Abstract
Obstructive sleep apnea (OSA) is associated with increased sympathetic nerve activity, endothelial dysfunction, and premature cardiovascular disease. To determine whether hypoxia is associated with impaired skeletal muscle vasodilation, we compared femoral artery blood flow (ultrasound) and muscle sympathetic nerve activity (peroneal microneurography) during exposure to acute systemic hypoxia (fraction of inspired oxygen 0.1) in awake patients with OSA (n = 10) and controls (n = 8). To assess the role of elevated sympathetic nerve activity, in a separate group of patients with OSA (n = 10) and controls (n = 10) we measured brachial artery blood flow during hypoxia before and after regional α-adrenergic block with phentolamine. Despite elevated sympathetic activity, in OSA the vascular responses to hypoxia in the leg did not differ significantly from those in controls [P = not significant (NS)]. Following regional phentolamine, in both groups the hypoxia-induced increase in brachial blood flow was markedly enhanced (OSA pre vs. post, 84 ± 13 vs. 201 ± 34 ml/min, P < 0.002; controls pre vs. post 62 ± 8 vs. 140 ± 26 ml/min, P < 0.01). At end hypoxia after phentolamine, the increase of brachial blood flow above baseline was similar (OSA vs. controls +61 ± 16 vs. +48 ± 6%; P = NS). We conclude that despite high sympathetic vasoconstrictor tone and prominent sympathetic responses to acute hypoxia, hypoxia-induced limb vasodilation is preserved in OSA.
Keywords: vascular function, sleep-disordered breathing, sympathetic nervous system
obstructive sleep apnea (OSA) is highly prevalent and is associated with increased cardiovascular risk. While the mechanisms of cardiovascular complications in OSA remain unclear, vascular dysfunction is thought to play an important role (12, 15, 33). Potential causes of vascular dysfunction in OSA include heightened sympathetic vasoconstrictor tone (5, 25, 44), reduced bioavailability of the endothelium-derived vasodilator nitric oxide (NO) (14, 42), elevated levels of endothelin (37), mediators of inflammation (43), prothrombotic factors (4, 9), and oxidative stress (21).
Surprisingly few investigations have examined the vascular responses to systemic hypoxia in humans with OSA, a disorder characterized by intermittent nocturnal hypoxia. In healthy humans, acute systemic hypoxia leads to skeletal muscle vasodilation despite an increase in sympathetic nerve activity (24). However, it has been reported that unlike controls, awake and regularly breathing patients with OSA exhibit a pressor response to brief bouts of isocapnic hypoxia (11). Furthermore, it has been reported that vasodilation evoked by exposure to hypoxia is attenuated in some patients with OSA (40). More recently, it was demonstrated that the sympathetic neural responses to systemic hypoxia are enhanced in OSA compared with controls and normalize in part following treatment with continuous positive-airway pressure therapy (13). Collectively, these findings suggest that skeletal muscle vasodilator capacity in response to acute hypoxia may be impaired in OSA possibly due to high sympathetic tone.
In this report we examined the vascular responses to acute hypoxia in the limb circulation in awake patients with OSA. In view of the known sympathetic (13) and vascular dysfunction (7, 12, 15, 33), we hypothesized that hypoxia-induced skeletal muscle vasodilation is impaired in OSA and is normalized by regional α-adrenergic block with phentolamine.
METHODS
Subjects
Twenty patients with newly diagnosed and untreated OSA and 15 overweight but otherwise healthy control subjects participated in two experimental protocols. Six of the OSA patients and four of the controls participated in a prior study on sympathetic neural control during hypoxia (13). Subject characteristics are shown in Table 1. Before testing, all participants underwent a history and physical examination and completed a standard full-night polysomnogram, and in female subjects pregnancy was excluded. None was a smoker. OSA patients were recruited through the Sleep Disorders Clinic of the Hershey Medical Center. An apnea-hypopnea index ≥ 20 events/h and daytime hypersomnolence were required for inclusion in the study. Control subjects were recruited locally and OSA was excluded via polysomogram (apnea-hypopnea index < 5 events/h). Subjects avoided caffeine, alcohol, and exercise for 24 h before testing. Two patients and one control were on a beta-blocker, three OSA patients and three controls were on an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, and one OSA patient and one control were on a calcium antagonist. Because of safety concerns, these medications were not withheld before the study. Three OSA patients and one control subject were on a diuretic that was held on the morning of the study. Of the seven women who participated in our study, three were postmenopausal, two others had previously undergone hysterectomy, and the only two menstruating women took part in protocol 1. In both protocols patients and controls were well matched with regards to age, sex, body mass index, and medication use, except for body mass index in protocol 2, which was lower in the controls (OSA vs. controls 34.3 ± 1.3 vs. 29.7 ± 1.6 kg/m2; P = 0.04). The experimental protocol was approved by the Institutional Review Board, and written informed consent was obtained. The studies were conducted in the General Clinical Research Center at the Milton S. Hershey Medical Center during the daytime hours. The studies were performed on awake subjects in the supine position at a room temperature of 21–24°C.
