following Injury Coincide with Changes in Transcriptional Activities of Myogenic Cells
Acute muscle injuries initiate a predictable series of responses by specific myeloid cell populations. As in other tissues, Ly6C+/F4/80- neutrophils are the first responders and begin to appear at elevated numbers within 2 h of muscle damage, typically peaking in concentration between 6 and 24 h postinjury and then rapidly declining in numbers. Following the onset of neutrophil invasion, phagocytic macrophages begin to invade, reaching significantly elevated concentrations at about 24 h postinjury and continue to increase in numbers until about 2 days postinjury, when their numbers begin to decline sharply (8, 16, 26, 80, 98, 109, 117). Their invasion precedes the elevation of a population of nonphagocytic macrophages that reaches peak concentrations in the muscle at about 4 days postinjury but remains significantly elevated for many days (Fig. 2).
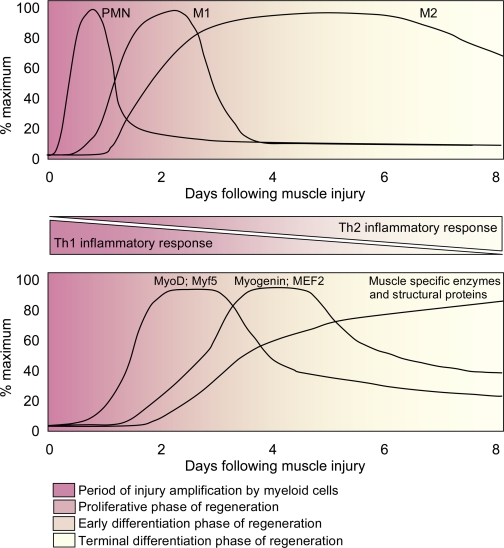
Fig. 2.
Generalized time course of changes in myeloid cell populations (top) and changes in the expression levels of muscle-specific transcription factors, enzymes, and structural proteins in muscle (bottom) following acute muscle injury. The transition from a Th1 inflammatory response to a Th2 inflammatory response coincides with a transition from the early proliferative stage of myogenesis to the early and terminal stages of myogenesis. Experimental perturbations that disrupt the Th1 to Th2 transition also disrupt the transition from proliferative to differentiation stages of myogenesis, suggesting a functional linkage between differentiation in the myeloid compartment and myogenic compartment. PMN, neutrophils; M1, M1 macrophages; M2, M2 macrophages.
Coincident with the flux of myeloid cells in muscle following acute injury, myogenic cells transition through a stereotypical pattern of expression of transcription factors and other genes that reflect muscle repair and regeneration (Fig. 2). For example, injection of cardiotoxin (CTX) into mouse soleus muscle induced an increase in MyoD expression in satellite cells by 2 days postinjury followed by an elevation of myogenin expression at 3 days postinjury (51, 136). In other injury models, MyoD expression was again highest at 2 to 3 days following muscle damage, while other transcripts that were upregulated during regeneration, such as developmental myosin heavy chain (dMyHC), appeared subsequently and continued to be expressed later in the regenerative process (65). Although these fluxes in the expression of developmentally regulated genes coincide with changes in the populations of myeloid cells in the regenerating muscle (Fig. 2), myeloid cells are not required for the process to occur in vitro. Assays of cultures that consist exclusively of myogenic cells show the same sequence of expression of developmentally regulated muscle genes. Studies of the activation of satellite cells on the surface of muscle fibers in culture showed that MyoD is expressed early in proliferating satellite cells, and myogenin and dMyHC appeared after the decline in MyoD expression and slowing of muscle cell proliferation (17, 134). Furthermore, elevated expression of myogenin was transient, while elevated expression of dMyHC was persistent, as occurs in injured muscle in vivo (136).
Although these in vitro studies show that the sequence of expression of developmentally regulated, myogenic genes occurs in the absence of myeloid cells, myeloid cells can clearly influence the magnitude and perhaps the timing of expression of at least some of these genes in vitro and in vivo. Factors released by cells of the monocyte/macrophage lineage can promote satellite cell proliferation in vitro (12, 62). Furthermore, conditioned media from J774 macrophage cultures increased MyoD expression in myoblasts but also increased myogenin expression when applied to cultured muscle cells later in differentiation (13), indicating that macrophages could promote both proliferation and differentiation of muscle cells. However, other studies show differing results. The addition of macrophages to cultures of myogenic cells yielded increased proliferation of myogenic cells but reduced the expression of myogenin (69), suggesting that macrophage-derived products could inhibit muscle differentiation by affecting the transition from the proliferative stage to the early differentiation stage of myogenesis. Although the explanation for these reported differences in the effects of macrophages on myogenic cell differentiation have not been identified with certainty, part of the explanation may lie in unknown differences in the phenotype of the macrophages used in the assays.
Phenotypic Diversity of Macrophages in Regenerative Skeletal Muscle
Skeletal muscle, as other tissues, initially responds to injury with an innate immune response driven by Th1 cytokines. Cytokines expressed during Th1-driven inflammatory responses, especially interferon-γ (IFN-γ) and TNF-α, drive the classical activation of macrophages to an M1 phenotype that is a proinflammatory population capable of perpetuating the inflammatory response (see Ref. 31 for review). M1 macrophages can also promote muscle damage in vitro and in vivo by the production of cytotoxic levels of nitric oxide (NO) generated by inducible nitric oxide synthase (iNOS) (77, 123). Early studies of muscle inflammation using the rodent hindlimb suspension/reloading model (HS/Rel) implicated M1 macrophages in the early stages of muscle injury (63, 64, 109). Mild muscle injury and inflammation are produced in rodent hindlimbs when the animals' hindlimbs are returned to normal weight bearing after a period of unloading and atrophy (45). M1 macrophages express CD68+, which is a valuable, macrophage-specific marker for the M1 phenotype. Although CD206-expressing M2 macrophages can also express CD68 under some conditions (58), this coexpression likely highlights the phenotypic and functional plasticity displayed by macrophages present in inflammatory microenvironments (73). CD68 is functionally important for M1 macrophages. CD68, also called macrosialin (87) or ED1 antigen (20), is a receptor for oxidized low-density lipoproteins (LDLs) and CD68 ligation of oxidized LDLs can activate phagocytosis by M1 macrophages and increase their production of proinflammatory cytokines (82, 89, 122). In particular, oxidative modification of LDLs by myeloperoxidase (MPO) (140) promotes LDL binding to CD68, thereby increasing activation of M1 macrophages. LDL modification by MPO may be particularly important in regulating the process of tissue repair and regeneration in skeletal muscle; neutrophils that invade injured muscle in advance of M1 macrophages induce muscle membrane damage through the release of MPO (78). This may be one of several important regulatory interactions that occur between neutrophils and M1 macrophages during the early stage of muscle inflammation. For example, neutrophils can also promote the cytolytic capacity of macrophages to lyse muscle cells (77). This elevated lysis of muscle cells can then provide positive feedback to further promote phagocytic activity of macrophages that is stimulated by myoblast lysis (97).
After M1 macrophages reach their peak concentration in injured and regenerative muscle, they are replaced by a population of M2 macrophages that can attenuate the inflammatory response and promote tissue repair. M2 macrophages are activated by Th2 cytokines; interleukin-4 (IL-4), IL-10, and IL-13 play particularly well-characterized roles in their activation (30). M2 macrophages are a complex phenotype that has been divided into three subcategories that reflect functional and molecular specializations (61). M2a macrophages are activated by IL-4 and IL-13 and can promote wound healing and tissue repair. M2b macrophages are activated by immune complexes or Toll-like receptors and release Th2, anti-inflammatory cytokines. M2c macrophages are activated by IL-10 and release cytokines that deactivate the M1 phenotype and can promote proliferation of nonmyeloid cells. Both M2a and M2c macrophages have been demonstrated in injured and regenerative muscle, indicated by their expression of CD206, present on both M2a and M2c macrophages, and their expression of CD163, which is present on M2c macrophages (123).
M2-macrophage-specific CD antigens have now been associated with functions that are important in regulating macrophage activity and phenotype. For example, CD163 is a macrophage-specific receptor for complexes of hemoglobin and haptaglobin (46, 95) and ligation of CD163 can contribute to regulating macrophage phenotype by increasing the expression of anti-inflammatory cytokines, especially IL-10 (86). Furthermore, internalization and breakdown of the hemaglobin/haptoglobin complex can contribute to returning extracellular hemoglobin to nontoxic levels, thereby reducing cellular damage following injury (71). Hemoglobin internalization and breakdown can also inhibit the production of cytolytic, free radicals by neutrophils and M1 macrophages because extracellular heme-Fe3+ can catalyze free radical production through the Fenton reaction (56, 93). Thus, CD163 ligation may contribute substantially to shifting macrophages from a M1 phenotype to an M2c phenotype, and it can reduce muscle damage mediated by free radicals. This transition could help set the stage for muscle regeneration following injury.
