Abstract
An acute injection of brain-derived neurotrophic factor (BDNF) in the hypothalamic paraventricular nucleus (PVN) reduces body weight by decreasing feeding and increasing energy expenditure (EE), in animals on standard laboratory chow. Animals have divergent responses to a high-fat diet (HFD) exposure, with some developing obesity and others remaining lean. In the current study, we tested two hypotheses: 1) BDNF in the PVN reverses HFD-induced obesity, and 2) animals with higher body fat have a greater physiological response to BDNF than those with less body fat. Eighty-four 10-wk old rats were allowed HFD ad libitum for 9 wk and then prepared with bilateral PVN cannulas. Animals were then divided into tertiles based on their body fat rank: high, intermediate, and low (H, I, and L). Each group was further divided into 2 subgroups and then PVN injected with BDNF or control (artificial cerebrospinal fluid, aCSF) every other day for 3 wk. Energy intake (EI), body weight, and body composition were measured. At study's end, rats were killed to allow measurement of other metabolic indices. In parallel, another 12 rats were fed control diet (CD), PVN-cannulated and injected with aCSF. HFD exposure induced obesity, particularly in the H body fat group, with a significant increase in EI, body weight, fat mass, liver size, and serum glucose, triglycerides, insulin, and leptin. BDNF significantly reduced EI, body weight, body fat, lean mass, and serum metabolic indices. These BDNF effects were greatest in the H body fat group. These data indicate that BDNF reduced HFD-induced obesity and metabolic syndrome-like measures, and the animals with the most body fat had the most significant response to BDNF.
Keywords: energy intake, body fat, metabolic syndrome
brain-derived neurotrophic factor (BDNF) has recently been reported to play an important role in regulating energy metabolism. Intracerebroventricular administration of BDNF decreases energy intake and body weight (42), and it reverses the hyperphagic and obese phenotype of BDNF heterozygous mutant mice (28). In animals with disruption of the regulatory locus of the BDNF gene (44), BDNF levels in central and peripheral tissues decrease to one-third of that in wild types. These animals exhibit increased body weight and adiposity; hepatic steatosis; elevated levels of serum LDL cholesterol, insulin, and leptin; impaired glucose tolerance; and age-related hyperglycemia. Similarly, human patients with mutations in the BDNF gene (22–23) and the BDNF receptor (TrkB) signal transduction pathway (21) exhibit obesity. This suggests that BDNF is necessary for maintaining normal body weight and may be protective against obesity and related diseases.
Obesity often is a multifactorial illness influenced by genetic, environmental, and lifestyle factors (20). Excess caloric intake, which can easily occur with consumption of calorically dense high-fat diets, likely contributes to the current obesity epidemic (14). Likewise, animals fed a high-fat diet (HFD) become obese (17, 19) and have high levels of blood glucose, LDL cholesterol, insulin, and leptin (17, 19), similar to that described in BDNF mutant mice (44). A HFD has been reported to reduce BDNF protein levels in the central nervous system (55, 56), and low levels of blood BDNF in obese patients have been reported in several studies (1, 18). These data suggest that reduced BDNF may be associated with adverse metabolic consequences; however, the effect of central BDNF on diet-induced obesity is of yet undetermined.
The paraventricular nucleus of the hypothalamus (PVN) is a brain area important to the regulation of energy metabolism. Several neuropeptides in the PVN influence feeding behavior and energy expenditure, including neuropeptide Y (5), agouti-related peptide (53), ghrelin (40), leptin (2), alpha-MSH (54), urocortin (12), and corticotropin-releasing hormone (CRH) (34). BDNF and the TrkB receptor are present in high concentrations in the PVN; and in BDNF heterozygous mice, low expression of BDNF was reported in the PVN (28). In our previous studies, an acute injection of BDNF in the PVN (50–51) inhibited feeding and elevated energy expenditure (EE), leading to a reduction in body weight gain. In the current study, we hypothesized that administration of BDNF in the PVN will reverse HFD-induced obesity.
It is well known that susceptibility to diet-induced obesity varies greatly among populations. Several animal studies (17, 19) and our previous work (unpublished observations) indicate a large variation in body weight gain in Sprague-Dawley (SD) rats after exposure to a HFD. Some animals develop obesity, defined as diet-induced obesity (DIO), while others have a very small change in body weight due to diet exposure, defined as obesity (diet) resistant (DR). Compared with DR animals, DIO animals have increased mRNA for neuropeptide Y and Agouti-related peptide in hypothalamus (17), reduced leptin receptor (9), and binding of labeled leptin and insulin in the hypothalamus (26), decreased sensitivity to leptin (31) and urocortin 2 (a member of CRH family) (11), and lower baseline and orexin A-induced spontaneous physical activity and mRNA for orexin 2 receptor (45). These findings suggest that DIO or DR may differ metabolically and physiologically and therefore may respond to treatments differently. In the current study, we tested the hypothesis that DIO and DR animals may respond differentially to BDNF treatment. To do this, we first induced obesity in SD rats by feeding them a HFD for 11 wk. As expected, there was wide variation in body weight and body fat change, and we divided the animals into three groups based on their body fat (high, intermediate, and low). Next, we chronically administered BDNF or a vehicle of artificial cerebrospinal fluid (aCSF) into the PVN of two subgroups of each group and measured energy intake, body weight, and body composition to determine whether BDNF reverses diet-induced obesity. At the end of the study, liver size, visceral fat, and serum metabolic indices were measured. The data show that BDNF in the PVN reverses HFD-induced obesity and related symptoms and that animals with greater body fat were more responsive to BDNF compared with those with intermediate or low body fat levels.
METHODS
Animals
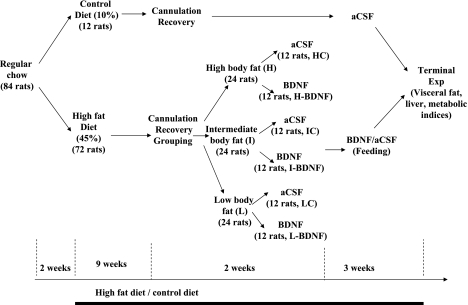
Eighty-four male SD rats (Charles River, Wilmington, MA) weighing 225–250 g were housed individually in cages with a 12:12-h light-dark cycle (lights on at 0700) in a room at 21–22°C. Teklad lab chow and water were allowed ad libitum; energy intake and body weight were measured 3 times a week, and body composition was measured with an EchoMRI-700 (Houston, TX) just before the HFD. The protocol was approved by the Veterans Affairs Medical Center Institutional Animal Care and Use Committee. An overview of the study design is shown in Fig. 1.
Fig. 1.
Flow chart of the study design, as described in the methods section. aCSF, artificial cerebrospinal fluid; BDNF, brain-derived neurotrophic factor; Exp, experiment.
Dietary Regimes
Fourteen days after arrival in the animal care facility, the rats were ranked based on body weight. Every fourth-ranked rat (animals with ranking number 4, 11, 18, 25, 32, 39, 46, 53, 60, 67, 74, and 81) was assigned to the control diet group (CD, D12450B from Research Diets, New Brunswick, NJ); the other 72 rats were assigned to the HFD group (45% of fat, D12451 from Research Diets, New Brunswick, NJ). Table 1 shows diet composition, and table 2 shows the body measurements and energy intake in both groups prior to receiving the new diets. The characteristics of rats in both groups were almost identical. All animals remained on the assigned diet until the terminal experiment.
Table 1.
Macronutrient and energy content of the control and high-fat diets
| Control Diet (D12450B) |
High-Fat Diet (D12451) |
|||
|---|---|---|---|---|
| g % | kcal % | g % | kcal % | |
| Protein | 19.2 | 20 | 24 | 20 |
| Carbohydrate | 67.3 | 70 | 41 | 35 |
| Fat | 4.3 | 10 | 24 | 45 |
| kcal/kg | 3.85 | 4.73 | ||
Table 2.