Table 1.
Subject characteristics
| n | Men/Women | Age, yr | Body Mass Index, kg/m2 | Apnea-Hypopnea Index, Events/h | SaO2 Nadir, % | |
|---|---|---|---|---|---|---|
| OSA | 20 | 17/3 | 47.6 ± 2.3 | 34.0 ± 1.2* | 43.2 ± 6.2* | 76.5 ± 2.6† |
| Controls | 15 | 11/4 | 42.3 ± 3.0 | 28.9 ± 1.2 | 2.6 ± 0.4 |
Data are means ± SE; n = no. of subjects. OSA, obstructive sleep apnea; SaO2 nadir, minimum oxyhemoglobin saturation.
P < 0.05, OSA compared with controls.
n = 18: data unavailable from 2 subjects who underwent protocol 1.
Measurements and Procedures
Blood pressure and heart rate.
Mean arterial pressure (MAP) was monitored beat-by-beat with a finger photoplethysmographic device (Finapres, model 2350; Ohmeda, Englewood, CO) (36) and was verified with an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL). Heart rate was monitored by two-lead electrocardiogram.
Oxygen saturation, minute ventilation, end-tidal CO2.
Oxyhemoglobin saturation (SaO2) was measured with an ear oximeter (Biox pulse oximeter, model 3740; Ohmeda, Boulder, CO). To monitor minute ventilation and end-tidal CO2, a tight-sealing face mask with separate inlet and outlet valves for inspired and expired air was placed and was connected to a respiratory gas monitor (Ohmeda RGM 5250; Ohmeda, Louisville, CO). The inspiratory line was connected to a reservoir bag containing the experimental gas mixture. Chest movements were monitored via a strain-gauge pneumograph.
Femoral and brachial arterial blood flow and vascular conductance.
Femoral or brachial artery (forearm) blood flow was determined by Doppler ultrasound (ATL 5000; Philips Medical Systems, Bothell, WA). A 5- to 12-MHz linear-array transducer was placed over the femoral artery (∼2 cm below the inguinal ligament) or the brachial artery (∼10 cm above the antecubital fossa). Mean blood velocity (MBV, cm/s) was measured at an insonation angle of ≤60° with the sample volume approximately equal to the size of the artery (32). Vessel diameter was measured at end diastole in a longitudinal view as the distance between the near- and far-wall intima-media interfaces, and cross-sectional area was calculated as πr2 (cm2), where r is vessel radius (cm). Blood flow (ml/min) was calculated as Q = πr2 × MBV × 60. Vascular conductance (C, ml·min−1·mmHg−1) was calculated as C = Q/MAP.
Skin blood flow.
To assess the contribution of skin blood flow to the total limb blood flow responses to the experimental interventions, skin blood flow was determined with a laser-Doppler diode (Laserflo BPM, Vasomedics, St. Paul, MN) positioned over the volar surface of the forearm and was expressed in arbitrary units. Skin vascular conductance was calculated as skin blood flow/MAP.
Microneurography.
Peroneal microneurography was used to determine muscle sympathetic nerve activity (MSNA) as described previously (25). The microneurographic recordings were made with tungsten microelectrodes that were inserted percutanously into the peroneal nerve to record from sympathetic efferent fibers innervating skeletal muscle. The filtered, amplified, rectified, and integrated nerve traffic signal was digitally stored on a computer (PowerLab, ADInstruments) at a sampling frequency of 400 Hz. MSNA was expressed as burst incidence (bursts/min) and units per minute (average burst amplitude × bursts/min).
Experimental Procedures
Protocol 1.