Similarly, CD206 mediates M2 macrophage functions that can lead to a reduction of muscle inflammation and damage. CD206 is a mannose receptor that binds and internalizes sugar moieties on molecules present at high levels in inflamed tissue. In the context of muscle damage and regeneration, MPO is an important ligand for CD206 (101) because it serves a prominent role in muscle membrane lysis that is caused by neutrophils (79), and its ligation and internalization by M2 macrophages would reduce cytotoxicity. CD206 expression by M2 macrophages is promoted by anti-inflammatory cytokines, and its binding increases the expression of anti-inflammatory cytokines, leading to positive feedback that can enable M2 macrophages to more rapidly deactivate Th1 cells that are capable of free radical-mediated damage of muscle cells.
Do Neutrophils or M1 Macrophages Promote Muscle Regeneration by Phagocytic Removal of Cellular Debris?
Although free radical production by neutrophils and M1 macrophages in injured muscle contributes to muscle membrane lysis, free radicals also target tissue debris for phagocytosis. However, the importance of clearing debris for the subsequent regeneration of muscle is uncertain. On one hand, depletion of phagocytes by administering liposome-encapsulated clodronate slows removal of cellular debris caused by freeze-injury of muscle, but reportedly has no effect on regeneration (111). In this case, regeneration was assessed by measuring relative levels of MyoD and myogenin expression in injured muscle at 3 days postinjury, which showed no difference between levels of these transcripts in depleted and nondepleted muscles. While that finding indicated that reductions in phagocyte numbers and their removal of cellular debris did not affect regeneration (111), the possibility remains open that other, untested indices of regeneration were affected by the phagocyte depletion, or perhaps treatment effects on regeneration would be evident at other sampling times.
In contrast, other investigators have concluded that reducing the numbers of phagocytic leukocytes from mice prior to muscle injury by toxin injection slows the removal of cellular debris and reportedly slows muscle regeneration (2, 114). In one case (114), depletion was achieved by targeting phagocytic cells with RB6–8C5, a monoclonal antibody that binds Ly6C and Ly6G and can deplete neutrophils and monocytes. In the second case, CD11b-expressing leukocytes were targeted by injecting diptheria toxin into transgenic mice that expressed the diptheria toxin receptor gene driven by the CD11b promoter (2); this strategy could deplete all CD11b-expressing cells, including neutrophils, monocytes, and macrophages. In these latter two studies, regeneration in phagocyte-depleted, toxin-injured muscle was evaluated by comparing relative quantities of central-nucleated muscle fibers (CNFs) in injured muscles of depleted and nondepleted muscles, finding more CNFs in muscles from nondepleted mice (2, 114). However, central nucleation is a consequence of fiber injury, and not an independent index of regeneration per se. Thus, an alternative interpretation would be that phagocyte depletion reduced injury and the reductions in CNFs reflected a decrease in fiber injury caused by the depletion of cytolytic neutrophils and M1 macrophages. This alternative interpretation is consistent with many published findings showing that neutrophils and M1 macrophages can cause muscle fiber damage in vitro and in vivo. For example, muscle injury following a puncture wound with a needle was reduced by administration of function-blocking, soluble CD11b or antibodies to CD11b prior to needle puncture, and the reduced injury was accompanied by a reduction in neutrophils entering the damaged tissue (138). Similarly, systemic administration of function-blocking, anti-CD11b before applying mechanical injury to muscle was sufficient to reduce muscle fiber damage (10). Thus, reductions of central nucleation in muscle fibers following injury of phagocyte-depleted mice may reflect both reductions in phagocyte-mediated damage and impaired regenerative capacity caused by slower removal of cellular debris.
Do Neutrophil-Derived or M1 Macrophage-Derived Molecules Modulate Muscle Growth and Regeneration?
Prostaglandins.
Impaired regeneration of injured muscle in phagocyte-depleted animals may reflect a loss of neutrophil- or M1 macrophage-derived products that can promote muscle regeneration. Much of the early evidence for a relationship between the secretory activities of phagocytes and muscle growth and regeneration was based upon the observation that administration NSAIDs to subjects or experimental animals could affect the course of muscle repair (70). NSAIDs are strong inhibitors of cycloxygenases (COX), and thereby impair the metabolism of arachidonic acid that is necessary for the synthesis of prostaglandins (8). Prostaglandins are able to affect muscle cell proliferation (142), differentiation (96), and fusion (141), and can also modulate muscle fiber growth and the synthesis and degradation of proteins in muscle (104, 121). Thus, perturbation of COX activity in either inflammatory cells or in muscle cells would be expected to have broad effects on muscle growth and regeneration following injury.
COX-1 and COX-2 are the most highly expressed members of the COX family in injured muscle (7). Despite the ability of each form to drive prostaglandin synthesis, their roles in muscle regeneration are distinct. Cytokines and mitogens that are released in injured and inflamed tissues promote the expression of COX-2 in inflammatory cells so that the elevation of prostaglandin synthesis in inflamed tissue is primarily reflective of COX-2 induction (29). In vivo studies show that COX-2 activation rather than COX-1 is primarily responsible for production of prostaglandins that affect muscle regeneration. For example, administration of a COX-2-selective inhibitor to mice experiencing muscle injury significantly slowed the growth of regenerating fibers during muscle repair, while COX-1 selective inhibition did not have a significant effect on fiber growth (7). Similarly, null mutation of COX-2 slowed muscle fiber growth in the same muscle freeze-injury model (7). Furthermore, COX-2 inhibition reduced MyoD expression in the regenerating muscle, suggesting a role for COX-2 in modulating muscle differentiation, as well as growth (7). However, COX-2 inhibition also reduced inflammation, so that the treatment effects could reflect either loss of inflammatory cell-derived substances that promoted regeneration or result from direct effects on the muscle cells themselves. Although the relative importance of inflammatory cell-derived prostaglandins and muscle-derived prostaglandins in regulating muscle regeneration in vivo is not known, in vitro studies show that perturbations of prostaglandin production by muscle cells can explain many of the effects observed in muscle injury models in vivo. For example, COX-2 selective inhibitors and COX-2 null mutation reduce muscle cell proliferation in vitro (8, 67), decrease myogenin expression (67, 100), decrease muscle cell fusion (67), and prevent stretch-activation of muscle cell proliferation (81) in the absence of inflammatory cells.
Cytokines.
Reductions in TNF-α signaling may be particularly important in contributing to impaired regeneration of neutrophil-depleted or M1 macrophage-depleted, injured muscles. However, predicting whether perturbing TNF-α functions in injured or diseased muscle will improve or worsen the progress of muscle regeneration is complicated by the myriad mechanisms through which TNF-α affects muscle inflammation and repair (Fig. 3).
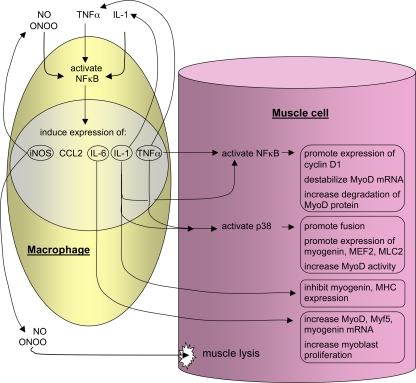
Fig. 3.
Activation of NF-κB in muscle cells or macrophages can directly or indirectly influence muscle cell proliferation and differentiation. Th1 cytokines or oxidative stress can increase NF-κB activation in muscle or macrophages. Those cytokines can then contribute to further activation of NF-κB or they can act on muscle cells to affect their proliferation or differentiation. In general, NF-κB activation increases proliferation and inhibits differentiation, increasing the expression of transcripts needed for cell cycle progression and by decreasing the expression or destabilizing transcripts needed for muscle to experience terminal differentiation. NO, nitric oxide; iNOS, inducible nitric oxide synthesis; ONOO, peroxynitrate; MEF2, myocyte enhancer binding factor-2; MHC, myson heavy chain.
