Abstract
Low birth weight humans often exhibit hypertension during adulthood. Studying the offspring of rat dams fed a maternal low-protein diet is one model frequently used to study the mechanisms of low birth weight-related hypertension. It remains unclear whether this model replicates key clinical findings of hypertension and increased blood pressure responsiveness to stress or high-salt diet. We measured blood pressure via radiotelemetry in 13-wk-old male offspring of maternal normal- and low-protein dams. Neither group exhibited hypertension at baseline; however, 1 h of restraint was accompanied by a significantly greater blood pressure response in low-protein compared with normal-protein offspring. To enhance the effect of a high-salt diet on blood pressure, normal- and low-protein offspring underwent right uninephrectomy, while controls underwent sham surgery. After 5 weeks on a high-salt diet (4% NaCl), mean arterial pressure in the Low-Protein+Sham offspring was elevated by 6 ± 2 mmHg (P < 0.05 vs. baseline), while it remained unchanged in the normal-protein offspring. In the two uninephrectomized groups, blood pressure increased further, but was of similar magnitude. Glomerular filtration rate in the low-protein uninephrectomized offspring was 50% less than that in normal-protein offspring with intact kidneys. These data indicate that, while male low-protein offspring are not hypertensive during young adulthood, their blood pressure is hyperresponsive to restraint stress and is salt sensitive, and their glomerular filtration rate is more sensitive to hypertension-causing insults. Collectively, these may predispose for the development of hypertension later in life.
Keywords: birth weight, salt sensitivity, Barker Hypothesis, prenatal programming
epidemiological studies have indicated that there is an inverse relationship between birth weight and the risk for cardiovascular diseases, such as coronary artery disease, stroke, and hypertension later in life (7, 8, 20, 34). The hypothesis (i.e., Barker Hypothesis) is that nutrient deprivation during discrete periods of organ development prenatally programs the offspring for cardiovascular disease later in life (6, 7, 44). With regard to hypertension, several animal models have been developed with the purpose of generating low birth weight offspring to determine the underlying mechanisms that contribute/cause the ensuing hypertension (39). One of the most frequently used models is maternal protein restriction, wherein pregnant animals (most commonly rats) are fed a low-protein diet during gestation. The phenotype of the offspring are low birth weight and elevated blood pressure as early as 4 wk of age (27), which progressively increases with age (32). Thus, this animal model has been thought to mimic some of the elements that occur clinically in low birth weight humans.
Another characteristic of low birth weight individuals is a reduced nephron number. This has been reported in humans (21, 22) and is replicated in maternal low-protein animal models (18, 19, 39). A leading theory is that reduced nephron endowment leads to impaired sodium excretion, and salt and water retention, and may thereby play a causal role in hypertension (57). In support of this, small-scale clinical studies have recently shown that that blood pressure in low birth weight children and adults is salt sensitive (14, 47). Studies in maternal low-protein offspring have been less consistent with two showing no effect of a high-salt diet (25, 58) and one showing salt sensitivity of blood pressure (56). This is troubling, as numerous previous studies have utilized this animal model to study low birth weight-related hypertension it could indicate that either the sodium retention theory is incorrect or else that this model does not replicate a key component of (i.e., sodium retention) that appears to exist in low birth weight humans.
A major limitation of previous studies that have investigated the mechanisms of hypertension in maternal low-protein offspring is that the baseline blood pressure measurement is frequently assessed with the tail cuff method, an indirect method that requires restraint and is sensitive to the arousal state of the animal. This method may be a problem because low-protein offspring have been shown to have an elevated response to stressors, such as inhaled ammonia (49). Clearly, if the same were true in response to restraint stress, it would render indirect measures of arterial pressure highly inaccurate. Little or no increase in baseline arterial pressure has been detected in two previous studies that have assessed blood pressure with radiotelemetry in maternal low-protein offspring (16, 49). This should be verified, as should the response to restraint stress, to assist in the interpretation of numerous previous studies in maternal low-protein offspring that have reported (27, 41, 51, 56) and continue to report hypertension (3, 11). Furthermore, differing methods of blood pressure measure are also a potential reason for the equivocal results of the effect of high-salt diet in this model (25, 56, 58), and the use of radiotelemetry may help determine this issue.
To address these issues, 13-wk-old normal- or low maternal-protein offspring were instrumented with radiotelemetry to measure arterial pressure in freely moving rats in their home cage to address the following questions. Do the offspring of rat dams fed a low gestational-protein diet have detectable hypertension when blood pressure is measured by radiotelemetry? Do the offspring have an increased response to stress? Finally, does blood pressure increase during a high-salt diet and does a further reduction in nephron number (via uninephrectomy) evoke/augment salt-sensitive hypertension?