Following instrumentation and obtaining a suitable MSNA site, the facemask was positioned securely and checked for leaks. After acclimatization, the Doppler probe was placed over the femoral artery of the opposite leg and measurements of hemodynamic and ventilatory parameters, MSNA, and femoral blood flow were made over ∼5 min. The facemask was then connected to a hypoxic gas mixture for 5 min [fraction of inspired oxygen FiO2 0.1], and hemodynamic and ventilatory parameters, MSNA and femoral blood flow were determined during the last 2 min of hypoxia. The subjects were then returned to room air.
Protocol 2.
A separate group of subjects was exposed to hypoxic stress before and following regional α-adrenergic block in the forearm with the nonspecific α-adrenoceptor antagonist phentolamine administered via Bier block technique as described previously (30). Following measurement of forearm volume by the water displacement technique, a catheter was placed retrogradely into a deep antecubital vein. Pneumatic cuffs (5 cm) were positioned at the wrist and just above the antecubital fossa. The facemask was then placed and brachial blood flow was determined by ultrasound as described above. Before each flow measurement, the wrist cuff was inflated to suprasystolic pressure (250 mmHg) to exclude blood flow to the hand, which predominantly consists of skin (23). Baseline measurements of brachial blood flow and ventilatory and hemodynamic parameters were obtained over ∼5 min. Hypoxia was then induced as described above and maintained for 10 min. Hemodynamic and blood flow measurements were made at minutes 4–5 and 9–10 of hypoxia. The facemask was then disconnected and the subjects breathed room air.
For the Bier block, the forearm was elevated and exsanguinated with a compressing bandage, and the upper arm cuff was inflated to 250 mmHg to occlude forearm blood flow. The bandage was removed, and phentolamine mesylate (0.12 mg/100 ml of forearm volume, dissolved in 40 ml of saline) was instilled into the venous cannula and allowed to diffuse into the forearm tissue while the forearm was kept ischemic for 20 min (30). The arm cuff was then released and following a 10-min recovery period and reestablishment of basal blood flow, the hypoxia exposure was repeated as described above. We demonstrated previously that this procedure markedly attenuates sympathetic vasoconstriction in the forearm (30). While the period of forearm ischemia is accompanied by moderate discomfort, this resolves promptly on restoration of blood flow.
Statistical Analysis
Comparisons of baseline characteristics, MSNA, blood flow, and vascular conductance between OSA and controls were made with the nonpaired t-test. Two-way repeated-measures ANOVA was used to determine effects of the interventions. Post hoc tests for simple effects were performed to compare OSA and control data at each time point. For multiple comparisons, P values were adjusted by the Bonferroni method (SAS version 6.12; SAS Institute, Cary, NC). The data were expressed as means ± SE. Statistical significance was defined as P < 0.05.
RESULTS
Protocol 1: Effects of Systemic Hypoxia (FiO2 0.1; 5 min) on Neurocirculatory Parameters in OSA (n = 10) and Controls (n = 8)
Hypoxia was associated with similar and statistically significant (P < 0.05) increases in heart rate (OSA: from 64 ± 3 to 75 ± 5 beats/min; controls: from 66 ± 3 to 78 ± 3 beats/min), minute ventilation (OSA: from 8.5 ± 0.8 to 11.4 ± 0.9 l/min; controls: from 8.0 ± 0.4 to 10.0 ± 0.5 l/min), and decreases of end-tidal CO2 (OSA: from 39 ± 1 to 36 ± 1 mmHg; controls: from 39 ± 1 to 36 ± 1 mmHg) and SaO2 (OSA: from 96 ± 1 to 84 ± 2%; controls: from 97 ± 1 to 83 ± 1%) while MAP did not change significantly (OSA: from 104 ± 3 to 107 ± 5 mmHg; controls: from 102 ± 5 to 106 ± 5 mmHg).
The effects of systemic hypoxia on MSNA and on leg blood flow and leg vascular conductance are shown in Table 2. Compared with normoxia, during hypoxia MSNA increased in both groups, and for bursts per minute this increase appeared to be more pronounced in OSA. Although mean leg blood flow and leg vascular conductance at baseline and during hypoxia were higher in OSA than in controls, these differences did not reach statistical significance.
Table 2.