TNF-α has long been viewed as the quintessential proinflammatory cytokine, capable of classical activation of macrophages to the M1 phenotype, and thereby inducing the production of other proinflammatory, Th1 cytokines. Following muscle injury, the early invading neutrophil and macrophage populations express TNF-α (15, 137), suggesting that the cytokine may contribute to the early inflammatory stages that precede muscle regeneration. TNF-α levels in muscle following acute injury peak at 24 h postinjury, which indicates that TNF-α production is most tightly coupled with the Th1 inflammatory response in injured muscle (126). Because findings show that TNF-α induces iNOS in myeloid cells and that myeloid cell-derived NO can cause muscle fiber damage that occurs during the early, Th1 inflammatory response following muscle injury, TNF-α activation of inflammatory cells has been associated with promoting muscle damage. However, TNF-α levels remain elevated for nearly 2 wk following acute injury, indicating that TNF-α may also modulate the regenerative process (126). Whether TNF-α release increases muscle damage or modulates regeneration appears to vary with the target of TNF-α. TNF-α stimulation of macrophages that are present in the early, Th1 inflammatory response can promote their ability to lyse muscle fibers. However, the expression of TNF-α receptors by muscle cells themselves is elevated as a later consequence of injury, during the regenerative process, and enables the direct influence of TNF-α on muscle cells to modulate their proliferation and differentiation (137).
Numerous experimental observations implicate the direct action of TNF-α on muscle cells in affecting muscle regeneration (Fig. 3). For example, TNF-α null mutants and TNF-α receptor mutants show lower levels of MyoD and MEF-2 expression than wild-type controls following acute injury (14, 126), suggesting that TNF-α may promote muscle regeneration by positively influencing both the proliferative and early differentiation stages of regeneration. However, the role of TNF-α in regeneration is not straightforward, and it appears that some aspects of the complex regenerative process may be inhibited by TNF-α. For example, although the application of exogenous TNF-α to myoblasts in vitro increases their proliferation (54); muscle cell fusion is inhibited by the cytokine, suggesting an inhibitory effect on the transition from the stage of early differentiation to terminal differentiation (35, 48, 49). In vivo data also support a role for TNF-α in inhibiting muscle differentiation following injury caused by modified muscle use. Expression of a lung-specific, TNF-α transgene that elevated systemic TNF-α attenuated the expression of dMyHC in mice subjected to hindlimb unloading followed by 3 to 5 days of muscle reloading (50). However, even these generally proproliferative, differentiation-suppressing effects of TNF-α are not always observed in muscle, and this variability may result from whether TNF-α-induced effects are mediated through NF-κB or other signaling pathways in muscle.
NF-κB is a transcription factor that is typically present in the cytosol in an inactive form, bound to endogenous inhibitors. Stimulation of cells with proinflammatory cytokines, such as TNF-α and IL-1, causes the inactive form to disassociate from its inhibitor and translocate to the cell nucleus (see Refs. 28 and 74 for reviews). Similarly, exposure of cells to oxidative stress, in particular, nitric oxide (NO) or peroxynitrite (ONOO), can activate NF-κB and cause its translocation. Once in the nucleus, NF-κB can induce the transcription of iNOS, TNF-α, and IL-1, which may then promote further NF-κB activation, as well as elevate the expression of other inflammatory mediators such as CCL2 and IL-6 (28, 74) (Fig. 3).
Activation of NF-κB can inhibit myogenesis through several processes, although activation through at least one alternative pathway may contribute to maintaining populations of myotubes (4). Importantly, NF-κB can promote the expression and stability of cyclin D1 in muscle (4, 35, 39, 132), leading to increased cell proliferation and inhibition of differentiation. Furthermore, NF-κB activation can cause destabilization of MyoD mRNA and degradation of MyoD protein (35, 49), which would further impede muscle differentiation. The inhibitory effects of NF-κB on muscle differentiation may also reflect its binding to the transcriptional repressor YY1, which suppresses the expression of myofibrillar genes (124).
TNF-α can activate signaling through other pathways independent of NF-κB to promote muscle differentiation instead of proliferation. Both IL-1 and TNF-α can activate p38 kinase (88), and activation of p38 can promote differentiation. In particular, inhibition of p38 in skeletal muscle cells in vitro inhibits myocytes from fusing to form myotubes and reduces the expression of MEF2, myogenin, and myosin light chain kinase (139), all of which indicate that p38 activation can promote muscle differentiation. Furthermore, increased activation of p38 increases the activity of MyoD (133, 139) (Fig. 2). The ability of p38 to promote myogenesis relies, in part, on its ability to phosphorylate and increase the transcriptional activity of MEF-2 (37, 139). However, p38 activation can also inhibit myogenesis when other myogenic regulatory factors are phosphorylated and activated. For example, myogenic regulatory factor-4 (MRF-4) is a p38 substrate that positively regulates muscle differentiation but is deactivated when phosphorylated by p38 (110). MRF-4, a member of the bHLH family of myogenic transcription factors, normally enhances expression of muscle-specific factors. However, the elevated expression and activity of p38 late in muscle differentiation leads to elevated MRF-4 phosphorylation and, as a consequence, a decline in desmin and skeletal α-actin expression (110). This negative effect of altered MRF-4 activation on muscle differentiation is also apparent in muscle regeneration in vivo. Overexpression of MRF4 in a transgenic mouse line caused defective muscle regeneration following injury (85). Presumably, any regulatory role played by p38 in muscle regeneration following injury can be attributable to the p38α isoform; genetic ablation of all other p38 isoforms had no effect on muscle fiber growth during regeneration following CTX injection (92).
TNF-α's function as a chemoattractant provides a further level of complexity in its function in muscle injury and regeneration. In vitro studies have shown that purified TNF-α can induce directional migration of myoblasts and satellite cells (120). More recently, investigators have demonstrated that the chemoattraction of stem cells toward M1 macrophages could be significantly inhibited by neutralizing antibodies to TNF-α (60). Chemoattraction could be further inhibited by the presence of inhibitors to a high-mobility group box 1 (HMGB1) to the assays (60). Stem cells used in these assays were vessel-associated mesangioblasts, which have been previously shown to be capable of contributing to muscle regeneration in dystrophic muscle (94). Thus, these data indicate that TNF-α release by neutrophils and M1 macrophages at the site of tissue damage soon after injury would attract satellite cells and mesangioblasts to the injury site, thereby further promoting muscle regeneration.
Induction of IL-6 expression by NF-κB activation is likely to provide an additional route through which the proproliferative, differentiation-suppressing effects of NF-κB are mediated. For example, application of IL-6 to myoblasts in culture increases their proliferation but not cell fusion (125). NF-κB induction of IL-6 expression can also have profound effects on muscle differentiation and play functionally important roles in muscle growth by acting on later stages of the differentiation process. Recent findings show that the genetic ablation of IL-6 greatly slows muscle growth in a model of compensatory muscle hypertrophy in mice where the gastrocnemius tendon was sectioned to increase loading and growth in the synergistic plantaris muscle (99). Although this treatment causes muscle inflammation (21), which could be a source of IL-6 in the model, the investigators showed that Pax7-expressing cells in the overloaded muscle also expressed IL-6, suggesting that both muscle cells and Th1 myeloid cells could provide IL-6 that is important for muscle growth. Apparently, slowed growth in the IL-6 null mutant muscle reflected loss of normal muscle cell differentiation, at least in part. Although IL-6 ablation had no effect on the number of MyoD+ cells, at 3 days of muscle overloading in vivo, the number of myogenin+ cells at that time point decreased significantly (99), suggesting that the transition from the proliferative stage to the early differentiation stage of myogenesis was impaired by the loss of IL-6 signaling. Thus, there are striking similarities in the functions of two of the major Th1 cytokines that are present at early stages of muscle regeneration and growth following injury or experimental perturbation that causes inflammation. Both IL-6 and TNF-α can increase myoblast proliferation, serve as chemoattractants for myoblasts and myeloid cells, inhibit myocyte fusion, and affect progression of activated satellite cells to the early differentiation stage.
Chemokines.
Chemokines function most prominently in regulating the trafficking of leukocytes in response to injury and disease, as well as during normal, physiological events. Chemokines are small soluble molecules that are typically released by immune cells to act as chemoattractants and influence the activation state of inflammatory cells. These signaling molecules are categorized according to the number and distribution of cysteine residues near their amino terminus, leading to their designation as C, CC, CXC, or CX3C chemokines. Receptor binding by chemokines shows variable specificity and reflects some overlap of function. However, expression-profiling studies showed that chemokines in the CC class and their receptors are expressed most highly following muscle injury and disease, suggesting that they may be especially important in affecting muscle regeneration.
Several thorough investigations have provided strong evidence that CC chemokines play a significant role in regulating muscle regeneration following injury. Work by Simeonova and colleagues (127, 128) has been especially valuable in demonstrating a role for CC-chemokine signaling in muscle regeneration in vivo. These investigators showed a rapid and coordinated elevation in CC ligand-3 (CCL3), CCL4 and CCL2 and their shared receptors, CCR5 and CCR2 in muscle following injury, which slowly returned to control levels over the course of muscle regeneration. Using recovery of muscle force production as an index of muscle repair and regeneration, the investigators found that force recovery was significantly slowed in injured mice that were null mutants for CCR2, but not in CCR5 mutants. Similarly, mice injected with anti-CCL2 to block interactions with its receptor CCR2 showed slower functional recovery than uninjured, untreated mice. Although this work showed a role for CCL2/CCR2 signaling in muscle repair, whether the treatment effect was attributable to perturbation of the inflammatory response or myogenic response to injury was unknown. The question was especially pertinent because CCR2 is expressed by macrophages, endothelial cells, and dendritic cells, as well as on myogenin-expressing cells in the injured muscle (128).