Body measurement and energy intake in the prospective control diet and high-fat diet groups prior to the new dietary regimes
| CD Group* | HFD Group | |
|---|---|---|
| Body weight, g | 326.7 ± 4.7 | 326.4 ± 1.8 |
| 14-day Body weight change, g | 110.1 ± 8.1 | 115.7 ± 1.9 |
| 14-day food intake, kcal | 1071.6 ± 32.1 | 1072.4 ± 9.0 |
| Body fat, g | 32.7 ± 1.6 | 34.2 ± 0.5 |
| Relative body fat, % | 10.3 ± 0.5 | 10.7 ± 0.1 |
| Lean mass, g | 249.6 ± 3.3 | 249.8 ± 1.5 |
| Relative lean mass, % | 78.3 ± 0.5 | 78.0 ± 0.2 |
Values are expressed as means ± SE.
After ranking body weight among 84 rats, we assigned animals with ranking numbers of 4, 11, 18, 25, 32, 39, 46, 53, 60, 67, 74, and 81 to receive the control diet (CD); the rest of the rats were given a high fat diet (HFD).
During the feeding period, energy intake and body weight were measured three times per week, and body composition was measured twice per week. These data are presented in the results section.
Cannulation and Verification of Placement
At the beginning of week 10 of the CD/HFD, all animals were prepared with bilateral PVN cannulas. The rats were first anesthetized with intramuscular injections of xylazine at 3.5 mg/kg and ketamine at 20 mg/kg and were fitted with a 28-gauge stainless-steel guide cannula placed just above the PVN bilaterally. Stereotaxic coordinates were determined from the rat brain atlas of Paxinos and Watson (41) and are as follows: 0.5 mm lateral and 1.9 mm posterior to bregma, and 7.4 mm below the skull surface. The injector extended 1 mm further than the end of the guide cannula. The animals were given at least 1 wk to recover following surgery. During this period, the animals were handled daily and received sham injections to habituate them to the injection process.
At the end of study, the rats were decapitated, and whole brain tissue was taken for histological examination. The brain tissue was sectioned with a cryostat at a thickness of 40 μm and mounted on gelatin-coated slides. All slides were stained with 0.1% thionin and treated with ethanol (from 10% to 100%) and clearing agent (Electron Microscopy Sciences, Hatfield, PA). After the slides were air dried, injection placement was determined microscopically at ×10, using the brain atlas of Paxinos and Watson (41) as a reference. A cannula was deemed correct if the histological examination indicated that the injection was within a 0.25-mm diameter from the targeted site. Data from animals with misplaced cannulas were excluded from the data analyses. In this study, eight animals were excluded from the statistical analysis due to incorrect cannula placement. In six animals the brain tissue became severely cracked in the quick freezing process with liquid nitrogen, which meant their cannula track could not be accurately identified. In these cases, the cannula placements were deemed correct as the statistical analyses indicated that the data resulting from these animals were not outliers (> mean ± 2 SD). With the exception of two rats that were excluded because of sickness, we did not find outliers in rest of the rats. We have extensive previous experience with PVN cannulations. Our success rate in targeting the PVN is >80%, which increases our confidence that the remaining four rats had correctly placed cannulas.
Assignment of HFD-Fed Rats Into Three Groups
Our previous experience with HFD and similar experiments (17, 19) indicate a large variation in feeding and body weight gain among SD rats on a HFD. Some rats have a large weight gain and develop severe obesity, while other rats remain lean. After 11 wk on the HFD, we also found variation among the rats: a range of 80–327 g of body fat and a range of 476–809 g of body weight. We took advantage of this variation to test our hypothesis that response to BDNF may be affected by level of body fatness.
After the animals recovered from cannulation (week 11), the HFD rats were ranked from high to low based on their body fat, with the top 24 assigned to the high body fat (H) group, the middle 24 assigned to the intermediate body fat (I) group, and the bottom 24 assigned to the low body fat (L) group. The rationale for distributing the rats among groups according to body fat (vs. body weight) is that body fat is more metabolically critical than body weight in disorders related to obesity. In support of this rationale, body fat changes after exposure to HFD were more robust than body weight changes. As indicated in the results, a statistically significant body weight increase [as compared with the CD group (Fig. 2A)] occurred after 4 wk of the HFD, while a significant body fat increase occurred after only 4 days of the HFD (Fig. 2B).
Fig. 2.
Comparisons between animals fed the high-fat diet (HFD) and animals fed the control diet (CD) for 11 wk. A: HFD group had a significant increase in body weight after about 4-wk of the HFD compared with the CD rats, with more than 10% of that in the CD rats (inset). B: HFD group increased their body fat from day 4 and had significantly more relative body fat (body fat/body weight × 100%, inset) than the CD rats. *P < 0.05.
Within each group, the rats were divided into two subgroups (12 rats/subgroup). The details of their body measurements are shown in Table 3, and there are no statistical differences between any two corresponding subgroups. One subgroup was assigned to aCSF treatment, and the other was assigned to BDNF treatment (1.0 μg). These divisions formed the following six subgroups: H-aCSF (HC), H-BDNF, I-aCSF (IC), I-BDNF, L-aCSF (LC), and L-BDNF. For the CD group, all rats were assigned to aCSF treatment and defined as CD-C.
Table 3.
Body measurements from control and high-fat diet-fed animals were assigned into high, intermediate, and low body fat groups prior to drug intervention
| High-Fat Diet* |
|||||||
|---|---|---|---|---|---|---|---|
| H† |
I† |
L† |
Control Diet |
||||
| aCSF | BDNF | aCSF | BDNF | aCSF | BDNF | aCSF | |
| Body weight, g | 702.7 ± 19.0 | 683.7 ± 16.4 | 638.5 ± 8.0 | 614.9 ± 10.6 | 580.9 ± 14.0 | 560.9 ± 11.9 | 577.4 ± 20.3 |
| Body fat, g | 205.2 ± 13.3 | 203.7 ± 11.0 | 148.2 ± 3.6 | 145.2 ± 3.7 | 107.4 ± 4.2 | 105.2 ± 4.1 | 101.4 ± 8.4 |
| Lean mass, g | 419.0 ± 9.99 | 409.4 ± 8.7 | 414.7 ± 5.6 | 399.4 ± 8.3 | 402.6 ± 12.2 | 387.8 ± 9.4 | 406.0 ± 11.0 |
| Relative body fat, % | 29.0 ± 1.3 | 28.7 ± 1.6 | 23.2 ± 0.4 | 23.6 ± 0.6 | 18.6 ± 0.8 | 18.8 ± 0.8 | 17.3 ± 0.99 |
| Relative lean mass, % | 59.8 ± 1.2 | 60.1 ± 1.4 | 65.0 ± 0.4 | 64.9 ± 0.6 | 69.2 ± 0.9 | 69.1 ± 0.7 | 70.6 ± 0.9 |
Values are expressed as means ± SE. aCSF, artificial cerebrospinal fluid; BDNF, brain-derived neurotrophic factor.
Rats ranked in the top 24, middle 24, and lowest 24 based on body fat were assigned to high (H), intermediate (I), and low (L) body fat groups, respectively.
Animals in each group were further subdivided into two subgroups (n = 12/group, aCSF or BDNF, 1 μg) with body fat level evenly distributed among subgroups. There were no significant differences between aCSF and BDNF subgroups in H, I, and L groups. There is no significant difference in any measurements between aCSF and BDNF subgroups in H, I, and L groups.
Drugs
BDNF was kindly provided by Regeneron Pharmaceuticals (Tarrytown, NY), and stored at −80°C in 10 mg/ml of 150 mM NaCl, 10 mM NaHPO3 buffer and 0.004% Tween-20 until use. Just before use, the BDNF was diluted with aCSF. The dose of 1 μg was selected based on a pilot experiment showing that 1 μg of BDNF reduced feeding, body fat, and body weight in rats fed with a HFD. Our previous studies indicate that BDNF in the PVN in the dose range of 0.5–3 μg does not induce taste aversion (51), suggesting that BDNF-inhibited feeding is not due to BDNF-induced illness at this dose.
Injections
A volume of 0.5 μl was injected slowly over 30 s, with the injector left in place an additional 15 s to ensure extrusion from the tip and to minimize distribution of drug up the cannula tract. In total, animals received 11 injections, and the injection sites were examined by light microscopy for tissue damage. Our previous observations indicate that repeated injection of BDNF does not induce gliosis (50).