Effects of systemic hypoxia (FiO2 0.1; 5 min) on MSNA, leg blood flow, and leg vascular conductance in patients with OSA (n = 10) and controls (n = 8)
| Baseline | Hypoxia | Statistics | |
|---|---|---|---|
| MSNA, bursts/min | |||
| OSA | 27.3 ± 2.9 | 35.7 ± 3.4 | Group 0.062; paradigm 0.001; interaction 0.018 |
| Control | 19.0 ± 4.2 | 22.3 ± 5.1 | |
| MSNA, units/min | |||
| OSA | 91 ± 13 | 112 ± 17 | Group 0.133; paradigm 0.002; interaction 0.163 |
| Control | 65 ± 13 | 79 ± 15 | |
| Leg blood flow, ml/min | |||
| OSA | 380 ± 87 | 419 ± 69 | Group 0.234; paradigm 0.040; interaction 0.568 |
| Control | 251 ± 26 | 318 ± 53 | |
| Leg vascular conductance, ml · min−1 · mmHg−1 | |||
| OSA | 3.65 ± 0.81 | 3.91 ± 0.61 | Group 0.293; paradigm 0.103; interaction 0.515 |
| Control | 2.52 ± 0.34 | 3.10 ± 0.61 |
Data are means ± SE. MSNA, muscle sympathetic nerve activity; FiO2, fraction of inspired oxygen.
Protocol 2: Effects of Systemic Hypoxia (FiO2 0.1; 10 min) on Brachial Artery Blood Flow and Forearm Vascular Conductance Before and Following Regional α-Adrenergic Block with Phentolamine in OSA (n = 10) and Controls (n = 10)
The effects of hypoxia on MAP, heart rate, minute ventilation, end-tidal CO2 and SaO2 are shown in Table 3. Similar to protocol 1, during hypoxia before and after phentolamine, both groups exhibited increased heart rate and minute ventilation, while end-tidal CO2 and SaO2 decreased and MAP did not change. However, following phentolamine, MAP increased in both OSA and controls.
Table 3.
Effects of systemic hypoxia (FiO2 0.1) on hemodynamic and ventilatory parameters in OSA and controls before and after regional α-adrenergic block with phentolamine
| OSA |
Controls |
|||
|---|---|---|---|---|
| Pre-Phentolamine | Post-Phentolamine | Pre-Phentolamine | Post-Phentolamine | |
| MAP, mmHg (n = 10) | ||||
| Baseline | 105 ± 3 | 113 ± 3† | 107 ± 4 | 112 ± 5† |
| Hypoxia (5 min) | 107 ± 3 | 115 ± 2† | 106 ± 5 | 112 ± 4† |
| Hypoxia (10 min) | 108 ± 3 | 113 ± 4 | 106 ± 4 | 109 ± 5 |
| Heart rate, beats/min (n = 10) | ||||
| Baseline | 71 ± 4 | 70 ± 4 | 64 ± 4 | 64 ± 4 |
| Hypoxia (5 min) | 83 ± 5* | 81 ± 5* | 74 ± 4* | 75 ± 4* |
| Hypoxia (10 min) | 81 ± 5* | 84 ± 4* | 76 ± 4* | 77 ± 4* |
| Minute ventilation, l/min (n = 8) | ||||
| Baseline | 7.3 ± 0.8 | 8.0 ± 0.9 | 7.4 ± 0.4 | 7.9 ± 0.4 |
| Hypoxia (5 min) | 10.5 ± 0.8* | 10.5 ± 0.8* | 10.5 ± 0.9* | 10.1 ± 0.5* |
| Hypoxia (10 min) | 12.9 ± 1.8* | 11.3 ± 0.9* | 9.8 ± 0.5* | 10.6 ± 0.7* |
| End-tidal CO2, mmHg (n = 8) | ||||
| Baseline | 41 ± 1 | 39 ± 1 | 40 ± 2 | 39 ± 1 |
| Hypoxia (5 min) | 36 ± 1* | 35 ± 1* | 37 ± 2* | 35 ± 2* |
| Hypoxia (10 min) | 35 ± 1* | 33 ± 1* | 35 ± 1* | 34 ± 2* |
| SaO2, % (n = 8) | ||||
| Baseline | 97 ± 0.4 | 97 ± 0.4 | 97 ± 0.4 | 98 ± 0.3 |
| Hypoxia (5 min) | 84 ± 2* | 85 ± 1* | 85 ± 1* | 86 ± 1* |
| Hypoxia (10 min) | 80 ± 3* | 80 ± 2* | 76 ± 2* | 78 ± 4* |
Data are means ± SE. MAP, mean arterial pressure.
P < 0.05, hypoxia compared with baseline.
P < 0.05, post- compared with pre-phentolamine.