More recent findings have shown that disruption of CCL2/CCR2 signaling in injured muscle may have multiple impacts on tissue response to injury that can lead to slower regeneration. Instead of the stereotypic inflammatory response in acutely injured muscle, CTX-injected muscles in CCR2-null mutant mice showed a greatly reduced invasion of macrophages into the injury site, while neutrophil numbers were elevated for a prolonged period (80). In addition, injured muscles in CCR2 mutants showed persistent necrosis of muscle fibers and a slowed growth of fiber diameter at the injury site. However, revascularization was also slowed, introducing the possibility that impaired repair of vasculature in the null mutant mice could contribute significantly to the delay in muscle regeneration (80). Defects in muscle regeneration of CTX-injured muscles in CCR2-null mice were reversed by bone marrow transplantation using marrow from wild-type donors into recipients receiving myeloablative irradiation before CTX-injection (112). Regeneration was assessed by measuring cross-sectional area of CNFs, and associating a larger cross-sectional area with improved regeneration (112). Central-nucleation has proven to be a valuable, morphological index of muscle regeneration, because the central position of myonuclei corresponds to their recapitulation of developmental programs of gene expression. The transplantation also restored macrophage numbers in the injured muscle to levels that were similar to those seen in injured, wild-type muscles (112).
Defects in muscle regeneration caused by disruptions of CCL2/CCR2 signaling may also result from direct effects on myogenic cells. Because satellite cells in vivo express both CCL2 (90) and CCR2 (5) disruptions of CCL2-mediated signaling could presumably have direct effects on the regenerative response of muscle cells. Recent work provides strong support for this additional possibility (135). The investigators showed that myoblasts constitutively express receptors for CCL2 (CCR2), CCL3 (CCR1 and CCR5), and CCL4 (CCR5), and that stimulation with either CCL2 or CCL4 was sufficient to promote myoblast proliferation. Furthermore, stimulation of myoblasts with CCL2, CCL3, or CCL4 was sufficient to induce phosphorylation and activation of ERK1/2. This outcome may be functionally important because ERK1/2 activation is a component of the pathway through which many mitogenic growth factors can stimulate cell proliferation. The investigators then continued by examining an in vitro injury model in which they scratched a row of muscle cells from a culture plate and found that application of CCL2, CCL3, or CCL4 to the preparation decreased the time needed for cells to repopulate the scratched surface (135). Although the treatment effect could be attributable to increased cell proliferation, mobility, size or spreading, any of these functional effects could contribute to more rapid muscle regeneration in vivo.
What Initiates the Th1 Inflammatory Response in Injured Muscle?
The speed with which the Th1 inflammatory response is initiated following acute muscle injury is nearly too fast to require de novo transcription of signaling molecules to activate the process. Because each injury model that produces the stereotypic inflammatory response involves damage to the muscle cell membrane, perhaps passive release of a stored chemoattractant initiates the inflammatory response. Desmin, a muscle-specific intermediate protein, is a candidate signaling molecule because it is rapidly lost from muscle fibers following injury (55), and it is able to activate the complement system (57), which provides an extremely rapid mechanism for initiating the innate immune response. Furthermore, blocking complement activation by systemic administration of soluble complement receptor-1 prior to muscle injury reduces muscle inflammation and damage (26).
More recent investigations indicate that direct, physiological perturbations applied to muscle cells may promote the rapid expression of chemokines that contribute to activation of the Th1 response to injured muscle. Application of periodic, electrical pulse stimulation or mechanical stretch to myotubes in vitro induced the release of CXCL1, CXCL5, and IL-6 (75, 76). Although these perturbations were not injury models per se, they cause elevations of cytosolic Ca2+, which is also a consequence of muscle injuries caused by modified loading. Several observations support the possibility that CXCL1 or CXCL5 release from activated or mechanically perturbed muscles may contribute to activating the Th1 response to injured muscle and subsequent muscle regeneration. First, induction and release of the chemokines is rapid; both were present at elevated concentrations in the media of stimulated muscles within 3 h of stimulation (76). In addition, both chemokines are chemoattractant to neutrophils, which become significantly elevated in muscle within a few hours of injury, and they would be sufficient to drive further the Th1 inflammatory response. Perhaps most provocative, the application of the CXCR2 antagonist SB-225002 to muscle cell cultures appeared to reduce levels of myogenin expression (76), which may reflect an autocrine role for CXCR2 in promoting muscle differentiation. Although testing whether injuries induce CXCL1 or CXCL5 signaling by muscle cells has not yet been performed, these chemokines are strong candidate molecules for serving as early activators of the stereotypic inflammatory response to muscle injury.
Do M2 Macrophages Promote Muscle Regeneration?
The shift in macrophage phenotype from M1 to M2 populations occurs at a major watershed event in muscle regeneration, when there is a change from the proliferative stage to the early differentiation phase of myogenesis. Because the change in macrophage phenotype coincides with the change in regenerative stage and because M2 macrophages lie in close proximity to regenerative muscle fibers, the possibility that M2 macrophages regulate muscle regeneration has been long suspected (109).
Several models substantiate a role for M2 macrophages in promoting muscle regeneration. For example, mice that were subjected to muscle damage in the HS/Rel model and were depleted of macrophages between days 2 and 4 of muscle reloading showed disruptions in muscle differentiation, repair, growth, and regeneration (119). Normally, M2 macrophages greatly increase in numbers and M1 macrophages decline in numbers during the period of 2 to 4 days of muscle reloading. Depletion of macrophages during this period prevented increases in muscle fiber diameter that normally occur during reloading, prevented muscle membrane repair that occurred in nondepleted controls, and eliminated the appearance of CNFs (119). Macrophage depletion also appeared to prevent myoblast differentiation. Normally, MyoD-expressing muscle cells decrease in number and myogenin-expressing muscle cells increase in numbers between days 2 and 4 reloading, reflecting the withdrawal of myogenic cells from the cell cycle, as they transition to early differentiation. Instead, numbers of MyoD-positive cells remained elevated, and myogenin expression did not increase in the macrophage-depleted muscles (119). Collectively, the consequences of depleting the late invading macrophage population that is dominated by M2 macrophages diminishes muscle repair, differentiation, regeneration, and growth following injury in vivo. However, a limitation to this investigation was that the depletion protocol could also reduce M1 macrophage populations that remained in the muscle between 2 and 4 days of reloading. Although the myeloid cell population is dominated by M2 macrophages during this period, it is feasible that loss of M1 macrophages at this stage of regeneration may have also contributed to the treatment effect.
Subsequent reports further emphasize the importance of the Th1 to Th2 transition in the normal progress of muscle regeneration. Following muscle injury by notexin toxin injection, myeloid cells that expressed relatively high levels of Gr-1 and low levels of CXCR1 invaded the injured muscle, reaching peak numbers at 1 day postinjury and then declining to nearly control levels by 2 days postinjury (2). The muscle then experienced invasion by a population of Gr-1low/CXCR1high myeloid cells, beginning at 2 days postinjury and continuing to increase and remain elevated for at least 10 days postinjury (2). Although the identity of the early invading, Gr-1high/CXCR1low cells was uncertain, several observations indicate that they may be neutrophils. One of the antibodies used for phenotyping, anti-Gr-1 binds Ly6G, which is expressed at high levels by neutrophils (19, 25, 38, 43, 103). In addition, other investigators have shown that the inflammatory infiltrate in toxin-injected muscle is dominated by neutrophils at 1 day postinjury, and their numbers decline by 2 to 3 days postinjury (16, 80, 102), as occurred for the Gr-1high/CXCR1low myeloid cells following notexin injection (2). Furthermore, the Gr-1high/CXCR1low cells were nonproliferative, another characteristic of mature, activated neutrophils (115). However, the early invading population expressed low levels of CXCR1, a receptor for IL-8 that is expressed constitutively in neutrophils but is also expressed by monocytes, cytotoxic T-cells, endothelial cells, and other cells in an inflammatory context to promote their activation and chemoattraction (9, 41). Although neutrophils express CXCR1 constitutively, expression levels vary with activation stage of the neutrophils (91), and the level of surface expression is greatly reduced following activation of the respiratory burst and induction of phagocytosis (22), so that the low levels of expression on the surface of early invading cells following toxin injection may reflect the stage of activation and differentiation of the cells, if they were neutrophils. However, it is feasible that the early invading population of Gr-1high/CXCR1low myeloid cells also included monocytes. Anti-Gr-1 also binds Ly6C, which is expressed by monocytes. In addition, monocytes do not express CXCR1 constitutively, which may explain the low levels of CXCR1 signal in the analysis. However, the kinetics of cell invasion is more similar to that of neutrophils in toxin-injected muscle (16, 80, 102), and monocytes are proliferative cells in inflamed tissues.