METHODS
Timed pregnant (4 days gestation) Wistar rats were obtained from Harlan (Indianapolis, IN). Once at our facility, the pregnant rats were housed individually under controlled conditions (21–23°C; 12:12-h light-dark cycle; lights on: 0700–1900, lights off: 1900–0700) with free access to water, and were placed on either a normal-protein diet (18% casein) or a low-protein diet (6% casein). The diets were isocaloric and contained a total of 0.5% methionine (base diet AIN-93, TestDiet; Purina Mills). Table 1 contains the diet composition. Food intake was weighed daily. In our preliminary studies, we found that the dams on a low-protein diet consumed more food than those on a normal-protein diet. To avoid the confounding effect of the dams on a low-protein diet increasing their protein intake via increased food intake, we pair-fed the dams on a low-protein diet to match the food intake to that of the normal-protein dams. Thus, the dams on a low-protein diet were given an equivalent mass of food that the dams on a normal-protein diet consumed the previous day. Dams were kept on their respective diet throughout gestation. Litter size and birth weight were recorded within 12 h of birth, and all litters were culled to eight pups to allow equal access to milk. All dams were maintained on a normal-protein diet (18% protein) from this point forward. Because in our preliminary studies, the blood pressure in female low-protein offspring appeared to be less responsive to a high-salt diet, and also because large differences in susceptibility to fetal programming between males and females have been observed by others (9, 17), we focused our studies on male offspring. The male offspring used for this study came from 11 different litters (5 normal protein and 6 low protein). Pups were weaned at 3 wk of age, sex was recorded, and they were maintained on the standard diet until adulthood. The rats were cared for in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were reviewed and approved by our Institutional Animal Investigation Committee.
Table 1.
Diet composition
| Diet |
||
|---|---|---|
| Normal Protein | Low Protein | |
| Caesin, % | 18.0 | 6.0 |
| Soybean oil, % | 7.1 | 7.1 |
| Corn starch, % | 51.1 | 63.7 |
| Maltodextrin, % | 13.2 | 13.2 |
| Cellulose, % | 3.7 | 5.1 |
| dl-Methionine, % | 0.5 | 0.5 |
| NaCl, % | 0.45 | 0.45 |
Surgical Preparation
Radiotransmitter implantation.
Eleven-week-old male offspring of dams fed either a normal-protein diet or low-protein diet during gestation were anesthetized with isoflurane, and a radiotransmitter (TA11PA-C40; Data Sciences International) was implanted to measure arterial blood pressure, as described previously (5, 31). Briefly, the left femoral artery was exposed, and the proximal end temporarily occluded; the gel-filled catheter attached to the transmitter device was inserted and advanced into the abdominal aorta so that the tip of the catheter remained below the renal arteries. The catheter was secured in place with two ligatures, and the transmitter was placed subcutaneously and adhered to the underlying muscle with tissue adhesive. The skin was closed with sutures.
Uninephrectomy.
Some rats underwent a right uninephrectomy (Normal Protein+Uninephrectomy, n = 6; Low Protein+Uninephrectomy, n = 6) or sham (Normal Protein+Sham, n = 6; Low Protein+Sham, n = 9). A right flank incision was made under isoflurane anesthesia; the renal artery, vein, and the ureter were ligated, and the right kidney was excised. The muscle was closed in layers, and the skin was closed with suture. At the end of both surgeries, each rat received butorphanol tartrate (0.075 mg sc) for analgesia.
Renal clearance experiments.
Some rats were anesthetized with thiobutabarbital sodium (100 mg/kg) (Normal Protein+Sham, n = 6; Low Protein+Sham, n = 6; Normal Protein+Uninephrectomy, n = 4; Low Protein+Uninephrectomy, n = 6). A 23-gauge polyethylene catheter was inserted into the left carotid artery for blood pressure monitoring and blood sampling and into the right jugular vein for infusion. To determine glomerular filtration rate, a bolus of FITC-inulin (Sigma-Aldrich) mixed in 0.9% saline was infused followed by continuous infusion at 3 ml/h to maintain a stable plasma inulin concentration and to account for fluid loss. The right and left ureters were cannulated with 27-gauge polyethylene catheters for urine sampling from each kidney. After a 30-min stabilization period, urine was collected for two consecutive 30-min time periods, and a arterial blood sample (0.2 ml) was collected from the carotid arterial catheter at the midpoint of each collection to assess plasma inulin. Rats were then euthanized with an overdose of pentobarbital (200 mg/kg iv). The kidneys were collected and weighed.
Experimental Protocol
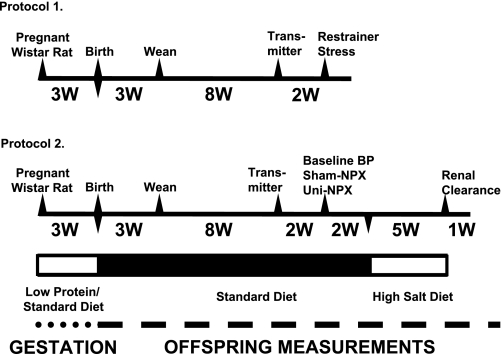
A time line of procedures, measurements, and experiments for protocols 1 and 2 is displayed in Fig. 1.
Fig. 1.
Time line for protocols 1 and 2. Transmitter refers to implant of radiotransmitter. High-salt diet was 4% NaCl. W, week(s); BP, blood pressure; Uni-NPX, uninephrectomy.
Protocol 1.
Approximately 10 days after the radiotransmitter was implanted, the recording of baseline blood pressure and heart rate was initiated. Data was sampled for a 10-s duration every 5 min. Sampling occurred for four consecutive days to compare baseline values between normal-protein and low-protein offspring. At 13 wk of age, we tested the acute stress response to cage restraint. On the morning of the experiment, at ∼0900, baseline blood pressure and heart rate were recorded continuously for 30 min while the rat moved freely in its home cage. Each rat was placed into a Plexiglas chamber (length, 21 cm; width, 7 cm; height, 6.5 cm), which was then placed back in the home cage. Hemodynamics were recorded continuously for 1 h, and then rats were removed from the chamber and placed back in their home cage. The rats used in this protocol were then used for unrelated studies.