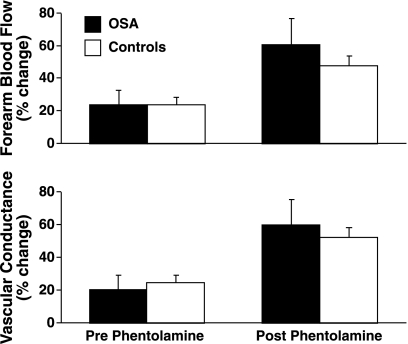
The effects of systemic hypoxia on forearm blood flow and forearm vascular conductance are shown in Table 4 and Fig. 1. Baseline forearm blood flow (P = 0.09) and vascular conductance (P = 0.06) tended to be higher in OSA than controls but this was less apparent when blood flow was normalized for forearm volume (P = 0.23 and 0.16, respectively). In both groups, before phentolamine, hypoxia was associated with statistically significant increases of forearm blood flow and vascular conductance. Furthermore, in both groups, following phentolamine, the forearm blood flow and conductance responses to hypoxia were markedly enhanced. However, the percent increase from baseline at 5 and 10 min of hypoxia in OSA was not significantly higher than in controls.
Table 4.
Effects of systemic hypoxia (FiO2 0.1) on forearm blood flow and vascular conductance before and after regional α-adrenergic block with phentolamine in patients with OSA and controls
| OSA (n = 10) |
Controls (n = 10) |
|||
|---|---|---|---|---|
| Pre-Phentolamine | Post-Phentolamine | Pre-Phentolamine | Post-Phentolamine | |
| Forearm blood flow, ml/min | ||||
| Baseline | 66 ± 6 | 129 ± 22† | 49 ± 6 | 93 ± 15† |
| Hypoxia (5 min) | 89 ± 12* | 180 ± 29*† | 57 ± 7* | 130 ± 28*† |
| Hypoxia (10 min) | 84 ± 13* | 201 ± 34*† | 62 ± 8* | 140 ± 26*† |
| Forearm vascular conductance, ml · min−1 · mmHg−1 | ||||
| Baseline | 0.632 ± 0.065 | 1.146 ± 0.195† | 0.463 ± 0.053 | 0.845 ± 0.141† |
| Hypoxia (5 min) | 0.850 ± 0.127* | 1.579 ± 0.255*† | 0.540 ± 0.062* | 1.160 ± 0.234*† |
| Hypoxia (10 min) | 0.782 ± 0.121* | 1.776 ± 0.283*† | 0.580 ± 0.071* | 1.306 ± 0.242*† |
Data are means ± SE.
P < 0.05, hypoxia compared with baseline.
P < 0.05, post- compared with pre-phentolamine.
Fig. 1.
The responses (expressed as percent change from baseline) of forearm blood flow and vascular conductance to acute systemic hypoxia [fraction of inspired oxygen (FiO2) 0.1; 10 min] before and following regional α-adrenergic block with phentolamine in patients with obstructive sleep apnea (OSA) and in controls. The differences between the groups were not significant.
Following regional α-adrenergic block, in both groups forearm skin blood flow and vascular conductance increased mildly but the differences did not reach statistical significance [P = not significant (NS)]. However, in OSA and controls, before and following regional α-adrenergic block, hypoxia had no effect on skin blood flow and vascular conductance (data not shown).
DISCUSSION
The principal new findings in this study are, first, that despite increased basal sympathetic nerve activity and sympathetic chemoreflex activity, limb vasodilation in response to acute hypoxia is preserved in OSA compared with controls. Second, in OSA and controls alike, regional α-adrenergic block enhances hypoxia-induced vasodilation in the forearm by a similar degree. Taken together, these findings suggest that during acute hypoxia in awake patients with OSA, neural vasoconstrictor activity is offset by enhanced metabolic vasodilator activation.