Further analysis of the inflammatory infiltrate in notexin-induced muscle injury confirmed the transition from a Th1 inflammatory response involving Gr-1high/CXCR1low myeloid cells to a Th2 response dominated by Gr-1low/CXCR1high myeloid cells. The latter population expressed the macrophage-specific antigen, F4/80, and showed many characteristics of M2 macrophages, including the expression of transforming growth factor-β and IL-10 (2). The characteristic expression of IL-10 in the late-invading macrophage population may be particularly significant in the context of muscle regeneration; administration of exogenous IL-10 to muscle cells in culture increases the proportion of cells that express myogenin and increases cell fusion (2). Thus, the transition from an early Th1 inflammatory response to a subsequent Th2 response involving M2 macrophages provided a myeloid cell population that could promote muscle differentiation that characterizes later stages of the inflammatory response.
Other injury models similarly implicate the late-invading macrophage population in promoting muscle growth and regeneration as the muscle inflammatory response transitions from a Th1 to a Th2 response. Macrophage invasion into muscle that experiences CTX injection (80) or ischemia (16, 102) was nearly obliterated between 3 and 7 days postinjury in mice that were null mutants for either CCR2 or CCL2. These dramatic reductions in macrophage numbers were associated with delayed appearance of regenerative fibers significantly slower growth of muscle fibers in the region of the muscle. More recently, strong experimental evidence has been provided to show that late-invading macrophage populations promote muscle growth and regeneration in vivo; transplantation of wild-type bone marrow into irradiated, CCR2-null mutant mice restored macrophage invasion and muscle growth following CTX-induced injury (112).
What Regulates the Shift of Macrophage Phenotype in Injured Muscle?
The shift in macrophages from a M1 to a M2 phenotype following muscle injury appears to be important in changing the environment in the muscle tissue from one that promotes the removal of cellular debris and increases satellite cell activation and proliferation to an environment that promotes muscle differentiation and growth. Although little is known concerning the mechanisms that regulate this transition in macrophage phenotype in injured muscle, in vitro studies support the view that the phagocytic removal of tissue debris by myeloid cells can play a significant role. For example, macrophages that were driven to a M1 phenotype by treatment with IFN-γ and lipopolysaccharide showed a reduction in TNF-α secretion and an increase in transforming growth factor-β (TGF-β) secretion after phagocytosis of necrotic muscle cells, reflecting a shift toward a M2 phenotype (2). Interestingly, the identity of the necrotic cells that are phagocytosed by the macrophage can affect whether the shift in macrophage phenotype occurs. Exposure of macrophages to lysed neutrophils greatly increases IL-10 production by macrophages, but exposure to lysed lymphocytes does not produce this effect, attributable to differences in the cytosolic proteins released by the two cell types following lysis (24). However, a more important variable in determining macrophage phenotype switching following phagocytosis may be whether the target of phagocytosis is a necrotic cell or an apoptotic cell. Phagocytosis of apoptotic neutrophils, but not opsonized neutrophils, suppressed expression of IL-1β, IL-10, and TNF-α and increased expression of TGF-β, indicating a shift toward an M2 phenotype (23). Similarly, apoptosis of apoptotic neutrophils, but not lysed neutrophils increased TGF-β secretion, while phagocytosis of lysed neutrophils but not apoptotic neutrophils, increased TNF-α secretion (24). The time course of myeloid cell apoptosis in muscle following acute injury coincides with the shift in macrophage phenotype, which supports the speculation that phagocytosis of apoptotic cells may contribute to regulating macrophage phenotype in injured muscle in vitro. Apoptosis of inflammatory cells in the HS/Rel model of muscle injury and repair peaked at 2 days of reloading, returning to control levels by 4 days reloading (116), while the shift from M1 to M2 macrophage phenotypes in this model occurred between days 2 and 4.
Do Immune Cells Regulate Muscle Repair and Regeneration in Chronic Myopathic Conditions?
Most current knowledge of immune cell function in muscle injury and regeneration is based on models in which muscle is subjected to acute damage, producing the well-conserved innate inflammatory response in which neutrophil invasion is followed by sequential waves of M1 and M2 macrophage populations. However, muscle inflammation that is dominated by a myeloid cell infiltrate is also a prominent feature of several, heritable, progressive muscle diseases, including Duchenne muscular dystrophy (DMD) (118), congenital muscular dystrophy (6, 47), and limb girdle muscular dystrophy 2B (66, 98). Although macrophages (123 129), mast cells (32), neutrophils (40), eosinophils (11, 131), and cytotoxic T-lymphocytes (106) all contribute to pathogenesis in at least some of these chronic myopathic conditions, only macrophages and perhaps eosinophils are known to contribute to muscle regeneration.
The mdx mouse is a genetic model of DMD that has provided a valuable system for studying the role of macrophage phenotype switching in chronic neuromuscular disease. Mdx mice, like DMD humans, are spontaneous null mutants of the dystrophin gene, which leads to progressive muscle wasting and necrosis that is exacerbated by muscle inflammation (118). Although dystrophin is normally expressed from the time of terminal differentiation of embryonic muscle throughout the life of a muscle fiber, neither muscle necrosis nor inflammation are apparent until 3 to 4 wk of age in mdx mice or about 3 years of age in DMD boys. In both DMD and mdx dystrophies, the onset of muscle histopathology coincides with the onset of muscle inflammation, which suggests that inflammatory cells may play a regulatory role in the pathology (129). That possibility was supported by data showing that depletion of macrophages from mdx mice before the onset of histopathology caused great reduction in muscle pathology in 4-wk-old mice (129). Similarly, the slowing of muscle pathology in DMD by systemic treatment with corticosteroids also supports a role for the immune system in affecting the course of muscular dystrophy (3, 44), presumably attributable to immunosuppressive effects of corticosteroids.
The course of inflammation at early stages of pathology in mdx dystrophy bears some similarities to the innate immune response that occurs in response to acute muscle injury. The initial population of myeloid cells invading mdx muscle is enriched in neutrophils and iNOS-expressing, phagocytic, M1 macrophages, reflecting their capacity to remove cellular debris produced by oxidative damage and produce Th1 cytokines capable of promoting the proliferative phase of myogenesis (123). Furthermore, the expression of iNOS by M1 macrophages enables them to induce further muscle damage through production of cytotoxic levels of NO (123). Unexpectedly, and unlike muscle inflammation following acute injury, the M1 macrophage influx is accompanied by contemporaneous invasion by M2a macrophages, which usually predominate at later stages of inflammation. Although M2a macrophages are typically associated with tissue repair, they appear to serve a different role in mdx muscle. In addition to elevated expression of transcripts such as IL-4 and CD206 that reflect M2 activation (30, 61), the M2a macrophages expressed high levels of arginase (123). Because iNOS in M1 macrophages and arginase in M2a macrophages share a common substrate, arginine, activity of the enzymes may feasibly be affected by substrate competition. In fact, the addition of M2a macrophages to M1 macrophage cocultures with muscle cells reduces the production of NO and greatly decreases M1 macrophage lysis of muscle cells (123). Thus, M2a macrophages may reduce muscle damage caused by M1 macrophages that invade dystrophic muscle.
Mdx muscle transitions from an early necrotic stage at 4 wk of age to a stage in which necrosis is reduced and regeneration is a dominant process in the muscle. This transition in mdx pathology is accompanied by a transition in macrophage phenotypes from the early stage dominated by iNOShigh/IFN-γ+ M1 macrophages and Arghigh/IL4R+/CD206+ M2a macrophages to a M2c population that is prevalent in the regenerative muscle (123). These M2c macrophages expressed elevated levels of IL-4 and IL-10, which may reduce muscle damage and promote regeneration. As shown previously, IL-10 can deactivate M1 macrophages (30, 31), which could reduce macrophage-mediated damage to mdx muscle (123). IL-4 can also contribute to shifting macrophages from the cytotoxic M1 phenotype to the M2c phenotype that promotes tissue repair (30, 31). However, IL-4 can also have direct effects on muscle regeneration, by promoting satellite cell activation and differentiation (42, 85). Thus, M2 macrophages can play a central role in regulating regeneration in mdx dystrophy by attenuating the potentially cytolytic M1 phenotype and acting directly on satellite cells to drive their function in muscle repair.