Body Composition
An EchoMRI-700 (Houston, TX) was used to measure the fat mass, lean mass, free water, and total water. Each animal was weighed and then placed into one of the plastic holders based on their body weight with limited restraint. Then the holder was placed in the EchoMRI machine for scanning. Each scan took 1–2 min.
Experimental Protocols
Feeding experiment.
Starting at week 12, all animals were injected with aCSF or BDNF at 1230–1430 every other day for a total of 11 injections. We administered BDNF every 48 h because we previously observed that BDNF inhibited feeding and body weight gain for up to 48 h after injection (52). Energy intake was measured daily, body weight was measured daily or every other day, and body composition was measured 2 or 3 times a week. The body fat and lean mass measured with MRI is consistent with those chemically analyzed, with almost 1:1 correlation (39).
Terminal experiment.
The terminal experiment was performed 44–48 h after the last injection. Food was removed at 0730-0800, and energy intake, body weight, and body composition were measured before death. Starting at 1030, the animals were decapitated, and perirenal white adipose tissue (pWAT), epididymal white adipose tissue (eWAT), and the liver were collected and weighed. Brain tissue was taken for cannula placement check and other analyses. Blood was collected for analyses of serum glucose, triglycerides, insulin, and leptin.
Serum metabolic measurements.
Blood was collected in vials, kept in room temperature for more than 30 min, centrifuged for 15 min at 1,000 g, and then aliquots of the serum were put in separate sets of vials and stored in −80°C. Serum glucose was analyzed with QuantiChrom Glucose Assay Kit (Hayward, CA), which is designed to measure glucose directly in serum without any pretreatment and utilizes a specific color reaction with glucose. Our test showed a very high correlation between the standard glucose concentrations and absorbance at OD620 nm (r2 = 0.9975). Serum triglycerides were measured with an Assay Kit from Cayman Chemical (Ann Arbor, MI), which utilizes the enzymatic hydrolysis of the triglycerides glycerol by lipase to produce glycerol and free fatty acids, which are subsequently measured by a coupled enzymatic reaction system with a colorimetric readout at 540 nm. Standard triglyceride concentrations and OD550 nm were highly correlated (r2 = 0.9998). Serum insulin and leptin were measured with an EIA kit from Alpco Diagnostics (Salem, NH), which uses peroxidase enzyme labeled monoclonal antibody and substrate for quantification of optical density (OD) after incubation. In our tests, the correlation between the standard protein concentrations and OD450 nm was r2 = 0.9992 for insulin and r2 = 0.9951 for leptin. All assays were performed according to the protocols provided by the manufacturers, and these assay kits have been used successfully in other studies (6, 16, 29, 35).
Liver histology.
Several lobes of the liver were removed from each animal and placed into liquid nitrogen and then into a −80°C freezer. A small portion of these tissue samples was cut and embedded with precooled optimal cutting temperature (OCT) compound (Torrance, CA) for cryostat sectioning at 10 μm. The sections were mounted on Fisher's SuperFrost Plus slides. The sections were postfixed with a 10% formalin solution for 2–3 min and then rinsed 3 times with PBS while shaking for 5 min. The following protocol for a hematoxylin and eosin (H&E) stain was used on all the slides: wash and stain with Richard Allen hematoxylin 7211 (Kalamazoo, MI); wash and remove background stain with Richard Allen Clarifier II; wash and stain with Richard Allen Bluing Reagent; wash and rinse in alcohol; counterstain with Richard Allen Eosin Y; dehydrate with increasing concentrations of alcohol; clear with xylene, and mount on a cover slip. The slides were left to dry for 24 h before viewing under a light microscope. Additionally, another set of slides was stained in Oil Red O, after postfixing, using the following protocol: rinse with 60% isopropanol; stain with Oil Red O for 15 min; rinse again with 60% isopropanol; lightly stain nuclei with a few dips in hematoxylin, and rinse with water and mount a cover slip.
Statistical analyses.
For the first 11 wk (starting with the CD/HFD and continuing until pre-BDNF treatment), one-factor ANOVA (diet = independent factor) was used to compare the daily data between the HFD and CD group. Two-factor (treatment and phenotype as group H, I, or L of body fat size) ANOVA was used in rats on HFD during the treatment period and terminal study for the analysis of energy intake, changes of body weight, body fat, lean mass, pWAT, eWAT, liver, and blood metabolic measurements. Post hoc analyses of the two-factor ANOVA did not reveal a significant difference between the two treatments within H, I, or L group when the main effect of treatment, phenotype, or interaction is identified, a further t-test was applied to compare BDNF with aCSF within each phenotype group. For multiple comparisons among HC, IC, and LC, or H-BDNF, I-BDNF, and L-BDNF (same treatment but different phenotype), the Benjamini-Hochberg (B-H) procedure (4) for controlling the false-positive rate was applied (13). One-factor (diet) ANOVA was used to compare the aCSF-treated HFD rats with the CD-C rats to determine differences between the two diets. ANOVA tests were analyzed using the StatView 5.0 program (Cary, NC) and were expressed as means ± SE. During the study, eight animals were sick (3 from HC, 1 from H-BDNF, 3 from IC, and 1 from LC), and eight died as a result of a possible infection or unknown cause (2 from CD-C, 1 from HC, 1 from IC, 2 from I-BDNF, and 2 from L-BDNF); the data for these animals were excluded. The sample size for each subgroup at the end of the study was 10 for CD-C, 8 for HC, 10 for H-BDNF, 6 for IC, 9 for I-BDNF, 9 for LC, and 8 for L-BDNF, respectively. Because of exclusion of data from sick and dead rats, there was a significant difference in body weight between LC and L-BDNF (554.6 ± 12.2 for L-BDNF vs. 593.8 ± 13.5 for LC, P = 0.04983). There were no significant differences in other body measurements between subgroups within each phenotype after excluding the data of sick and dead animals.
RESULTS
HFD Exposure, Pre-BDNF Intervention
Energy intake.
HFD exposure significantly increased cumulative energy intake beginning on day 2 (P ≤ 0.015) and was more than 13% above that of the CD group by end of the period (7,997 ± 114 kcal for HFD vs. 7,026 ± 204 kcal for CD).
Body weight.
In contrast to the rapid increase in energy intake after the start of the diets, it took 4 wk for the HFD group to show a significant increase in body weight compared with the CD group (P ≤ 0.025 from day 29, Fig. 2A). By the end of the period, the HFD group showed a significant >10% increase in body weight compared with the CD group (of Fig. 2A, inset).
Body fat.
In contrast to body weight, HFD-fed rats had significantly increased body fat starting on day 4 of diet exposure. This significant difference (P ≤ 0.00198, Fig. 2B) remained throughout the period compared with the CD group, with more than 50% of the body fat of controls at the end. HFD rats also had significantly increased relative body fat (P ≤ 0.00177, Fig. 2B, inset), with an increase of >37% of that of the CD group at the end of the period (23.72 ± 0.63% for HFD vs. 17.33 ± 0.99% for CD).
Lean mass.
Despite the increase in body weight (52.36 g, a difference between 629.78 g in HFD rats and 577.42 g in CD rats) and fat mass (51.14 g, a difference between 152.56 g in HFD rats and 101.42 g in CD rats) when comparing the rats on the HFD to those on the CD, these two groups had an almost identical lean mass (404.98 g for HFD vs. 405.97 g for CD). Their lean mass was similar throughout the 11 wk (data not shown).
BDNF Intervention
Energy intake.
Two-factor ANOVA indicated a significant main effect of treatment from day 1 to the end (P ≤ 0.0123). Post hoc t-tests indicated that in HFD-fed animals, BDNF significantly inhibited energy intake in H (P ≤ 0.0106 from day 1, Fig. 3), I (P = 0.0496 on day 6 and P ≤ 0.0389 from day 13) and L subgroups (P ≤ 0.0193 from day 5) compared with the corresponding aCSF-treated subgroups. Cumulatively, BDNF significantly reduced energy intake in H (996.7 ± 99.3 vs. 1,640.2 ± 99.5 kcal for HC, P < 0.0001), I (1,257.1 ± 110.3 vs. 1,609.8 ± 26.0 kcal for IC, P < 0.0001), and L groups (1,234.1 ± 88.6 vs. 1,666.2 ± 37.5 kcal for LC, P < 0.0001), respectively (Fig. 3, inset). BDNF inhibited feeding the most in H group since H-BDNF rats ate the least compared with I- and L-BDNF rats. However, the B-H statistical procedure revealed no significant difference between H-, I- and L-BDNF subgroups. As indicated in Fig. 3, inset, H-BDNF rats ate 60% of the energy intake of HC animals, while those in the I-BDNF and L-BDNF subgroups ate about 76–78% of aCSF-treated rats in corresponding subgroups.