Protocol 2.
Approximately 10 days after the radiotransmitter was implanted, and blood pressure and heart rate were recorded for 2–4 consecutive days. At 13 wk of age, the rats then underwent either a right uninephrectomy or sham surgery. At this point, there were four experimental groups: Normal Protein, Normal Protein+Uninephrectomy, Low Protein, and Low Protein+Uninephrectomy. Two weeks were allowed to determine whether the removal of one kidney impacted arterial blood pressure. At 15 wk of age, all rats were placed on a high-salt diet (4% NaCl, base diet AIN-93; TestDiet, Purina Mills) for 5 wk to determine the salt sensitivity of blood pressure. Rats were then maintained on the high-salt diet, and renal clearance experiments were performed the following week.
Analytical Measurements
Plasma and urinary inulin concentrations were determined using a spectraflurometer. Plasma and urinary sodium concentrations were determined with flame photometry.
Data and Statistical Analysis.
Baseline hemodynamics and restrainer stress experiments (protocol 1).
The data collected over four consecutive days for baseline blood pressure and heart rate were averaged every 12 h to give average daytime (0700–1900) and nighttime values (1900–0700). For the restrainer experiments, the last 10 min of the 30-min baseline recording was averaged to give a single value for mean arterial pressure and heart rate. The hemodynamic data collected while the animals were in the restrainer was averaged in 4-min bins, and then the change from baseline was calculated for each time point. Differences between the normal-protein and low-protein offspring were analyzed using a two-way ANOVA for repeated measures. When appropriate, a Student-Newman-Keuls post hoc test was performed.
Salt sensitivity and renal clearance experiments (protocol 2).
Mean arterial blood pressure values for baseline, following nephrectomy (or sham) and for each time point on high-salt diet were 24-h averages of the last day for each given time period. Salt sensitivity was defined as a statistically significant increase in mean arterial pressure compared with the baseline measurement on a standard diet. Glomerular filtration rate and fractional excretion of sodium were calculated using standard formulas. A one-way ANOVA was used to compare baseline parameters. Time course data were compared using a two-way ANOVA for repeated measures. When appropriate, a Student-Newman-Keuls post hoc test was performed. All data are presented as means ± SE. A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of Maternal Protein Restriction on Litter Size, Birth Weight, and Growth in Offspring
The number of pups per litter tended to be less in the low-protein (13 ± 1 pups) compared with the normal-protein offspring (16 ± 1 pups), although this did not reach statistical significance (P = 0.057, two tailed). Birth weights were reduced by 15% in the low-protein offspring (5.13 ± 0.05 and 5.92 ± 0.06 g, low protein vs. normal protein, respectively, P < 0.001). This trend of a significantly low body weight in the low-protein offspring gradually decreased, and by the end of the study, the body weights were no longer different (normal protein, 0.58 ± 0.02 kg vs. low protein, 0.54 ± 0.02 kg).
Effect of Maternal Protein Restriction on Baseline Blood Pressure and Response to Restrainer Stress in Offspring
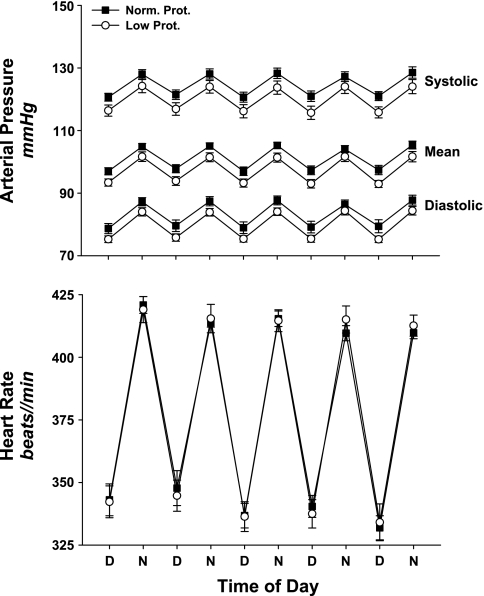
Baseline arterial blood pressure and heart rate, recorded via radiotelemetry over four consecutive days are displayed in Fig. 2. Systolic, mean, and diastolic arterial pressure were not different when low-protein and normal-protein offspring were compared (systolic, P = 0.092; diastolic, P = 0.095) (Fig. 2). Thus, the offspring of maternal low-protein diet dams did not develop hypertension during the time frame studied. Baseline heart rates were virtually identical (P = 0.970).
Fig. 2.
Baseline systolic, mean, and diastolic arterial pressure (top) and heart rate (bottom) measured with radiotelemetry in normal-protein (Norm. Prot.; ■, n = 11) and low-protein (Low Prot.; ○, n = 11) offspring. Data were collected over 4 consecutive days and are averaged every 12 h to display the daytime (D) and nighttime (N) variation in arterial pressure and heart rate. No significant differences were detected.