Activation of the Th2 inflammatory response in mdx muscle may also affect muscle regeneration by induction of eosinophilia. Eosinophils are myeloid cells that are activated by the Th2 cytokine IL-5 and drive the innate immune response to parasitic infections or allergy. Although eosinophilia occurs only rarely and inexplicably in muscle disease, DMD and mdx dystrophies were found recently to involve muscle invasion by eosinophils (131). Despite the original expectation that eosinophilia of dystrophic muscle was a nonspecific consequence of the Th2 inflammatory environment, eosinophils were shown to modulate injury and regeneration. In particular, genetic ablation of major basic protein-1 (MBP-1), a cytolytic protein expressed by eosinophils in dystrophic muscle, caused an increase in the numbers of cytotoxic T-lymphocytes in the muscle (131). That finding is significant to mdx dystrophy because a Th1-driven, cellular immune response in which cytotoxic T-cells promote apoptosis of mdx muscle fibers is an early feature of the disease that is attenuated as muscle regeneration begins (106). Thus, eosinophils may mediate the regeneration of dystrophic muscle by promoting the transition from a Th1 to Th2 inflammatory environment.
Why Does Muscle Inflammation Differ in Acute and Chronic Muscle Injuries?
While the inflammatory response to acute muscle injury is a stereotypical, innate immune response, the more complex response to chronic injury presents many unanswered questions (Fig. 4). For example, why does inflammation of mdx muscle involve a cellular immune response that is not seen following a single crush, strain, freeze, or toxin-induced injury? Although speculative, the differences may reflect a breakdown in peripheral tolerance to self-molecules that can develop in chronic myopathic conditions but is highly unlikely to occur following a single, acute injury. Autoreactive T-lymphocytes are normally negatively selected in the thymus; however, if they escape to the periphery, they are normally eliminated by induction of apoptosis or rendered nonfunctional by the induction of anergy (108). However, if cytotoxic T cells escape these mechanisms for producing peripheral tolerance to self-antigens, a cellular immune response will accompany the innate immune response to tissue damage. The presence of alloreactive cytotoxic T cells in mdx muscle (106), the ability to transfer pathology from mdx mice to healthy mice by adoptive transfer of immune cells primed with muscle homogenates (107), and the presence of a well-concerned peptide in the hypervariable domain of the T-cell receptor of cytotoxic T cells from DMD patients (34), all support the possibility that a breakdown of peripheral tolerance occurs in muscular dystrophy. Alternatively, the lack of a cellular immune response following acute injury while cellular immunity is a component of muscular dystrophy may reflect the low traffic of cytotoxic T cells in healthy or acutely injured muscle. If an injury-associated antigen were presented by an antigen-presenting cell following an acute injury, the low T-cell traffic through the muscle parenchyma would make the probability of presentation to a T cell low. However, if the damage and antigen presentation persisted as in chronic injuries, successful presentation of the antigen and activation of a cellular immune response would be more likely. Further work is needed to distinguish between these or other speculations.
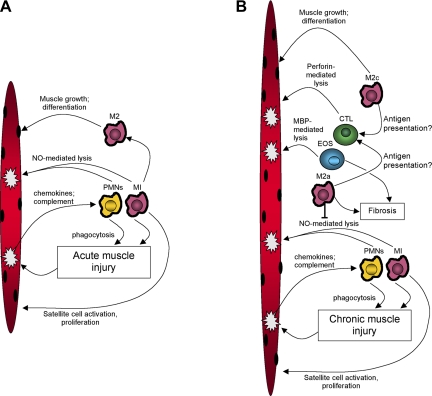
Fig. 4.
Summary of general interactions between immune cells and muscle cells following acute muscle injury (A) or during chronic muscle injury that occurs in mdx dystrophy (B). A: acute damage causes release of chemoattractant molecules that initially attract neutrophils (PMNs) or M1 macrophages (M1) into the muscle. Neutrophils and M1 macrophages promote further damage through nitric oxide (NO)-mediated processes. M1 macrophages also release cytokines that can promote satellite cell activation and proliferation. Neutrophils and M1 macrophages are then replaced by M2 macrophages that then promote muscle repair, differentiation and growth. B: during chronic muscle injury, the initial inflammatory response is similar to the response to acute damage, typified by neutrophil and M1 macrophage invasion. However, M2a macrophages invade contemporaneously and inhibit NO-mediated lysis by M1 macrophages and promote muscle fibrosis through arginase metabolism of arginine. M2 macrophages may also participate in activation of cytotoxic T-cells that are then able to promote muscle damage through perforin-mediated processes. The Th2 inflammatory environment also increases the activation of eosinophils (EOS) that contribute to muscle lysis through major basic protein-1 (MBP-1) mediated lysis. Eosinophils also increase muscle fibrosis through MBP-dependent processes and negatively regulate the cellular immune response to chronically injured muscle.
Perspectives and Significance
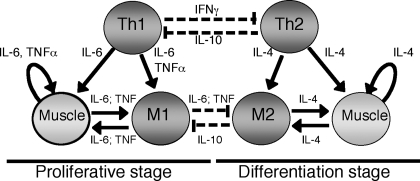
Recent investigations into the interactions between muscle and myeloid cells now support a general model in which the central role for myeloid cells in muscle regeneration has become apparent. In particular, the transition between macrophages of the M1 phenotype to the M2 phenotype is a key event for the normal progression of muscle regeneration (Fig. 5). Collectively, findings show that the initial invasion of muscle by neutrophils and M1 macrophages is necessary for normal occurrence of the proliferative stage of myogenesis at the onset of regeneration. Release of cytokines during the Th1 inflammatory response, especially TNF-α and IL-6, has a strong influence on the normal progression of the proliferative stage, and is apparently necessary for the transition to the early differentiation stage. Strong experimental evidence also shows that signaling via CCL2/CCR2 and perhaps IL-6 is required for normal recruitment of myeloid cells and satellite cells to the site of regeneration. However, in the absence of transition from the Th1 response dominated by neutrophils and M1 macrophages, to the Th2 response dominated by M2 macrophages, the regenerative process is arrested at a stage at which satellite cells are activated to proliferate and express early bHLH transcription factors, such as MyoD. Furthermore, failure to transition from the Th1 to Th2 inflammatory response is associated with greatly diminished ability of muscle cells to withdraw from the cell cycle and fuse to form myotubes following muscle injuries in vivo. Whether this arrest reflects the prolonged influence of differentiation-suppressing effects of Th1-derived factors or the absence of differentiation-promoting effects of Th2-derived factors remains unknown.
Fig. 5.
Generalized diagram of the interactions between macrophages and muscle cells during the proliferative stage and differentiation stages of myogenesis in injured muscle. “Th1” and “Th2” represent cells that produce Th1 or Th2 cytokines, that include helper T-cells or macrophages. “M1” and “M2” represent macrophage populations. Solid lines indicate activating effects on target cells. Dotted lines represent deactivating effects.
The complex and occasionally antagonistic phenotypes of myeloid cells in injured muscles emphasize the need to target specific populations of myeloid cells in therapeutic approaches that employ anti-inflammatory medications. This may be particularly important for the treatment of chronic muscle inflammation, such as DMD or mdx muscular dystrophy. DMD is most commonly treated with the anti-inflammatory corticosteroid, prednisone, which can reduce the extravasation of macrophages into the injured muscle (130). However, the treatment effect may not be specific for a particular macrophage phenotype and therefore has the potential to impair regenerative processes, as well as reduce inflammatory cell-mediated damage. Indeed, administration of anti-inflammatory medications after acute muscle injury can delay muscle repair and regeneration under some circumstances (70). Thus, improved therapeutics that rely on manipulating inflammation of injured or diseased muscle will be most effective when specific macrophage phenotypes are targeted at specific stages of the injury and repair process. However, those therapeutic advances will require an improved understanding to the functional diversity of myeloid cells in injured and diseased muscle.
Perhaps the most provocative question concerning the role of myeloid cells in muscle regeneration is: why are they so important for normal muscle regeneration? Satellite cells in vitro proceed rapidly through the proliferative and early differentiation stages of myogenesis, in the absence of myeloid cells or myeloid cell products, which contrasts with the surprising importance of myeloid cells in normal muscle regeneration in vivo. Presumably, this distinction reflects unique features of the microenvironment for satellite cells in injured muscle and illustrates limitations to extrapolating processes that regulate muscle proliferation and differentiation in vitro to regulatory processes that may be important in injured tissues in vivo. Discovery of the key molecules that distinguish the myogenic environment in vitro from the myogenic environment in injured muscle will provide an important advance in understanding the role of myeloid cells in muscle regeneration and growth.