Fig. 3.
Time course of energy intake during BDNF intervention. The energy intake in the animals treated with BDNF in H (clear blue square), I (clear brown triangle), and L (clear maroon diamond) subgroups was significantly reduced compared with the corresponding aCSF-treated subgroups (corresponding solid color symbols). The H-BDNF rats had the lowest levels of energy intake and also consumed the smallest percentage of its corresponding aCSF-treated group when compared with the other subgroups (inset). The energy intake in the CD-C rats (solid green square) was lower than the rats treated with aCSF in the H, I, and L subgroups (P = 0.0756 for main effect). H, high body fat group; I, intermediate body fat group; L, low body fat group; HC, H subgroup treated with aCSF; H-BDNF, H subgroup treated with BDNF; IC, I subgroup treated with aCSF; I-BDNF, I subgroup treated with BDNF; LC, L subgroup treated with aCSF; L-BDNF, L subgroup treated with BDNF; CD, control diet; CD-C, CD group treated with aCSF. *P < 0.05, comparison between BDNF and aCSF treatment in H (blue), I (brown), and L (maroon) group.
Energy intake in CD-C was lower than the HFD rats treated with aCSF in the H, I, and L subgroups, but without a significant difference (P = 0.0756 for main effect, Fig. 3).
Body weight.
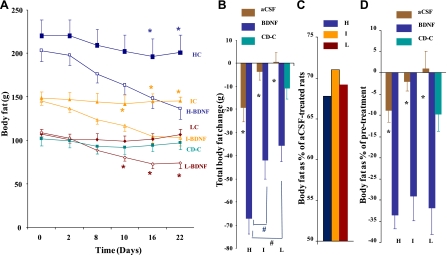
Two-factor ANOVA for the time course of body weight indicated significant main effects of treatment (P < 0.01) and phenotype from day 2 to the end (P < 0.01). Post hoc t-test indicated that BDNF significantly decreased body weight in H (P ≤ 0.0337 from day 6, Fig. 4A), I (P ≤ 0.0395 from day 4, Fig. 4A) and L subgroups (P ≤ 0.0331 from day 2, Fig. 4A), compared with their corresponding aCSF-treated rats. The analysis of change of body weight (difference between pre and 48 h postinjection) with two-factor ANOVA showed a significant effect of treatment (from day 2 to day 16, P ≤ 0.0497) and phenotype (on days 4, 14 and 16, P ≤ 0.0428). Post hoc t-test showed a BDNF-induced significant reduction in H (on days 2, 6, 8, 12, and 14; P ≤ 0.0337), I (on days 4, 10, 12, and 14; P ≤ 0.0382), and L groups (on days 4, 6, 10, 14, and 16; P ≤ 0.0219), respectively (data not shown). Two-factor ANOVA for the total body weight change indicated a significant main effect of treatment (P < 0.0001) and phenotype (P < 0.0257). The cumulative body weight change by BDNF in the H, I, and L subgroup (Fig. 4B) was −101.6 g (vs. −21.4 g for HC, P = 0.0007), −80.4 g (vs. −6.1 g for IC, P = 0.0036), and −67.6 g (vs. +16.7 g for LC, P = 0.00007), respectively. H-BDNF rats lost more body weight compared with I- and L-BDNF rats, but without a significant difference, according to the B-H statistical procedure. In comparing HC, IC, and LC using the B-H procedure, body weight was significantly higher in HC than IC (P = 0.007846, and less than the B-H critical value of 0.016667) and LC (P = 0.000153, and less than the B-H critical value of 0.008333), and body weight in IC was also significantly higher in IC than LC (P = 0.0215, and less than B-H critical value of 0.025). By the end of the experiment, BDNF treatment reduced body weight to 80.3%, 84.4%, and 81.2% of the aCSF-treated animals in corresponding subgroups (Fig. 4C). On the basis of body weight at pretreatment (Fig. 4D), BDNF significantly decreased body weight by 16.7% in H (vs. −2.7% for HC, P = 0.00037), 12.8% in I (vs. −0.7% for IC, P = 0.00185), and 11.4% in L rats (vs. 1.9% for LC, P = 0.00085), respectively. This may suggest a greater sensitivity to BDNF in H-BDNF rats compared with I-BDNF and L-BDNF subgroups.
Fig. 4.
Body weight during BDNF intervention. In the time course (A), body weight in the animals treated with BDNF in H (clear blue square), I (clear brown triangle), and L (clear maroon diamond) subgroups was significantly reduced compared with the corresponding aCSF-treated subgroups (corresponding solid color symbols). H-BDNF rats showed the greatest body weight reduction (B), the lowest percentage of the body weight of the aCSF-treated animals (C), and the lowest percentage of their own pretreatment weight (D). The body weight change in animals from the CD-C group (green solid square; A, B, D) was significantly lower than LC and comparable with HC and IC. H: high body fat group; I: intermediate body fat group; L: low body fat group; HC: H subgroup treated with aCSF; H-BDNF: H subgroup treated with BDNF; IC: I subgroup treated with aCSF; I-BDNF: I subgroup treated with BDNF; LC: L subgroup treated with aCSF; L-BDNF: L subgroup treated with BDNF; CD: control diet; CD-C: CD group treated with aCSF. *P < 0.05, comparison between BDNF and aCSF treatment in H (blue), I (brown) and L (maroon) group. #P < 0.05, comparison between CD-C and LC.
The time course of body weight for CD-C is shown in Fig. 4A. Total body weight change in CD-C animals was lower than LC rats (P = 0.041) (Fig. 4B).
Body fat.
Two-factor ANOVA for the time course of body fat indicated a significant main effect of treatment (on days 8, 10, 16, and 22) and phenotype (on days 0, 2, 8, 10, 16, and 22) (P < 0.01). Post hoc t-test showed that BDNF significantly decreased body fat in H (P ≤ 0.05 from day 16, Fig. 5A), I (P ≤ 0.03914 from day 10, Fig. 5A), and L subgroups (P ≤ 0.02697 from day 10, Fig. 5A), compared with their corresponding aCSF-treated rats. Comparisons using the B-H procedure indicated that body fat was significantly higher in HC than IC (P = 0.016092, and a less than B-H critical value of 0.025) and LC (P = 0.0000536, and a less than B-H critical value of 0.008333), and body weight in IC was also significantly higher in IC than LC (P = 0.016092, and a less than B-H critical value of 0.025). The analysis of change of body fat (difference between pre- and 48-h postinjection) with two-factor ANOVA showed a significant effect of treatment (from day 2 to day 16, P ≤ 0.009) and phenotype (on day 10, P < 0.0001). Post hoc t-test showed that BDNF significantly reduced body fat in H (on days 2, 8, 10, 14, and 20; P ≤ 0.045), I (on days 4, 8, 10, and 16; P ≤ 0.01947), and L groups (on days 4, 8, 10, and 16; P ≤ 0.0211), respectively (data not shown). Two-factor ANOVA for the total body fat change indicated a significant main effect of treatment (P < 0.0001) and phenotype (P < 0.0006). Cumulatively, BDNF-induced total body fat change (Fig. 5B) in the H, I, and L subgroups was −67.1 ± 6.9 g (vs. −19.2 ± 6.2 g for HC, P = 0.0001), −41.9 ± 8.2 g (vs. −3.7 ± 3.5 g for IC, P = 0.0031), and −35.4 ± 7.4 g (vs. +0.4 ± 4.0 g for LC, P = 0.0005), respectively. The B-H procedure indicated that H-BDNF rats had significantly reduced body fat relative to I-BDNF (P = 0.014779, and a less than B-H critical value of 0.016667) and L-BDNF rats (P = 0.003139, and a less than B-H critical value of 0.008333), suggesting a greater response to BDNF by H rats. By the end of the experiment, BDNF treatment reduced body fat to 67.7%, 71.0%, and 69.2% of that in aCSF-treated animals in the H, I, and L subgroups, respectively (Fig. 5C). On the basis of body fat at pretreatment, BDNF significantly decreased body fat by 33.6% in the H (vs. −8.9% for HC, P < 0.001), 29.1% in I (vs. −2.1% for IC, P < 0.001) and 31.8% in L rats (vs. +1.0% for LC, P < 0.001; Fig. 5D). To determine the relationship between initial body fat and the amount of reduced body fat in the animals treated with BDNF, we performed a regression analysis using data from all BDNF-treated rats. As indicated in Fig. 6, there is a significant association between the initial body fat and amount of reduced body fat (r = 0.576; r2 = 0.331, and P = 0.0017), further indicating that PVN BDNF was especially effective at reducing body fat in animals with higher initial body fat.