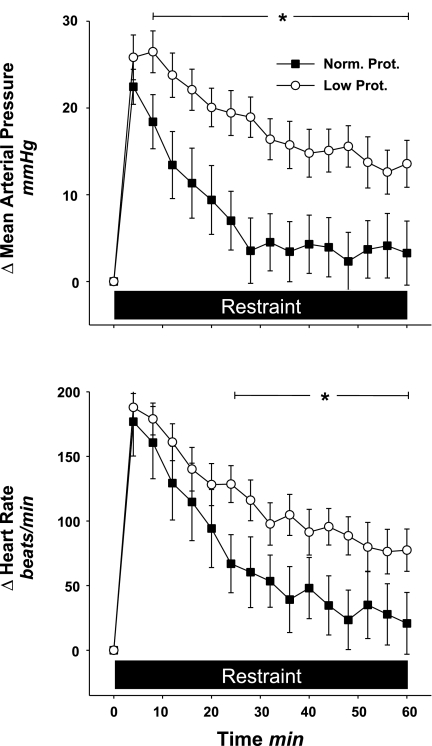
The change in mean arterial pressure and heart rate from baseline during 1 h of cage restraint for both groups is displayed in Fig. 3. In the normal-protein offspring, restraint caused an initial increase in mean arterial pressure and heart rate that then returned toward baseline during approximately the last 30 min. While the initial change in mean arterial pressure in the low-protein offspring was similar to that seen in the normal-protein offspring, the return toward baseline was significantly blunted and remained elevated by ∼15 mmHg above basal level throughout the restraint period. The heart rate response to restraint in the low-protein offspring followed a similar pattern, and was significantly different from that seen in the normal-protein offspring. ANOVA detected a statistically significant interaction (diet × time) for mean arterial pressure and heart rate (P < 0.001 for both).
Fig. 3.
Change in mean arterial pressure and heart rate from baseline during 1 h of restraint stress in normal-protein (n = 6) and low-protein (n = 11) offspring. Note that the change in mean arterial pressure and heart rate in the low-protein offspring was significantly higher compared with the normal-protein offspring. Black bar, time period of restraint stress. *P < 0.05 vs. Norm. Prot.
Effect of Maternal Protein Restriction on Sensitivity of Blood Pressure to Salt in Offspring
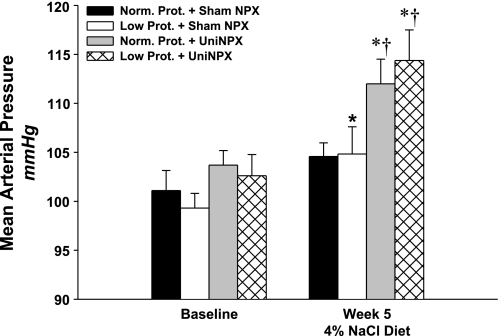
Mean arterial pressure at baseline, 2 wk following uninephrectomy or sham surgery and during five consecutive weeks on a high-salt diet are shown in Table 2. Baseline pressures were not different between the four groups, and the surgery had no measurable effect. Within the normal-protein group, the high-salt diet had no significant effect on mean arterial pressure (P = 0.404). However, within the low-protein offspring, the high-salt diet significantly increased mean arterial pressure by 6 ± 2 mmHg (P < 0.05, week 5 vs. baseline). The effect of the high-salt diet had an even greater impact on both uninephrectomized groups, as mean arterial pressure increased similarly and progressively in each. The magnitude of the increase when comparing the 5th week of high-salt diet vs. baseline was 8 ± 3 mmHg in the Normal-Protein+Uninephrectomized offspring and 12 ± 4 mmHg in the Low-Protein+Uninephrectomized offspring (P < 0.05 for both, Fig. 4). During weeks 2–5 on high-salt, the increase in mean arterial pressure in both uninephrectomized groups was significantly greater than both the normal-protein and low-protein offspring that had two intact kidneys.
Table 2.
Mean arterial pressure at baseline, 2 wk postuninephrectomy (Post-Uni-NPX; or sham), and during 5 wk on a 4% NaCl diet in male offspring of dams fed either a normal (18%) or low (6%) protein diet during gestation
| Sham |
Uninephrectomy |
|||
|---|---|---|---|---|
| Normal Maternal Protein | Low Maternal Protein | Normal Maternal Protein | Low Maternal Protein | |
| n | 6 | 9 | 6 | 6 |
| Baseline | 101 ± 2 | 99 ± 2 | 104 ± 1 | 103 ± 2 |
| 2 wk Post-Uni-NPX (or sham) | 102 ± 1 | 100 ± 2 | 105 ± 1 | 104 ± 1 |
| 4% NaCl Diet | ||||
| Week 1 | 101 ± 2 | 100 ± 2 | 107 ± 1 | 108 ± 1*† |
| Week 2 | 102 ± 1 | 101 ± 2 | 109 ± 2*† | 108 ± 2*† |
| Week 3 | 103 ± 2 | 103 ± 2* | 109 ± 2*† | 110 ± 2*† |
| Week 4 | 104 ± 2 | 104 ± 2* | 109 ± 3*† | 112 ± 2*† |
| Week 5 | 105 ± 1 | 105 ± 3* | 112 ± 3*† | 114 ± 3*† |
Values are means ± SE.
P < 0.05 vs. Baseline;
P < 0.05 vs. normal and low maternal protein.
Fig. 4.
Mean arterial pressure at baseline and then during the 5th week on high-salt (4% NaCl diet) in normal protein+sham nephrectomy (Norm. Prot.+Sham NPX; black bar, n = 6); low protein+sham nephrectomy (Low Prot.+Sham NPX; white bar, n = 9); normal protein+uninephrectomy (Norm. Prot.+Uni-NPX, gray bar, n = 6); and low protein+uninephrectomy, (Low Prot.+Uni-NPX, hatched bar, n = 6). Note that mean arterial pressure in the Low Prot. group was salt sensitive and that uninephrectomy exacerbated salt sensitivity in both uninephrectomized groups to a similar degree. *P < 0.05 vs. baseline; †P < 0.05 vs. both Norm. and Low Prot.