Several prior reports demonstrated that sympathetic nerve activity is chronically elevated in OSA compared with nonapneic controls (5, 25, 44). It has been postulated that this is due to a carryover effect of intermittent hypoxia that results in resetting of the arterial baroreflex (6, 29) and/or altered activity of the arterial chemoreflex (13, 27, 31). Several other reports suggest that vascular function is impaired in OSA. Carlson et al. (7) and Kato et al. (17) reported attenuated forearm blood flow responses to acetylcholine in OSA, a characteristic feature of endothelial dysfunction. Reactive hyperemia, an index of maximal vasodilator capacity, was reduced in OSA, and improved following continuous positive-airway pressure therapy (12). Similarly, OSA was found to be associated with decreased flow-mediated dilation of the brachial artery, a biomarker of endothelial dysfunction, as a function of the apnea-hypopnea index (15, 33). Potential explanations for vascular dysfunction in OSA are downregulation of nitric oxide (NO), or, alternatively, enhanced vasoconstrictor tone. Indeed, decreased circulating NO (14, 42), increased levels of the endogenous NO synthase inhibitor asymmetric NG-dimethylarginine (ADMA) (35), the vasoconstrictors endothelin-1 (37) and thromboxane A2 (20), activation of the renin-angiotensin-aldosterone system (1), and increased vascular oxidative stress (21) have been reported in OSA, all of which could contribute to impaired responses to vasodilator stimuli. In view of increased sympathetic nerve activity and vascular dysfunction, one might therefore expect that the capacity of skeletal muscle arterioles to vasodilate in response to hypoxia is reduced in OSA.
Blood flow and vascular conductance in the leg and forearm during hypoxia tended to be higher in OSA than in controls although, possibly due to the limited sample size, these differences did not reach statistical significance. Importantly, in contrast to our hypothesis, our data suggest that during moderate systemic hypoxia limb vasodilation is preserved in OSA and in the forearm is at least equal to that reported previously in acutely hypoxic healthy young subjects (24, 30, 47). Because we compared relative rather than absolute changes of blood flow and vascular conductance during hypoxia, it is very unlikely that this conservative analytical approach led us to overestimate the vasodilator response to hypoxia in OSA. Indeed, when expressed as absolute changes of forearm blood flow and vascular conductance, the vasodilator responses to hypoxia were slightly larger in OSA than controls [OSA vs. controls: +23 ± 7 vs. +8 ± 2 ml/min, and +18 ± 7 vs. +12 ± 3 ml/min at 5 and 10 min, respectively (P = 0.11 and P > 0.45, respectively), and +0.219 ± 0.078 vs. +0.077 ± 0.017 ml·min−1·mmHg−1 and +0.151 ± 0.073 vs. +0.116 ± 0.024 ml·min−1·mmHg−1 at 5 and 10 min, respectively (P = 0.18 and P > 0.65, respectively)] but these differences did not reach statistical significance. Thus it is conceivable that a larger study sample might suggest enhanced vasodilator responses to hypoxia in OSA. The lack of skin vasodilation in OSA and controls suggests that the hypoxia-induced increase in limb blood flow is primarily directed to skeletal muscle. Although the level of hypoxia was less severe in protocol 1, the simultaneous sympathetic nerve and blood flow data from the leg suggest that the forearm vasodilation during hypoxia in protocol 2 occurred despite an increase in sympathetic nerve activity.
Our finding of preserved hypoxia-induced skeletal muscle vasodilation in OSA is at variance with a report of reduced vasodilation during hypoxia in some but not all patients with OSA (40). The discrepancy between those data and ours likely relates to methodological differences and subject selection, including a shorter duration of hypoxia (3–4 min), maintenance of isocapnia, the younger age and smaller body mass of the controls, and the method employed to measure blood flow (plethysmography vs. ultrasound) in the study by Remsburg et al. (40).
Because in healthy humans and in patients with OSA, hypoxia activates sympathetic vasoconstrictor nerves (13, 24), the vasodilator signal evoked by hypoxia in skeletal muscle is thought to be metabolic in origin. In healthy humans, adenosine (26), and NO (3) have been shown to play important roles, and in animal models other factors, including prostacyclin (28), and endothelium-derived hyperpolarizing factors (EDHF) (46), may also contribute to hypoxia-induced vasodilation.
In accordance with prior studies, regional administration (by Bier block) of the α-adrenoceptor antagonist phentolamine unmasked substantial vasodilation during hypoxia, suggesting that sympathetic vasoconstrictor tone markedly restrains the increase in limb blood flow during hypoxic stress (30, 47). Consistent with regional but not systemic α-adrenergic block, regional administration of phentolamine increased basal blood flow approximately twofold and did not lower blood pressure (30). This effect was similar to that achieved by regional α-adrenergic block produced by intra-arterial infusion of α-adrenoceptor antagonists (47), regional (Bier block) administration of the sympatholytic agent bretylium tosylate (22), or regional anesthesia (16).