Fig. 5.
Body fat during BDNF intervention. In the time course (A), body fat in the animals treated with BDNF in H (clear blue square), I (clear brown triangle), and L (clear maroon diamond) subgroups was significantly reduced compared with the corresponding aCSF-treated subgroups (corresponding solid color symbols). The H-BDNF rats showed the significant reduction of body fat reduction (B) compared with I- and L-BDNF rats. The body fat in the BDNF-treated rats is displayed as a percentage of that in the aCSF-treated animals (C) and the percentage of their own pretreatment level (D). The body fat change in the animals from CD-C group (green solid square) is displayed in A, B, and D. Same groups studied as in Fig. 4. *P < 0.05, comparison between BDNF and aCSF treatment in H (blue), I (brown), and L (maroon) group. #P < Benjamini-Hochberg critical value P (comparison between H-BDNF and I-BDNF or L-BDNF).
Fig. 6.
Association between initial body fat and amount of body fat loss in BDNF-treated and HFD-fed rats. n = 27.
The time course of body fat change for CD-C rats is comparable to the LC rats, with no significant difference at any time point (Fig. 5A). Although there was a significant difference in total body fat change (P = 0.0411) among CD-C, HC, IC and LC rats, post hoc analysis indicated that the body fat change in the CD-C group (−11.4 ± 4.1 g) was comparable with aCSF-treated rats in the H, I, and L subgroups (P = 0.211, 0.3357, and 0.092, respectively).
Lean mass.
Two-factor ANOVA for the time course of lean mass indicated significant main effect of treatment (P ≤ 0.0094) for the entire period. Post-hoc t-test revealed that BDNF significantly reduced lean mass in H (P ≤ 0.044 from day 6), I (P ≤ 0.05 from day 4), and L (P ≤ 0.031 from day 2, data not shown) groups, respectively. Two-factor ANOVA for lean mass change indicated significant main effect of treatment (P ≤ 0.0275) on days 2, 8, 10, and 16 (data not shown). Post hoc t-test revealed that BDNF significantly reduced lean mass in H (P = 0.0126 on day 14), I (P = 0.0046 on day 16), and L (P ≤ 0.023 on days 8, 10, and 16; data not shown) groups, respectively. BDNF-induced cumulative change in lean mass was −33.8 ± 5.6 g (vs. −0.5 ± 8.2 g for HC, P = 0.0008) in H, −33.5 ± 7.6 g (vs. 9.6 ± 7.2 g for IC, P = 0. 0095) in I and −26.2 ± 7.7 g (vs. 11.1 ± 5.5 g for LC, P = 0.0017) in L subgroups. On the basis of the lean mass at pretreatment, BDNF significantly changed lean mass by −8.7 ± 1.5% in the H (vs. 0.13 ± 1.9% for HC, P = 0.0095), −8.2 ± 1.8% in I (vs. 2.3 ± 1.5% for IC, P = 0.0344) and −6.8 ± 2.0% in the L rats (vs. 2.8 ± 1.3% for LC, P = 0.0008), respectively.
There were no significant differences in lean mass among CD-C, HC, IC, and LC at any time point. Cumulative lean mass change in CD-C animals (−10.2 ± 5.3 g) was also comparable with HFD rats treated with aCSF in all subgroups, with no significant difference between groups (P = 0.1608).
Terminal Experiment
All animals were killed 44–48 h after the last injection, and their liver, pWAT, and eWAT were collected and weighed.
Two-factor ANOVA suggested significant main effects of treatment (P = 0.0002 and P = 0.0073) and phenotype (P < 0.0001 and P = 0.0002) on pWAT and eWAT weight, respectively. Post-hoc t-test indicated that BDNF reduced pWAT and eWAT in the H (P = 0.0215 and P = 0.0197), I (P = 0.0772 and P = 0.0122), and L subgroups (P = 0.00294 and P = 0.0497, Table 4), respectively. The B-H procedure indicated that HC rats had significantly higher pWAT and eWAT than IC (P = 0.005681 and 0.010884, and a less than B-H critical value of 0.016667) and LC (P = 0.000202 and 0.0000545, and a less than B-H critical value of 0.008333), respectively.
Table 4.
Perirenal and epididymal white adipose tissue and liver mass
| High-Fat Diet |
|||||||
|---|---|---|---|---|---|---|---|
| H |
I |
L |
Control Diet |
||||
| aCSF | BDNF | aCSF | BDNF | aCSF | BDNF | aCSF | |
| pWAT, g | 36.9 ± 4.2§ | 25.0 ± 2.5* | 21.9 ± 1.1 | 17.3 ± 1.8 | 18.6 ± 1.5 | 11.5 ± 1.2* | 14.5 ± 1.4† |
| eWAT, g | 21.2 ± 1.2§ | 16.3 ± 1.4* | 16.1 ± 1.7 | 10.6 ± 1.1* | 12.1 ± 1.3 | 8.8 ± 0.7* | 11.7 ± 0.8† |
| Liver, g | 20.7 ± 1.0§ | 15.4 ± 0.9* | 16.9 ± 0.5 | 13.9 ± 0.7* | 15.5 ± 0.4 | 12.0 ± 0.5* | 16.5 ± 1.0‡ |
pWAT, perirenal white adipose tissue; eWAT, epididymal white adipose tissue. Please refer to the detailed statistic analyses described in the methods and results sections.
P < 0.05, compared with aCSF-treated rats within each phenotype group with t-test;
P < 0.05, compared to aCSF-treated H and I;
P < 0.05, compared to aCSF-treated H;
P < Benjamini-Hochberg critical values (0.01667 or 0.00833), compared to aCSF-treated I and L.
In comparisons among the aCSF-treated rats, pWAT and eWAT weights in CD-C animals were significantly lower than HC (P < 0.0001 and P <0.0001) and IC rats (P = 0.0472 and P = 0.0228, respectively) but there was no difference relative to LC rats.
There were significant main effects of treatment (P < 0.0001) and phenotype (P < 0.0001) on liver weight. Post hoc t-tests indicated a significant reduction of liver mass in BDNF-treated H (P = 0.0009), I (P = 0.0092), and L (P = 0.0004, Table 4) rats. The B-H procedure indicated that HC rats had larger livers than IC (P = 0.005369, and a less than B-H critical value of 0.016667) and LC (P = 0.0000523, and a less than B-H critical value of 0.008333; Table 4), respectively.
H-BDNF rats also had significantly lower relative liver weight (2.7 ± 0.04% vs. 2.93 ± 0.09% for HC, P = 0.0308). Liver weight in CD-C was significantly lower than that of HC rats (P = 0.0012) but showed no difference from IC and LC rats (Table 4).
Serum Metabolic Measurements
Two-factor ANOVA showed a significant interaction between treatment and phenotype on blood glucose (P = 0.0023) and triglycerides (P = 0.0436). Post hoc t-tests indicated that H-BDNF had significantly lower levels of glucose (P = 0.000249) and triglycerides (P = 0.015186) compared with HC rats (Table 5).
Table 5.