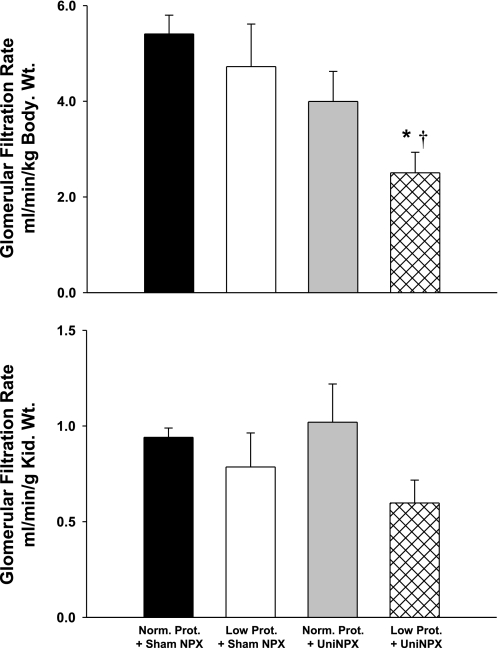
Figure 5 contains glomerular filtration rate normalized to either body weight or kidney weight for all four groups. When normalized to body weight, glomerular filtration rate in the uninephrectomized, low-protein offspring was ∼50% lower than that seen in either group with intact kidneys (P < 0.05). When the glomerular filtration rate was normalized to kidney weight, although it tended to be lower in the uninephrectomized, low-protein offspring, no significant difference was detected. The left kidney weight and left kidney weight-to-body weight ratios were similar between the normal and low-protein offspring (Table 3). However, within the uninephrectomized groups, as expected, the left kidney weights and the left kidney weight-to-body weight ratios were significantly higher compared with the groups with both native kidneys. There were no significant differences between plasma sodium, sodium excretion, and fractional excretion of sodium across any of the four groups (Table 3).
Fig. 5.
Glomerular filtration rate normalized to body weight (top) and kidney (Kid.) weight (bottom) following 5 wk of high-salt diet in Norm. Prot.+Sham (black bar, n = 6), Low Prot.+Sham (white bar, n = 6), Norm. Prot.+Uni-NPX (gray bar, n = 4), and Low Prot.+Uni-NPX (hatched bar, n = 6). Note that glomerular filtration rate in the Low Prot.+Uni-NPX group was ∼50% lower than that seen in the other groups. *P < 0.05 vs. Norm. Prot.+Sham NPX; †P < 0.05 vs. Low Prot.+Sham NPX.
Table 3.
Body and total kidney weights, plasma Na (PNa), Na excretion (UNaV), and fractional excretion of Na in 20-wk-old offspring of dams fed either a normal (18% protein)- or low (6% protein)-protein diet during gestation
| Sham |
Uninephrectomy |
|||
|---|---|---|---|---|
| Normal Maternal Protein | Low Maternal Protein | Normal Maternal Protein | Low Maternal Protein | |
| n | 6 | 6 | 4 | 6 |
| Body weight, kg | 0.58 ± 0.02 | 0.54 ± 0.02 | 0.58 ± 0.01 | 0.53 ± 0.02 |
| Left kidney weight, g | 1.66 ± 0.04 | 1.64 ± 0.07 | 2.31 ± 0.08* | 2.34 ± 0.19* |
| Left kidney weight-to-body weight ratio, % | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.40 ± 0.02* | 0.44 ± 0.04* |
| PNa, mM | 144 ± 1 | 143 ± 1 | 145 ± 1 | 142 ± 1 |
| UNaV, μmol·min−1·kg body wt−1 | 0.99 ± 0.15 | 0.87 ± 0.15 | 4.40 ± 2.66 | 1.53 ± 0.26 |
| UNaV, μmol·min−1·g kidney wt−1 | 0.17 ± 0.03 | 0.13 ± 0.03 | 1.19 ± 0.75 | 0.35 ± 0.06 |
| Fractional excretion of Na, % | 0.21 ± 0.03 | 0.25 ± 0.05 | 0.37 ± 0.19 | 0.28 ± 0.07 |
Values are means ± SE.
P < 0.05 vs. sham.
DISCUSSION
Fetal programming of hypertension, which is based on the notion that some adverse event occurs in utero that leads to low birth weight and an increased likelihood of developing hypertension as an adult, is supported by abundant epidemiological evidence (8, 13, 20, 34, 37). Determining the mechanisms of hypertension that may result from fetal programming is critical in reducing the incidence of future hypertension, as the US infant mortality rate, which is an index of overall fetal health, ranks a dismal 30th in the world (30). The maternal low-protein rat model has been frequently used to study such mechanisms in the offspring. Contrary to a large body of literature, we have confirmed two previous studies that demonstrate that adult male offspring of maternal low-protein dams do not have detectable hypertension when blood pressure is assessed with radiotelemtry (16, 49). The major new findings of this study are that the low-protein offspring 1) have an increased response to restraint stress, 2) have mild salt-sensitive hypertension, and 3) have a glomerular filtration rate 50% lower in the uninephrectomized, low-protein offspring on high-salt diet compared with all other groups.