GRANTS
During the preparation of this work, support was received from the Muscular Dystrophy Association, USA (Grants 157881 and 4031 to J. G. Tidball) and the National Institutes of Health (Grants R01 AR47721, RO1 AR47855, and R01, AR/AG054451 to J. G. Tidball and F31 AR054724 to S. A. Villalta).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Abood EA, Jones MM. Macrophages in developing mammalian skeletal muscle: evidence for muscle fibre death as a normal developmental event. Acta Anat (Basel) 140: 201–212, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backman E, Henriksson KG. Low-dose prednisolone treatment in Duchenne and Becker muscular dystrophy. Neuromusc Disord 5: 233–241, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-κB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol 180: 787–802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol (Berl) 102: 385–392, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Biancheri R, Falace A, Tessa A, Pedemonte M, Scapolan S, Cassandrini D, Aiello C, Rossi A, Broda P, Zara F, Santorelli FM, Minetti C, Bruno C. POMT2 gene mutation in limb-girdle muscular dystrophy with inflammatory changes. Biochem Biophys Res Commun 363: 1033–1037, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 287: C475–C483, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bondesen BA, Mills ST, Pavlath GK. COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 290: C1651–C1659, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bonecchi R, Facchetti F, Dusi S, Luini W, Lissandrini D, Simmelink M, Locati M, Bernasconi S, Allavena P, Brandt E, Rossi F, Mantovani A, Sozzani S. Induction of functional IL-8 receptors by IL-4 and IL-13 in human monocytes. J Immunol 164: 3862–3869, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Brickson S, Ji LL, Olabisi R, Schneider BSP, Best TM. M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J Appl Physiol 95: 969–976, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Cai B, Spencer MJ, Nakamura G, Tseng-Ong L, Tidball JG. Eosinophilia of dystrophin-deficient muscle is promoted by perforin-mediated cytotoxicity by T cell effectors. Am J Pathol 156: 1789–1796, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol 54: 121–128, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni Vittiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci 23: 189–194, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Chen SE, Gerken E, Zhang Y, Zhan M, Mohan RK, Li AS, Reid MB, Li YP. Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. Am J Physiol Cell Physiol 289: C1179–C1187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins RA, Grounds MD. The role of tumor necrosis factor-alpha (TNF-α) in skeletal muscle regeneration. Studies in TNF-α(−/−) and TNF-α(−/−)/LT-α(−/−) mice. J Histochem Cytochem 49: 989–1001, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Contreras-Shannon V, Ochoa O, Reyes-Reyna SM, Sun D, Michalek JE, Kuziel WA, McManus LM, Shireman PK. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol 292: C953–C967, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol 224: 122–137, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83: 64–70, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Dijkstra CD, Döpp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54: 589–599, 1985 [PMC free article] [PubMed] [Google Scholar]
- 21.DiPasquale DM, Cheng M, Billich W, Huang SA, van Rooijen N, Hornberger TA, Koh TJ. Urokinase-type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol 293: C1278–C1285, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Doroshenko T, Chaly Y, Savitskiy V, Maslakova O, Portyanko A, Gorudko I, Voitenok NN. Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood 100: 2668–2671, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166: 6847–6854, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol 151: 2399–2408, 1993 [PubMed] [Google Scholar]
- 26.Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol 156: 2103–2110, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Füchtbauer EM, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn 193: 34–39, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225–260, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 5: 698–701, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–64, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Gorospe JR, Tharp M, Demitsu T, Hoffman EP. Dystrophin-deficient myofibers are vulnerable to mast cell granule-induced necrosis. Neuromuscul Disord 4: 325–333, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res 267: 99–104, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Gussoni E, Pavlath GK, Miller RG, Panzara MA, Powell M, Blau HM, Steinman L. Specific T cell receptor gene rearrangements at the site of muscle degeneration in Duchenne muscular dystrophy. J Immunol 153: 4798–4805, 1994 [PubMed] [Google Scholar]
- 35.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19: 5785–5799, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386: 296–299, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SEW, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J Immunol 147: 22–28, 1991 [PubMed] [Google Scholar]
- 39.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol 19: 2690–2698, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFα function with Etanercept in mdx mice. Neuromuscul Disord 16: 591–602, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science 253: 1278–1280, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113: 483–94, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Jutila MA, Kroese FG, Jutila KL, Stall AM, Fiering S, Herzenberg LA, Berg EL, Butcher EC. Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-γ. Eur J Immunol 18: 1819–1826, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Kinali M, Mercuri E, Main M, Muntoni F, Dubowitz V. An effective, low-dosage, intermittent schedule of prednisolone in the long-term treatment of early cases of Duchenne dystrophy. Neuromuscul Disord 12Suppl 1: S169–S174, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve 16: 99–108, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Lamperti C, Cagliani R, Ciscato P, Moroni I, Viri M, Romeo A, Fagiolari G, Prelle A, Comi GP, Bresolin N, Moggio M. Congenital muscular dystrophy with muscle inflammation α dystroglycan glycosylation defect and no mutation in FKRP gene. J Neurol Sci 243: 47–51, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-κB. FASEB J 15: 1169–1180, 2001, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Langen RC, Van der Velden JL, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Tumor necrosis factor-α inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18: 227–237, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen-Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol 35: 689–696, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Launay T, Armand AS, Charbonnier F, Mira JC, Donsez E, Gallien CL, Chanoine C. Expression and neural control of myogenic regulatory factor genes during regeneration of mouse soleus. J Histochem Cytochem 49: 887–899, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 19: 628–633, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factor α. FASEB J 12: 871–880, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Li YP. TNF-α is a mitogen in skeletal muscle. Am J Physiol Cell Physiol 285: C370–C376, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Lieber RL, Schmitz MC, Mishra DK, Friden J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J Appl Physiol 77: 1926–1934, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Lim SK, Kim H H, Lim SK, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92: 1870–1877, 1998 [PubMed] [Google Scholar]
- 57.Linder E, Lehto VP, Stenman S. Activation of complement by cytoskeletal intermediate filaments. Nature 278: 176–178, 1979 [DOI] [PubMed] [Google Scholar]
- 58.Linehan SA. The mannose receptor is expressed by subsets of APC in non-lymphoid organs. BMC Immunol 6: 4, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lluis F, Perdiguero E, Nebreda AR, Munoz-Canoves P. Regulation of skeletal muscle gene expression by p38 MAP Kinases. Trends Cell Biol 16: 36–44, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA, Brunelli S, Rovere-Querini P. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol 85: 779–87, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Massimino M, Rapizzi E, Cantini M, Libera L, Mazzoeni F, Arsian P, Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun 235: 754–759, 1997 [DOI] [PubMed] [Google Scholar]
- 63.McLennan IS. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res 272: 193–196, 1993 [DOI] [PubMed] [Google Scholar]
- 64.McLennan IS. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J Anat 188: 17–28, 1996 [PMC free article] [PubMed] [Google Scholar]
- 65.McLoon LK, Nguyen LT, Wirtschafter J. Time course of the regenerative response in bupivacaine injured orbicularis oculi muscle. Cell Tissue Res 294: 439–47, 1998 [DOI] [PubMed] [Google Scholar]
- 66.McNally EM, Ly CT, Rosenmann H, Mitrani Rosenbaum S, Jiang W, Anderson LV, Soffer D, Argov Z. Splicing mutation in dysferlin produces limb-girdle muscular dystrophy with inflammation. Am J Med Genet 91: 305–312, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Mendias CL, Tatsumi R, Allen RE. Role of cyclooxygenase-1 and -2 in satellite cell proliferation, differentiation, and fusion. Muscle Nerve 30: 497–500, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Merchant SH, Gurule DM, Larson RS. Amelioration of ischemia-reperfusion injury with cyclic peptide blockade of ICAM-1. Am J Physiol Heart Circ Physiol 284: H1260–H1268, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22: 724–732, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Mishra DK, Friden J, Schmitz M, Lieber RL. Anti-inflammatory medication after muscle injury. J Bone Joint Surg 77: 1410–1519, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med 36: 347–354, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83: 1125–1136, 1995 [DOI] [PubMed] [Google Scholar]