Serum metabolic indices
| High-Fat Diet |
|||||||
|---|---|---|---|---|---|---|---|
| H |
I |
L |
Control Diet |
||||
| aCSF | BDNF | aCSF | BDNF | aCSF | BDNF | aCSF | |
| Glucose, mg/dl | 141.5 ± 8.1** | 90.9 ± 7.1* | 108.9 ± 5.7 | 119.4 ± 10.5 | 116.3 ± 9.4 | 124.2 ± 11.7 | 120.5 ± 2.8‡ |
| Triglycerides, mg/dl | 80.3 ± 9.4 | 48.0 ± 7.3* | 46.2 ± 7.0 | 52.3 ± 6.8 | 53.1 ± 7.7 | 51.4 ± 4.8 | 97.1 ± 20.1† |
| Insulin, ng/ml | 3.83 ± 0.32§ | 1.41 ± 0.27* | 1.67 ± 0.24 | 1.38 ± 0.35 | 1.73 ± 0.22 | 1.00 ± 0.13* | 2.50 ± 0.37‡ |
| Leptin, pg/ml | 3.20 ± 0.27§ | 1.61 ± 0.43* | 1.79 ± 0.19 | 1.29 ± 0.32 | 1.37 ± 0.22 | 0.83 ± 0.14* | 1.58 ± 0.20‡ |
Please refer to the detailed statistical analysis described in the methods and results sections.
P < 0.05, compared with aCSF-treated rats in each phenotype group with t-test;
P < 0.05, compared to aCSF-treated rats in I and L;
P < 0.05, compared to aCSF-treated H;
P < Benjamini-Hochberg critical value (0.01667 or 0.00833), compared to aCSF-treated I and L.
P < Benjamini-Hochberg critical value (0.00833), compared to aCSF-treated I.
There were significant main effects of treatment (P < 0.0001), phenotype (P < 0.0001), and interactions between the two (P = 0.0007) on serum insulin. Post hoc t-test indicated BDNF significantly reduced serum insulin in H (P = 0.000234) and L (P = 0.01698) groups. Two-factor ANOVA also indicated a main effect of treatment (P = 0.0012) and phenotype (P = 0.0003) on serum leptin level, and post-hoc t-tests indicated that BDNF significantly decreased leptin in H (P = 0.00937), and L (P = 0.0411) groups (Table 5).
After two-factor ANOVA, the B-H procedure was used to compare differences among HC, IC, and LC rats. HC rats showed significantly higher levels of glucose than that in IC rats (P = 0.0004913, a less than B-H critical value of 0.00833), higher levels of insulin than that in IC rats (P = 0.00013, a less than B-H critical value of 0.01667), and LC rats (P = 0.0000198, a less than B-H critical value of 0.00833), and higher levels of leptin than that in IC rats (P = 0.00089, a less than B-H critical value of 0.01667) and LC rats (P = 0.000053, a less than B-H critical value of 0.00833), respectively (Table 5).
In comparison between the CD-C and HC, IC and LC, CD-C rats had significantly lower serum glucose, insulin, and leptin than HC (P = 0.0442, P = 0.0037, and P < 0.0001, respectively), and higher triglycerides than IC (P = 0.0137) and LC (P = 0.0181), respectively (Table 5).
Liver Histology
Figure 7 shows the representative liver histology of rats in the HC, H-BDNF, and CD-C group. Figure 7B shows balloon-like cells and infiltration from white blood cells in the liver of a HC rat, possibly consistent with fat deposits and inflammation in the liver. Fig. 7C (from an H-BDNF rat) and Fig. 7A (from a CD-C rat) display normal tissue structure with no fat deposit. All eight HC rats displayed histology similar to Fig. 7B; and among 10 H-BDNF rats, two had liver structure change but with no detectable inflammation, and 8 of 10 rats had normal histology similar to Fig. 7C. With the Oil Red O staining, Fig. 7D shows fat deposits in the liver of a HC rat, and Fig. 7E shows no detectable fat deposits in the liver of a H-BDNF rat (Fig. 7E).
Fig. 7.
Histology of liver from CD-C, HC, and H-BDNF. aCSF/BDNF was injected in the PVN every other day for 22 days. At the end of experiment, the liver from CD-C, HC, and H-BDNF was harvested, cryostat sectioned, fixed with 10% formalin, and stained with H&E (A: for CD-C, B: for HC, and C: for H-BDNF) and Red Oil O (D: for HC and E: for H-BDNF). Representative example of each group is shown. Magnification is ×20. The scale bar represents 50 μM.
DISCUSSION
The above results demonstrate four key points. First, provision of the HFD generally increased energy intake, body weight gain, and body fat, without affecting lean mass (Fig. 2). Second, there was a large variation in body size among the HFD-fed animals, and only a small number of rats developed obesity with large size of visceral adipose tissue and liver, as well as increased concentrations of serum glucose, triglycerides, insulin, and leptin, as seen in HC animals. Third, BDNF reversed HFD-induced obesity by significantly reducing energy intake; BDNF also decreased levels of serum glucose, triglycerides, insulin, and leptin in the obese animals. Fourth, the response to BDNF treatment was greater in the H group compared with I and L groups since the H-BDNF rats displayed a more significant reduction in body fat.
After 11 wk on the diets, HFD animals had more than a 10% increase in energy intake and body weight and a 50% increase in body fat compared with CD rats (Fig. 2), but lean mass was similar between these groups. This is likely due to the similar intake of dietary protein (20%) and the similar biological variability (indicated by comparable body weight, body fat, lean mass, and energy intake) between groups prior to the dietary regimes (Table 2). The observation that HFD and CD rats have similar amounts of lean mass, has also been reported in another study (19). Compared with CD rats, the increased average body weight (52.4 g) and body fat (51.1 g) without an increase in lean mass in the HFD rats, indicates that the increase in body weight was mainly due to adipose tissue.
Eleven weeks of HFD resulted in a huge variation in body weight among these SD rats, and this confirms that the animals responded to the HFD differently, with some developing diet-induced obesity (as seen in H) and some maintaining obesity resistance (as seen in L). The large variability allowed us to divide the rats into distinct tertiles based on body fatness, which allowed us to test the effect of BDNF on diet-induced obesity. This division also further allowed us to test another hypothesis that rats with varying body fatness levels respond more or less robustly to BDNF.
There are several reasons for selecting body fat instead of body weight as the criteria for dividing the groups. Response of adiposity to BDNF treatment was a primary goal of the study, and as the response of body fat is more robust than body weight since a significant increase for body fat occurred on day 4 (Fig. 2B, inset), whereas a change in body weight was not observed until day 29 (Fig. 2A). In a late-on regression test, the r2 value from the analysis of body fat vs. serum leptin (0.529) was higher than that in the analysis of body weight vs. serum leptin (0.274; data not shown). This suggests that body fat measurements more accurately represent adiposity than body weight. Thus, using body fat as the criteria for dividing groups is a better choice.
After the HFD rats were divided into H, I, and L groups, half of each group were chronically injected with aCSF, while the other half received BDNF. Compared with the corresponding aCSF-treated animals, the BDNF-treated rats in all three groups had significantly decreased energy intake (Fig. 3), body weight gain (Fig. 4), and body fat gain (Fig. 5). The H-BDNF rats seemed to show the greatest response to the BDNF treatment. Although the H-BDNF group was not significantly different from I-BDNF and L-BDNF rats even though it had the lowest energy intake and the highest body weight reduction, it had significantly reduced body fat compared with I-BDNF and L-BDNF rats. A further regression analysis between initial body fat and the amount of reduced body fat in the BDNF-treated animals (Fig. 6) revealed that animals with greater body fat tend to lose a greater proportion of body fat after BDNF treatment.
All BDNF-treated animals also showed a significant decrease in lean mass; suggesting that besides decreases in body fat, loss of lean mass is also accompanied with a reduction of body weight. However, the amount of body fat and lean mass reduced by BDNF differed among the H, I, and L groups. Body weight loss due to body fat loss was 66.1%, 52.1% and 49.4%, respectively, and body weight loss due to lean mass loss was 33.2%, 41.7%, and 36.6% for H- (101.7 g as body weight loss), I- (80.5 g as body weight loss) and L-BDNF (71.7 g as body weight loss), respectively. This suggests that BDNF reduced relatively more body fat and less lean mass in the H-BDNF subgroup compared with both the I-BDNF and L-BDNF subgroups.