The maternal low-protein model has been used for > 15 yr to study fetal programming of hypertension. Blood pressure in low-protein offspring is reported to be 20–30 mmHg higher than that in control animals (24, 26, 41, 46, 51). However, when blood pressure has been assessed via radiotelemetry, no hypertension has been detected in low-protein offspring (16, 49). Our data confirm and extend these findings with longer radiotelemetric tracings of baseline blood pressure, which allowed us to determine diurnal variation. We found that systolic, mean, and diastolic arterial pressure, as well as day-night changes in blood pressure in the low-protein offspring, were no different than that observed in the normal-protein offspring. Thus, male offspring of maternal low-protein dams are not hypertensive, at least not at ∼3–4 mo of age. These findings are similar to those observed in another common low birth weight model (prenatal administration of the glucocorticoid dexamethasone) in which the offspring were previously thought to be hypertensive until baseline blood pressure was accurately determined via radiotelemetry and they were actually found to be hypotensive relative to control offspring (38). The lack of hypertension in these low birth weight models when blood pressure is assessed with radiotelemetry suggests some underlying difference either in control of blood pressure or in the methods of blood pressure measurement that have been utilized or both.
Aside from the two studies mentioned above that utilized radiotelemetry to measure blood pressure (16, 49), all previous studies we have found that reported hypertension in low-protein offspring assessed blood pressure either indirectly via tail cuff (11, 26, 27, 51, 58) or via indwelling catheters (3, 4, 10, 41, 56). The tail cuff method requires restraint and heating, and both methods require handling of the animals. The major strength of using radiotelemetry is that blood pressure can be determined continuously in conscious, freely moving animals in their home cage, without the necessity of handling (50). Clearly, there is the potential to influence baseline blood pressure if an experimental group of animals is hyperresponsive to stresses such as handling or restraint, compared with control. Indeed, we found that when male low-protein offspring were placed in a restrainer, their mean arterial pressure and heart rate remained significantly elevated throughout the restraint period, while that in the normal-protein offspring returned to baseline. Low-protein offspring have also been shown to have elevated mean arterial pressure response to olfactory stress with ammonia (49). Furthermore, there appears to be a generalized affect of fetal programming response to acute stress as low birth weight rats in which the dams were fed a prenatal high-salt diet also have an elevated blood pressure and heart rate response to restraint stress (42). Similarly, low birth weight humans have elevated blood pressure and urinary catecholamine response to mental stress (23, 43). Although our study was not designed to determine the mechanisms of the elevated stress response, several studies indicate a role for sympathoadrenal over activity in low birth weight offspring (2, 12, 23, 40), and more specifically, neurons in regions of the brain involved in stress are over active in low-protein offspring (45). Taken together, the differential responses to stress between low-protein and normal-protein offspring make the use of tail cuff plethysmography both inappropriate and inadequate to accurately determine differences in resting blood pressure. With this in mind, the strengths of using telemetry are clear (50), but it is important to consider that resting blood pressure measured with telemetry in a rodent's home cage under highly regulated conditions may not be the best representation of resting blood pressure in humans living in the real world. Thus, despite the lack of increased resting blood pressure, chronic increased responsiveness to stress in low birth weight offspring is an important finding, as it may contribute to stress-related spikes in blood pressure that, over time, could contribute to end-organ damage (28).
Despite there being no evidence of hypertension in our low-protein offspring, we wanted to test salt sensitivity of blood pressure in our rats. Following 5 weeks of the high-salt diet, blood pressure values between the normal- and low-protein offspring with native kidneys were nearly identical. However, because the baseline blood pressure in the low-protein group tended to be less compared with the normal-protein group, we detected a statistically significant increase of 6 mmHg in the low-protein offspring. While the increase in blood pressure is much smaller than is typically reported in genetic models of salt sensitivity (36), it is similar to that which is reported clinically in response to a high-salt diet (48). Thus, even mild salt sensitivity of blood pressure is an important finding that is likely attributed to an effect of prenatal programming, considering that these studies were performed in Wistar rats, a strain with a genetic background that is not known to have sensitivity of blood pressure to salt. Further studies to pursue whether low-protein offspring of dams genetically predisposed to salt sensitivity may be more profoundly affected are warranted. Two previous studies in low-protein offspring did not detect exacerbation of salt sensitivity of blood pressure in low-protein offspring compared with control rats (25, 58); however, blood pressure was measured with the tail cuff method. Our results are generally in line with those of Woods et al. (56), although the magnitude of the rise in blood pressure in low-protein offspring during high-salt diet in their study was greater (∼15 mmHg increase) than what we observed. Differing methods of blood pressure measure (indwelling catheter vs. radiotelemetry) may account for these differences. Consistent with our studies in the low maternal protein-low birth weight model, two recent clinical studies (one in children and one in adults) associated birth weight with salt sensitivity (14, 47).