During regional α-adrenergic block, in OSA we found substantial increases in blood flow during hypoxia that were at least equal to those seen in controls. Thus substantial sympathetic restraint of hypoxia-mediated vasodilation was present in OSA and controls. Because blood pressure does not drop during hypoxia in OSA, the increase in systemic vascular conductance appears to be fully matched by the increase in blood flow.
The finding of preserved vasodilation in response to hypoxia would seem to contradict earlier reports of decreased reactive hyperemia (12) and reduced flow-mediated vasodilation in OSA (15, 33). However, the vasodilator stimuli of limb ischemia and systemic hypoxia differ in important ways. Whereas systemic hypoxia may primarily activate metabolic vasodilator mechanisms, reactive hyperemia represents in part a myogenic phenomenon (8), and flow-mediated vasodilation may in large part reflect NO bioavailability (15, 33). The sharp increase in basal forearm blood flow following phentolamine, and the pronounced augmentation of the responses to hypoxia following phentolamine in OSA argue against attenuated vascular responsiveness to sympathetic tone in OSA. Prior studies on vasoconstrictor responsiveness in OSA are conflicting. In one report the forearm vascular response to ANG II infusion was enhanced (19) while findings from another study suggested functional downregulation of vascular α- and β-receptors in OSA (10).
A number of reports are consistent with the notion that metabolic vasodilator pathways are altered in OSA. NO bioavailability is reduced in OSA (14, 42) possibly due to intermittent hypoxia resulting in oxidative stress (14, 38, 42), and elevated C-reactive protein (43) may decrease NO bioavailability through decreased expression of endothelial NO synthase (45). Preserved hypoxia-mediated vasodilation despite downregulated NO expression and decreased flow-mediated brachial reactivity (15, 33) raises the possibility that NO-independent vasodilation could be upregulated in OSA. Indeed, the ratio of vasodilator to vasoconstrictor prostaglandins was found to be increased in OSA and normalized after continuous positive-airway pressure therapy (18). Furthermore, hypoxia may increase endothelial prostaglandin production, possibly through enhanced release of adenosine (39). Alternative adaptive mechanisms induced by hypoxia in OSA might include increased production of vascular endothelial growth factor (41). Last, EDHF could contribute to the preserved hypoxia-mediated vasodilation in OSA. In support of this possibility, animal models have shown EDHF upregulation in NO-deficient states (2) and, conversely, an inhibitory effect of NO on EDHF has been reported (34). Whether EDHF is upregulated in OSA remains to be determined.
Several limitations of our study need to be acknowledged. Despite our efforts, on average our control subjects were less overweight and slightly younger than the patients with OSA. However, because the vasodilator responses to hypoxia were not reduced in OSA, differences in age and body mass between the two groups cannot account for the lack of a difference in the vascular responses to hypoxia. Another issue is that in both protocols, some subjects were on vasoactive drugs. However, because this affected the OSA and control groups in both protocols similarly, and because the individual responses of these subjects varied similarly around the mean values in each group, we believe it is highly unlikely to have influenced our conclusions. In addition, in OSA and controls alike, β-adrenoceptor stimulation could have contributed to the vasodilation noted during hypoxia. Whether this mechanism would have differential effects in the two groups, some of whom were on beta-blocker therapy, is not known. Because we measured total limb and skin blood flow, our data do not allow us to rule out the possibility that blood flow was preserved in OSA secondary to flow redistribution within skeletal muscle. Last, we should acknowledge that our protocols did not model physiological events that occur during obstructive apnea during sleep. Instead, our intent was to examine whether the frequent nocturnal episodes of hypoxia that occur in OSA, and are thought to raise sympathetic nerve activity and chemoreflex sensitivity, over time might alter the vascular responses to hypoxic stress.
In conclusion, our data demonstrate that despite high basal sympathetic vasoconstrictor tone and prominent neural responses to acute hypoxia, hypoxia-induced skeletal muscle vasodilation is preserved during wakefulness in sleep apnea. Because regional α-adrenergic block enhanced the vasodilator effect of hypoxia similarly in OSA and controls, we postulate that metabolic vasodilator mechanisms are upregulated in OSA. The precise nature of this effect remains unclear and will require further investigation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants P01-HL-077670 and R01-HL-068699, National Center for Research Resources Grant M01-RR-010732 and C06-RR-016499, and Pennsylvania Tobacco Settlement Funds-Pennsylvania State College of Medicine.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the authors.