Effect of BDNF on DIO has been tested in mice on 4 mo of HFD, and BDNF was given subcutaneously at a dose of 10 mg·kg−1·day−1 for 6 days (36). The BDNF-treated DIO mice in that report had decreased energy intake (52% of vehicle-treated) and body weight (90% of vehicle-treated). The author did not report body fat measurements. Peripheral BDNF effects on the energy intake and body weight were also tested in other animal models, such as diabetic mice (37, 38) and genetic leptin-resistant mice (36); and the doses were 20–70 mg/kg. Although the results in our study may not be comparable with these reports due to different species, dietary fat content, approach of drug administration, dose, and duration, the findings are consistent in reduction of energy intake and body weight induced by BDNF. However, our study used a dose of 1 μg BDNF, much lower than that used in these reports, suggesting high efficiency for PVN administration. Our study also had an advantage of monitoring dynamics of body composition during dietary regimes and drug intervention.
The reduced body weight and fat mass in HFD rats are likely the combined result of the observed decreased food intake and increased energy expenditure (EE). In our previous work, we found that BDNF in the PVN significantly increased EE and resting metabolic rate (50), but the large study design and feasibility issues precluded the measurement of such metabolic variables in the current study. A recent study with HFD in mice reported that delivery of BDNF gene into the hypothalamus increased heat production, resting metabolism, and respiration exchange ratio (7), suggesting BDNF increases EE in HFD-induced obesity models.
Consistent with the decreased body fat measured by MRI scanning, the weights of the pWAT and eWAT depots revealed a dramatic decrease in all BDNF-treated subgroups compared with their corresponding aCSF-treated rats (Table 4). These findings validate that BDNF treatment significantly reduced body fat, and additionally confirm the reliability of the MRI data. The terminal experiment also showed a significant decrease in the liver weight in all of the BDNF-treated subgroups compared with the aCSF-treated rats. The reductions in the H-BDNF, I-BDNF, and L-BDNF subgroups were 25.8%, 17.4%, and 22.6%, respectively. Moreover, the H-BDNF subgroup had a significantly lower relative liver weight compared with the HC animals.
The histology of the liver from all HC rats revealed balloon-like cells spreading throughout the liver sections along with infiltration of some inflammatory cells (Fig. 7B), suggesting fat deposits and inflammation. The Oil Red O staining further indicated fat deposits in the balloon-like structure of the liver from HC rats (Fig. 7D). These findings are consistent with a study in which livers from rats fed with a HFD for 12 wk showed diffused steatosis, mononuclear cell infiltration, necrosis, fibrosis, accumulation of fat droplets, and intra-cytoplasm vacuoles (30). The histology findings suggest that the hepatomegaly seen in HC rats is due to fat deposit and inflammation in the liver. In contrast to the HC subgroup, the liver histology from the majority of the animals in the H-BDNF subgroup looked fairly normal, without signs of fat deposits and inflammation, which was similar to structure from the CD-C animals. This suggests that 3-wk of BDNF injections improved the HFD-induced histological change in the liver.
Circulating glucose, triglycerides, insulin, and leptin were measured to determine whether a HFD would induce metabolic syndrome-like symptoms (obesity as well as hyperglycemia, hyperlipidemia, hyperinsulinemia, hyperleptinemia, insulin resistance, and leptin resistance). Compared with IC and/or LC groups, the HC rats had significantly higher levels of serum glucose, insulin and leptin.
HFD suppresses glucose transporter 4 (GLUT4) expression in skeletal muscle (27) and impairs GLUT4 translocation to the cell surface (24), which may explain the high glucose levels in HFD-fed rats. The presence of high levels of glucose and insulin in the HC rats suggest insulin resistance, which often leads to type II diabetes, and high insulin levels may facilitate fat storage.
HFD has been reported to produce leptin resistance. One report found DIO and leptin resistance developed after 15 days of a cafeteria diet (43). Other studies reported leptin resistance anywhere from 16 days to 12 wk (48) (49) of HFD. Lin et al. (32) found that the feeding response to leptin was decreased after a HFD and that the longer the duration of the HFD, the lower the response to leptin. Although the current study did measure response to leptin, other evidence that 14-wk HFD (which is within the reported timing of leptin resistance) produced significantly high level of circulatory leptin and large amounts of body fat implies that the HC subgroup was possibly leptin resistant. In addition, HC rats had significantly higher body weight, total body fat, pWAT, eWAT, and liver mass, compared with IC and LC. Together, all of these findings indicate that HFD-fed HC group might develop the symptoms of metabolic syndrome.
The metabolic measurements also suggest that BDNF reverses metabolic syndrome-like symptoms. In contrast to the findings in the HC subgroup, chronic injections of BDNF in the PVN significantly decreased serum levels of glucose, triglycerides, insulin, and leptin in the H-BDNF rats. Several studies indicate that peripheral/ICV BDNF regulates glucose metabolism in diabetic animals and prevents diabetes in prediabetic animals (25, 37, 57). BDNF ameliorates glucose metabolism by enhancing glucose utilization in muscle and brown adipose tissue (58), increasing pancreatic insulin concentrations (57) and decreasing the secretion of glucagon (25). In one study, peripheral BDNF for 3 wk significantly decreased liver, but not circulating, triglycerides (47), whereas in the current study, PVN-BDNF results in a significant decrease in blood triglyceride in H-BDNF animals. Studies have reported a significantly increased circulatory leptin in the obese (3) and/or BDNF-deficient animal models (44) compared with lean or wild-typeanimals. Our findings suggest that PVN BDNF reduces HFD-induced obesity, hyperglycemia, hyperlipidemia, and resistance to insulin and leptin.
During treatment, we found that the CD-C rats were mostly comparable to the LC rats in energy intake, body fat and lean mass, pWAT, eWAT, and liver weight. Although CD-C rats had similar body measurements as the LC subgroup prior to treatment (Table 3), their biological characteristics were different. The LC rats had the lowest body fat after 11-wk on HFD, and they tended to resist DIO. By design, the CD-C group comprised rats with differing susceptibility to DIO, and some of those would have developed obesity if they had been given a HFD. This suggests that with a low-fat diet (10%), a population that is susceptible to DIO may not develop obesity and related symptoms. There was a substantial increase of triglyceride levels in CD-C rats compared with the IC and LC (Table 4). In the control diet, 35% of calories come from sucrose. Several reports indicate that diets containing high sucrose increases the level of triglycerides (15, 46), as a consequence of de novo lipogenesis from the sugar (8).
Animals with reduced BDNF expression (BDNF-mutated and heterozygous) (10, 21–23, 33, 44) develop hyperphagia, obesity, and resistance to insulin and leptin. The HFD-fed animals in our study also showed elevated levels of energy intake, body fat, and body weight gain, as well as increased serum glucose, triglycerides, insulin, and leptin. Three weeks of PVN BDNF treatment significantly reduced energy intake, body weight gain, body fat, eWAT, pWAT, and liver weight. PVN BDNF also dramatically reduced the levels of serum glucose, triglycerides, leptin, and insulin, especially in the H-BDNF subgroup. These findings indicate that BDNF in the PVN reversed HFD-induced obesity and related symptoms, especially in H animals. The reversal of obesity by PVN BDNF suggests that BDNF is a necessity for the central regulation of energy balance and can serve as a candidate for obesity therapy. The attenuation of HFD-induced insulin and leptin resistance by PVN BDNF also suggests that BDNF may be a candidate for the therapy of Type II diabetes and leptin resistance.