Impaired sodium excretion resulting from glomerulogenesis retardation (53) and reduced nephron endowment, which has been shown to occur in both low birth weight animals (18, 19, 39) and humans (21, 22), is hypothesized to play a causal role in low birth weight-related hypertension (57). Nephron number was not assessed in our study and the final left kidney weights were not different between low-protein and normal-protein rats with both native kidneys, which could suggest that there was no difference in nephron number. However, this coincides with data from McMullen and Langley-Evans (35), who used a similar maternal protein diet (9% protein) and the same strain of rat, and found that 20-wk-old male low-protein offspring had kidney weights that were similar to control but had ∼30% fewer nephrons. Other studies have reported reduced nephron endowment in low-protein offspring as well, although they did find that kidney weights were lower in low-protein offspring (51, 55). Since fewer nephrons are available to filter the same amount of plasma, hyperfiltration would be expected to occur, but is apparently not enough to compensate, as our data and that of Woods et al. (56) show a reduced glomerular filtration rate in low-protein offspring. However, we did not find any differences in either sodium excretion or fractional excretion of sodium. Furthermore, a 50% reduction in nephron number with uninephrectomy plus a high-salt diet evoked roughly equivalent increases in blood pressure and again, no difference in sodium excretion or fractional excretion of sodium. The sodium excretion and fractional excretion of sodium values in the uninephrectomized rats actually tended to be higher compared with control, although there was a high amount of variability for unknown reasons. If impaired sodium excretion indeed plays a causal role in low birth weight-related hypertension, then removal of one kidney plus a high-salt diet should have exacerbated blood pressure in the low-protein offspring. A recent study found that 4-wk-old male, maternal low-protein rats actually have increased, not decreased, sodium excretion (3). Thus, our data in concordance with that of Alwasel and Ashton (3), do not support the hypothesis that low birth weight-related hypertension in maternal low-protein offspring is caused by reduced sodium excretion resulting from reduced nephron endowment.
However, although the effect of high-salt on blood pressure was not significantly different between normal- and low-protein offspring that were uninephrectomized, glomerular filtration rate when normalized to body weight in the uninephrectomized, low-protein group was significantly lower compared with all other groups. We believe this indicates that a single insult, such as maternal low protein, can be compensated for, as evidenced by lack of hypertension in low-protein offspring. A recent study in the same low maternal protein model demonstrated that chronic infusion of advanced glycation end products, led to increased expression of several early molecular markers of renal injury, which did not occur in normal-protein offspring when infused with the same compound (59). Furthermore, the kidneys of low-protein offspring show increased gene and protein expression for markers of senescence (29, 33). Finally, catch-up growth, which is known to occur in low birth weight offspring (and did occur in our offspring, as the low-protein offspring birth weights were less but adult body weights were not different from that in the normal-protein offspring) may predispose for renal dysfunction (9) and hypertension (1). Thus, as the number of insults mount (i.e., uninephrectomy, high dietary sodium, increase in advanced glycation end products with aging or just aging per se, postnatal overfeeding, etc.) the ability of the kidneys to further compensate may become limited and manifest as reduced renal function and hypertension.
Perspectives and Significance
Although 3- to 4-mo-old male offspring of low-protein dams had low birth weight, they do not have hypertension when blood pressure is measured via radiotelemetry. However, low-protein offspring do have an increased response to restraint stress and mild salt sensitivity of blood pressure. Whether or not the long-term effects of these predispose for the development of hypertension in older animals remains to be determined, but salt sensitivity of blood pressure in known to increase with age (54). The fact that glomerular filtration rate in the uninephrectomized low-protein offspring on a high-salt diet was only 50% of that seen in control rats, provides further evidence that the kidneys of low-protein offspring are more vulnerable to hypertension-causing insults. This provides basic science evidence supporting not only the relationship between low birth weight and hypertension but also that between low birth weight and kidney disease (15, 52).
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant HL-079102 and a Department of Veterans Affairs Merit Award (to N. F. Rossi).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 41: 451–456, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Alwasel SH, Ashton N. Prenatal programming of renal sodium handling in the rat. Clin Sci (Lond) 117: 75–84, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Ashton N, Al-Wasil SH, Bond H, Berry JL, Denton J, Freemont AJ. The effect of a low-protein diet in pregnancy on offspring renal calcium handling. Am J Physiol Regul Integr Comp Physiol 293: R759–R765, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Augustyniak RA, Picken MM, Leonard D, Zhou XJ, Zhang W, Victor RG. Sympathetic nerves and the progression of chronic kidney disease during 5/6 nephrectomy: studies in sympathectomized rats. Clin Exp Pharmacol Physiol 37: 12–18 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ. The origins of the developmental origins theory. J Intern Med 261: 412–417, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1: 1077–1081, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ 297: 134–135, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boubred F, Daniel L, Buffat C, Feuerstein JM, Tsimaratos M, Oliver C, Dignat-George F, Lelievre-Pegorier M, Simeoni U. Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. Am J Physiol Renal Physiol 297: F943–F951, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Le NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol 292: R1236–R1245, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dagan A, Habib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low-protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol 297: R93–R99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol 295: F29–F34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies AA, Smith GD, May MT, Ben-Shlomo Y. Association between birth weight and blood pressure is robust, amplifies with age, and may be underestimated. Hypertension 48: 431–436, 2006 [DOI] [PubMed] [Google Scholar]