ACKNOWLEDGMENTS
We express gratitude to our study subjects, the nursing staff in the General Clinical Research Center, Stephen Gugoff for technical assistance, and Jennifer Stoner for excellent administrative help. We are grateful to Allen Kunselman for assistance with the statistical analyses.
REFERENCES
- 1. Barceló A, Elorza MA, Barbé F, Santos C, Mayoralas LR, Agusti AG. Angiotensin converting enzyme in patients with sleep apnoea syndrome: plasma activity and gene polymorphisms. Eur Respir J 17: 728–732, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Blitzer ML, Lee SD, Creager MA. Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol 271: H1182–H1185, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bokinsky G, Miller M, Ault K, Husband P, Mitchell J. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. Chest 108: 625–630, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154: 1490–1496, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Carlson JT, Rångemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens 14: 577–584, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Carlsson I, Sollevi I, Wennmalm A. The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperemia. J Physiol 389: 147–161, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chin K, Ohi M, Kita H, Noguchi T, Otsuka N, Tsuboi T, Mishima M, Kuno K. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 153: 1972–1976, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Grote L, Kraiczi H, Hedner J. Reduced α- and β2-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med 162: 1480–1487, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE. A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis 146: 1240–1245, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Imadojemu VA, Gleeson K, Quraishi S, Kunselman AR, Sinoway LI, Leuenberger UA. Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure therapy. Am J Respir Crit Care Med 165: 950–953, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131: 1406–1413, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, Lam WK. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med 162: 2166–2171, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169: 348–353, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol 263: H1078–H1083, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 102: 2607–2610, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kimura H, Niijima M, Abe Y, Edo H, Sakabe H, Kojima A, Hasako K, Masuyama S, Tatsumi K, Kuriyama T. Compensatory excretion of prostacyclin and thromboxane metabolites in obstructive sleep apnea syndrome. Intern Med 37: 127–133, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Kraiczi H, Hedner J, Peker Y, Carlson J. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol 89: 493–498, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Krieger J, Benzoni D, Sforza E, Sassard J. Urinary excretion of prostanoids during sleep in obstructive sleep apnoea patients. Clin Exp Pharmacol Physiol 18: 551–555, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lee F, Shoemaker JK, McQuillan PM, Kunselman AR, Smith MB, Yang QX, Smith H, Gray K, Sinoway LI. Effects of forearm Bier block with bretylium on the hemodynamic and metabolic responses to handgrip. Am J Physiol Heart Circ Physiol 279: H586–H593, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Lenders J, Janssen GJ, Smits P, Thien T. Role of the wrist cuff in forearm plethysmography. Clin Sci 80: 413–417, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol Heart Circ Physiol 261: H1659–H1664, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79: 581–588, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol 87: 2218–2224, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Leuenberger UA, Hogeman CS, Quraishi S, Linton-Frazier L, Gray KS. Short-term intermittent hypoxia enhances sympathetic responses to continuous hypoxia in humans. J Appl Physiol 103: 835–842, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Messina EJ, Sun D, Koller A, Wolin MS, Kaley G. Role of endothelium-derived prostaglandins in hypoxia-elicited arteriolar dilation in rat skeletal muscle. Circ Res 71: 790–796, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 574: 605–613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moradkhan R, McQuillan P, Hogeman C, Leuenberger A, Linton-Frazier L, Leuenberger UA. Metabolic forearm vasodilation is enhanced following Bier block with phentolamine. Am J Physiol Heart Circ Physiol 293: H2289–H2295, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99: 1183–1189, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 556: 1001–1011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med 169: 354–360, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol 279: H459–H465, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Ohike Y, Kozaki K, Iijima K, Eto M, Kojima T, Ohga E, Santa T, Imai K, Hashimoto M, Yoshizumi M, Ouchi Y. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure—possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ J 69: 221–226, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens 17: 61–66, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol 96: 359–366, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544: 195–209, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Remsburg S, Launois SH, Weiss JW. Patients with obstructive sleep apnea have an abnormal peripheral vascular response to hypoxia. J Appl Physiol 87: 1148–1153, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med 165: 67–70, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Schulz R, Schmidt D, Blum A, Lopes-Ribeiro X, Lucke C, Mayer K, Olschewski H, Seeger W, Grimminger F. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax 55: 1046–1051, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105: 2462–2464, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 106: 1439–1441, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int 70: 840–853, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]