Perspectives and Significance
BDNF is a neurotrophin recently identified as being important in the regulation of energy balance. In the study reported herein, BDNF was given into the hypothalamic PVN, a site of action for BDNF and an area important to coordinating signals important to energy balance. The goal was to determine whether BDNF injection into this region in young (3–6 mo) rats, could reverse obesity produced by high-fat diet feeding. We found high variability in response to the high-fat diet, with some animals becoming obese, and insulin and leptin resistant, and others not. In the obese animals, a 3-wk treatment with BDNF reversed their obesity and related symptoms, whereas there was modest effect of BDNF in the animals with less adiposity. Thus amelioration of obesity and related symptoms by BDNF was greatest in animals with the highest amount of body fat, suggesting a potential therapeutic role of PVN BDNF in treating obesity, and insulin and leptin resistance. In future studies, it would be informative to assess changes in energy expenditure and determine effects in older rats, as much of human obesity is late-onset. More studies are also needed to explore the effect of central BDNF on energy balance in animals on different dietary regimes and in different animal models.
GRANTS
This work was supported by the Department of Veterans Affairs, and in part by National Institutes of Health grant 1R01DK080782.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Regeneron Pharmaceuticals Inc (Tarrytown, NY) for providing BDNF, Martha Grace, Jen Teske, Anaya Mitra, Heather Bainter, Joseph Herron, and Mark Margosian for their lab tasks, and Dr. Michael Kuskowski for providing statistical consultation.
REFERENCES
- 1.Araya AV, Orellana X, Espinoza J. Evaluation of the effect of caloric restriction on serum BDNF in overweight and obese subjects: preliminary evidences. Endocrine 33: 300–304, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bagnasco M, Dube MG, Katz A, Kalra PS, Kalra SP. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obes Res 11: 1463–1470, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bahceci M, Tuzcu A, Akkus M, Yaldiz M, Ozbay A. The effect of high-fat diet on the development of obesity and serum leptin level in rats. Eat Weight Disord 4: 128–132, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B, 57: 289–300, 1995 [Google Scholar]
- 5.Billington CJ, Briggs JE, Harker S, Grace M, Levine AS. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am J Physiol Regul Integr Comp Physiol 266: R1765–R1770, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci USA 105: 16314–16319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med 15: 447–454, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong MF, Fielding BA, Frayn KN. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc 66: 52–59, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Coppola V, Tessarollo L. Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport 15: 2665–2668, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol 583: 487–504, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res 916: 222–228, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false-positive rate in multiple comparisons. J Educ Behav Stat 27: 77–83, 2002 [Google Scholar]
- 14.Dreon DM, Frey-Hewitt B, Ellsworth N, Williams PT, Terry RB, Wood PD. Dietary fat:carbohydrate ratio and obesity in middle-aged men. Am J Clin Nutr 47: 995–1000, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Frayn KN, Kingman SM. Dietary sugars and lipid metabolism in humans. Am J Clin Nutr 62: 250S–261S; discussion 261S–263S, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Gandhi A, Beam HA, O'Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone 37: 482–490, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Ghibaudi L, van Heek M, Hwa JJ. Characterization of diet-induced obese rats that develop persistent obesity after 6 months of high-fat followed by 1 month of low-fat diet. Brain Res 936: 87–90, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Geroldi D, Minoretti P, Emanuele E. Brain-derived neurotrophic factor and the metabolic syndrome: more than just a hypothesis. Med Hypotheses 67: 195–196, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ. Fat intake affects adiposity, comorbidity factors, and energy metabolism of Sprague-Dawley rats. Obes Res 10: 956–963, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am 48: 931–953, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gray J, Yeo G, Hung C, Keogh J, Clayton P, Banerjee K, McAulay A, O'Rahilly S, Farooqi IS. Functional characterization of human NTRK2 mutations identified in patients with severe early-onset obesity. Int J Obes (Lond) 31: 359–364, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, Yanovski JA, El Gharbawy A, Han JC, Tung YC, Hodges JR, Raymond FL, O'Rahilly S, Farooqi IS. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55: 3366–3371, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med 359: 918–927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen PA, Han DH, Marshall BA, Nolte LA, Chen MM, Mueckler M, Holloszy JO. A high fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. J Biol Chem 273: 26157–26163, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Hanyu O, Yamatani K, Ikarashi T, Soda S, Maruyama S, Kamimura T, Kaneko S, Hirayama S, Suzuki K, Nakagawa O, Nawa H, Aizawa Y. Brain-derived neurotrophic factor modulates glucagon secretion from pancreatic alpha cells: its contribution to glucose metabolism. Diabetes Obes Metab 5: 27–37, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin, and melanocortin binding associated with moderate-fat diet and predisposition to obesity. Endocrinology 148: 310–316, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kahn BB, Pedersen O. Suppression of GLUT4 expression in skeletal muscle of rats that are obese from high fat feeding but not from high carbohydrate feeding or genetic obesity. Endocrinology 132: 13–22, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19: 1290–1300, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kevorkova O, Ethier-Chiasson M, Lafond J. Differential expression of glucose transporters in rabbit placenta: effect of hypercholesterolemia in dams. Biol Reprod 76: 487–495, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kiki I, Altunkaynak BZ, Altunkaynak ME, Vuraler O, Unal D, Kaplan S. Effect of high fat diet on the volume of liver and quantitative feature of Kupffer cells in the female rat: a stereological and ultrastructural study. Obes Surg 17: 1381–1388, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res 875: 89–95, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 96: 15239–15244, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masaki T, Yoshimichi G, Chiba S, Yasuda T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H. Corticotropin-releasing hormone-mediated pathway of leptin to regulate feeding, adiposity, and uncoupling protein expression in mice. Endocrinology 144: 3547–3554, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA 102: 2537–2542, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa T, Ogawa Y, Ebihara K, Yamanaka M, Tsuchida A, Taiji M, Noguchi H, Nakao K. Anti-obesity and anti-diabetic effects of brain-derived neurotrophic factor in rodent models of leptin resistance. Int J Obes Relat Metab Disord 27: 557–565, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa T, Ono-Kishino M, Sugaru E, Yamanaka M, Taiji M, Noguchi H. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab Res Rev 18: 185–191, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 49: 436–444., 2000 [DOI] [PubMed] [Google Scholar]
- 39.Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-Fos immunoreactivity. Peptides 24: 919–923, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic, 1998, p. xxvi, [236] of plates [Google Scholar]
- 42.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol 131: 229–238, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Perez-Echarri N, Perez-Matute P, Martinez JA, Marti A, Moreno-Aliaga MJ. Serum and gene expression levels of leptin and adiponectin in rats susceptible or resistant to diet-induced obesity. J Physiol Biochem 61: 333–342, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Sha H, Xu J, Tang J, Ding J, Gong J, Ge X, Kong D, Gao X. Disruption of a novel regulatory locus results in decreased BDNF expression, obesity, and type 2 diabetes in mice. Physiol Genomics 31: 252–263, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 291: R889–R899, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Truswell AS. Food carbohydrates and plasma lipids—an update. Am J Clin Nutr 59: 710S–718S, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Tsuchida A, Nonomura T, Nakagawa T, Itakura Y, Ono-Kishino M, Yamanaka M, Sugaru E, Taiji M, Noguchi H. Brain-derived neurotrophic factor ameliorates lipid metabolism in diabetic mice. Diabetes Obes Metab 4: 262–269, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Tulipano G, Vergoni AV, Soldi D, Muller EE, Cocchi D. Characterization of the resistance to the anorectic and endocrine effects of leptin in obesity-prone and obesity-resistant rats fed a high-fat diet. J Endocrinol 183: 289–298, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Vanheek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol 293: R992–R1002, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1003–R1012, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1037–R1045, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Wirth MM, Giraudo SQ. Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides 21: 1369–1375, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Wirth MM, Olszewski PK, Yu C, Levine AS, Giraudo SQ. Paraventricular hypothalamic alpha-melanocyte-stimulating hormone and MTII reduce feeding without causing aversive effects. Peptides 22: 129–134, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience 119: 365–375, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol 197: 309–317, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Yamanaka M, Itakura Y, Ono-Kishino M, Tsuchida A, Nakagawa T, Taiji M. Intermittent administration of brain-derived neurotrophic factor (BDNF) ameliorates glucose metabolism and prevents pancreatic exhaustion in diabetic mice. J Biosci Bioeng 105: 395–402, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Yamanaka M, Tsuchida A, Nakagawa T, Nonomura T, Ono-Kishino M, Sugaru E, Noguchi H, Taiji M. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes Metab 9: 59–64, 2007 [DOI] [PubMed] [Google Scholar]