- 73.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mourkioti F, Rosenthal N. NF-κB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med 86: 747–759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295: E1191–E1204, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Nedachi T, Hatakeyama H, Kono T, Sato M, Kanzaki M. Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am J Physiol Endocrinol Metab 297: E866–E878, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of muscle cells in vitro. J Physiol 547: 125–132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen HX, Tidball JG. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol 553: 833–841, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen HX, Lusis AJ, Tidball JG. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J Physiol 565: 403–413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Otis JS, Burkholder TJ, Pavlath GK. Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Exp Cell Res 310: 417–425, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Ottnad E, Parthasarathy S, Sambrano GR, Ramprasad MP, Quehenberger O, Kondratenko N, Green S, Steinberg D. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low-density lipoprotein: partial purification and role in recognition of oxidatively damaged cells. Proc Natl Acad Sci USA 92: 1391–1395, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279: L1029–L1037, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Pavlath GK, Dominov JA, Kegley KM, Miller JB. Regeneration of transgenic skeletal muscles with altered timing of expression of the basic helix-loop-helix muscle regulatory factor MRF4. Am J Pathol 162: 1685–1691, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: anti-inflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 94: 119–126, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Rabinowitz SS, Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med 174: 827–836, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270: 7420–7426, 1995 [DOI] [PubMed] [Google Scholar]
- 89.Ramprasad MP, Fischer W, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D. The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc Natl Acad Sci USA 92: 9580–9584, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reyes-Reyna SM, Krolick KA. Chemokine production by rat myocytes exposed to interferon-γ. Clin Immunol 94: 105–113, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J Biol Chem 279: 24372–24386, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Ruiz-Bonilla V, Perdiguero E, Gresh L, Serrano AL, Zamora M, Sousa-Victor P, Jardí M, Wagner EF, Muñoz-Cánoves P. Efficient adult skeletal muscle regeneration in mice deficient in p38β, p38γ and p38ζ MAP kinases. Cell Cycle 7: 2208–2214, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton Hemoglobin JW. A biologic Fenton reagent. J Biol Chem 259: 14354–14356, 1984 [PubMed] [Google Scholar]
- 94.Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444: 574–579, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Schaer DJ, Boretti FS, Hongegger A, Poehler D, Linnscheid P, Staege H, Muller C, Schoedon G, Schaffner A. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics 53: 170–177, 2001 [DOI] [PubMed] [Google Scholar]
- 96.Schutzle UB, Wakelam MJ, Pette D. Prostaglandins and cyclic AMP stimulate creatine kinase synthesis but not fusion in cultured embryonic chick muscle cells. Biochim Biophys Acta 805: 204–210, 1984 [DOI] [PubMed] [Google Scholar]
- 97.Schwab N, Waschbisch A, Wrobel B, Lochmüller H, Sommer C, Wiendl H. Human myoblasts modulate the function of antigen-presenting cells. J Neuroimmunol 200: 62–70, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Selva-O'Callaghan A, Labrador-Horrillo M, Gallardo E, Herruzo A, Grau-Junyent JM, Vilardell-Tarres M. Muscle inflammation, autoimmune Addison's disease and sarcoidosis in a patient with dysferlin deficiency. Neuromuscul Disord 16: 208–209, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Shen W, Li Y, Tang Y, Cummins J, Huard J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol 167: 1105–1117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shepherd VL, Hoidal JR. Clearance of neutrophil-derived myeloperoxidase by the macrophage mannose receptor. Am J Respir Cell Mol Biol 2: 335–340, 1990 [DOI] [PubMed] [Google Scholar]
- 102.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81: 775–785, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Shortman K, Naik SH. Steady-state and inflammatory dendritic cell development. Nat Rev Immunol 7: 19–30, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Smith RH, Palmer RM, Reeds PJ. Protein synthesis in isolated rabbit forelimb muscles. The possible role of metabolites of arachidonic acid in the response to intermittent stretching. Biochem J 214: 153–161, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith J, Grisham M, Granger N, Korthuis R. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol Heart Circ Physiol 256: H789–H793, 1989 [DOI] [PubMed] [Google Scholar]
- 106.Spencer MJ, Walsh CM, Dorshkind KA, Rodriguez EM, Tidball JG. Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest 99: 2745–2751, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4+) and cytotoxic (CD8+) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 98: 235–243, 2001 [DOI] [PubMed] [Google Scholar]
- 108.Srinivasan M, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine 46: 147–159, 2009 [DOI] [PubMed] [Google Scholar]
- 109.St. Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol 77: 290–297, 1994 [DOI] [PubMed] [Google Scholar]
- 110.Suelves M, Lluís F, Ruiz V, Nebreda AR, Muñoz-Cánoves P. Phosphorylation of MRF4 transactivation domain by p38 mediates repression of specific myogenic genes. EMBO J 23: 365–375, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol 290: R1488–R1495, 2006 [DOI] [PubMed] [Google Scholar]
- 112.Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J 23: 382–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, Sugawara K, Yamaya K, Sato K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol 87: 1360–1367, 1999 [DOI] [PubMed] [Google Scholar]
- 114.Teixeira CFP, Zamuner SR, Zuliani JP, Fernandes CM, Cruz-Hofling MA, Fernandes I, Chaves F, Gutierrez JM. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 28: 449–459, 2003 [DOI] [PubMed] [Google Scholar]
- 115.Theilgaard-Mönch K, Porse BT, Borregaard N. Systems biology of neutrophil differentiation and immune response. Curr Opin Immunol 18: 54–60, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Tidball JG, St. Pierre BA. Apoptosis of macrophages during the resolution of muscle inflammation. J Leukoc Biol 59: 380–388, 1996 [DOI] [PubMed] [Google Scholar]
- 117.Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol 65: 492–498, 1999 [PubMed] [Google Scholar]
- 118.Tidball JG, Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res 56: 831–841, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 578: 327–336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torrente Y, El Fahime E, Caron NJ, Del Bo R, Belicchi M, Pisati F, Tremblay JP, Bresolin N. Tumor necrosis factor-alpha (TNF-α) stimulates chemotactic response in mouse myogenic cells. Cell Transplant 12: 91–100, 2003 [DOI] [PubMed] [Google Scholar]
- 121.Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: influence of ibuprofen acetaminophen. J Clin Endocrinol Metab 86: 5067–5070, 2001 [DOI] [PubMed] [Google Scholar]
- 122.Van Velzen AG, Da Silva RP, Gordon S, Van Berkel TJ. Characterization of a receptor for oxidized low-density lipoproteins on rat Kupffer cells: similarity to macrosialin. Biochem J 322: 411–415, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 18: 482–496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC. NF-κB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol 27: 4374–4387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang X, Wu H, Zhang Z, Liu S, Yang J, Chen X, Fan M, Wang X. Effects of interleukin-6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell Mol Neurobiol 28: 113–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP. Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB J 16: 1630–1632, 2002 [DOI] [PubMed] [Google Scholar]
- 127.Warren GL, O'Farrell L, Summan M, Hulderman T, Mishra D, Luster MI, Kuziel WA, Simeonova PP. Role of CC chemokines in skeletal muscle functional restoration after injury. Am J Physiol Cell Physiol 286: C1031–C1036, 2004 [DOI] [PubMed] [Google Scholar]
- 128.Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J 19: 413–415, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol 155: 123–131, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wehling-Henricks M, Lee JJ, Tidball JG. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul Disord 14: 483–490, 2004 [DOI] [PubMed] [Google Scholar]
- 131.Wehling-Henricks M, Sokolow S, Lee JJ, Myung KH, Villalta SA, Tidball JG. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet 17: 2280–2292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS., Jr The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition. Mol Cell Biol 21: 8428–8436, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol 20: 3951–3964, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol 164: 588–603, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yahiaoui L, Gvozdic D, Danialou G, Mack M, Petrof BJ. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J Physiol 586: 3991–4004, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Zádor E, Mendler L, Takács V, de Bleecker J, Wuytack F. Regenerating soleus and extensor digitorum longus muscles of the rat show elevated levels of TNF-α and its receptors, TNFR-60 and TNFR-80. Muscle Nerve 24: 1058–1067, 2001 [DOI] [PubMed] [Google Scholar]
- 138.Zerria K, Jerbi E, Hammami S, Maaroufi A, Boubaker S, Xiong JP, Arnaout MA, Fathallah DM. Recombinant integrin CD11b A-domain blocks polymorphonuclear cells recruitment and protects against skeletal muscle inflammatory injury in the rat. Immunology 1194: 431–440, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem 274: 5193–5200, 1999 [DOI] [PubMed] [Google Scholar]
- 140.Zouaoui Boudjeltia K, Moguilevsky N, Legssyer I, Babar S, Guillaume M, Delree P, Vanhaeverbeek M, Brohee D, Ducobu J, Remacle C. Oxidation of low density lipoproteins by myeloperoxidase at the surface of endothelial cells: an additional mechanism to subendothelium oxidation. Biochem Biophys Res Commun 3252: 434–438, 2004 [DOI] [PubMed] [Google Scholar]
- 141.Zalin RJ. Prostaglandins and myoblast fusion. Dev Biol 59: 241–248, 1977 [DOI] [PubMed] [Google Scholar]
- 142.Zalin RJ. The role of hormones and prostanoids in the in vitro proliferation and differentiation of human myoblasts. Exp Cell Res 172: 265–281, 1987 [DOI] [PubMed] [Google Scholar]