- 14.de Boer MP, Ijzerman RG, de Jongh RT, Eringa EC, Stehouwer CD, Smulders YM, Serne EH. Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension 51: 928–932, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Fan ZJ, Lackland DT, Lipsitz SR, Nicholas JS. The association of low birthweight and chronic renal failure among Medicaid young adults with diabetes and/or hypertension. Public Health Rep 121: 239–244, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Twinn DS, Ekizoglou S, Wayman A, Petry CJ, Ozanne SE. Maternal low-protein diet programs cardiac β-adrenergic response and signaling in 3-mo-old male offspring. Am J Physiol Regul Integr Comp Physiol 291: R429–R436, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 5, Suppl A: S121–S132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoppe CC, Evans RG, Bertram JF, Moritz KM. Effects of dietary protein restriction on nephron number in the mouse. Am J Physiol Regul Integr Comp Physiol 292: R1768–R1774, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hoppe CC, Evans RG, Moritz KM, Cullen-McEwen LA, Fitzgerald SM, Dowling J, Bertram JF. Combined prenatal and postnatal protein restriction influences adult kidney structure, function, and arterial pressure. Am J Physiol Regul Integr Comp Physiol 292: R462–R469, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hovi P, Andersson S, Raikkonen K, Strang-Karlsson S, Jarvenpaa AL, Eriksson JG, Pesonen AK, Heinonen K, Pyhala R, Kajantie E. Ambulatory blood pressure in young adults with very low birth weight. J Pediatr 156: 54.e1–59.e1, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hughson MD, Gobe GC, Hoy WE, Manning RD, Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpee M. Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J Intern Med 261: 480–487, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol 110: 223–228, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Langley-Evans SC, Jackson AA. Rats with hypertension induced by in utero exposure to maternal low-protein diets fail to increase blood pressure in response to a high salt intake. Ann Nutr Metab 40: 1–9, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 91: 607–615, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Luyckx VA, Compston CA, Simmen T, Mueller TF. Accelerated senescence in kidneys of low-birth-weight rats after catch-up growth. Am J Physiol Renal Physiol 297: F1697–F1705, 2009 [DOI] [PubMed] [Google Scholar]
- 30.MacDorman MF, Mathews TJ. Behind international rankings of infant mortality: how the United States compares with Europe. NCHS Data Brief 23: 1–8, 2009 [PubMed] [Google Scholar]
- 31.Maliszewska-Scislo M, Chen H, Augustyniak RA, Seth D, Rossi NF. Subfornical organ differentially modulates baroreflex function in normotensive and two-kidney, one-clip hypertensive rats. Am J Physiol Regul Integr Comp Physiol 295: R741–R750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol 16: 417–422, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Martin-Gronert MS, Tarry-Adkins JL, Cripps RL, Chen JH, Ozanne SE. Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am J Physiol Regul Integr Comp Physiol 294: R494–R500, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Martyn CN, Barker DJ. Reduced fetal growth increases risk of cardiovascular disease. Health Rep 6: 45–53, 1994 [PubMed] [Google Scholar]
- 35.McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension 46: 1374–1380, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M. Alterations of circadian expressions of clock genes in Dahl salt-sensitive rats fed a high-salt diet. Hypertension 42: 189–194, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Mzayek F, Hassig S, Sherwin R, Hughes J, Chen W, Srinivasan S, Berenson G. The association of birth weight with developmental trends in blood pressure from childhood through mid-adulthood: the Bogalusa Heart study. Am J Epidemiol 166: 413–420, 2007 [DOI] [PubMed] [Google Scholar]
- 38.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. J Endocrinol 196: 343–352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojeda NB, Grigore D, Alexander BT. Developmental programming of hypertension: insight from animal models of nutritional manipulation. Hypertension 52: 44–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol 34: 1212–1216, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res 55: 1042–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Porter JP, King SH, Honeycutt AD. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol 293: R334–R342, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Pyhala R, Raikkonen K, Feldt K, Andersson S, Hovi P, Eriksson JG, Jarvenpaa AL, Kajantie E. Blood pressure responses to psychosocial stress in young adults with very low birth weight: Helsinki study of very low birth weight adults. Pediatrics 123: 731–734, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 315: 396–400, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosene DL, Lister JP, Schwagerl AL, Tonkiss J, McCormick CM, Galler JR. Prenatal protein malnutrition in rats alters the c-Fos response of neurons in the anterior cingulate and medial prefrontal region to behavioral stress. Nutr Neurosci 7: 281–289, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 98: 269–275, 2000 [PubMed] [Google Scholar]
- 47.Simonetti GD, Raio L, Surbek D, Nelle M, Frey FJ, Mohaupt MG. Salt sensitivity of children with low birth weight. Hypertension 52: 625–630, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Skrabal F, Herholz H, Neumayr M, Hamberger L, Ledochowski M, Sporer H, Hortnagl H, Schwarz S, Schonitzer D. Salt sensitivity in humans is linked to enhanced sympathetic responsiveness and to enhanced proximal tubular reabsorption. Hypertension 6: 152–158, 1984 [PubMed] [Google Scholar]
- 49.Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension 32: 108–114, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Van Vliet BN, Chafe LL, Antic V, Schnyder-Candrian S, Montani JP. Direct and indirect methods used to study arterial blood pressure. J Pharmacol Toxicol Methods 44: 361–373, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19: 151–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villar-Martini VC, Carvalho JJ, Neves MF, Aguila MB, Mandarim-de-Lacerda CA. Hypertension and kidney alterations in rat offspring from low protein pregnancies. J Hypertens 27, Suppl 6: S47–S51, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension 18: 67–71, 1991 [DOI] [PubMed] [Google Scholar]
- 55.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int 74: 187–195, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension 47: 502–508, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Zimanyi MA, Bertram JF, Black MJ. Does a nephron deficit in rats predispose to salt-sensitive hypertension? Kidney Blood Press Res 27: 239–247, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Zimanyi MA, Denton KM, Forbes JM, Thallas-Bonke V, Thomas MC, Poon F, Black MJ. A developmental nephron deficit in rats is associated with increased susceptibility to a secondary renal injury due to advanced glycation end-products. Diabetologia 49: 801–810, 2006 [DOI] [PubMed] [Google Scholar]